Carbon Dot/Naphthalimide Based Ratiometric Fluorescence Biosensor for Hyaluronidase Detection
Abstract
1. Introduction
2. Material and Methods
2.1. Chemicals and Reagents
2.2. Instrumentation
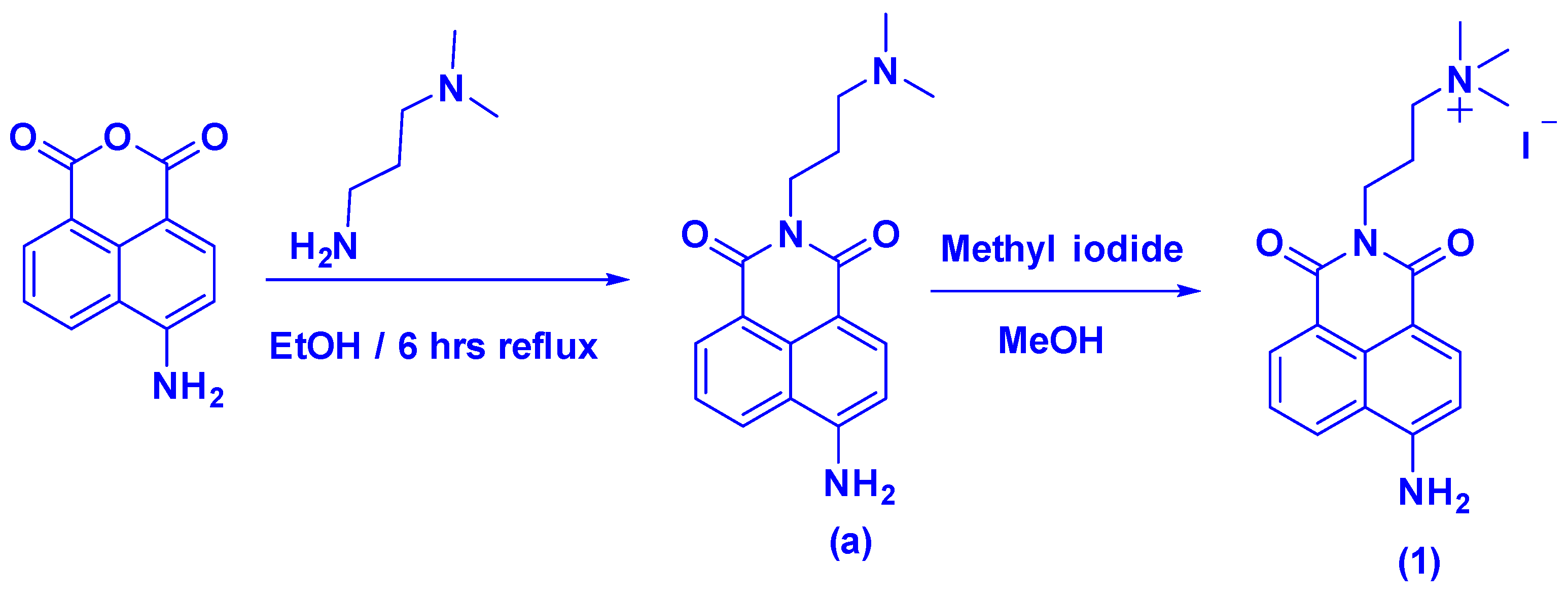
2.3. Synthesis of Naphthalimide Probe 1
2.3.1. Compound (a)
2.3.2. Probe 1
2.4. Synthesis of Cationic Carbon Dots (CQDs)
2.5. Detection of HAase
3. Result and Discussions
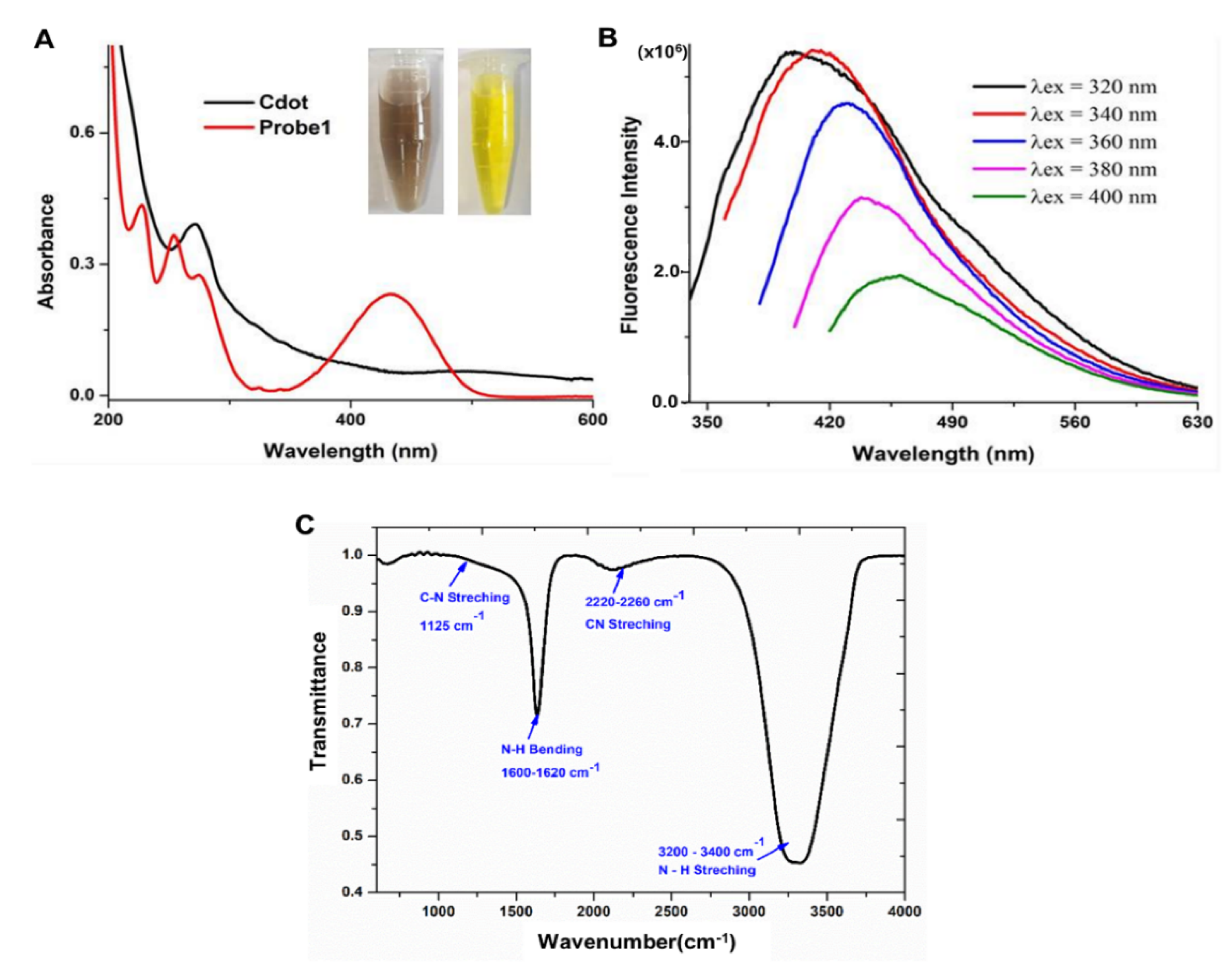
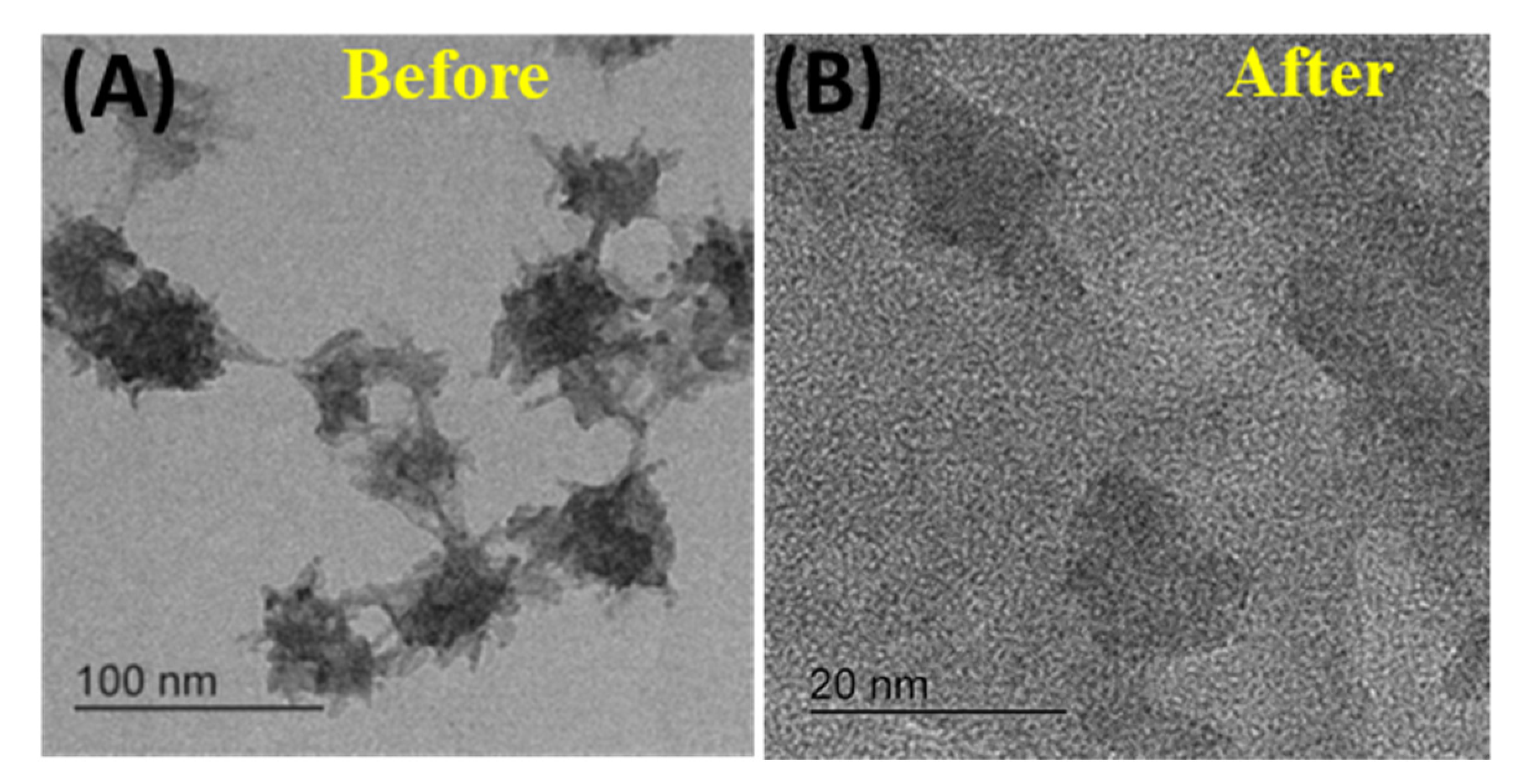
3.1. Synthesis and Characterization of Carbon Dots
3.2. Synthesis of Naphthalimide Probe 1
3.3. Sensing Mechanism
3.4. Fluorescence Sensing
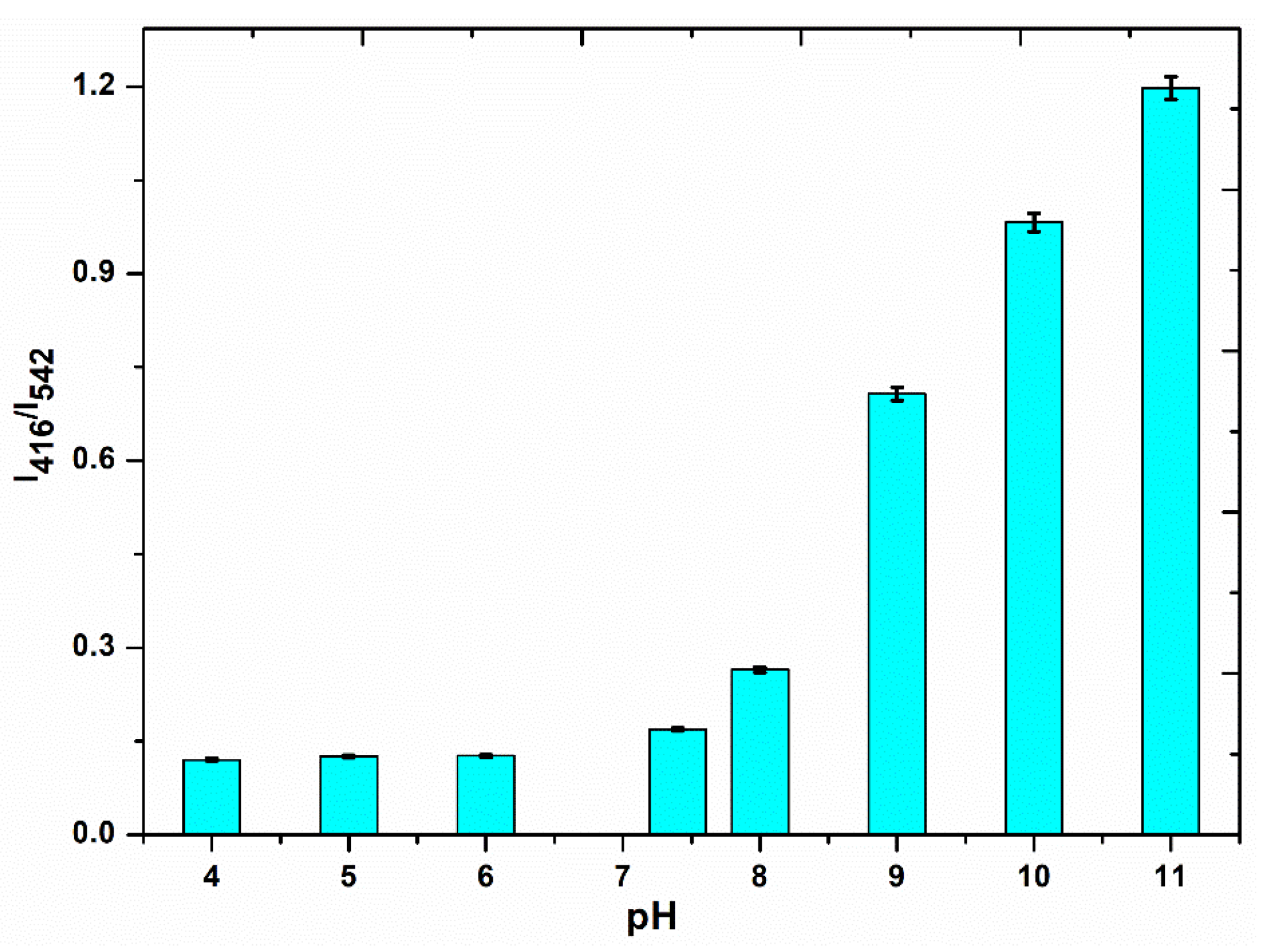
3.4.1. Optimization of Experimental Conditions
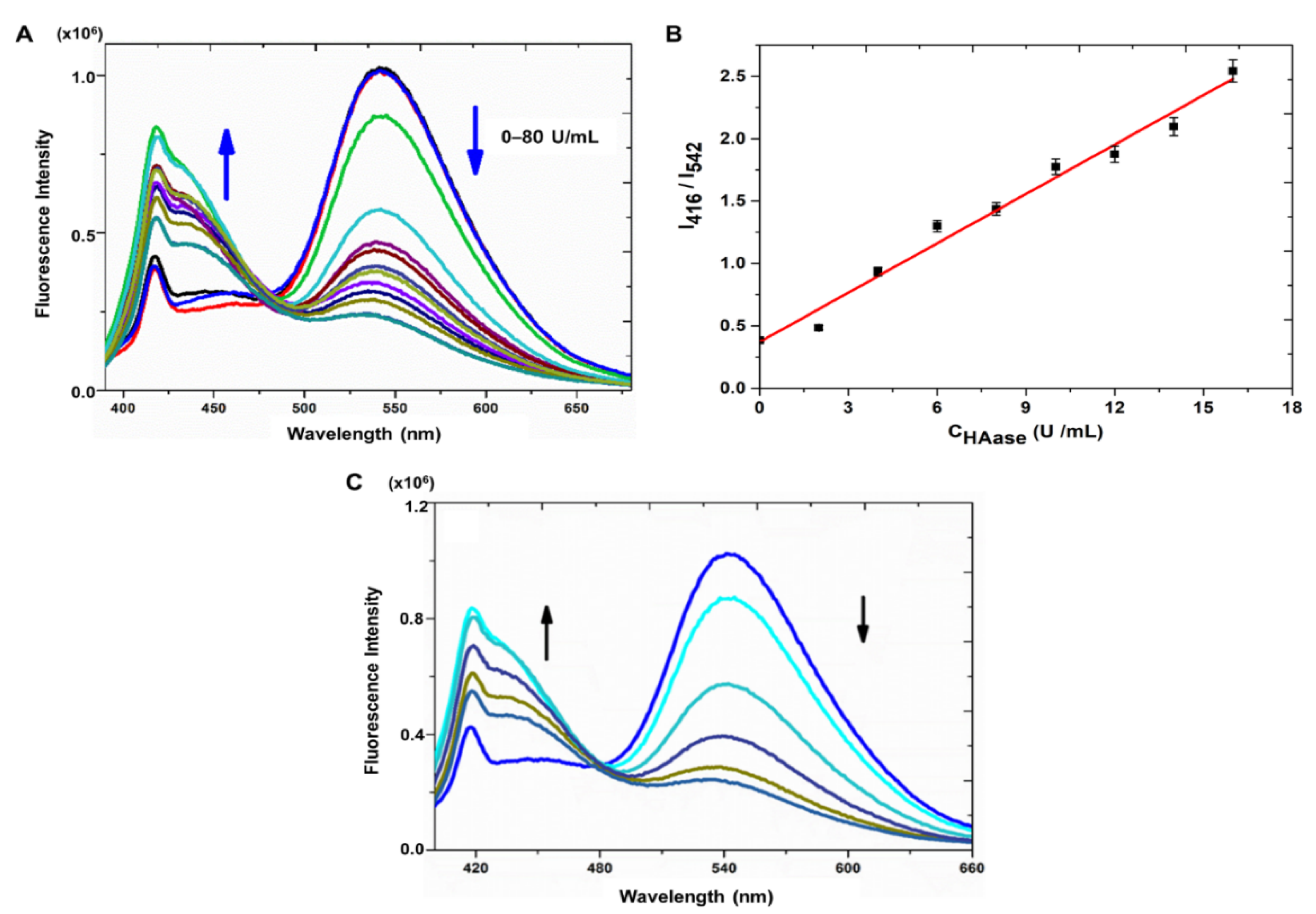
3.4.2. Performance of Sensor System
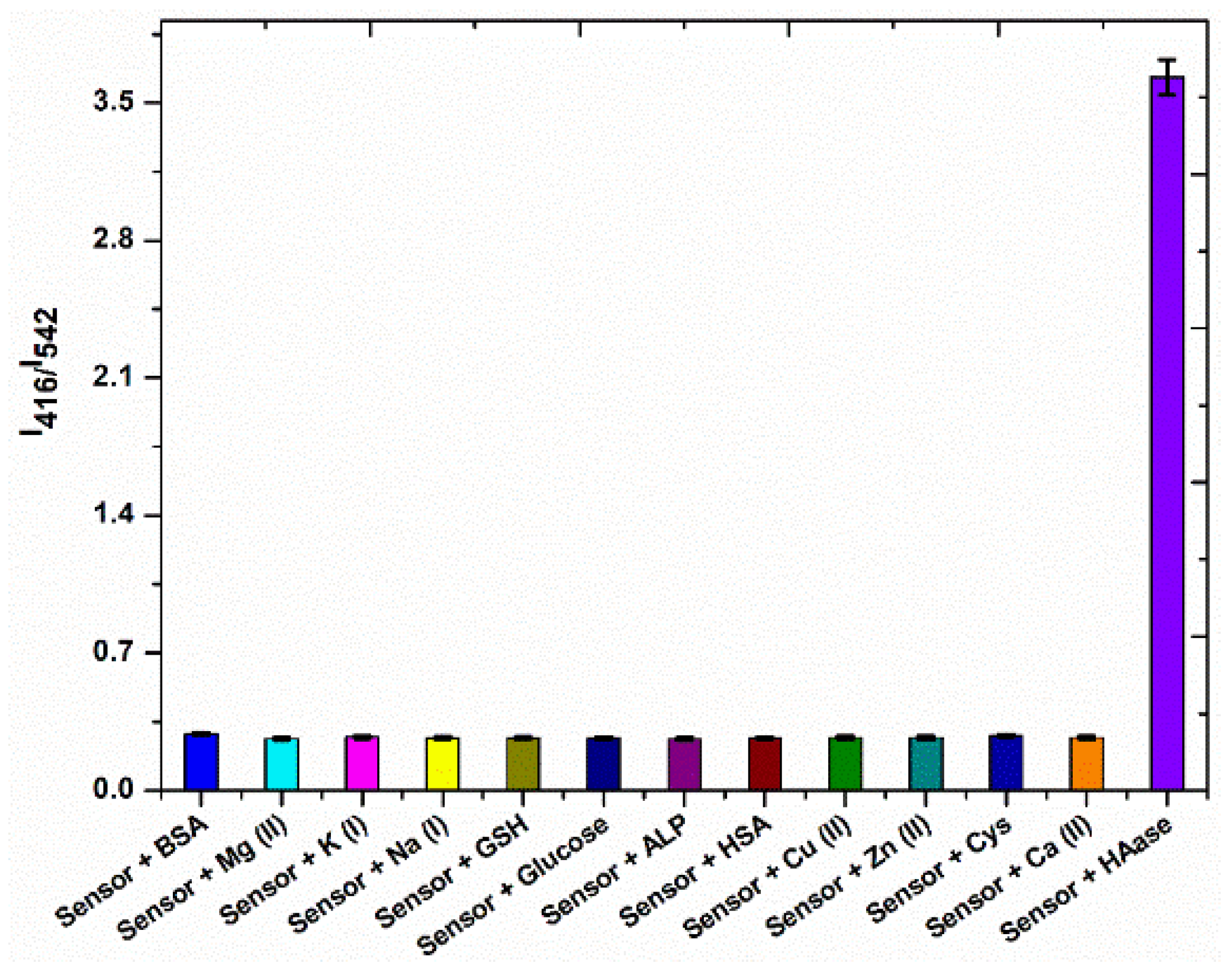
3.5. Selectivity Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lapčík, L.; Lapčík, L.; De Smedt, S.; Demeester, J.; Chabreček, P. Hyaluronan: Preparation, Structure, Properties, and Applications. Chem. Rev. 1998, 98, 2663–2684. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Song, C.; Li, H.; Zhang, R.; Jiang, R.; Liu, X.; Zhang, G.; Fan, Q.; Wang, L.; Huang, W. Cationic Conjugated Polymer/Hyaluronan-Doxorubicin Complex for Sensitive Fluorescence Detection of Hyaluronidase and Tumor-Targeting Drug Delivery and Imaging. ACS Appl. Mater. Interfaces 2015, 7, 21529–21537. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Gao, W.; Han, X.; Qu, F.; Xia, L.; Kong, R.-M. A label-free and fluorescence turn-on assay for sensitive detection of hyaluronidase based on hyaluronan-induced perylene self-assembly. New J. Chem. 2019, 43, 3383–3389. [Google Scholar] [CrossRef]
