Evaluation of the Hydration Characteristics and Anti-Washout Resistance of Non-Dispersible Underwater Concrete with Nano-SiO2 and MgO
Abstract
1. Introduction
2. Experimental Details
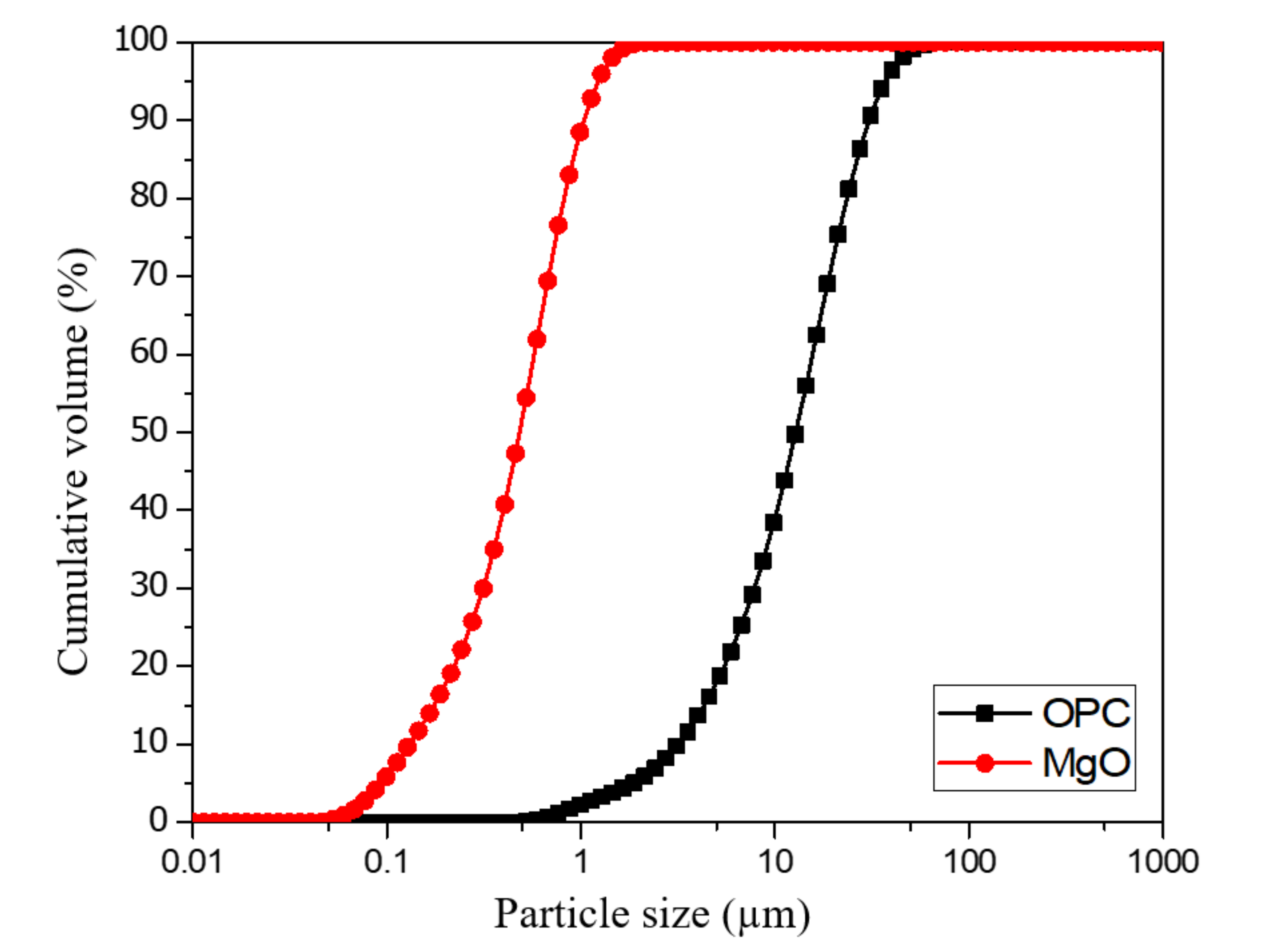
2.1. Materials
2.2. Mix Proportions and Fabrication of Specimens
2.3. Experiment Methods
3. Results and Discussion
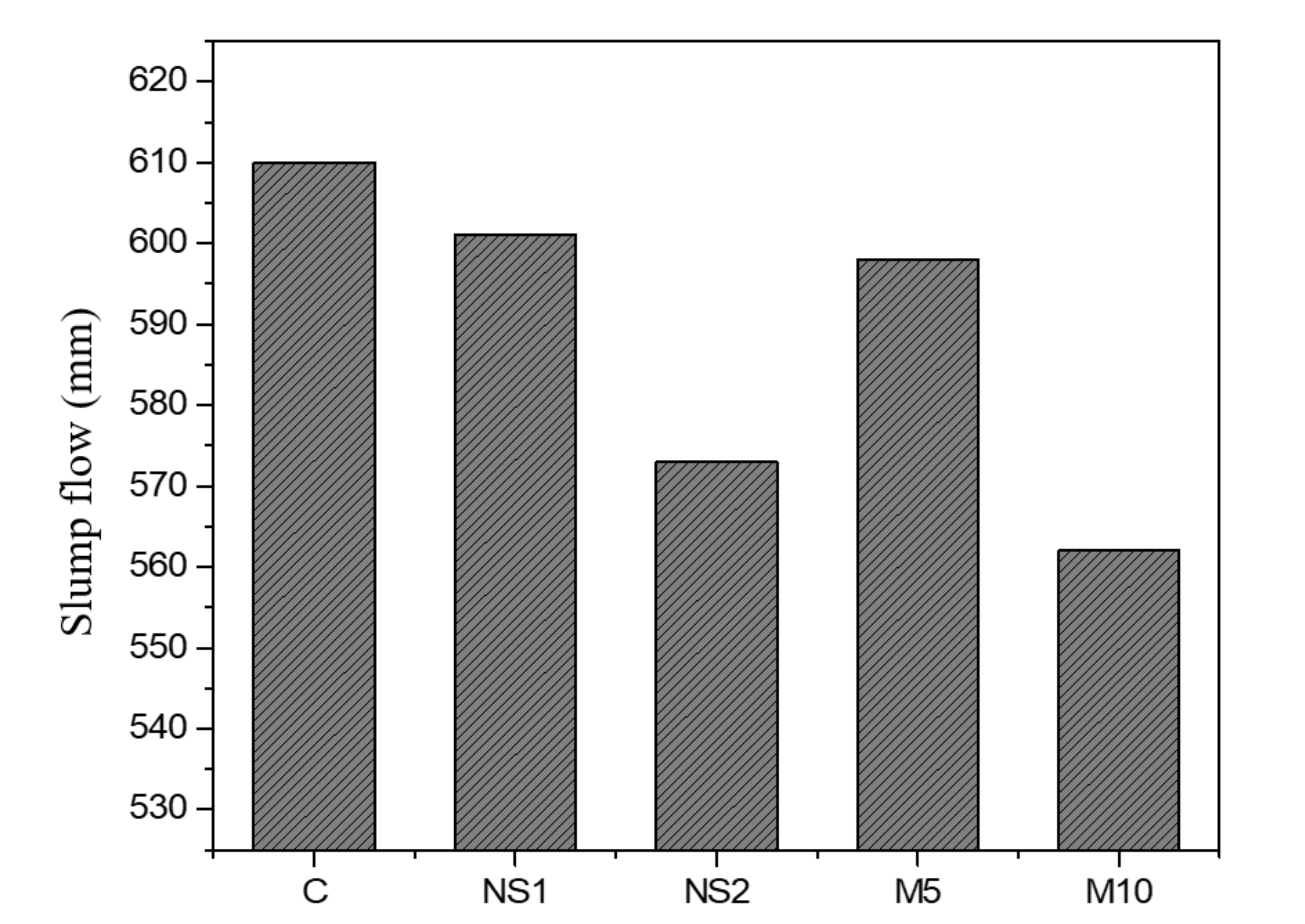
3.1. Slump Flow Test
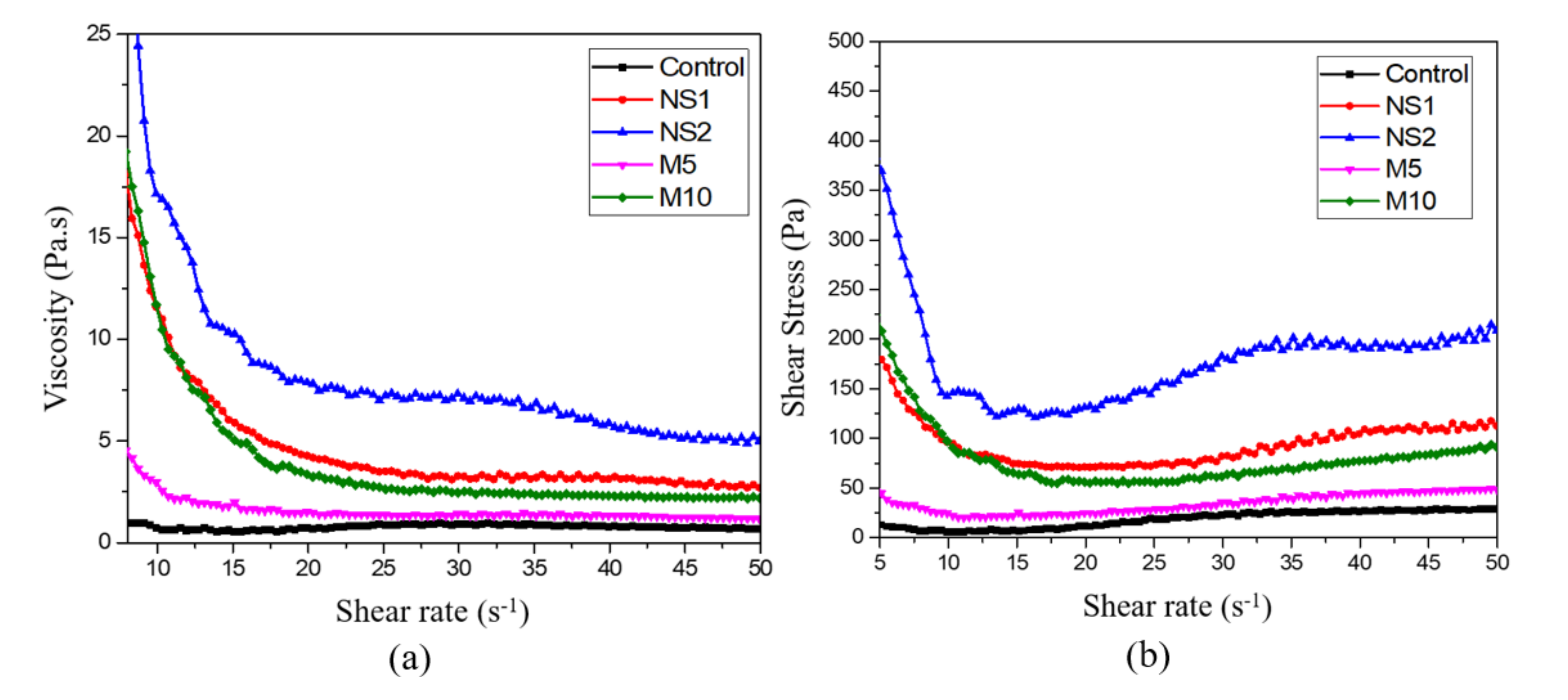
3.2. Viscosity Test
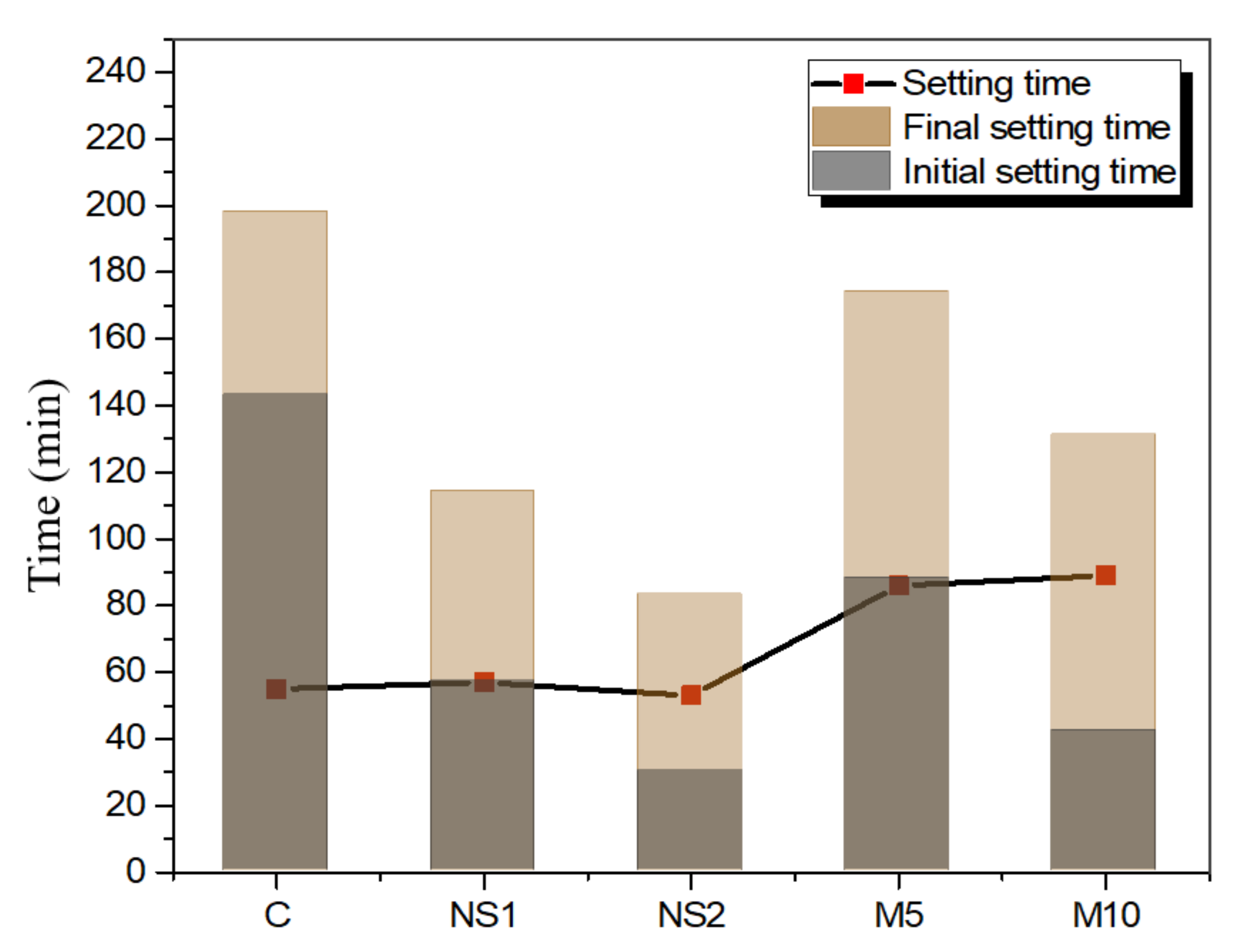
3.3. Setting Time
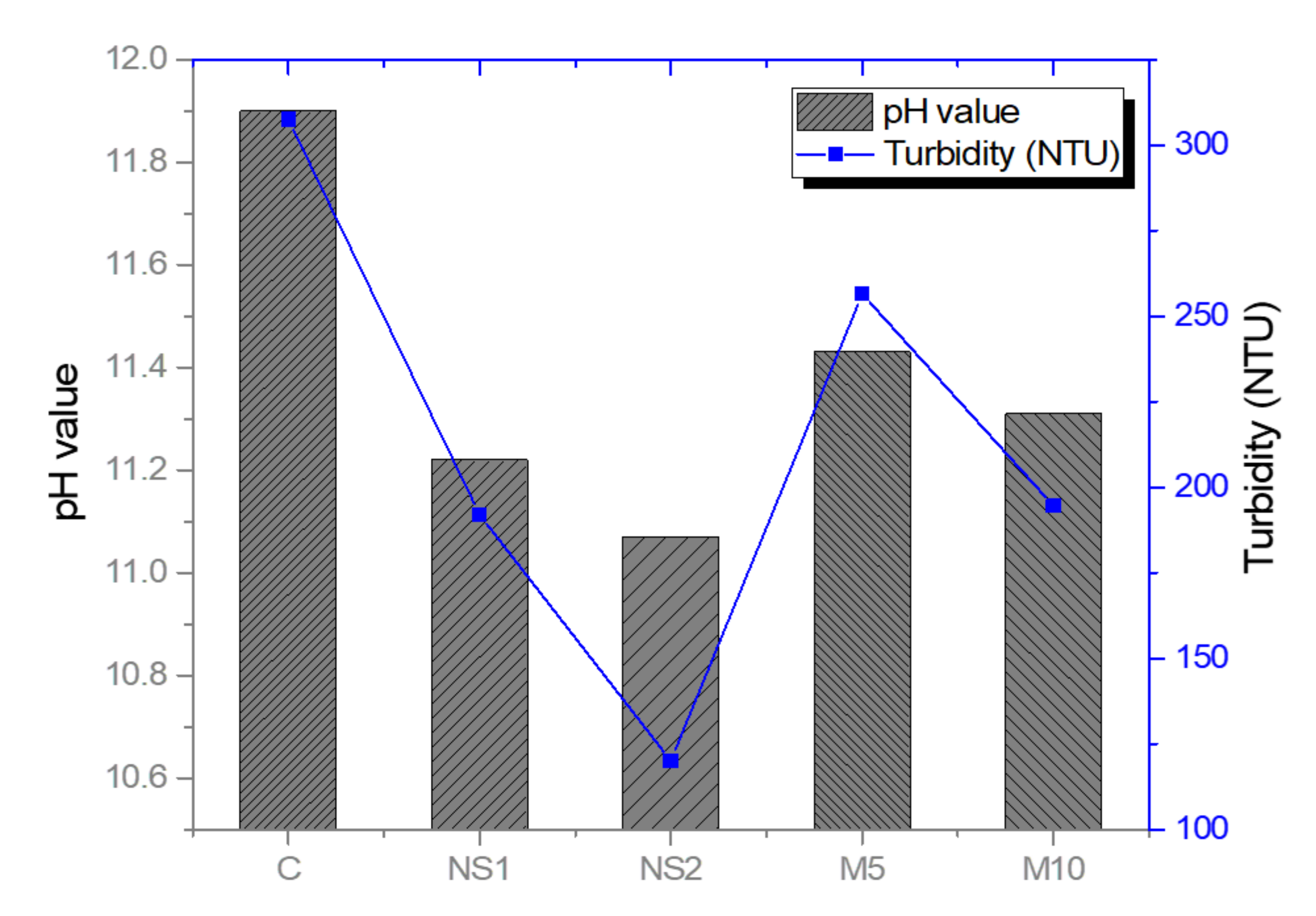
3.4. Anti-Washout Resistance
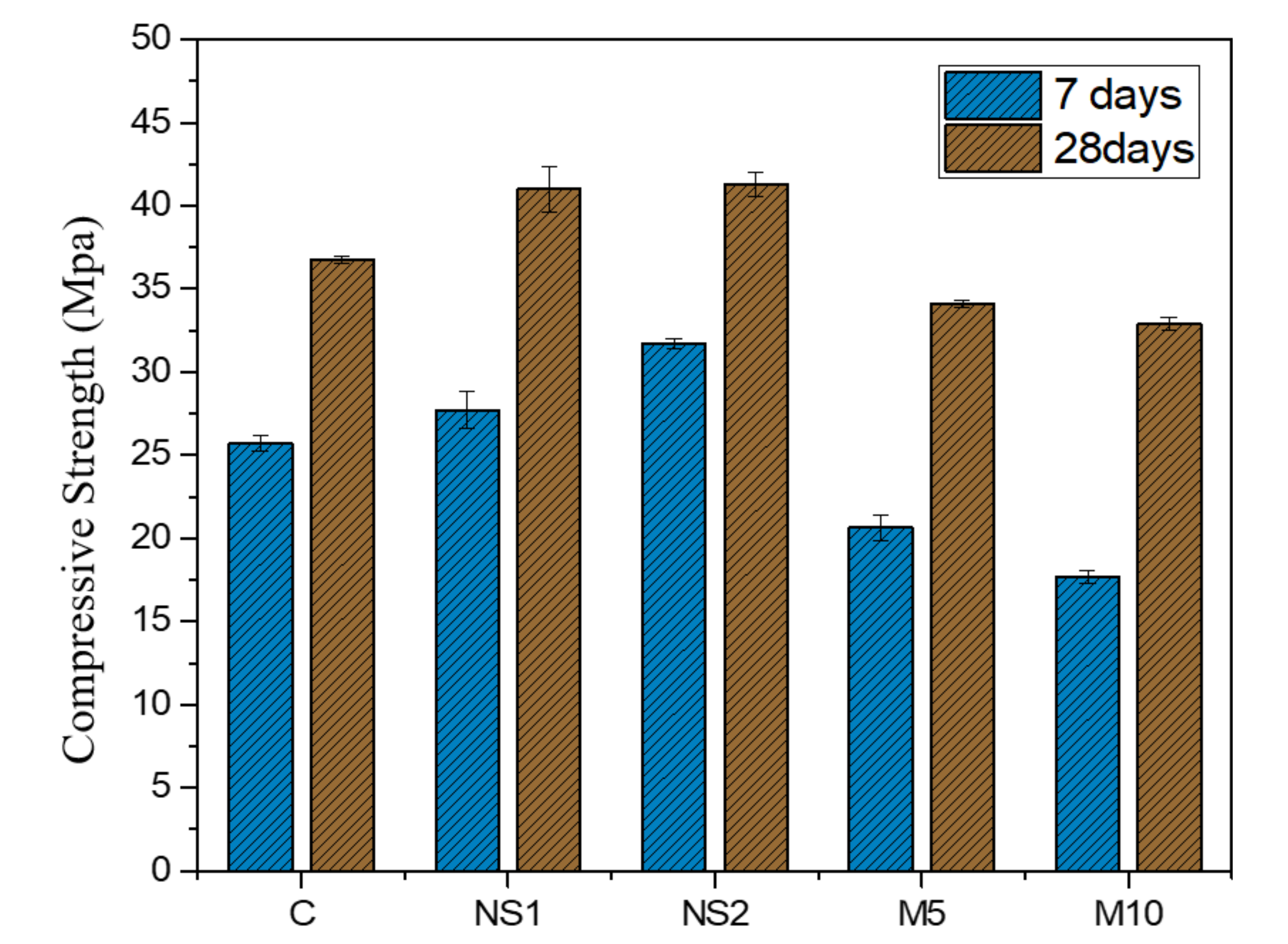
3.5. Compressive Strength Test
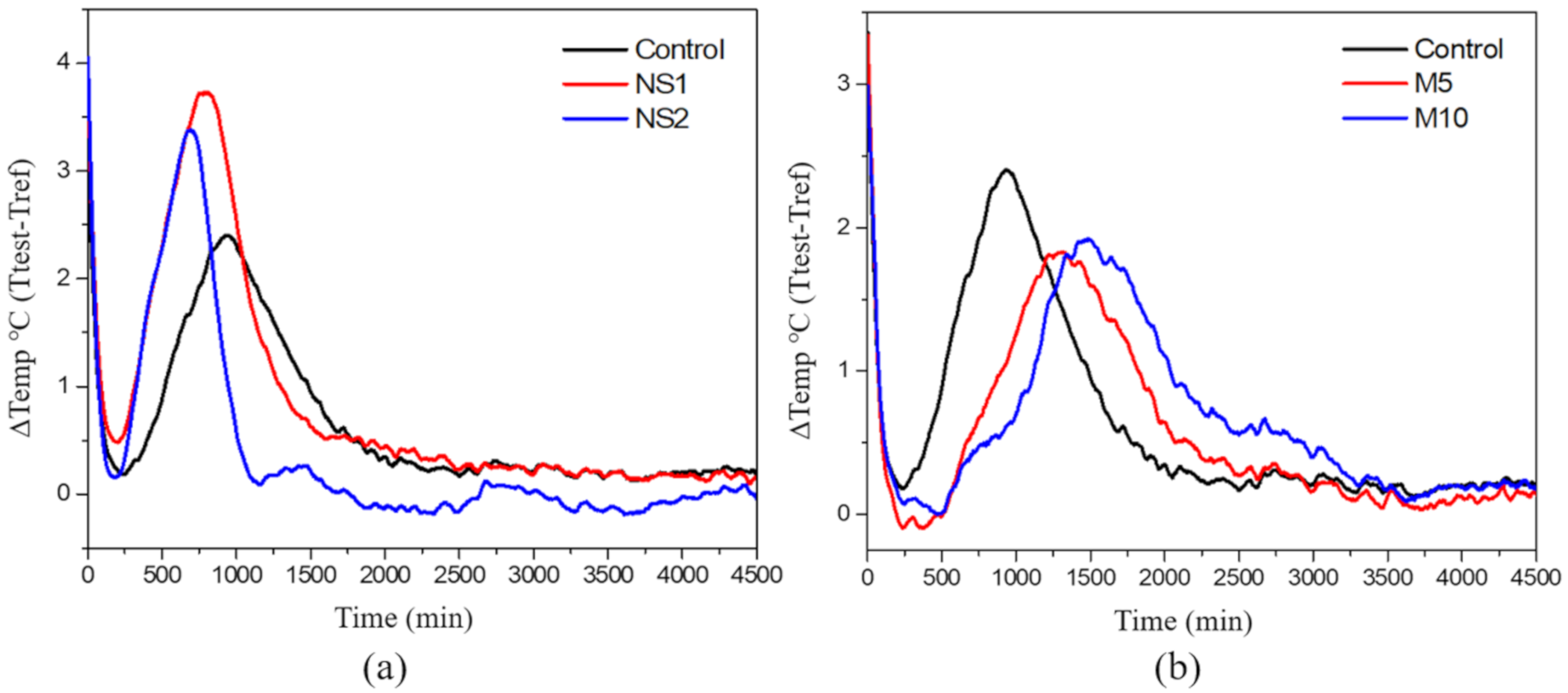
3.6. Hydration Heat
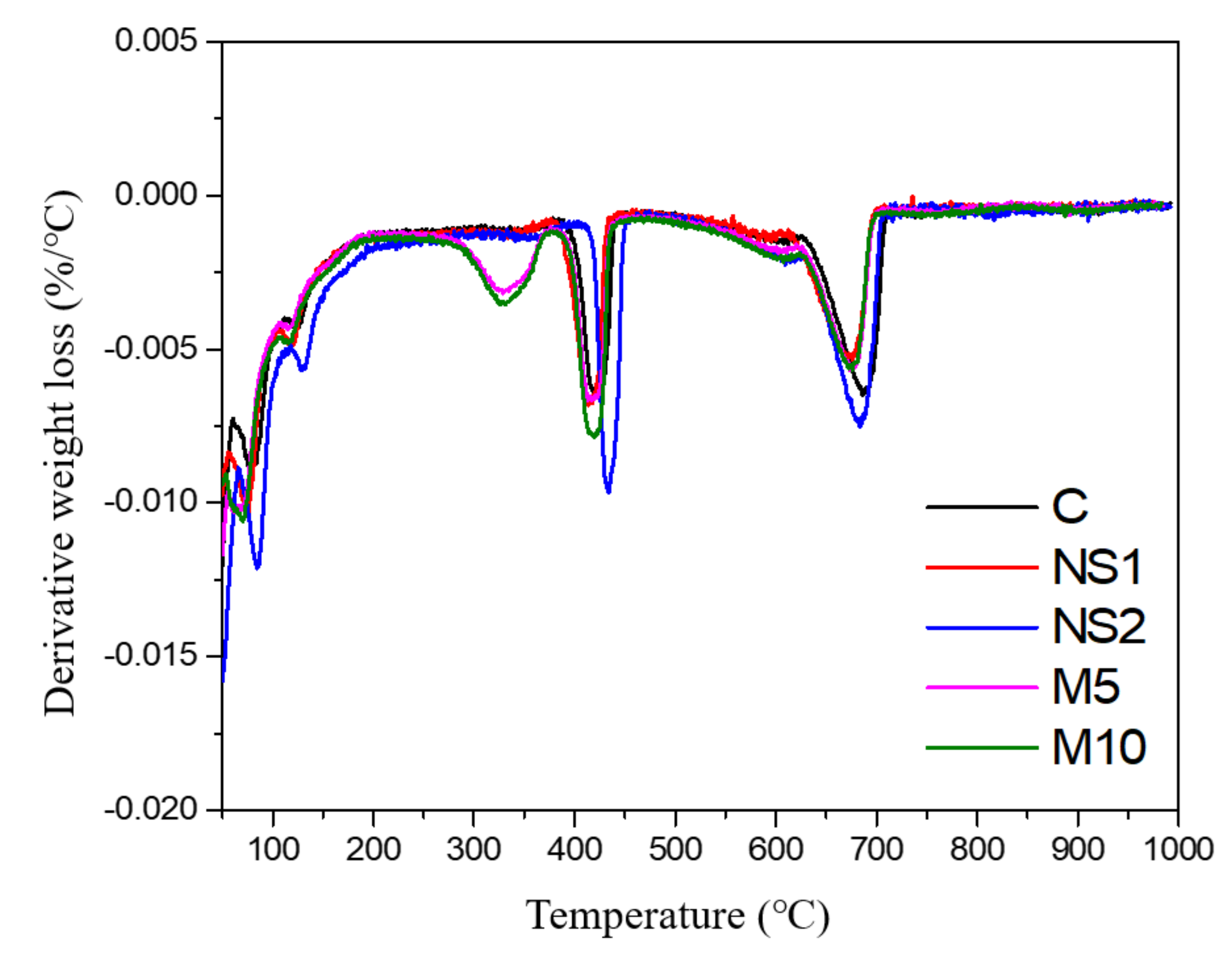
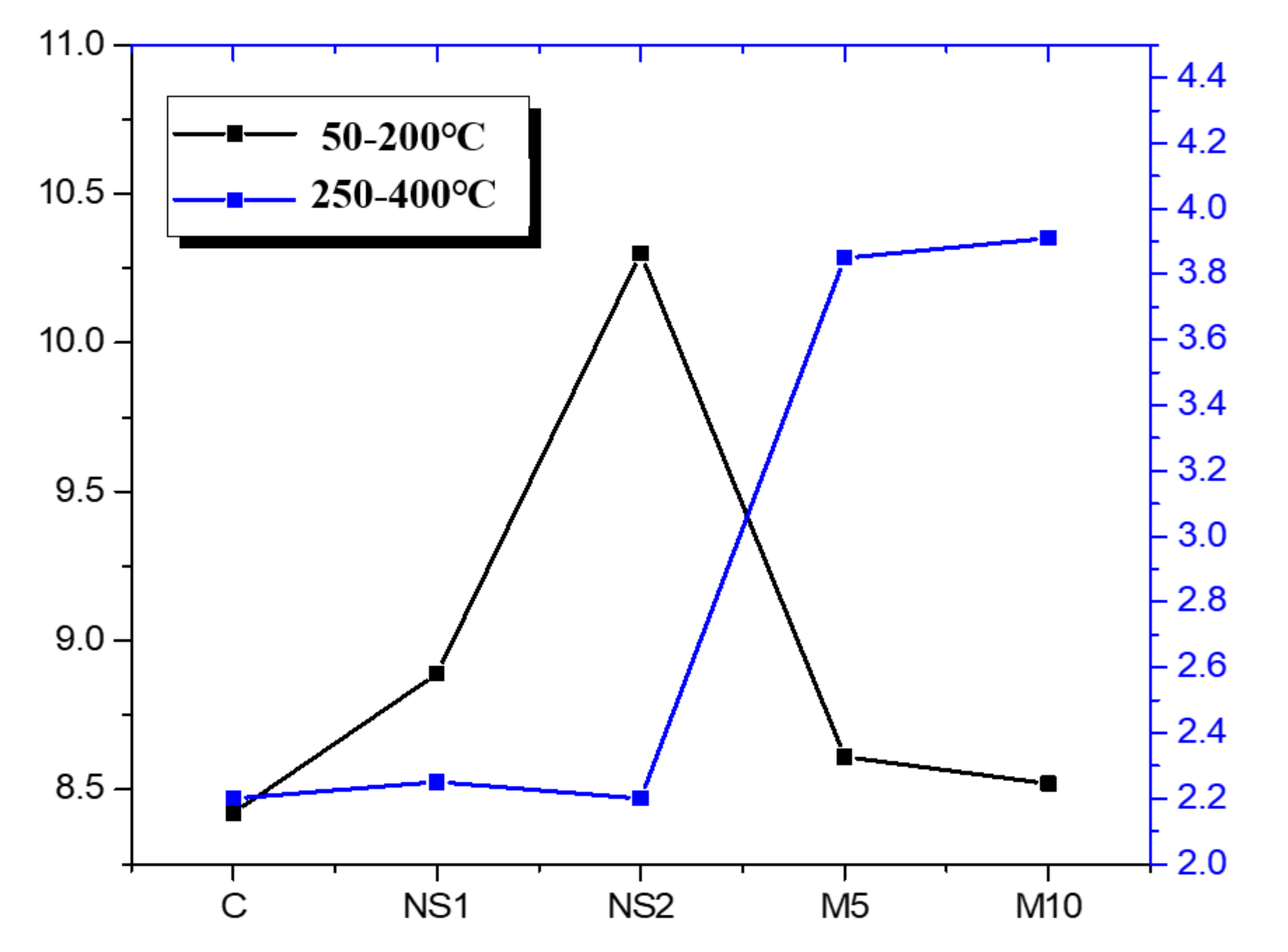
3.7. Thermogravimetric Analysis
4. Conclusions
- The results of the rheological experiments revealed that the fine particles and large specific areas of NS and MgO reduced the flowability and increased the viscosity of UWC. However, the impact of the smaller NS on the rheological properties was greater than that of MgO;
- Both NS and MgO shortened the initial and final setting times compared to the control specimens due to their fine particle sizes. However, MgO prolonged the setting time (the duration between the initial and final setting times) due to the formation of an insoluble hydration product. The hydration heat analysis showed that NS accelerated the hydration process and increased the hydration heat, whereas MgO delayed the peak time and reduced the temperature;
- The anti-washout performance results showed similar pH and turbidity tendencies in all the mixes. Both the addition of NS and replacement OPC with MgO reduced the pH and turbidity values, demonstrating that both NS and MgO increased the cohesion of the UWC mixes due to their finer particle size compared to that of OPC, which enhanced the anti-washout resistance of the UWC;
- The addition of NS slightly increased the compressive strength of the UWC because the high pozzolanic activity of NS generated extra nucleation sites and accelerated the hydration process. However, MgO decreased the compressive strength of M5 and M10 by 7.13% and 10.48%, respectively, compared to that of the control specimen due to the formation of brucite;
