Simple Model for Alkali Leaching from Geopolymers: Effects of Raw Materials and Acetic Acid Concentration on Apparent Diffusion Coefficient
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Geopolymer Specimen Preparation and Basic Properties
2.3. Leaching (in Acid) Experimental Setup
2.4. Mathematical Modeling
- CFLn is the cumulative fraction of species leached for a period n including all individual fractions i = 1 to n;
- IFLn is the incremental fraction of species leached during test interval n;
- ci is the concentration of the species in eluate for the incremental test interval n in mg units (related to the leaching test setup, i.e., the leaching solution volume);
- C0 is the (total) concentration of the species in the specimen at the beginning of the test, in mg units (per specimen).
- t is the duration of exposure in s;
- S is the surface area of the specimen in cm2;
- V is the specimen volume in cm3.
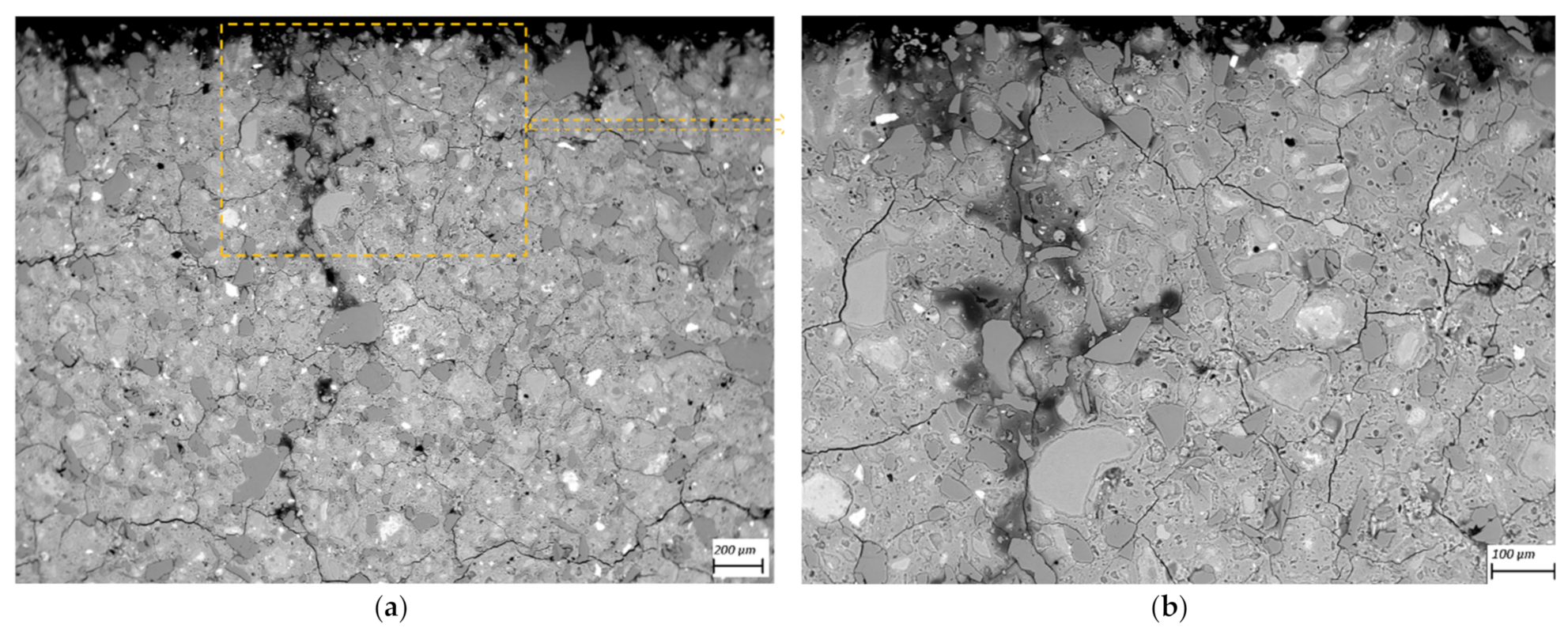
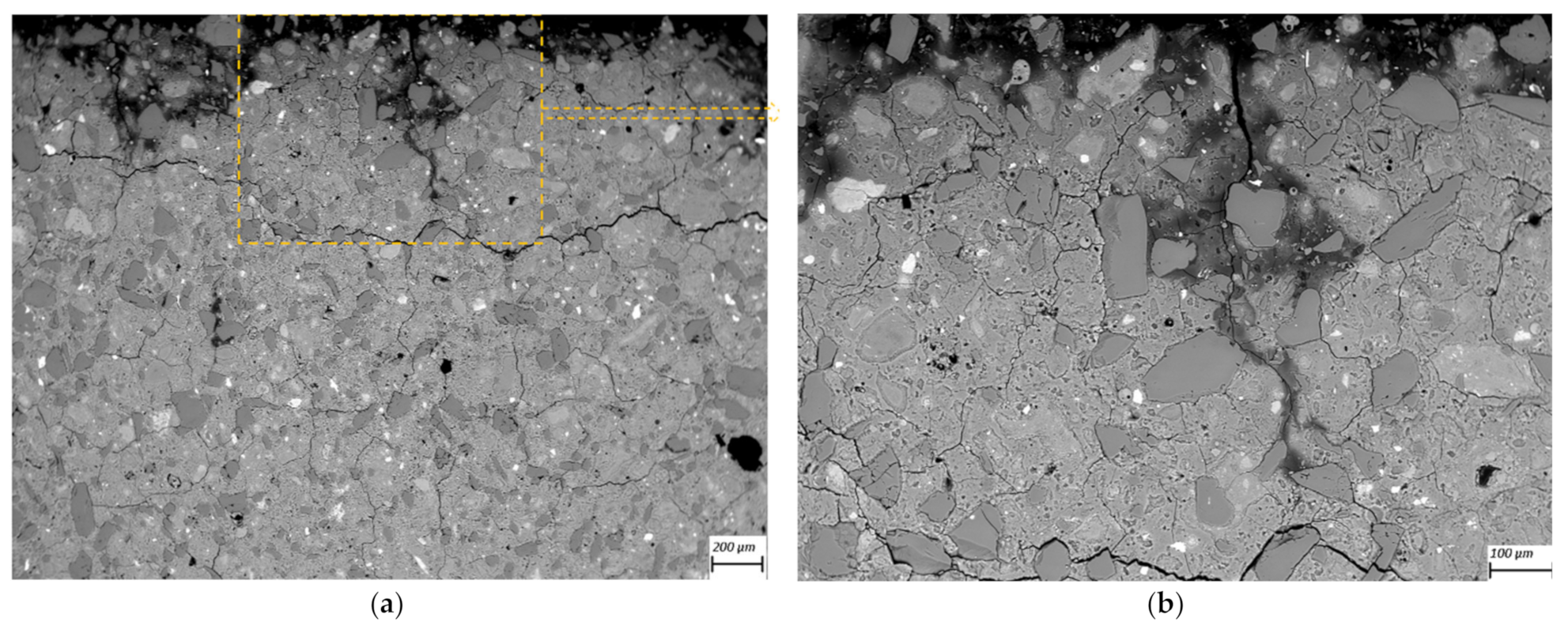
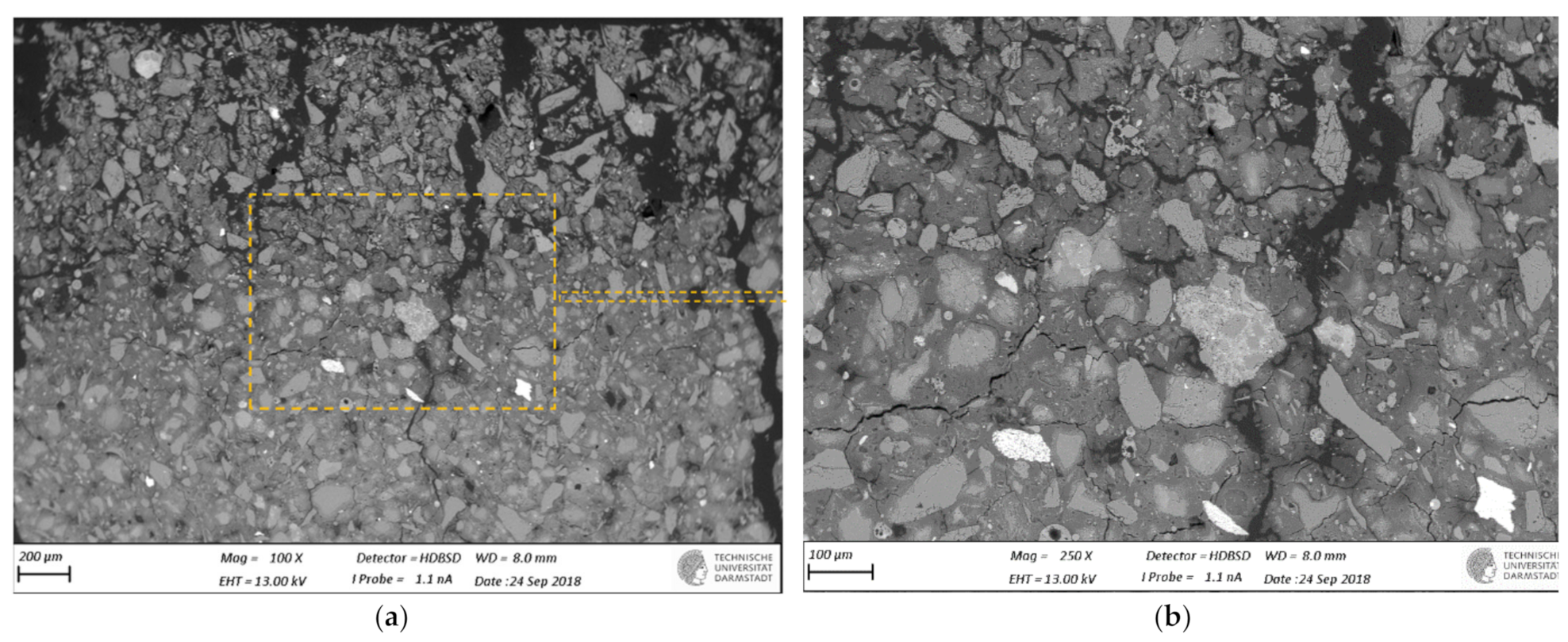
2.5. SEM-BSE Microscopy on Polished Cross-Sections
3. Results
3.1. Basic Properties of Geopolymer Pastes
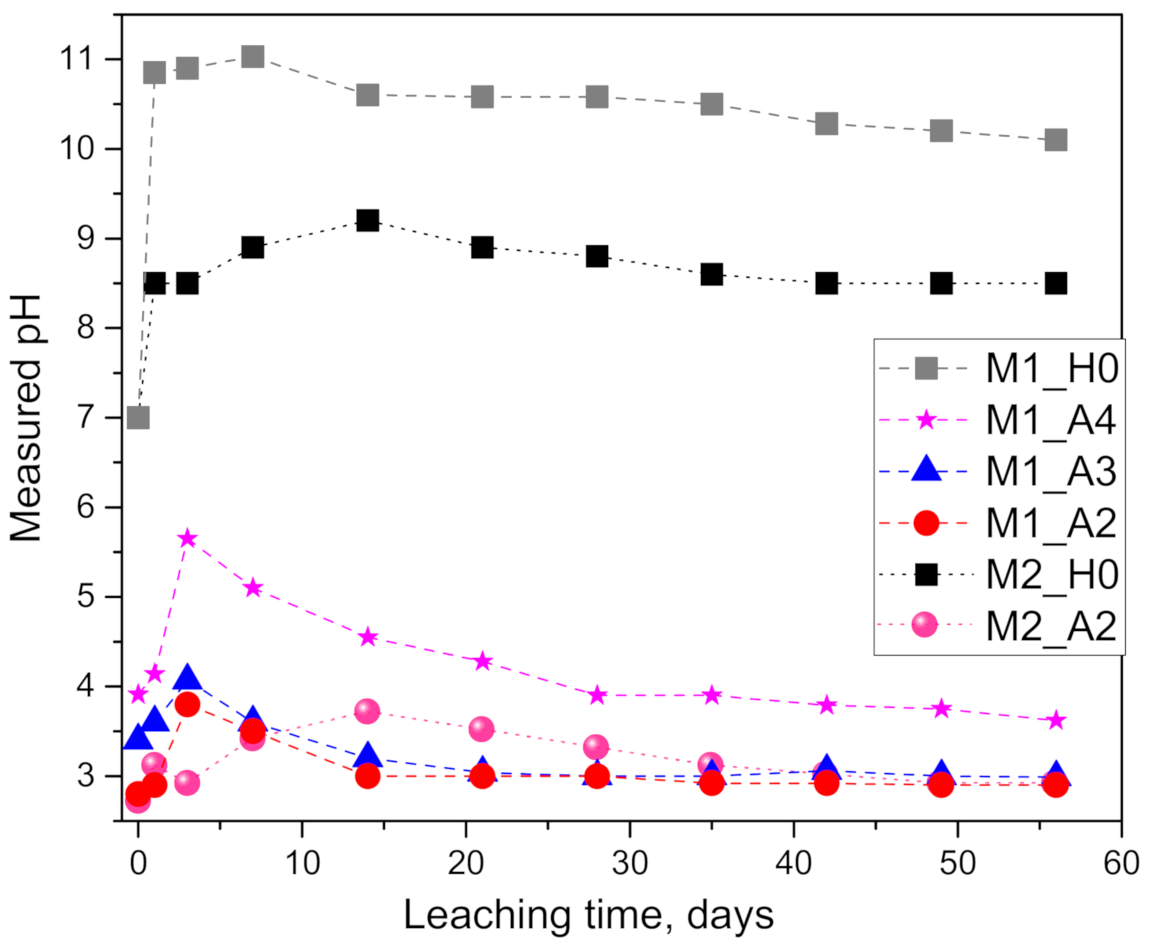
3.2. pH and Alkali Leaching
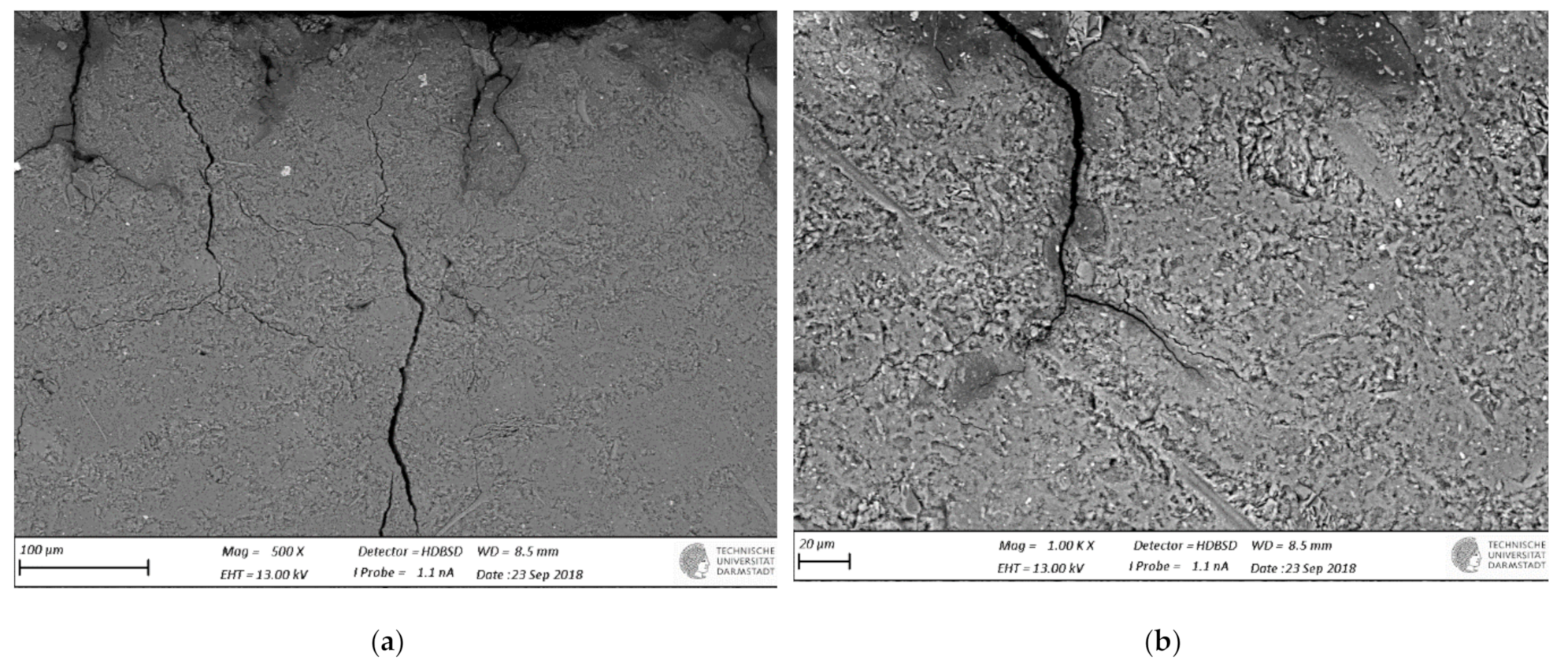
3.3. SEM-BSE Microscopy
4. Discussion
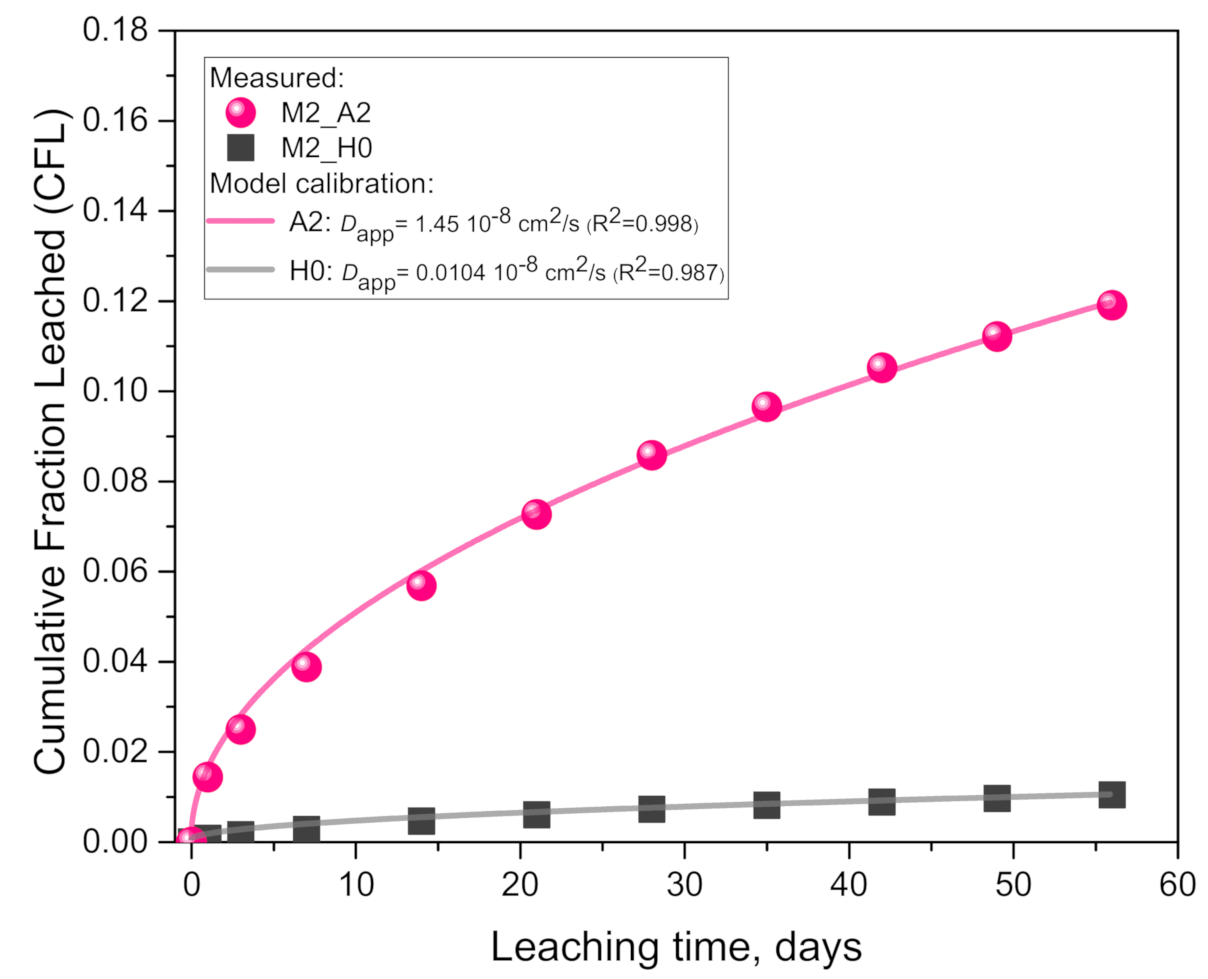
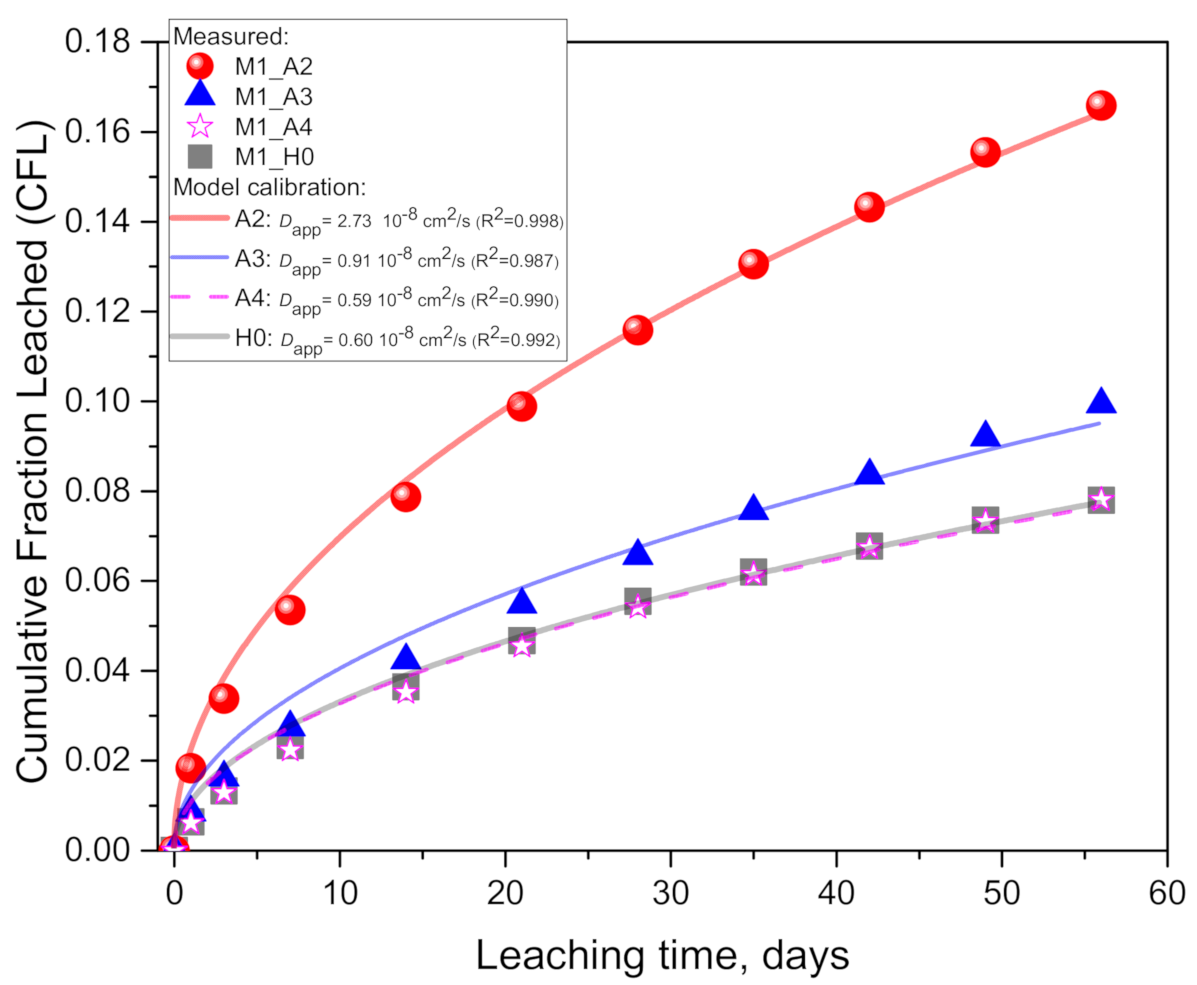
4.1. Experimentally Supported Modeling Approach
4.2. Geopolymer Pastes M1 vs. M2 and Effect of Acid Concentration
- Ion exchange reaction between the cation X+ and the charge-compensating cations (Na+ or K+) of the geopolymer framework:
[Si–O–Al…]−X+ + Si(OH)4(aq) + K(H2O)n+
- Partial dealumination dissolution of the aluminosilicate framework;