- Yang, W.; Ni, J.; Luo, F.; Weng, W.; Wei, Q.; Lin, Z.; Chen, G. Cationic carbon dots for modification-free detection of hyaluronidase via an electrostatic-controlled ratiometric fluorescence assay. Anal. Chem. 2017, 89, 8384–8390. [Google Scholar] [CrossRef] [PubMed]
- Turley, E.A.; Noble, P.W.; Bourguignon, L.Y.W. Signaling properties of hyaluronan receptors. J. Biol. Chem. 2002, 277, 4589–4592. [Google Scholar] [CrossRef]
- Lokeshwar, V.B.; Selzer, M.G. Hyaluronidase: Both a tumor promoter and suppressor. In Hyaluronan in Cancer Biology; Elsevier: Amsterdam, The Netherlands, 2009; pp. 189–206. [Google Scholar]
- Whatcott, C.J.; Han, H.; Posner, R.G.; Hostetter, G.; Von Hoff, D.D. Targeting the tumor microenvironment in cancer: Why hyaluronidase deserves a second look. Cancer Discov. 2011, 1, 291–296. [Google Scholar] [CrossRef]
- Lokeshwar, V.B.; Rubinowicz, D.; Schroeder, G.L.; Forgacs, E.; Minna, J.D.; Block, N.L.; Nadji, M.; Lokeshwar, B.L. Stromal and epithelial expression of tumor markers hyaluronic acid and HYAL1 hyaluronidase in prostate cancer. J. Biol. Chem. 2001, 276, 11922–11932. [Google Scholar] [CrossRef]
- Tan, J.; Wang, X.; Li, H.; Su, X.; Wang, L.; Ran, L.; Zheng, K.; Ren, G. HYAL1 overexpression is correlated with the malignant behavior of human breast cancer. Int. J. Cancer 2011, 128, 1303–1315. [Google Scholar] [CrossRef]