- The TGA results showed that NS promoted the generation of C–S–H gel due to its pozzolanic activity and nucleation effect. In addition, the specimens that contained MgO showed enhanced mass loss fractions at 25 to 400 °C, which generated larger amounts of hydrotalcite.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ren, W.; Sneed, L.H.; Gai, Y.; Kang, X. Test results and nonlinear analysis of RC T-beams strengthened by bonded steel plates. Int. J. Concr. Struct. Mater. 2015, 9, 133–143. [Google Scholar] [CrossRef]
- Lehman, D.E.; Gookin, S.E.; Nacamuli, A.M.; Moehle, J.P. Repair of earthquake-damaged bridge columns. Struct. J. 2001, 98, 233–242. [Google Scholar]
- Parghi, A.; Alam, M.S. Seismic behavior of deficient reinforced concrete bridge piers confined with FRP–A fractional factorial analysis. Eng. Struct. 2016, 126, 531–546. [Google Scholar] [CrossRef]
- Saeed, H.Z.; Ahmed, A.; Ali, S.M.; Iqbal, M. Experimental and finite element investigation of strengthened LSC bridge piers under Quasi-Static Cyclic Load Test. Comp. Struct. 2015, 131, 556–564. [Google Scholar] [CrossRef]
- Jun, A.; Liyuan, S. Discussion of design and construction method on extraneous prestressed strengthening technique for bridge. J. Southeast Univ. 2002, 32, 771–7741. [Google Scholar]
- Zhu, J.-H.; Su, M.-H.; Huang, J.-Y.; Ueda, T.; Xing, F. The ICCP-SS technique for retrofitting reinforced concrete compressive members subjected to corrosion. Construct. Build. Mater. 2018, 167, 669–679. [Google Scholar] [CrossRef]
- Yi, N.H.; Nam, J.W.; Kim, S.B.; Kim, I.S.; Kim, J.-H.J. Evaluation of material and structural performances of developed Aqua-Advanced-FRP for retrofitting of underwater concrete structural members. Construct. Build. Mater. 2010, 24, 566–576. [Google Scholar] [CrossRef]
- Menkulasi, F.; Baghi, H.; Hall, D.; Farzana, N. Rehabilitation of Deteriorated Timber Piles with Fiber Reinforced Polymer Composites. In Proceedings of the IABSE Symposium Report, Nantes, France, 19–21 September 2018; pp. 381–388. [Google Scholar]
- Wei, Y.; Wu, G.; Wu, Z. Several innovative strengthening technologies for underwater piers. Build. Struct. 2010, 40, 683–686. [Google Scholar]
- Yamaguchi, M.; Tsuchida, T. Development of high-viscosity underwater concrete for marine structures. Marine Concrete. In Proceedings of the International Conference on Concrete in the Marine Environment, Concrete Society, London, UK, 22–24 September 1986; pp. 235–245. [Google Scholar]
- Han, B.; Zhang, L.; Ou, J. Non-Dispersible Underwater Concrete. In Smart and Multifunctional Concrete Toward Sustainable InfraStructures; Springer: Berlin/Heidelberg, Germany, 2017; pp. 369–377. [Google Scholar]