- Precipitation of acid anion salts (which, here in the acetic case, are highly soluble);
- Crystallization of (Na-based) zeolites causing a decrease in material strength;
- Dissolution and re-crystallization of the Si-rich aluminosilicate framework.
4.3. Mortars vs. Pastes and Fly Ash- vs. Metakaolin-Based Geopolymers
5. Conclusions
- Geopolymer paste M2 (pure metakaolin-based) exhibited an order of magnitude (1041%) higher alkali leaching in 0.1 molar acetic acid than in pure water, while the calibrated Dapp was 139 times higher.
- In the M1 (quartz-rich metakaolin-based) geopolymer paste, the potassium leaching in acetic acid increased by 113% (100 mM acid), 27.7% (10 mM) and 0% (1 mM) relative to the pure water case. The corresponding Dapp for the acid case was 4.5 (100 mM) and 1.51 (10 mM) times higher than in the pure water case while having no significant difference for the 1 mM case.
- Geopolymers based on less pure metakaolin M1 resulted in 23.8% (in 100 mM acid) and as much as 564.3% (in pure water) higher alkali leaching compared to the (pure) M2 case. The Dapp value for the M1 geopolymer is 1.9 (in 100 mM acid) and 58.1 (in pure water) times higher than for M2.
- SEM-BSD microscopy revealed occurrence of degradation and dissolution zones across the outermost surface depths, the estimated average thicknesses being for the M2 case 1 mm (in 100 mM acid) and 20 μm (water), while 100 μm (water and 1mM), 200 μm (10 mM) and 1.2 mm (100 mM) in the M1 case. Especially under acid attack, geopolymer surface layers exhibited long cracks that connect the top surface layer with the sound inner zone.
- Lower leaching rates in (pure) M2 compared to (quartz-rich) M1 were discussed in terms of the differences in the mix designs and (mechanical, porosity and chemical) properties of the geopolymers. Here, the major importance of the alkali-binding capacity on Dapp overruled the effect of increased porosity for M2 compared to M1 on the leaching rates. This also explains why the observed cracks and increased porosity due to matrix dissolution had no effect on the time dependency of Dapp, which led to the conclusion that the Dapp is being governed by the chemistry (of the cation exchange release of bonded alkalis, Equation (5)) and not by Deff (porosity and pore morphology).
- The diffusion model can accurately represent the experimental data for both pastes and mortars, evaluated by rel. error (Equation (3) for a goodness of calibration), being much less than the critical value of 0.5% (according to [29]).