- Martinez-Quintanilla, J.; He, D.; Wakimoto, H.; Alemany, R.; Shah, K. Encapsulated stem cells loaded with hyaluronidase-expressing oncolytic virus for brain tumor therapy. Mol. Ther. 2015, 23, 108–118. [Google Scholar] [CrossRef]
- Yang, K.; Liu, M.; Wang, Y.; Wang, S.; Miao, H.; Yang, L.; Yang, X. Carbon dots derived from fungus for sensing hyaluronic acid and hyaluronidase. Sens. Actuators B Chem. 2017, 251, 503–508. [Google Scholar] [CrossRef]
- Eissa, S.; Kassim, S.K.; Labib, R.A.; El-Khouly, I.M.; Ghaffer, T.M.; Sadek, M.; Razek, O.A.; El-Ahmady, O. Detection of bladder carcinoma by combined testing of urine for hyaluronidase and cytokeratin 20 RNAs. Cancer 2005, 103, 1356–1362. [Google Scholar] [CrossRef] [PubMed]
- Thompson, C.B.; Shepard, H.M.; O’Connor, P.M.; Kadhim, S.; Jiang, P.; Osgood, R.J.; Bookbinder, L.H.; Li, X.; Sugarman, B.J.; Connor, R.J. Enzymatic depletion of tumor hyaluronan induces antitumor responses in preclinical animal models. Mol. Cancer Ther. 2010, 9, 3052–3064. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Yan, X.; Lai, Q.; Su, X. Bimetallic gold/silver nanoclusters-gold nanoparticles based fluorescent sensing platform via the inner filter effect for hyaluronidase activity detection. Sens. Actuators B Chem. 2019, 282, 45–51. [Google Scholar] [CrossRef]
- Hu, P.; Chen, Y.; Liu, Y. Hyaluronan/Ru(ii)-cyclodextrin supramolecular assemblies for colorimetric sensor of hyaluronidase activity. RSC Adv. 2015, 5, 99240–99244. [Google Scholar] [CrossRef]
- Magalhães, M.R.; da Silva, N.J., Jr.; Ulhoa, C.J. A hyaluronidase from Potamotrygon motoro (freshwater stingrays) venom: Isolation and characterization. Toxicon 2008, 51, 1060–1067. [Google Scholar] [CrossRef]