- Khayat, K.H. Effects of antiwashout admixtures on fresh concrete properties. Mater. J. 1995, 92, 164–171. [Google Scholar]
- Heniegal, A.M.; Maaty, A.A.E.S.; Agwa, I.S. Simulation of the behavior of pressurized underwater concrete. Alexand. Eng. J. 2015, 54, 183–195. [Google Scholar] [CrossRef]
- Park, J.-J.; Moon, J.-H. An Estimation on the Performance of High Fluidity Anti-washout Underwater Concrete. Adv. Mater. Res. 2014, 577–578, 501–504. [Google Scholar] [CrossRef]
- Kumar, V.; Kumar, R.; Mandal, S.; Sinha, A. Admixtures for underwater concreteing for repair of cracks in the structure. In Proceedings of the 30th Conference on Our World in Concrete & Structures, Singapore, 29–30 August 2002. [Google Scholar]
- Khayat, K.H.; Sonebi, M. Effect of mixture composition on washout resistance of highly flowable underwater concrete. Mater. J. 2001, 98, 289–295. [Google Scholar]
- Grzeszczyk, S.; Jurowski, K.; Bosowska, K.; Grzymek, M. The role of nanoparticles in decreased washout of underwater concrete. Construct. Build. Mater. 2019, 203, 670–678. [Google Scholar] [CrossRef]
- Sonebi, M.; Khayat, K. Effect of mixture composition on relative strength of highly flowable underwater concrete. Mater. J. 2001, 98, 233–239. [Google Scholar]
- Kojouri, S.J.; Abang Ali, A.A.; Farzadnia, N. Effect of Colloidal Nano Silica as a Viscosity Modifying Agent in Self Compacting Concrete with High Volume POFA. Adv. Mater. Res. 2016, 1133, 491–495. [Google Scholar] [CrossRef]
- Meng, T.; Hong, Y.; Wei, H.; Xu, Q. Effect of nano-SiO2 with different particle size on the hydration kinetics of cement. Thermochim. Acta 2019, 675, 127–133. [Google Scholar] [CrossRef]
- Kumar, S.; Ali, N.; Begum, S.; Rahman, Z. Characterization, properties and microstructure studies of cement mortar incorporating nano-SiO2. Mater. Today Proceed. 2020, 37, 425–430. [Google Scholar] [CrossRef]
- Zhou, J.; Zheng, K.; Liu, Z.; He, F. Chemical effect of nano-alumina on early-age hydration of Portland cement. Cem. Concr. Res. 2019, 116, 159–167. [Google Scholar] [CrossRef]
- Berra, M.; Carassiti, F.; Mangialardi, T.; Paolini, A.; Sebastiani, M. Effects of nanosilica addition on workability and compressive strength of Portland cement pastes. Construct. Build. Mater. 2012, 35, 666–675. [Google Scholar] [CrossRef]
- Nazari, A.; Riahi, S. The effects of SiO2 nanoparticles on physical and mechanical properties of high strength compacting concrete. Compos. Part B Eng. 2011, 42, 570–578. [Google Scholar] [CrossRef]
