Reduced Carbonation, Sulfate and Chloride Ingress Due to the Substitution of Cement by 10% Non-Precalcined Bentonite
Abstract
:1. Introduction
2. Materials and Methods
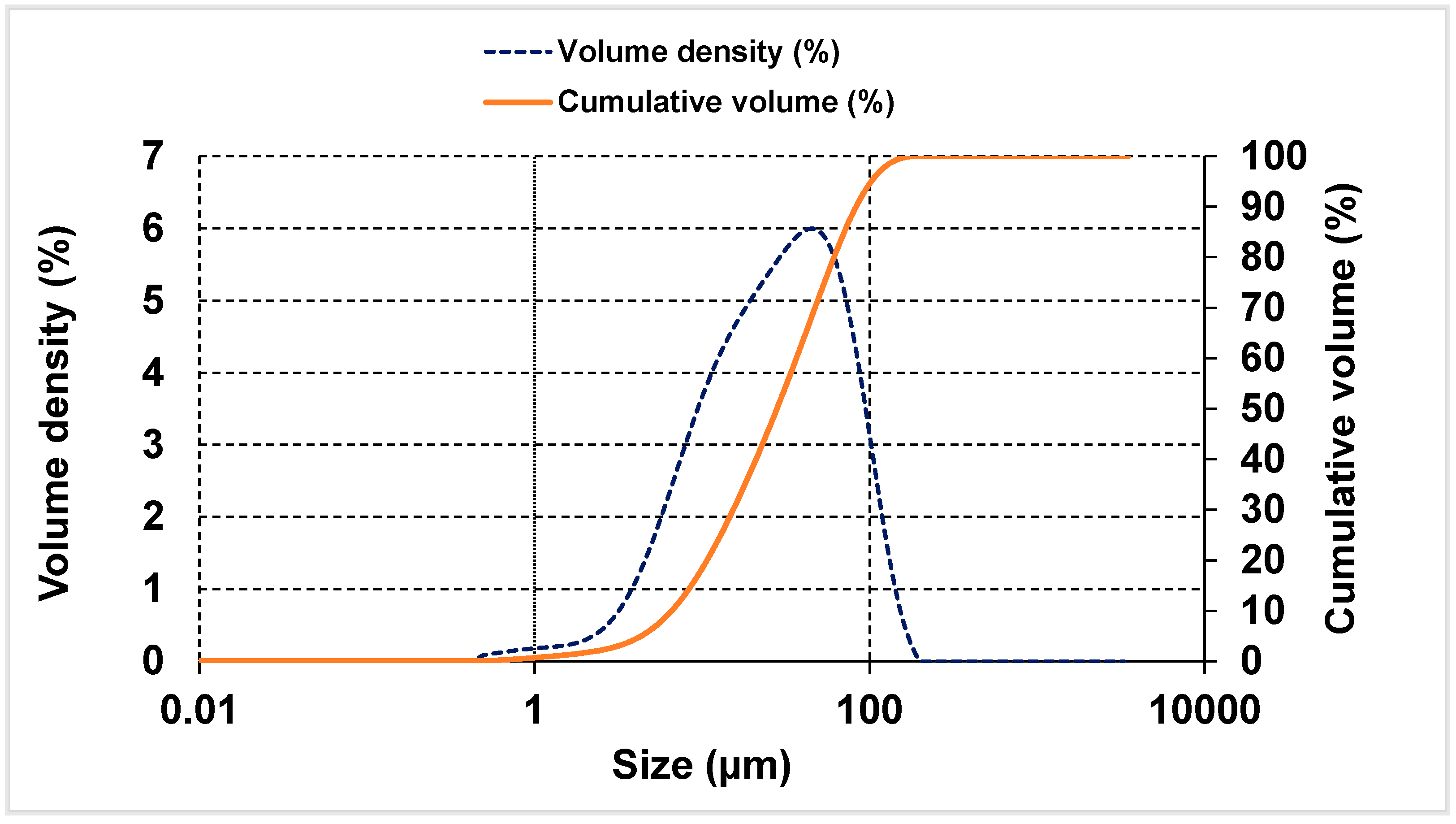
2.1. Materials
2.2. Specimen Types
- -
- For mechanical strength and carbonation resistance testing, 10 × 10 × 60 mm cement paste specimens bearing 10%, 20% or 30% bentonite additions were prepared at a water/cement ratio of 0.5. They were cured in a climatic chamber at 90% relative humidity, first in the moulds for 24 h and after removal for 28 d prior to testing.
