Heat Treatment-Induced Microstructure and Property Evolution of Mg/Al Intermetallic Compound Coatings Prepared by Al Electrodeposition on Mg Alloy from Molten Salt Electrolytes
Abstract
1. Introduction
2. Materials and Methods
2.1. Zn Immersion
2.2. Electrodeposition and Heat Treatment
2.3. Characterization
3. Results and Discussion
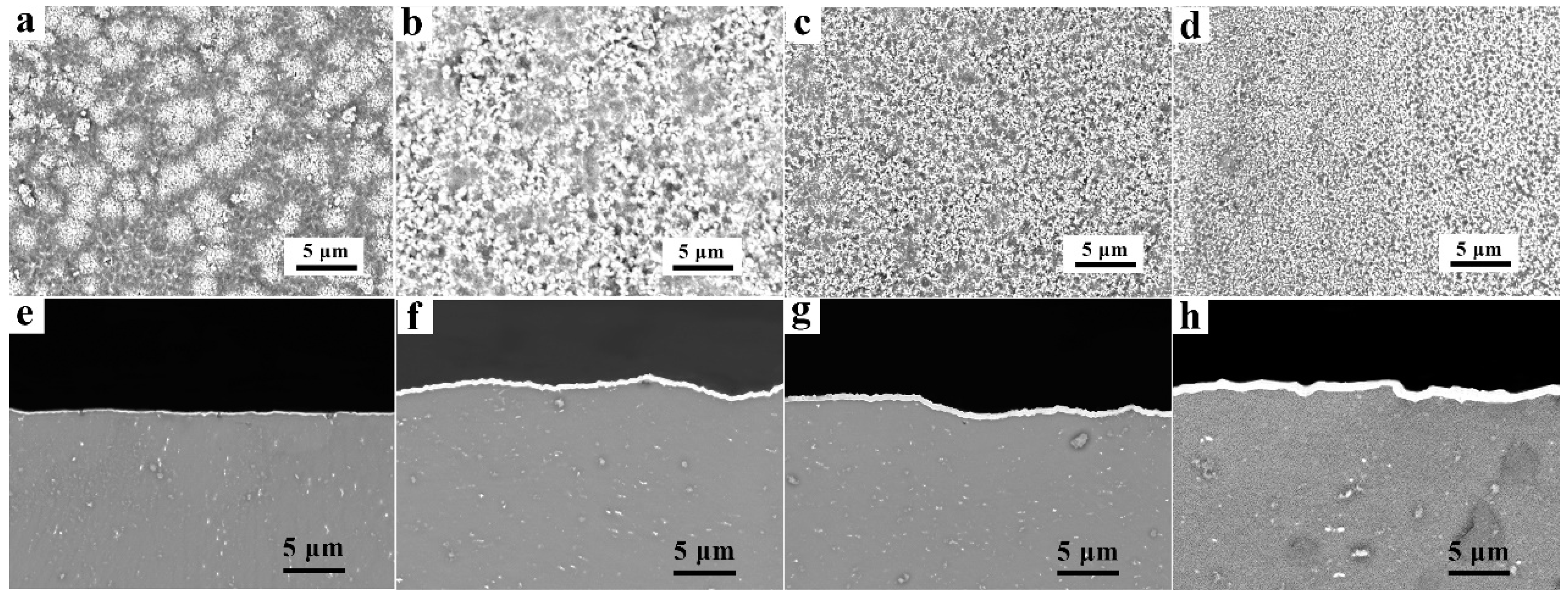
3.1. As-Deposited Microstructures
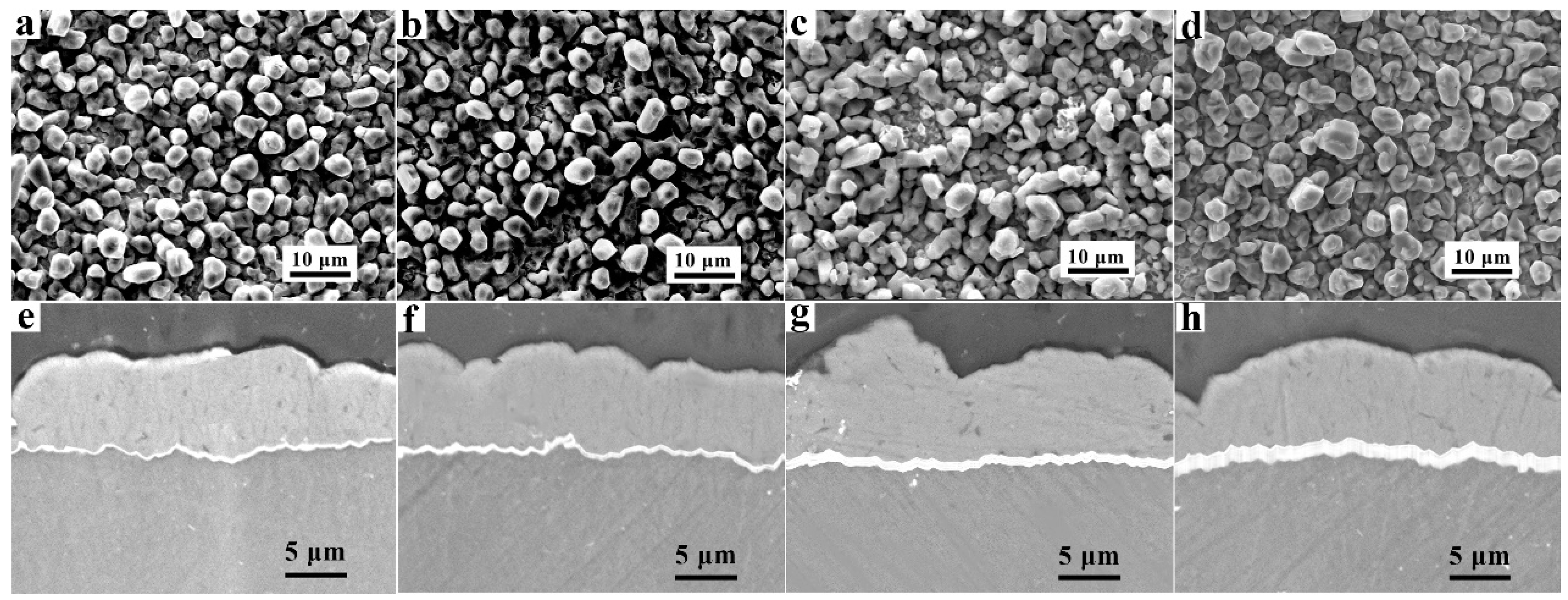
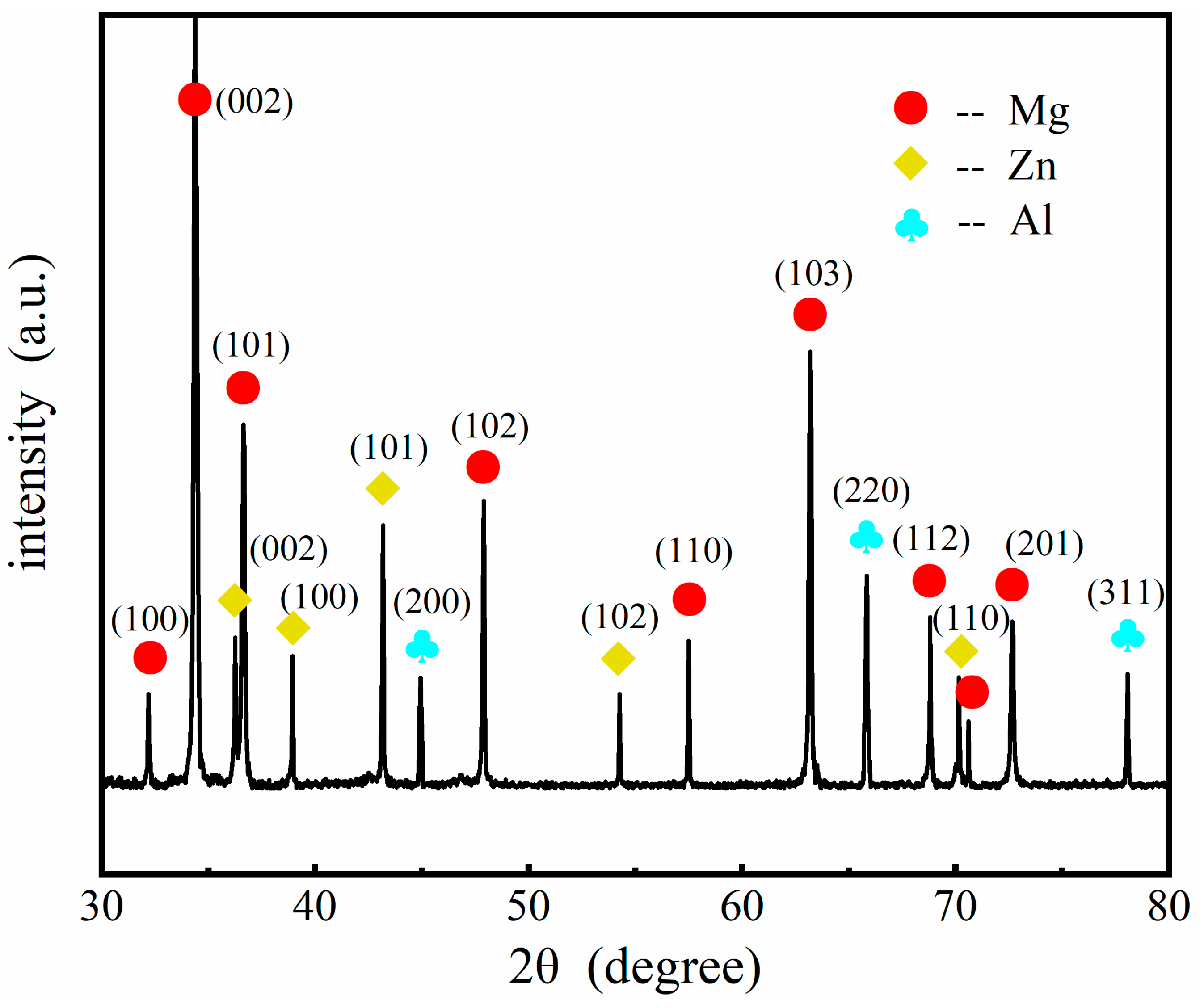
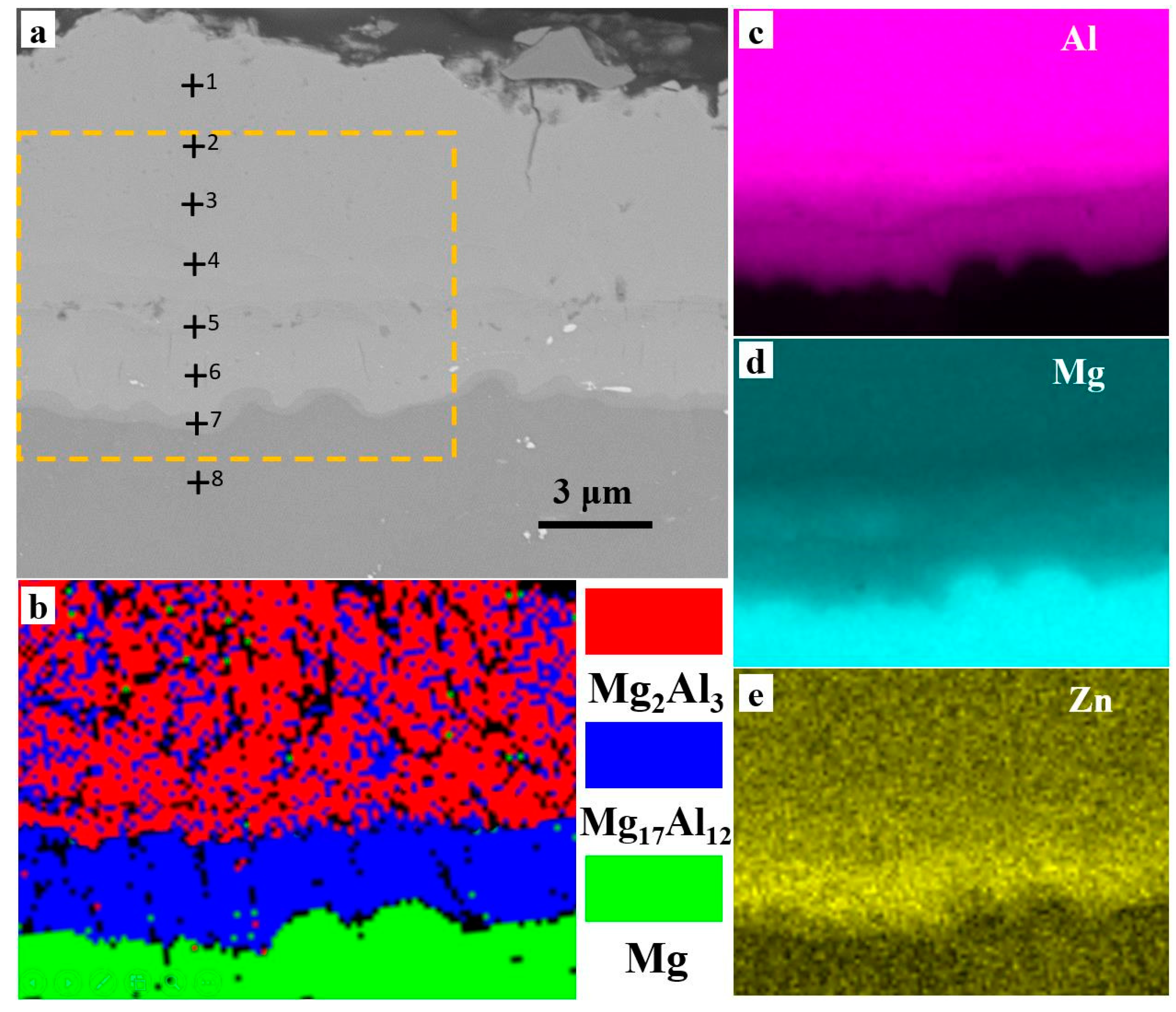