- Yu, R.; Spiesz, P.; Brouwers, H. Effect of nano-silica on the hydration and microstructure development of Ultra-High Performance Concrete (UHPC) with a low binder amount. Construct. Build. Mater. 2014, 65, 140–150. [Google Scholar] [CrossRef]
- Yeşilmen, S.; Al-Najjar, Y.; Balav, M.H.; Şahmaran, M.; Yıldırım, G.; Lachemi, M. Nano-modification to improve the ductility of cementitious composites. Cem. Concr. Res. 2015, 76, 170–179. [Google Scholar] [CrossRef]
- Behfarnia, K.; Salemi, N. The effects of nano-silica and nano-alumina on frost resistance of normal concrete. Construct. Build. Mater. 2013, 48, 580–584. [Google Scholar] [CrossRef]
- Madani, H.; Bagheri, A.; Parhizkar, T.; Raisghasemi, A. Chloride penetration and electrical resistivity of concretes containing nanosilica hydrosols with different specific surface areas. Cem. Concr. Compos. 2014, 53, 18–24. [Google Scholar] [CrossRef]
- Lingling, X.; Min, D. Dolomite used as raw material to produce MgO-based expansive agent. Cem. Concr. Res. 2005, 35, 1480–1485. [Google Scholar] [CrossRef]
- Jang, B.-S.; Kwon, Y.-G.; Choi, S.-W.; Lee, K.-M. Fundamental properties of cement composites containing lightly burnt MgO powders. J. Korea Concr. Inst. 2011, 23, 225–233. [Google Scholar] [CrossRef]
- Mo, L.; Deng, M.; Tang, M. Potential approach to evaluating soundness of concrete containing MgO-based expansive agent. ACI Mater. J. 2010, 107, 99. [Google Scholar]
- Gao, P.-w.; Wu, S.-x.; Lin, P.-h.; Wu, Z.-r.; Tang, M.-s. The characteristics of air void and frost resistance of RCC with fly ash and expansive agent. Construct. Build. Mater. 2006, 20, 586–590. [Google Scholar] [CrossRef]
- Yousri, K. Self-flowing underwater concrete mixtures. Mag. Concr. Res. 2008, 60, 1–10. [Google Scholar] [CrossRef]
- Yao, S.X.; Berner, D.E.; Gerwick, B.C. Assessment of Underwater Concrete Technologies for in-the-Wet Construction of Navigation Structures; GERWICK (BEN C) INC: San Francisco, CA, USA, 1999. [Google Scholar]
- American Society for Testing and Materials. C150/C150M-17, Standard Specification for Portland Cement; American Society for Testing and Materials: West Conshohocken, PA, USA, 2017. [Google Scholar]
- Specimens, C.T. ASTM C 31/C 31M. a. Cast and Laboratory Cure Two Sets of Two Standard Cylinder Specimens for Each Composite Sample. b. Cast and Field Cure Two Sets of Two Standard Cylinder Specimens for Each Composite Sample; American Society for Testing and Materials: West Conshohocken, PA, USA, 2019. [Google Scholar]