- Vercruysse, K.P.; Lauwers, A.R.; Demeester, J.M. Absolute and empirical determination of the enzymatic activity and kinetic investigation of the action of hyaluronidase on hyaluronan using viscosimetry. Biochem. J. 1995, 306, 153–160. [Google Scholar] [CrossRef]
- Kim, J.-W.; Kim, J.H.; Chung, S.J.; Chung, B.H. An operationally simple colorimetric assay of hyaluronidase activity using cationic gold nanoparticles. Analyst 2009, 134, 1291–1293. [Google Scholar] [CrossRef]
- Nossier, A.I.; Eissa, S.; Ismail, M.F.; Hamdy, M.A.; Azzazy, H.M.E.-S. Direct detection of hyaluronidase in urine using cationic gold nanoparticles: A potential diagnostic test for bladder cancer. Biosens. Bioelectron. 2014, 54, 7–14. [Google Scholar] [CrossRef]
- Steiner, B.; Cruce, D. A zymographic assay for detection of hyaluronidase activity on polyacrylamide gels and its application to enzymatic activity found in bacteria. Anal. Biochem. 1992, 200, 405–410. [Google Scholar] [CrossRef]
- Jayadev, C.; Rout, R.; Price, A.; Hulley, P.; Mahoney, D. Hyaluronidase treatment of synovial fluid to improve assay precision for biomarker research using multiplex immunoassay platforms. J. Immunol. Methods 2012, 386, 22–30. [Google Scholar] [CrossRef]
- Ai, H. Fluorescent sensors for biological applications. Sensors 2014, 14, 17829–17831. [Google Scholar] [CrossRef]
- Li, X.; Wu, T.; Fu, Y.; Ding, X.; Li, Z.; Zhu, G.; Fan, J. A high sensitivity background eliminated fluorescence sensing platform for hyaluronidase activity detection based on Si QDs/HA-δ-FeOOH nanoassembly. Biosens. Bioelectron. 2020, 150, 111928. [Google Scholar] [CrossRef]