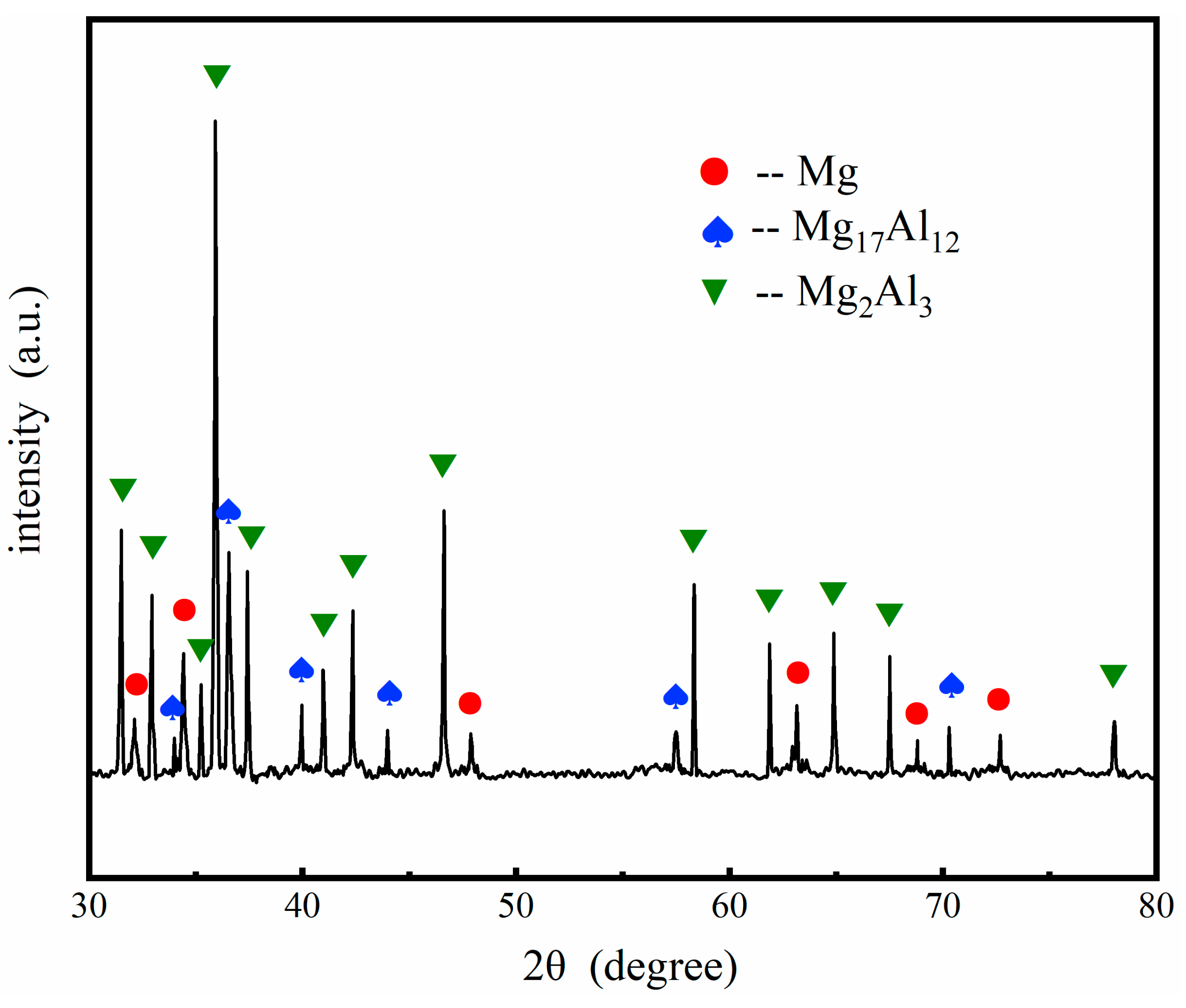
3.2. Microstructures after Heat Treatment
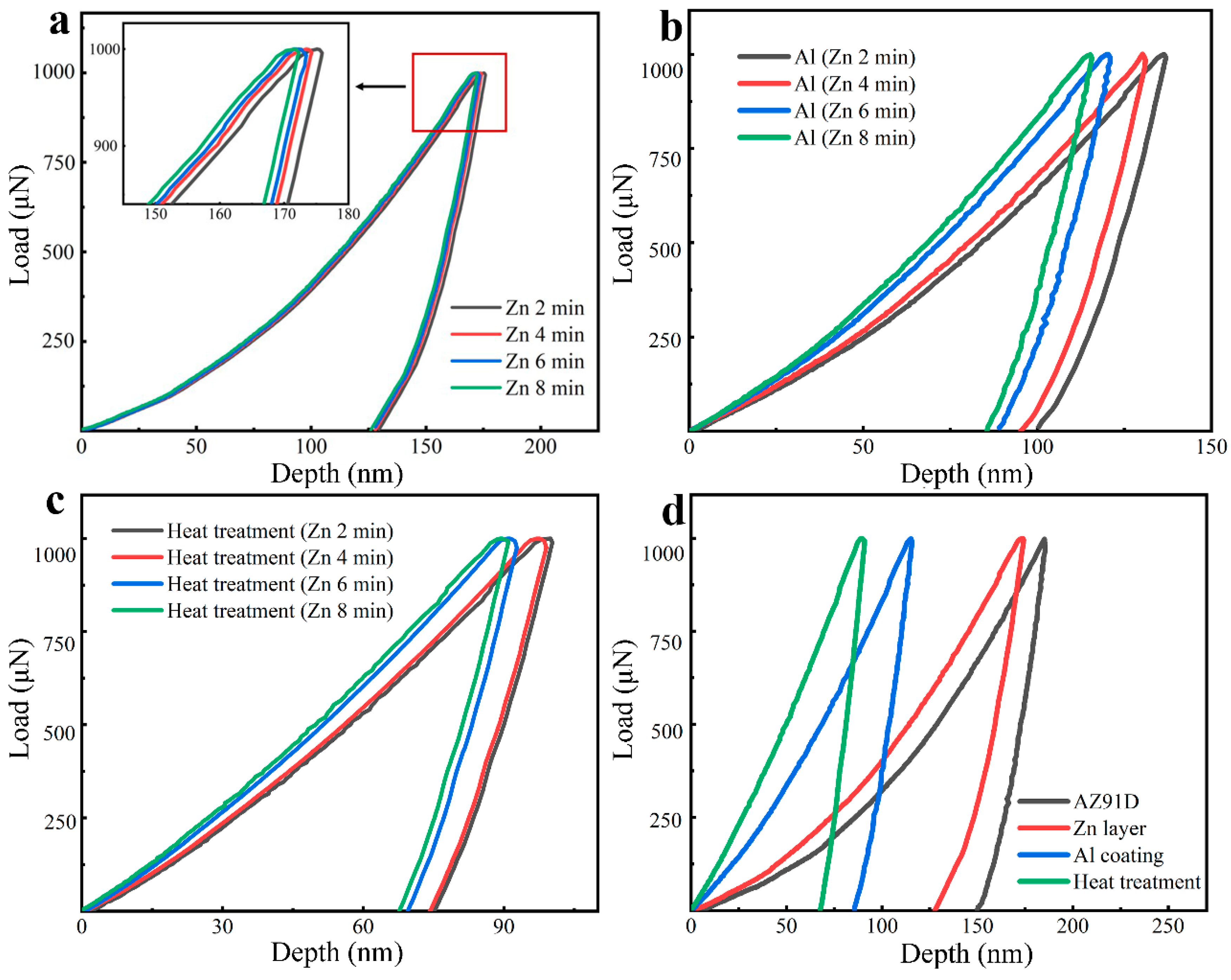
3.3. Nanomechanical Coating Properties
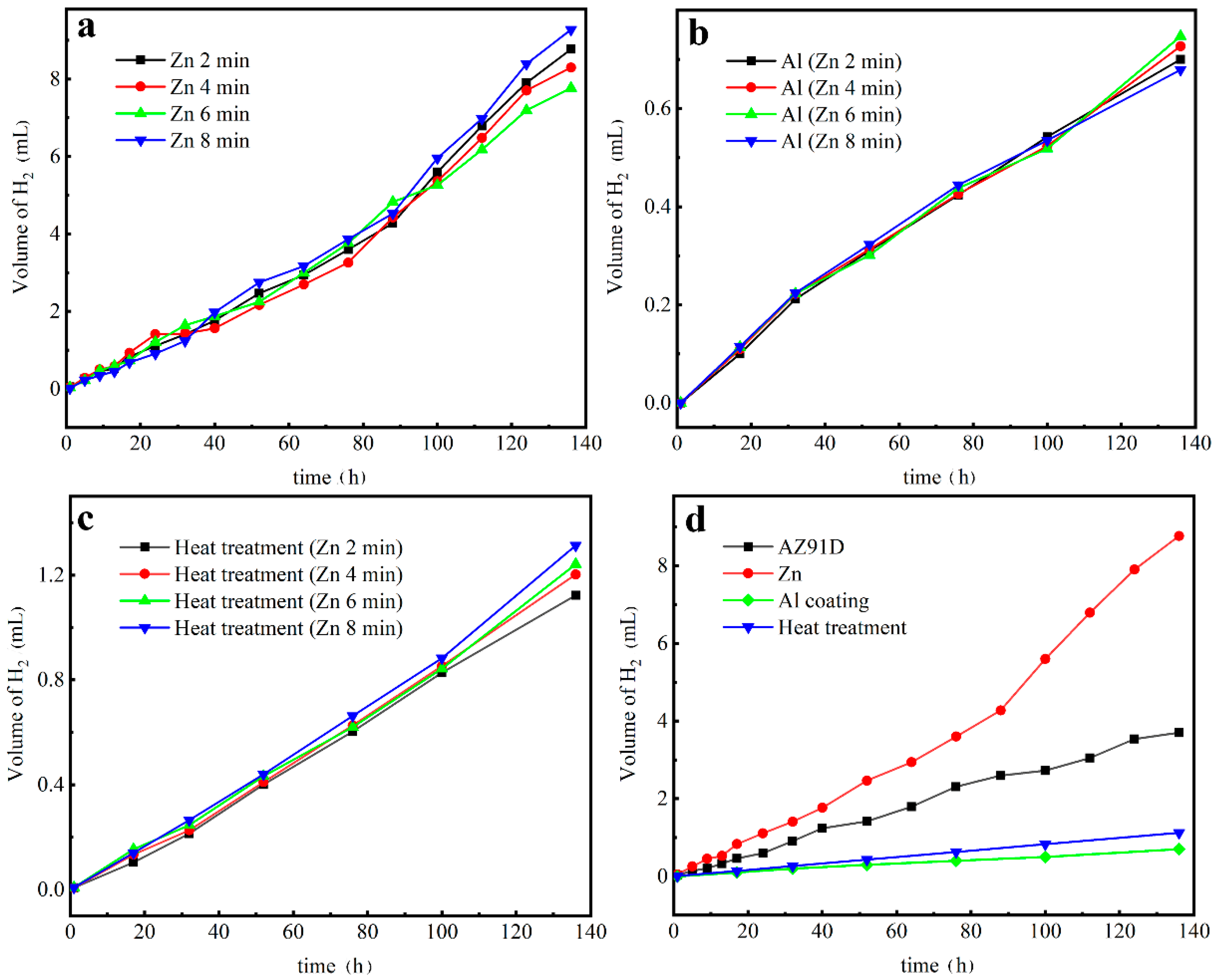
3.4. Hydrogen Evolution Tests
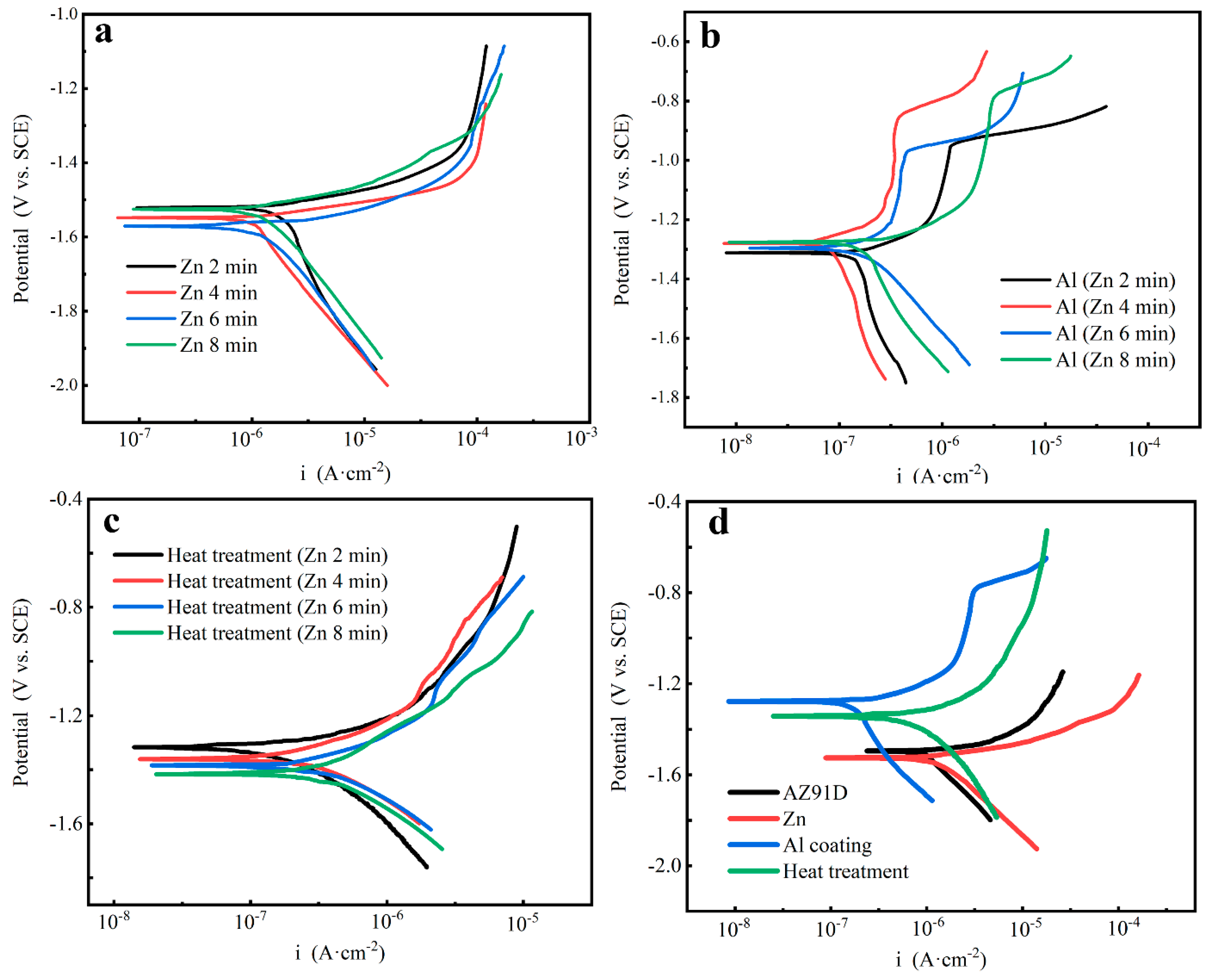
3.5. Potentiodynamic Testing
3.6. Comprehensive Assessment
4. Conclusions
- (1)
- Different thicknesses of Zn films immersed on AZ91 alloy that varied from 0.2 μm to 1.8 μm were achieved by controlling the immersion times from 2 min to 8 min. The compact and uniform Al coating with a thickness of 7 ± 3 μm was electrodeposited on the Zn film, which exhibited no clear relationship with the thickness of the Zn layer. After heat treatment at 350 °C, the Al coating and the Zn layer were transformed to Mg/Al intermetallic compounds β-phase (Mg17Al12) and γ-phase (Mg2Al3).