- -
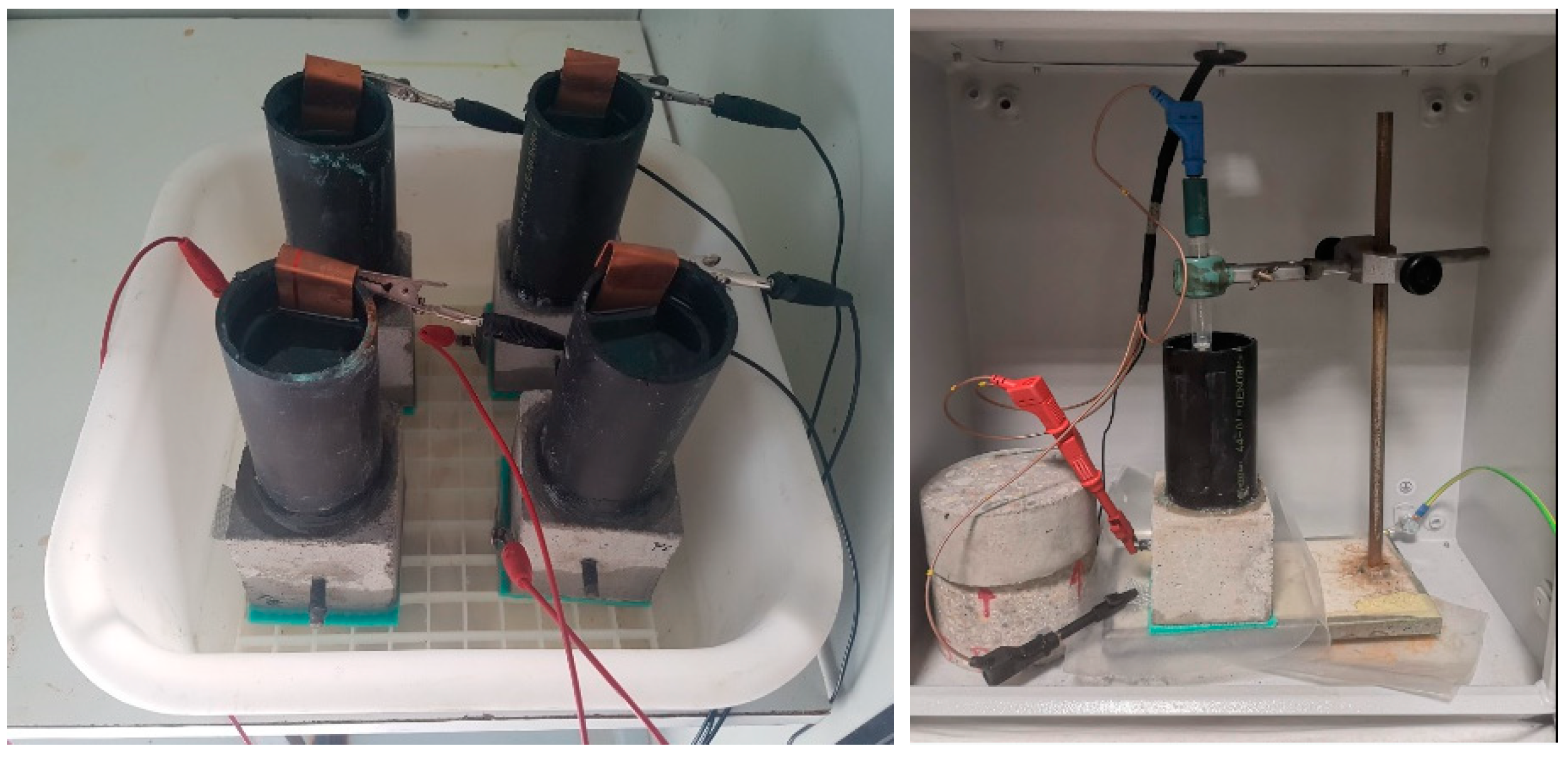
- For chloride diffusions, the 70 cubic mm cement mortar specimens used were prepared with a water/cement ratio of 0.5. They were cured in a climatic chamber at 90% relative humidity, first in the moulds for 24 h and after removal for 28 d prior to application of an electric current to test for chloride diffusion.