- Gao, N.; Yang, W.; Nie, H.; Gong, Y.; Jing, J.; Gao, L.; Zhang, X. Turn-on theranostic fluorescent nanoprobe by electrostatic self-assembly of carbon dots with doxorubicin for targeted cancer cell imaging, in vivo hyaluronidase analysis, and targeted drug delivery. Biosens. Bioelectron. 2017, 96, 300–307. [Google Scholar] [CrossRef]
- Panagiotopoulou, M.; Kunath, S.; Medina-Rangel, P.X.; Haupt, K.; Tse Sum Bui, B. Fluorescent molecularly imprinted polymers as plastic antibodies for selective labeling and imaging of hyaluronan and sialic acid on fixed and living cells. Biosens. Bioelectron. 2017, 88, 85–93. [Google Scholar] [CrossRef]
- Hu, Q.; Zeng, F.; Wu, S. A ratiometric fluorescent probe for hyaluronidase detection via hyaluronan-induced formation of red-light emitting excimers. Biosens. Bioelectron. 2016, 79, 776–783. [Google Scholar] [CrossRef]
- Gu, W.; Yan, Y.; Zhang, C.; Ding, C.; Xian, Y. One-Step Synthesis of Water-Soluble MoS2 Quantum Dots via a Hydrothermal Method as a Fluorescent Probe for Hyaluronidase Detection. ACS Appl. Mater. Interfaces 2016, 8, 11272–11279. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Zeng, F.; Wu, S. Ratiometric Fluorescent Biosensor for Hyaluronidase with Hyaluronan As Both Nanoparticle Scaffold and Substrate for Enzymatic Reaction. Biomacromolecules 2014, 15, 3383–3389. [Google Scholar] [CrossRef]