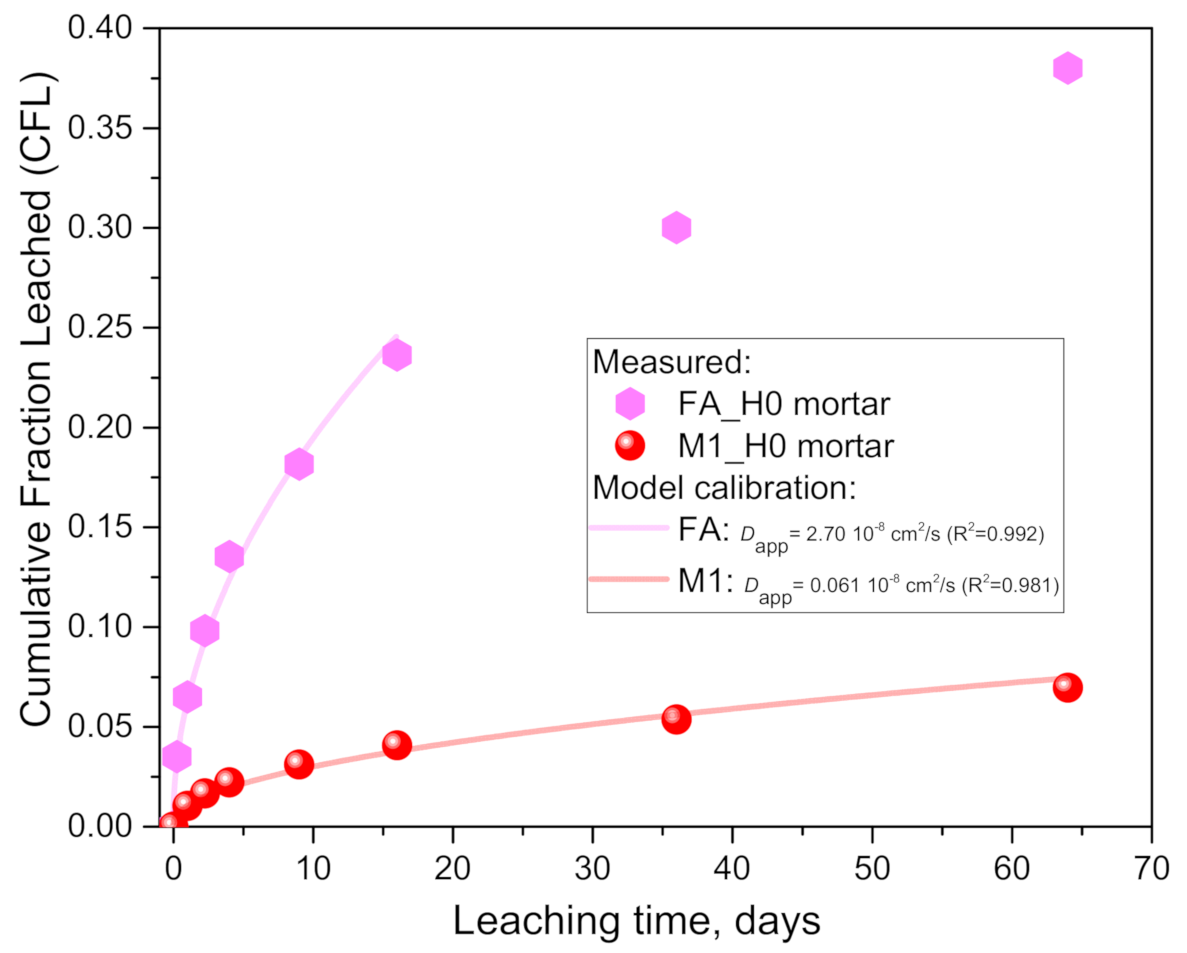
- Dapp for fly ash mortar was 44.6 times higher than for the M1 mortar, in agreement with conclusion point 5 that a lower geopolymerization reaction degree (of fly ash [20]) results in more free alkalis in the pore solution.
- The ten times higher Dapp for the leaching of the M1 paste than the mortar (in water) could be explained by a) the lower porosity due to the aggregate inclusion (pore tortuosity, etc.) and matrix dilution effects, and b) the higher alkali-binding capacity expected from the higher geopolymerization reaction degree (and Al/Si) due to the use of purer M1 metakaolin in the paste case (in agreement with conclusion points 5 and 8).
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pacheco-Torgal, F.; Labrincha, J.A.; Leonelli, C.; Palomo, A.; Chindaprasirt, P. Handbook of Alkali-activated Cements, Mortars and Concretes; Elsevier: Heidelberg, Germany, 2015; pp. 1–16. [Google Scholar]
- Provis, J.L.; Palomo, A.; Shi, C. Advances in understanding alkali-activated materials. Cem. Concr. Res. 2015, 78, 110–125. [Google Scholar] [CrossRef]
- Davidovits, J. Environmental implications of geopolymers. Mater. Today, 2015. Available online: https://www.materialstoday.com/polymers-soft-materials/features/environmental-implications-of-geopolymers/ (accessed on 15 March 2021).