- Moon, H.Y.; Shin, K.J. Evaluation on steel bar corrosion embedded in antiwashout underwater concrete containing mineral admixtures. Cem. Concr. Res. 2006, 36, 521–529. [Google Scholar] [CrossRef]
- Standard, A. C143, 2010. Standard Test Method for Slump of Hydraulic-Cement Concrete; ASTM International: West Conshohocken, PA, USA, 2012. [Google Scholar]
- Ding, Z.; Li, H.; Wei, J.; Li, R.; Yan, Y. Developing a novel magnesium glycerophosphate/silicate-based organic-inorganic composite cement for bone repair. Mater. Sci. Eng. C 2018, 87, 104–111. [Google Scholar] [CrossRef]
- Jeon, I.K.; Ryou, J.S.; Jakhrani, S.H.; Kim, H.G. Effects of Light-Burnt Dolomite Incorporation on the Setting, Strength, and Drying Shrinkage of One-Part Alkali-Activated Slag Cement. Materials 2019, 12, 2874. [Google Scholar] [CrossRef] [PubMed]
- American Society for Testing and Materials. 191. Standard Test Method for Time of Setting of Hydraulic Cement by Vicat Needle; American Society for Testing and Materials: West Conshohocken, PA, USA, 2006. [Google Scholar]
- Sikandar, M.A.; Wazir, N.R.; Khan, M.A.; Nasir, H.; Ahmad, W.; Alam, M. Effect of various anti-washout admixtures on the properties of non-dispersible underwater concrete. Construct. Build. Mater. 2020, 245, 118469. [Google Scholar] [CrossRef]
- ISO. International Standards ISO 7027—Water Quality—Determination of Turbidity; International Organization for Standards: Geneva, Switzerland, 1990. [Google Scholar]
- American Society for Testing and Materials. ASTM C39/C39M-12 Standard Test Method for Compressive Strength of Cylindrical Concrete Specimens; American Society for Testing and Materials: West Conshohocken, PA, USA, 2012. [Google Scholar]
- Moon, H.; Ramanathan, S.; Suraneni, P.; Shon, C.-S.; Lee, C.-J.; Chung, C.-W. Revisiting the effect of slag in reducing heat of hydration in concrete in comparison to other supplementary cementitious materials. Materials 2018, 11, 1847. [Google Scholar] [CrossRef]
- Quercia, G.; Hüsken, G.; Brouwers, H. Water demand of amorphous nano silica and its impact on the workability of cement paste. Cem. Concr. Res. 2012, 42, 344–357. [Google Scholar] [CrossRef]
- Zhang, R.; Panesar, D.K. New approach to calculate water film thickness and the correlation to the rheology of mortar and concrete containing reactive MgO. Construct. Build. Mater. 2017, 150, 892–902. [Google Scholar] [CrossRef]