- Wang, Z.; Li, X.; Song, Y.; Li, L.; Shi, W.; Ma, H. An Upconversion Luminescence Nanoprobe for the Ultrasensitive Detection of Hyaluronidase. Anal. Chem. 2015, 87, 5816–5823. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Cai, R.; Yang, L.; Zhang, L.; Jiang, Y.; Yang, Y.; Cui, C.; Wan, S.; Chu, X.; Tan, W. Core–Shell HA-AuNPs@SiNPs Nanoprobe for Sensitive Fluorescence Hyaluronidase Detection and Cell Imaging. ACS Sustain. Chem. Eng. 2018, 6, 16555–16562. [Google Scholar] [CrossRef]
- Ge, M.; Sun, J.; Chen, M.; Tian, J.; Yin, H.; Yin, J. A hyaluronic acid fluorescent hydrogel based on fluorescence resonance energy transfer for sensitive detection of hyaluronidase. Anal. Bioanal. Chem. 2020, 412, 1915–1923. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Ding, F.; Wang, Y.; Lu, Z.; Zou, P.; Wang, X.; Zhao, Q.; Rao, H. A dual-readout nanosensor based on biomass-based C-dots and chitosan@AuNPs with hyaluronic acid for determination of hyaluronidase. Luminescence 2020, 35, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Veale, E.B.; Phelan, C.M.; Murphy, S.A.; Tocci, G.M.; Gillespie, L.J.; Frimannsson, D.O.; Kelly, J.M.; Gunnlaugsson, T. Recent advances in the development of 1,8-naphthalimide based DNA targeting binders, anticancer and fluorescent cellular imaging agents. Chem. Soc. Rev. 2013, 42, 1601–1618. [Google Scholar] [CrossRef] [PubMed]
- Duke, R.M.; Veale, E.B.; Pfeffer, F.M.; Kruger, P.E.; Gunnlaugsson, T. Colorimetric and fluorescent anion sensors: An overview of recent developments in the use of 1,8-naphthalimide-based chemosensors. Chem. Soc. Rev. 2010, 39, 3936–3953. [Google Scholar] [CrossRef] [PubMed]