- (2)
- Evaluated by nanoindentation testing, the nano-hardness properties of the AZ91D increased from 1.1 ± 0.1 GPa to 1.5 ± 1.0 GPa by Zn immersion, and to 2.4 ± 0.5 GPa by the electrodeposition of Al coatings. In addition, the obtained Mg/Al intermetallic compounds induced by heat treatment led to a further improvement to reach 3.5 ± 0.1 GPa.
- (3)
- The hydrogen evolution tests and the potentiodynamic polarization proved that the Zn layer could not protect the Mg alloy matrix, while the electrodeposition Al coatings and heat treatment could improve corrosion resistance effectively. Compared with the Al coatings, the corrosion resistance of the Mg/Al intermetallic compounds β-phase (Mg17Al12) and γ-phase (Mg2Al3) was a little bit worse.
- (4)
- With the increase of the Zn immersion times, the thickness of the Zn layer increased and the mechanical properties were gradually improved, while their corrosion resistance got worse. When the Zn immersion time was 4 min, the obtained intermetallic compounds had relatively optimal comprehensive properties.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tokunaga, T.; Ohno, M.; Matsuura, K. Coatings on Mg alloys and their mechanical properties: A review. J. Mater. Sci. Technol. 2018, 34, 1119–1126. [Google Scholar] [CrossRef]
- Gray, J.E.; Luan, B.J. Protective Coatings on Magnesium and Its Alloys—A Critical Review. J. Alloys Compd. 2002, 336, 88–113. [Google Scholar] [CrossRef]
- Shigematsu, I.; Nakamura, M.; Saitou, N.; Shimojima, K. Surface treatment of AZ91D magnesium alloy by aluminum diffusion coating. J. Mater. Sci. Lett. 2000, 19, 473–475. [Google Scholar] [CrossRef]
- Christoglou, C.; Voudouris, N.; Angelopoulos, G.N.; Pant, M.; Dahl, W. Deposition of aluminum on magnesium by a CVD process. Surf. Coat. Technol. 2004, 184, 149–155. [Google Scholar] [CrossRef]
- Meng, F.P.; Peng, S.; Xu, G.B.; Wang, Y.; Ge, F.F.; Huang, F. Structure of uniform and high-quality Al-doped ZnO films by magnetron sputter deposition at low temperatures. Thin Solid Films 2018, 665, 109–116. [Google Scholar] [CrossRef]
- Zhang, R.F. Film formation in the second step of micro-arc oxidation on magnesium alloys. Corrosion Sci. 2010, 52, 1285–1290. [Google Scholar] [CrossRef]
- Belova, I.V.; Heuskin, D.; Sondermann, E.; Ignatzi, B.; Kargl, F.; Murch, G.E.; Meyer, A. Combined interdiffusion and self-diffusion analysis in Al-Cu liquid diffusion couple. Scr. Mater. 2018, 143, 40–43. [Google Scholar] [CrossRef]
- Cho, L.; Seo, E.J.; Sulistiyo, D.H.; Jo, K.R.; Kim, S.W.; Oh, J.K.; Cho, Y.R.; De Cooman, B.C. Influence of vanadium on the hydrogen embrittlement of aluminized ultra-high strength press hardening steel. Mater. Sci. Eng. A 2018, 735, 448–455. [Google Scholar] [CrossRef]
- Rocca, E.; Juers, C.; Steinmetz, J. Corrosion behaviour of chemical conversion treatments on as-cast Mg–Al alloys: Electrochemical and non-electrochemical methods. Corros. Sci. 2010, 52, 2172–2178. [Google Scholar] [CrossRef]
- Luo, X.X.; Yao, Z.J.; Zhang, P.Z.; Gu, D.D. Al2O3 nanoparticles reinforced Fe-Al laser cladding coatings with enhanced mechanical properties. J. Alloys Compd. 2018, 755, 41–45. [Google Scholar] [CrossRef]
- Zhu, L.; Song, G. Improved corrosion resistance of AZ91D magnesium alloy by an aluminium-alloyed coating. Surf. Coat. Technol. 2006, 200, 2834–2840. [Google Scholar] [CrossRef]
- Bu, H.; Yandouzi, M.; Lu, C.; Jodoin, B. Effect of heat treatment on the intermetallic layer of cold sprayed aluminum coatings on magnesium alloy. Surf. Coat. Technol. 2011, 205, 4665–4671. [Google Scholar] [CrossRef]
- Bu, H.; Yandouzi, M.; Lu, C.; Jodoin, B. Post-heat Treatment Effects on Cold-Sprayed Aluminum Coatings on AZ91D Magnesium Substrates. J. Therm. Spray Technol. 2012, 21, 731–739. [Google Scholar] [CrossRef]