- Cheng, B.; Yao, C.; Xiong, J.; Liu, X.; Zhang, H.; Zhang, S. Effects of Sodium Hexametaphosphate Addition on the Dispersion and Hydration of Pure Calcium Aluminate Cement. Materials 2020, 13, 5229. [Google Scholar] [CrossRef]
- Chougan, M.; Ghaffar, S.H.; Jahanzat, M.; Albar, A.; Mujaddedi, N.; Swash, R. The influence of nano-additives in strengthening mechanical performance of 3D printed multi-binder geopolymer composites. Construct. Build. Mater. 2020, 250, 118928. [Google Scholar] [CrossRef]
- Zhang, M.-H.; Islam, J. Use of nano-silica to reduce setting time and increase early strength of concretes with high volumes of fly ash or slag. Construct. Build. Mater. 2012, 29, 573–580. [Google Scholar] [CrossRef]
- Li, W.; Shaikh, F.U.; Wang, L.; Lu, Y.; Wang, B.; Jiang, C.; Su, Y. Experimental study on shear property and rheological characteristic of superfine cement grouts with nano-SiO2 addition. Construct. Build. Mater. 2019, 228, 117046. [Google Scholar] [CrossRef]
- Polat, R.; Demirboğa, R.; Karagöl, F. The effect of nano-MgO on the setting time, autogenous shrinkage, microstructure and mechanical properties of high performance cement paste and mortar. Construct. Build. Mater. 2017, 156, 208–218. [Google Scholar] [CrossRef]
- Senff, L.; Labrincha, J.A.; Ferreira, V.M.; Hotza, D.; Repette, W.L. Effect of nano-silica on rheology and fresh properties of cement pastes and mortars. Construct. Build. Mater. 2009, 23, 2487–2491. [Google Scholar] [CrossRef]
- Scrivener, K.L.; Kirkpatrick, R.J. Innovation in use and research on cementitious material. Cem. Concr. Res. 2008, 38, 128–136. [Google Scholar] [CrossRef]
- Xu, J.; Wang, B.; Zuo, J. Modification effects of nanosilica on the interfacial transition zone in concrete: A multiscale approach. Cem. Concr. Compos. 2017, 81, 1–10. [Google Scholar] [CrossRef]
- Bernard, E.; Lothenbach, B.; Chlique, C.; Wyrzykowski, M.; Dauzeres, A.; Pochard, I.; Cau-Dit-Coumes, C. Characterization of magnesium silicate hydrate (MSH). Cem. Concr. Res. 2019, 116, 309–330. [Google Scholar] [CrossRef]
- Bernard, E.; Lothenbach, B.; Rentsch, D.; Pochard, I.; Dauzères, A. Formation of magnesium silicate hydrates (MSH). Phys. Chem. Earth Parts A B C 2017, 99, 142–157. [Google Scholar] [CrossRef]
- Unluer, C. Carbon dioxide sequestration in magnesium-based binders. In Carbon Dioxide Sequestration in Cementitious Construction Materials; Elsevier: Amsterdam, The Netherlands, 2018; pp. 129–173. [Google Scholar]