- Meher, N.; Iyer, P.K. Functional group engineering in naphthalimides: A conceptual insight to fine-tune the supramolecular self-assembly and condensed state luminescence. Nanoscale 2019, 11, 13233–13242. [Google Scholar] [CrossRef]
- Lim, S.Y.; Shen, W.; Gao, Z. Carbon quantum dots and their applications. Chem. Soc. Rev. 2015, 44, 362–381. [Google Scholar] [CrossRef]
- Liu, M.L.; Chen, B.B.; Li, C.M.; Huang, C.Z. Carbon dots: Synthesis, formation mechanism, fluorescence origin and sensing applications. Green Chem. 2019, 21, 449–471. [Google Scholar] [CrossRef]
- Wang, X.; Feng, Y.; Dong, P.; Huang, J. A Mini Review on Carbon Quantum Dots: Preparation, Properties, and Electrocatalytic Application. Front. Chem. 2019, 7, 671. [Google Scholar] [CrossRef]
- Jian, H.-J.; Wu, R.-S.; Lin, T.-Y.; Li, Y.-J.; Lin, H.-J.; Harroun, S.G.; Lai, J.-Y.; Huang, C.-C. Super-Cationic Carbon Quantum Dots Synthesized from Spermidine as an Eye Drop Formulation for Topical Treatment of Bacterial Keratitis. ACS Nano 2017, 11, 6703–6716. [Google Scholar] [CrossRef]
- Wang, S.; Cole, I.S.; Zhao, D.; Li, Q. The dual roles of functional groups in the photoluminescence of graphene quantum dots. Nanoscale 2016, 8, 7449–7458. [Google Scholar] [CrossRef]
- Gan, Z.; Xu, H.; Hao, Y. Mechanism for excitation-dependent photoluminescence from graphene quantum dots and other graphene oxide derivates: Consensus, debates and challenges. Nanoscale 2016, 8, 7794–7807. [Google Scholar] [CrossRef]