2.3. Test Methods
2.3.1. X-ray Diffraction
2.3.2. Twenty-Eight Day Flexural and Compressive Strength
2.3.3. Carbonation in Natural Environments
2.3.4. Sulfate Resistance: The Koch–Steinegger Method
2.3.5. Accelerated Chloride Ingress
3. Results
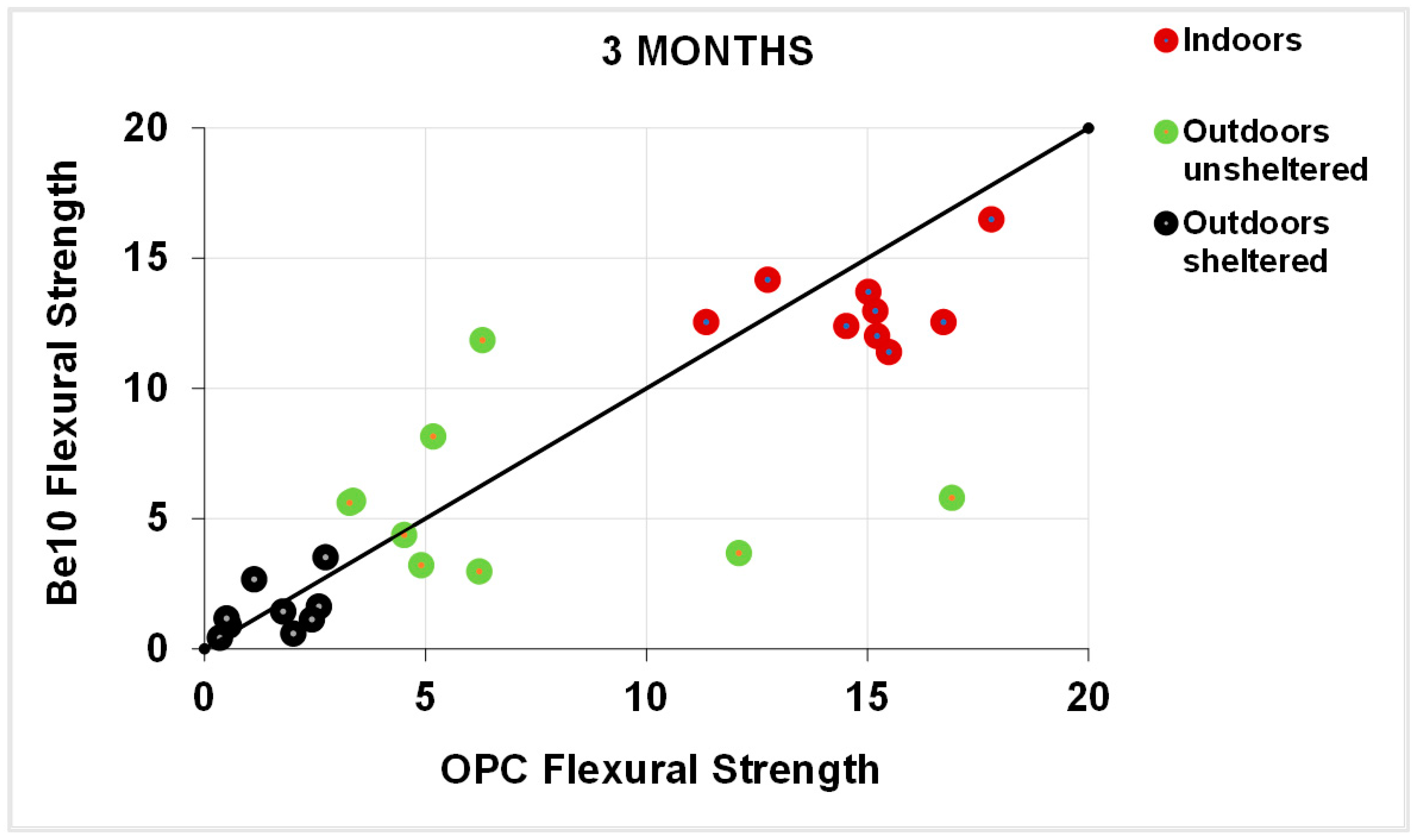