- Koenig, A.; Herrmann, A.; Overmann, S.; Dehn, F. Resistance of alkali-activated binders to organic acid attack: Assessment of evaluation criteria and damage mechanisms. Constr. Build. Mater. 2017, 151, 405–413. [Google Scholar] [CrossRef]
- Ukrainczyk, N.; Muthu, M.; Vogt, O.; Koenders, E. Geopolymer, Calcium Aluminate, and Portland Cement-Based Mortars: Comparing Degradation Using Acetic Acid. Materials 2019, 12, 3115. [Google Scholar] [CrossRef]
- Aiken, T.A.; Kwasny, J.; Sha, W. Resistance of fly ash geopolymer binders to organic acids. Mater. Struct. 2020, 53, 1–18. [Google Scholar] [CrossRef]
- Ukrainczyk, N.; Vogt, O. Geopolymer leaching in water and acetic acid. RILEM Tech. Lett. 2021, 5, 163–173. [Google Scholar] [CrossRef]
- Gao, X.X.; Michaud, P.; Joussein, E.; Rossignol, S. Behavior of metakaolin-based potassium geopolymers in acidic solutions. J. Noncryst. Sol. 2013, 380, 95–102. [Google Scholar] [CrossRef]
- Bakharev, T. Resistance of geopolymer materials to acid attack. Cem. Concr. Res. 2005, 35, 658–670. [Google Scholar] [CrossRef]
- Sturm, P.; Gluth, G.; Jäger, C.; Brouwers, H.; Kühne, H.-C. Sulfuric acid resistance of one-part alkali-activated mortars. Cem. Concr. Res. 2018, 109, 54–63. [Google Scholar] [CrossRef]
- Grengg, C.; Ukrainczyk, N.; Koraimann, G.; Mueller, B.; Dietzel, M.; Mittermayr, F. Long-term in situ performance of geopolymer, calcium aluminate and Portland cement-based materials exposed to microbially induced acid corrosion. Cem. Concr. Res. 2020, 131, 106034. [Google Scholar] [CrossRef]
- Grengg, C.; Mittermayr, F.; Ukrainczyk, N.; Koraimann, G.; Kienesberger, S.; Dietzel, M. Advances in concrete materials for sewer systems affected by microbial induced concrete corrosion: A review. Water Res. 2018, 134, 341–352. [Google Scholar] [CrossRef]
- Drugă, B.; Ukrainczyk, N.; Weise, K.; Koenders, E.; Lackner, S. Interaction between wastewater microorganisms and geopolymer or cementitious materials: Biofilm characterization and deterioration characteristics of mortars. Int. Biodeterior. Biodegradation 2018, 134, 58–67. [Google Scholar] [CrossRef]
- Vogt, O.; Ballschmiede, C.; Ukrainczyk, N.; Koenders, E. Evaluation of Sulfuric Acid-Induced Degradation of Potassium Silicate Activated Metakaolin Geopolymers by Semi-Quantitative SEM-EDX Analysis. Materials. 2020, 13, 4522. [Google Scholar] [CrossRef]
- Grengg, C.; Gluth, G.J.; Mittermayr, F.; Ukrainczyk, N.; Bertmer, M.; Buzanich, A.G.; Radtke, M.; Leis, A.; Dietzel, M. Deterioration mechanism of alkali-activated materials in sulfuric acid and the influence of Cu: A micro-to-nano structural, elemental and stable isotopic multi-proxy study. Cem. Concr. Res. 2021, 142, 106373. [Google Scholar] [CrossRef]
- Škvára, F.R.; Šmilauer, V.; Hlaváček, P.E.; Kopecký, L.U.; Cilova, Z. A weak alkali bond in (N,K)-A-S-H gels: Evidence from leaching and modeling. Ceramics Silikaty 2012, 56, 374–382. [Google Scholar]
- Ukrainczyk, N.; Vogt, O.; Koenders, E.A.B. Reactive Transport Numerical Model for Durability of Geopolymer Materials. Adv. Chem. Eng. Sci. 2016, 6, 355–363. [Google Scholar] [CrossRef][Green Version]
- Aly, Z.; Vance, E.; Perera, D. Aqueous dissolution of sodium aluminosilicate geopolymers derived from metakaolin. J. Nucl. Mater. 2012, 424, 164–170. [Google Scholar] [CrossRef]
- Lloyd, R.R.; Provis, J.L.; van Deventer, J.S. Pore solution composition and alkali diffusion in inorganic polymer cement. Cem. Concr. Res. 2010, 40, 1386–1392. [Google Scholar] [CrossRef]
- Sun, Z.; Vollpracht, A. Leaching of monolithic geopolymer mortars. Cem. Concr. Res. 2020, 136, 106161. [Google Scholar] [CrossRef]
- Deng, N.; An, H.; Cui, H.; Pan, Y.; Wang, B.; Mao, L.; Zhai, J. Effects of gamma-ray irradiation on leaching of simulated 133 Cs + radionuclides from geopolymer wasteforms. J. Nucl. Mater. 2015, 459, 270–275. [Google Scholar] [CrossRef]
- Larreur-Cayol, S.; Bertron, A.; Escadeillas, G. Degradation of cement-based materials by various organic acids in agro-industrial waste-waters. Cem. Concr. Res. 2011, 41, 882–892. [Google Scholar] [CrossRef]
- Koenig, A.; Dehn, F. Main considerations for the determination and evaluation of the acid resistance of cementitious materials. Mater. Struct. 2015, 49, 1693–1703. [Google Scholar] [CrossRef]
- ASTM C267-01(2012). Standard Test Methods for Chemcial Resistance of Mortars, Grouts, and Monolithic Surfacings and Polymer Concretes; ASTM International: West Conshohocken, PA, USA, 2020. [Google Scholar]
- De Silva, P.; Sagoe-Crenstil, K.; Sirivivatnanon, V. Kinetics of geopolymerization: Role of Al2O3 and SiO2. Cem. Concr. Res. 2007, 37, 512–518. [Google Scholar] [CrossRef]