- Zhao, M.C.; Liu, M.; Song, G.; Atrens, A. Influence of the β-phase morphology on the corrosion of the Mg alloy AZ91. Corros. Sci. 2008, 50, 1939–1953. [Google Scholar] [CrossRef]
- Gamburg, Y.D.; Zangari, G. Theory and Practice of Metal Electrodeposition; Springer: Heidelberg, Germany, 2011; pp. 1–2. [Google Scholar]
- Yao, T.Y.; Yang, H.Y.; Wang, K.; Jiang, H.Y.; Chen, X.B.; Liu, H.Z.; Wang, Q.D.; Ding, W.J. Effects of additive NaI on electrodeposition of Al coatings in AlCl3-NaCl-KCl molten salts. Front. Chem. Sci. Eng. 2020, 1, 1–10. [Google Scholar] [CrossRef]
- Yang, H.Y.; Guo, X.W.; Wu, G.H.; Ding, W.J.; Birbilis, N. Electrodeposition of chemically and mechanically protective Al-coatings on AZ91D Mg alloy. Corros. Sci. 2011, 53, 381–387. [Google Scholar] [CrossRef]
- Liu, C.; Yang, H.; Wan, P.; Wang, K.H.; Tian, L.; Yang, K. Study on biodegradation of the second phase Mg17Al12 in Mg–Al–Zn Alloys: In vitro experiment and thermodynamic calculation. Mater. Sci. Eng. C 2014, 35, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.B.; Tang, G.Y.; Shek, C.H.; Zhu, Y.H.; Xu, Z.H. On the thermodynamics and kinetics of electropulsing induced dissolution of β-Mg17Al12 phase in an aged Mg–9Al–1Zn alloy. Acta Mater. 2009, 57, 4797–4808. [Google Scholar] [CrossRef]
- Song, G.; Shen, Q.X.; Zhu, M.L. A novel method for the preparation of Al–Mg intermetallic coating. Mater. Lett. 2011, 65, 480–482. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. Measurement of hardness and elastic modulus by instrumented indentation: Advances in understanding and refinements to methodology. J. Mater. Res. 2004, 19, 3–20. [Google Scholar] [CrossRef]
- Huang, H.; Winchester, K.; Liu, Y.; Hu, X.Z.; Musca, C.A.; Dell, J.M.; Faraone, L. Determination of mechanical properties of PECVD silicon nitride thin films for tunable MEMS Fabry–Pérot optical filters. J. Micromech. MicroEng. 2005, 15, 608–614. [Google Scholar] [CrossRef]
- Yang, H.Y.; Chen, X.B.; Guo, X.W.; Wu, G.H.; Ding, W.J.; Birbilis, N. Coating pretreatment for Mg alloy AZ91D. Appl. Surf. Sci. 2012, 258, 5472–5481. [Google Scholar] [CrossRef]
- Zhang, M.X.; Huang, H.; Spencer, K.; Shi, Y.N. Nanomechanics of Mg-Al intermetallic compounds. Surf. Coat. Technol. 2010, 204, 2118–2122. [Google Scholar] [CrossRef]
- To, S.; Zhu, Y.H.; Lee, W.B. Microstructural Changes at the Ultra-Precision Raster Milled Surface of Zn-Al Based Alloy. Mater. Trans. 2008, 49, 698–703. [Google Scholar] [CrossRef]
- Sun, H.Q.; Shi, Y.N.; Zhang, M.X.; Lu, K. Surface alloying of an Mg alloy subjected to surface mechanical attrition treatment. Surf. Coat. Technol. 2008, 202, 3947–3953. [Google Scholar] [CrossRef]
- Yang, H.; Guo, X.; Wu, G.; Wu, G.H.; Ding, W.J.; Birbilis, N. Continuous intermetallic compounds coatings on AZ91D Mg alloy fabricated by diffusion reaction of Mg–Al couples. Surf. Coat. Technol. 2011, 205, 2907–2913. [Google Scholar] [CrossRef]
- Fini, M.H.; Amadeh, A. Improvement of wear and corrosion resistance of AZ91 magnesium alloy by applying Ni-SiC nanocomposite coating via pulse electro-deposition. Trans. Nonferrous Met. Soc. China 2013, 23, 2914–2922. [Google Scholar] [CrossRef]
- Zhang, J.; Yan, C.; Wang, F. Electrodeposition of Al-Mn alloy on AZ31B magnesium alloy in molten salts. Appl. Surf. Sci. 2009, 255, 4926–4932. [Google Scholar] [CrossRef]
- Birbilis, N.; Buchheit, R.G. Electrochemical Characteristics of Intermetallic Phases in Aluminum Alloys An Experimental Survey and Discussion. J. Electrochem. Soc. 2005, 152, 467–472. [Google Scholar] [CrossRef]
- Searles, J.L.; Gouma, P.I.; Buchheit, R.G. Stress corrosion cracking of sensitized AA5083(Al-4.5Mg-1.0Mn). Metall. Mater. Trans. A 2001, 32, 2859–2867. [Google Scholar] [CrossRef]