- Bernal, S.A.; de Gutierrez, R.M.; Provis, J.L.; Rose, V. Effect of silicate modulus and metakaolin incorporation on the carbonation of alkali silicate-activated slags. Cem. Concr. Res. 2010, 40, 898–907. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, H.; Provis, J.L.; Bullen, F.; Reid, A.; Zhu, Y. Quantitative kinetic and structural analysis of geopolymers. Part 1. The activation of metakaolin with sodium hydroxide. Thermochim. Acta 2012, 539, 23–33. [Google Scholar] [CrossRef]
- Thomas, J.J.; Jennings, H.M.; Chen, J.J. Influence of nucleation seeding on the hydration mechanisms of tricalcium silicate and cement. J. Phys. Chem. C 2009, 113, 4327–4334. [Google Scholar] [CrossRef]
- Gu, Y.; Ran, Q.; Shu, X.; Yu, C.; Chang, H.; Liu, J. Synthesis of nanoSiO2@ PCE core-shell nanoparticles and its effect on cement hydration at early age. Construct. Build. Mater. 2016, 114, 673–680. [Google Scholar] [CrossRef]
- Feng, P.; Chang, H.; Liu, X.; Ye, S.; Shu, X.; Ran, Q. The significance of dispersion of nano-SiO2 on early age hydration of cement pastes. Mater. Design 2020, 186, 108320. [Google Scholar] [CrossRef]
- Mehta, P.K.; Monteiro, P.J. Concrete Microstructure, Properties and Materials; McGraw-Hill Education: New York, NY, USA, 2017. [Google Scholar]
- Duxson, P.; Lukey, G.C.; van Deventer, J.S. Physical evolution of Na-geopolymer derived from metakaolin up to 1000 °C. J. Mater. Sci. 2007, 42, 3044–3054. [Google Scholar] [CrossRef]
- Rivera, O.; Long, W.; Weiss, C., Jr.; Moser, R.; Williams, B.; Torres-Cancel, K.; Gore, E.; Allison, P. Effect of elevated temperature on alkali-activated geopolymeric binders compared to portland cement-based binders. Cem. Concr. Res. 2016, 90, 43–51. [Google Scholar] [CrossRef]
- Lothenbach, B.; Le Saout, G.; Gallucci, E.; Scrivener, K. Influence of limestone on the hydration of Portland cements. Cem. Concr. Res. 2008, 38, 848–860. [Google Scholar] [CrossRef]
- Bernal, S.A.; Provis, J.L.; Walkley, B.; San Nicolas, R.; Gehman, J.D.; Brice, D.G.; Kilcullen, A.R.; Duxson, P.; van Deventer, J.S. Gel nanostructure in alkali-activated binders based on slag and fly ash, and effects of accelerated carbonation. Cem. Concr. Res. 2013, 53, 127–144. [Google Scholar] [CrossRef]
- Rozov, K.; Berner, U.; Taviot-Gueho, C.; Leroux, F.; Renaudin, G.; Kulik, D.; Diamond, L.W. Synthesis and characterization of the LDH hydrotalcite–pyroaurite solid-solution series. Cem. Concr. Res. 2010, 40, 1248–1254. [Google Scholar] [CrossRef]