- Vogt, O.; Ukrainczyk, N.; Ballschmiede, C.; Koenders, E. Reactivity and Microstructure of Metakaolin Based Geopolymers: Effect of Fly Ash and Liquid/Solid Contents. Materials 2019, 12, 3485. [Google Scholar] [CrossRef] [PubMed]
- EN 15863:2015. Characterization of Waste-Leaching behaviour Test for Basic Characterization-Dynamic Monolithic Leaching Test with Periodic Leachant Renewal, under Fixed Conditions; CEN-CENELEC: Brussels, Belgium, 2015. [Google Scholar]
- CEN/TS 16637-2:2014. Construction Products-Assessment of Release of Dangerous Substances-Part 2: Horizontal Dynamic Surface Leaching Test; CEN-CENELEC: Brussels, Belgium, 2014. [Google Scholar]
- ASTM C1308-08. Standard Test Method for Accelerated Leach Test for Diffusive Releases from Solidified Waste and a Computer Program to Model Diffusive, Fractional Leaching from Cylindrical Waste Forms; ASTM International: West Conshohocken, PA, USA, 2017. [Google Scholar]
- ANSI/ANS-16.1. Measurement of the Leachability of Solidified Low-level Radioactive Waste by a Short-term Test Procedure; American Nuclear Society: La Grange Park, IL, USA, 2003. [Google Scholar]
- Electron Microscopy. A Practical Guide to Microstructural Analysis of Cementitious Materials, 1st ed.; Scrivener, K., Snellings, R., Lothenbach, B., Eds.; CRC Press Taylor and Francis Group: Boca Raton, FL, USA, 2016; Chapter 8. [Google Scholar]
- Pescatore, C. Improved expressions for modeling diffusive, fractional cumulative leaching from finite-size waste forms. Waste Manag. 1990, 10, 155–159. [Google Scholar] [CrossRef]
- Jasielec, J.J.; Stec, J.; Szyszkiewicz-Warzecha, K.; Łagosz, A.; Deja, J.; Lewenstam, A.; Filipek, R. Effective and Apparent Diffusion Coefficients of Chloride Ions and Chloride Binding Kinetics Parameters in Mortars: Non-Stationary Diffusion–Reaction Model and the Inverse Problem. Materials 2020, 13, 5522. [Google Scholar] [CrossRef]
- Perko, J.; Ukrainczyk, N.; Šavija, B.; Phung, Q.T.; Koenders, E.A.B. Influence of Micro-Pore Connectivity and Micro-Fractures on Calcium Leaching of Cement Pastes—A Coupled Simulation Approach. Materials 2020, 13, 2697. [Google Scholar] [CrossRef]
- Hartman, R.L.; Fogler, H.S. Understanding the Dissolution of Zeolites. Langmuir 2007, 23, 5477–5484. [Google Scholar] [CrossRef]
- Ukrainczyk, N.; Koenders, E.A.B. Representative elementary volumes for 3D modeling of mass transport in cementitious materials. Model. Simul. Mater. Sci. Eng. 2014, 22, 035001. [Google Scholar] [CrossRef]


| Material | SiO2 | Al2O3 | CaO | TiO2 | Fe2O3 | MgO | Na2O | K2O | H2O |
|---|---|---|---|---|---|---|---|---|---|
| Metakaolin MK2 | 50.4 | 40.5 | 0.1 | 1.5 | 2.0 | 0.1 | 0.1 | 0.1 | – |
| Metakaolin MK1 | 67.0 | 27.0 | 1.0 | 1 | 4 | 0.1 | 0.1 | 0.2 | – |
| K-waterglass | 22 | – | – | – | – | – | – | 23 | 55 |
| Specimen Notation | AcH Concentration, mM | Solution Replenishment (pH and K+ Measured), Days | SEM-BSE Imaging after, Days |
|---|---|---|---|
| M1_H0 | 0 (ultra-pure water) | 1, 3, 7, 14, 21, 28, 35, 42, 49, 56 | 56 |
| M1_A2 | 100 | ||
| M1_A3 | 10 | ||
| M1_A4 | 1 | ||
| M2_H0 | 0 (ultra-pure water) | ||
| M2_A2 | 100 |
| Sample Name | Dapp 108, cm2/s | Std. Error 108, cm2/s | R-Square | Rel. Error% | S, cm2 | V, cm3 | C0, mg |
|---|---|---|---|---|---|---|---|
| M2_H0 | 0.0104 | 0.0004 | 0.987 | 0.017 | 0.567 | 1.418 | 270.8 |
| M2_A2 | 1.45 | 0.023 | 0.998 | 0.033 | |||
| M1_H0 | 0.604 | 0.018 | 0.992 | 0.081 | 241.0 | ||
| M1_A4 | 0.592 | 0.020 | 0.990 | 0.104 | |||
| M1_A3 | 0.912 | 0.036 | 0.987 | 0.159 | |||
| M1_A2 | 2.73 | 0.042 | 0.998 | 0.044 |
| Sample Name | Dapp 108 (cm2/s) | Std. Error 108 (cm2/s) | R-Square | Rel. Error (%) | S (cm2) | V (cm3) | c0 (mg) |
|---|---|---|---|---|---|---|---|
| M1H0_mortar | 0.0606 | 0.0036 | 0.981 | 0.101 | 288 | 256 | 9780 |
| FAH0_mortar | 2.70 | 0.113 | 0.992 | 0.085 | 288 | 256 | 7040 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ukrainczyk, N. Simple Model for Alkali Leaching from Geopolymers: Effects of Raw Materials and Acetic Acid Concentration on Apparent Diffusion Coefficient. Materials 2021, 14, 1425. https://doi.org/10.3390/ma14061425
Ukrainczyk N. Simple Model for Alkali Leaching from Geopolymers: Effects of Raw Materials and Acetic Acid Concentration on Apparent Diffusion Coefficient. Materials. 2021; 14(6):1425. https://doi.org/10.3390/ma14061425
Chicago/Turabian StyleUkrainczyk, Neven. 2021. "Simple Model for Alkali Leaching from Geopolymers: Effects of Raw Materials and Acetic Acid Concentration on Apparent Diffusion Coefficient" Materials 14, no. 6: 1425. https://doi.org/10.3390/ma14061425
APA StyleUkrainczyk, N. (2021). Simple Model for Alkali Leaching from Geopolymers: Effects of Raw Materials and Acetic Acid Concentration on Apparent Diffusion Coefficient. Materials, 14(6), 1425. https://doi.org/10.3390/ma14061425