- Maiti, S.; Das, K.; Das, P.K. Label-free fluorimetric detection of histone using quaternized carbon dot–DNA nanobiohybrid. Chem. Commun. 2013, 49, 8851–8853. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Li, C.; Ren, Y.; Sun, X.; Pan, W.; Li, Y.; Wang, J.; Wang, W. Carbon dots: Surface engineering and applications. J. Mater. Chem. B 2016, 4, 5772–5788. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Fujii, M.; Yamaji, M.; Okamoto, H. Fluorescence behaviour of 2-, 3- and 4-amino-1,8-naphthalimides: Effects of the substitution positions of the amino functionality on the photophysical properties. Photochem. Photobiol. Sci. 2018, 17, 1319–1328. [Google Scholar] [CrossRef] [PubMed]
- Marei, W.F.A.; Salavati, M.; Fouladi-Nashta, A.A. Critical role of hyaluronidase-2 during preimplantation embryo development. Mol. Hum. Reprod. 2013, 19, 590–599. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raj, P.; Lee, S.-y.; Lee, T.Y. Carbon Dot/Naphthalimide Based Ratiometric Fluorescence Biosensor for Hyaluronidase Detection. Materials 2021, 14, 1313. https://doi.org/10.3390/ma14051313
Raj P, Lee S-y, Lee TY. Carbon Dot/Naphthalimide Based Ratiometric Fluorescence Biosensor for Hyaluronidase Detection. Materials. 2021; 14(5):1313. https://doi.org/10.3390/ma14051313
Chicago/Turabian StyleRaj, Pushap, Seon-yeong Lee, and Tae Yoon Lee. 2021. "Carbon Dot/Naphthalimide Based Ratiometric Fluorescence Biosensor for Hyaluronidase Detection" Materials 14, no. 5: 1313. https://doi.org/10.3390/ma14051313
APA StyleRaj, P., Lee, S.-y., & Lee, T. Y. (2021). Carbon Dot/Naphthalimide Based Ratiometric Fluorescence Biosensor for Hyaluronidase Detection. Materials, 14(5), 1313. https://doi.org/10.3390/ma14051313