3.1. Flexural and Comprenssive Strength
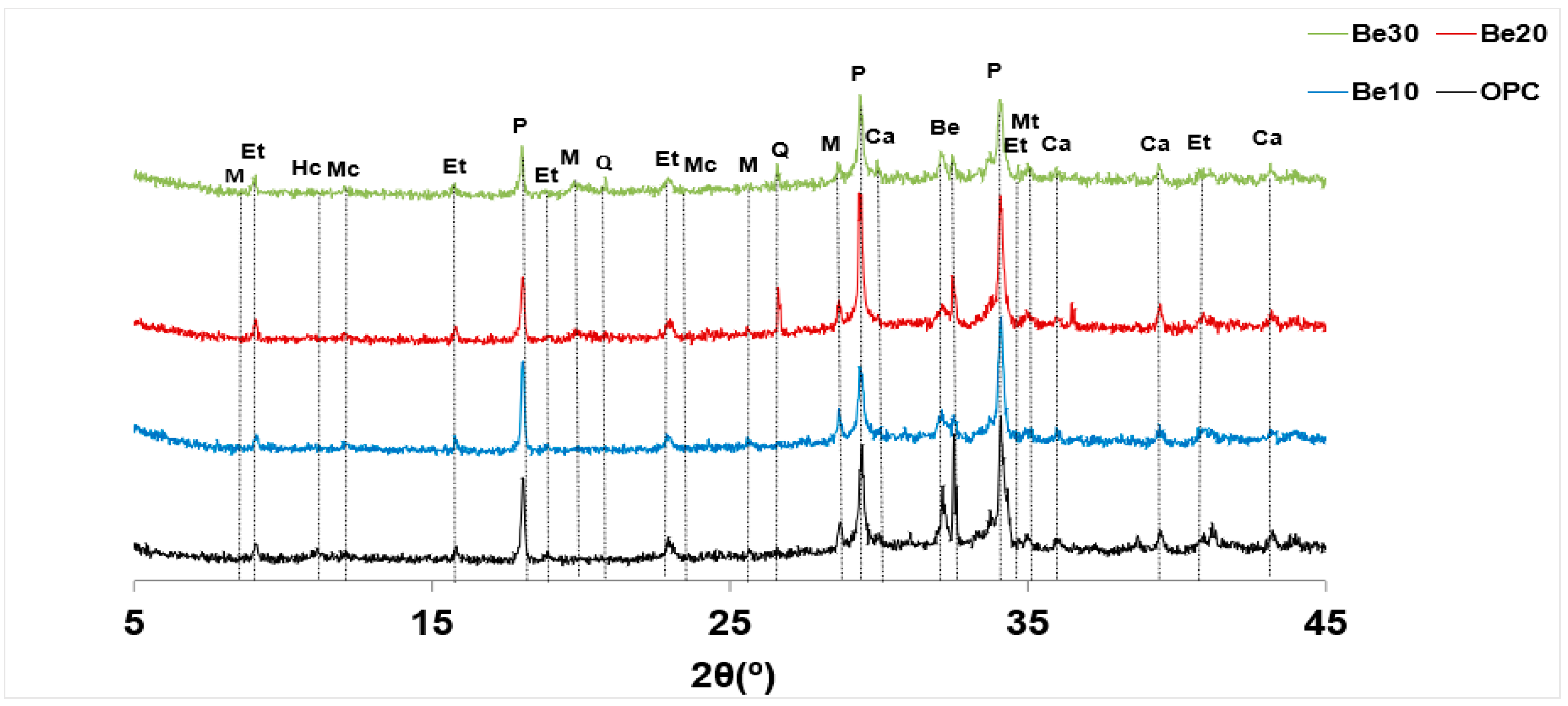
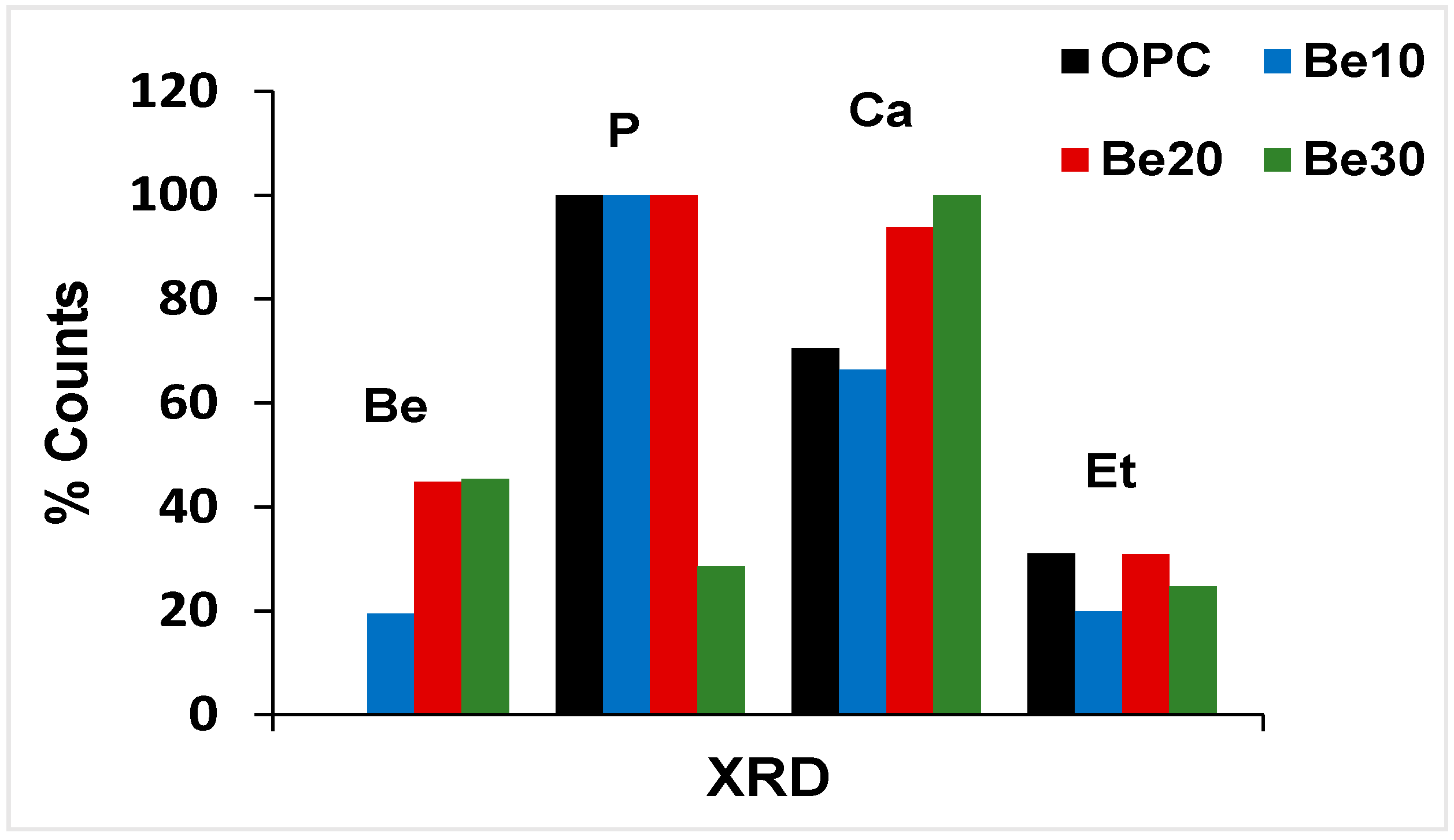