- Zhang, J.F.; Zhang, W.; Yan, C.; Du, K.; Wang, F.H. Corrosion behaviors of Zn/Al–Mn alloy composite coatings deposited on magnesium alloy AZ31B (Mg–Al–Zn). Electrochim. Acta 2009, 55, 560–571. [Google Scholar] [CrossRef]




| Process | Bath Compositions | Content | Conditions | Time |
|---|---|---|---|---|
| Pickling | C6H8O7 | 40 g/L | Room Temperature | 2 min |
| Conditioning | NaOH | 200 g/L | Room Temperature | 10 min |
| Activation | C6H8O7 | 20 g/L | Room Temperature | 40 s |
| Zn immersion | ZnSO4·7H2O | 30 g/L | 80 °C | 2~8 min |
| Na4P2O7·10H2O | 120 g/L | |||
| NaF | 5.5 g/L | |||
| Na2CO3 | 5.5 g/L | |||
| Point | Al wt. % | Zn wt. % | Mg wt. % | Al at. % | Zn at. % | Mg at. % |
|---|---|---|---|---|---|---|
| 1 | 62.1 | 0.0 | 37.9 | 59.3 | 0.0 | 40.7 |
| 2 | 61.9 | 0.0 | 38.1 | 59.1 | 0.0 | 40.9 |
| 3 | 61.3 | 0.0 | 38.8 | 58.4 | 0.0 | 41.6 |
| 4 | 61.1 | 0.0 | 38.9 | 58.3 | 0.0 | 41.7 |
| 5 | 39.8 | 0.9 | 59.3 | 41.4 | 2.8 | 55.8 |
| 6 | 36.9 | 1.5 | 61.6 | 38.5 | 3.7 | 57.8 |
| 7 | 0.0 | 0.0 | 100.0 | 0.0 | 0.0 | 100.0 |
| 8 | 0.0 | 0.0 | 100.0 | 0.0 | 0.0 | 100.0 |
| Unit | AZ91D | – | Zn | Al | β-Phase (Mg17Al12) | γ-Phase (Mg2Al3) |
|---|---|---|---|---|---|---|
| nHV (GPa) | 1.12 ± 0.09 | Zn 2 min | 1.40 ± 0.04 | 2.35 ± 0.06 | 3.51 ± 0.05 | 3.49 ± 0.10 |
| Zn 4 min | 1.45 ± 0.02 | 2.40 ± 0.08 | 3.55 ± 0.04 | 3.52 ± 0.08 | ||
| Zn 6 min | 1.53 ± 0.05 | 2.41 ± 0.07 | 3.56 ± 0.04 | 3.57 ± 0.07 | ||
| Zn 8 min | 1.57 ± 0.03 | 2.44 ± 0.06 | 3.58 ± 0.06 | 3.59 ± 0.08 | ||
| EIT (GPa) | 40.20 ± 8.40 | Zn 2 min | 49.72 ± 0.03 | 66.73 ± 0.05 | 65.91 ± 0.08 | 63.15 ± 0.06 |
| Zn 4 min | 51.54 ± 0.04 | 67.51 ± 0.06 | 66.32 ± 0.07 | 63.89 ± 0.05 | ||
| Zn 6 min | 52.71 ± 0.04 | 67.76 ± 0.05 | 66.67 ± 0.06 | 64.28 ± 0.08 | ||
| Zn 8 min | 55.31 ± 0.02 | 68.25 ± 0.07 | 66.78 ± 0.06 | 64.81 ± 0.06 |
| Unit | AZ91D | Zn layer Zn 2 min | Zn layer Zn 4 min | Zn layer Zn 6 min | Zn layer Zn 8 min |
| Ecorr (V) | −1.51 | −1.52 | −1.55 | −1.56 | −1.52 |
| icorr (μA·cm−2) | 103.81 | 125.18 | 118.07 | 120.90 | 122.52 |
| Unit | Al-coated Zn 2 min | Al-coated Zn 4 min | Al-coated Zn 6 min | Al-coated Zn 8 min | |
| Ecorr (V) | −1.31 | −1.27 | −1.29 | −1.27 | |
| icorr (μA·cm−2) | 35.79 | 33.44 | 41.38 | 37.49 | |
| Unit | Heat Treatment Zn 2 min | Heat Treatment Zn 4 min | Heat Treatment Zn 6 min | Heat Treatment Zn 8 min | |
| Ecorr (V) | −1.35 | −1.37 | −1.39 | −1.41 | |
| icorr (μA·cm−2) | 42.19 | 49.90 | 55.67 | 60.57 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, T.; Wang, K.; Yang, H.; Jiang, H.; Wei, J.; Wu, W.; Liu, H.; Wang, Q.; Ding, W. Heat Treatment-Induced Microstructure and Property Evolution of Mg/Al Intermetallic Compound Coatings Prepared by Al Electrodeposition on Mg Alloy from Molten Salt Electrolytes. Materials 2021, 14, 1407. https://doi.org/10.3390/ma14061407
Yao T, Wang K, Yang H, Jiang H, Wei J, Wu W, Liu H, Wang Q, Ding W. Heat Treatment-Induced Microstructure and Property Evolution of Mg/Al Intermetallic Compound Coatings Prepared by Al Electrodeposition on Mg Alloy from Molten Salt Electrolytes. Materials. 2021; 14(6):1407. https://doi.org/10.3390/ma14061407
Chicago/Turabian StyleYao, Tianyu, Kui Wang, Haiyan Yang, Haiyan Jiang, Jie Wei, Weiping Wu, Hezhou Liu, Qudong Wang, and Wenjiang Ding. 2021. "Heat Treatment-Induced Microstructure and Property Evolution of Mg/Al Intermetallic Compound Coatings Prepared by Al Electrodeposition on Mg Alloy from Molten Salt Electrolytes" Materials 14, no. 6: 1407. https://doi.org/10.3390/ma14061407
APA StyleYao, T., Wang, K., Yang, H., Jiang, H., Wei, J., Wu, W., Liu, H., Wang, Q., & Ding, W. (2021). Heat Treatment-Induced Microstructure and Property Evolution of Mg/Al Intermetallic Compound Coatings Prepared by Al Electrodeposition on Mg Alloy from Molten Salt Electrolytes. Materials, 14(6), 1407. https://doi.org/10.3390/ma14061407