- Myers, R.J.; Lothenbach, B.; Bernal, S.A.; Provis, J.L. Thermodynamic modelling of alkali-activated slag cements. Appl. Geochem. 2015, 61, 233–247. [Google Scholar] [CrossRef]
- Jeon, I.K.; Qudoos, A.; Jakhrani, S.H.; Kim, H.G.; Ryou, J.-S. Investigation of sulfuric acid attack upon cement mortars containing silicon carbide powder. Powder Technol. 2020, 359, 181–189. [Google Scholar] [CrossRef]
- Zhang, B.; Tan, H.; Shen, W.; Xu, G.; Ma, B.; Ji, X. Nano-silica and silica fume modified cement mortar used as Surface Protection Material to enhance the impermeability. Cem. Concr. Compos. 2018, 92, 7–17. [Google Scholar] [CrossRef]
- Yoon, H.; Park, S.M.; Lee, H.-K. Effect of MgO on chloride penetration resistance of alkali-activated binder. Construct. Build. Mater. 2018, 178, 584–592. [Google Scholar] [CrossRef]
| Concrete Mix | OPC (kg/m3) | MgO (kg/m3) | Nano-SiO2 (kg/m3) | Fine Aggregate (kg/m3) | Coarse Aggregate (kg/m3) | AWA (kg/m3) | SP (kg/m3) | W (kg/m3) |
|---|---|---|---|---|---|---|---|---|
| Control | 440 | – | – | 708 | 889 | 2.4 | 7.92 | 198 |
| NS1 | 4.4 | |||||||
| NS2 | 8.8 | |||||||
| M5 | 418 | 22 | – | |||||
| M10 | 396 | 44 |
| Concrete Mix | Peak Time (min) | Peak Temperature (∆ °C) |
|---|---|---|
| Control | 940 | 2.40 |
| NS1 | 784 | 3.73 |
| NS2 | 696 | 3.38 |
| M5 | 1222 | 1.81 |
| M10 | 1492 | 1.92 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeon, I.K.; Woo, B.H.; Yoo, D.H.; Ryou, J.S.; Kim, H.G. Evaluation of the Hydration Characteristics and Anti-Washout Resistance of Non-Dispersible Underwater Concrete with Nano-SiO2 and MgO. Materials 2021, 14, 1328. https://doi.org/10.3390/ma14061328
Jeon IK, Woo BH, Yoo DH, Ryou JS, Kim HG. Evaluation of the Hydration Characteristics and Anti-Washout Resistance of Non-Dispersible Underwater Concrete with Nano-SiO2 and MgO. Materials. 2021; 14(6):1328. https://doi.org/10.3390/ma14061328
Chicago/Turabian StyleJeon, In Kyu, Byeong Hun Woo, Dong Ho Yoo, Jae Suk Ryou, and Hong Gi Kim. 2021. "Evaluation of the Hydration Characteristics and Anti-Washout Resistance of Non-Dispersible Underwater Concrete with Nano-SiO2 and MgO" Materials 14, no. 6: 1328. https://doi.org/10.3390/ma14061328
APA StyleJeon, I. K., Woo, B. H., Yoo, D. H., Ryou, J. S., & Kim, H. G. (2021). Evaluation of the Hydration Characteristics and Anti-Washout Resistance of Non-Dispersible Underwater Concrete with Nano-SiO2 and MgO. Materials, 14(6), 1328. https://doi.org/10.3390/ma14061328