3.2. X-ray Diffraction-Based Characterisation
3.3. Sulfate Attack
3.4. Chloride Resistance Test
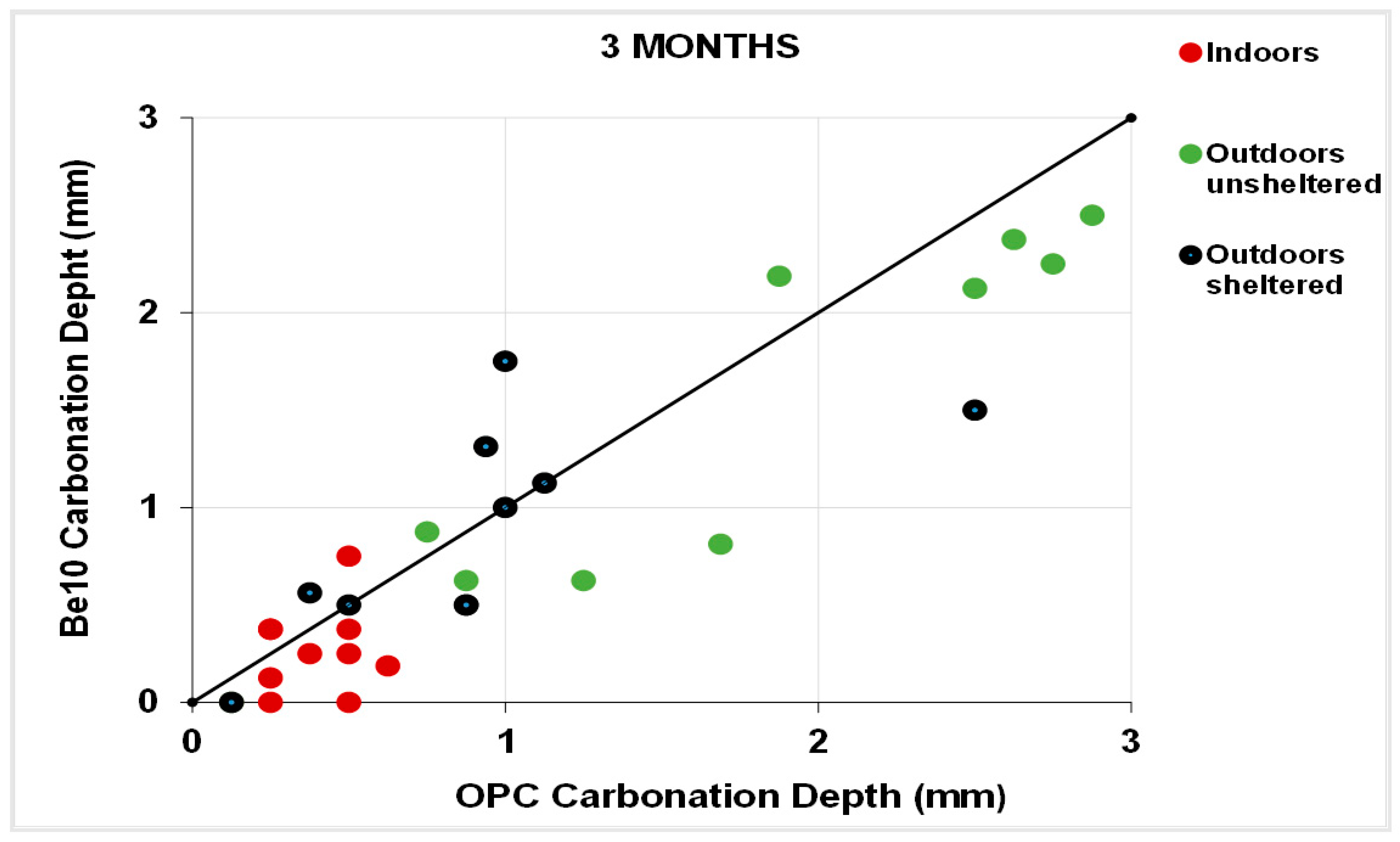
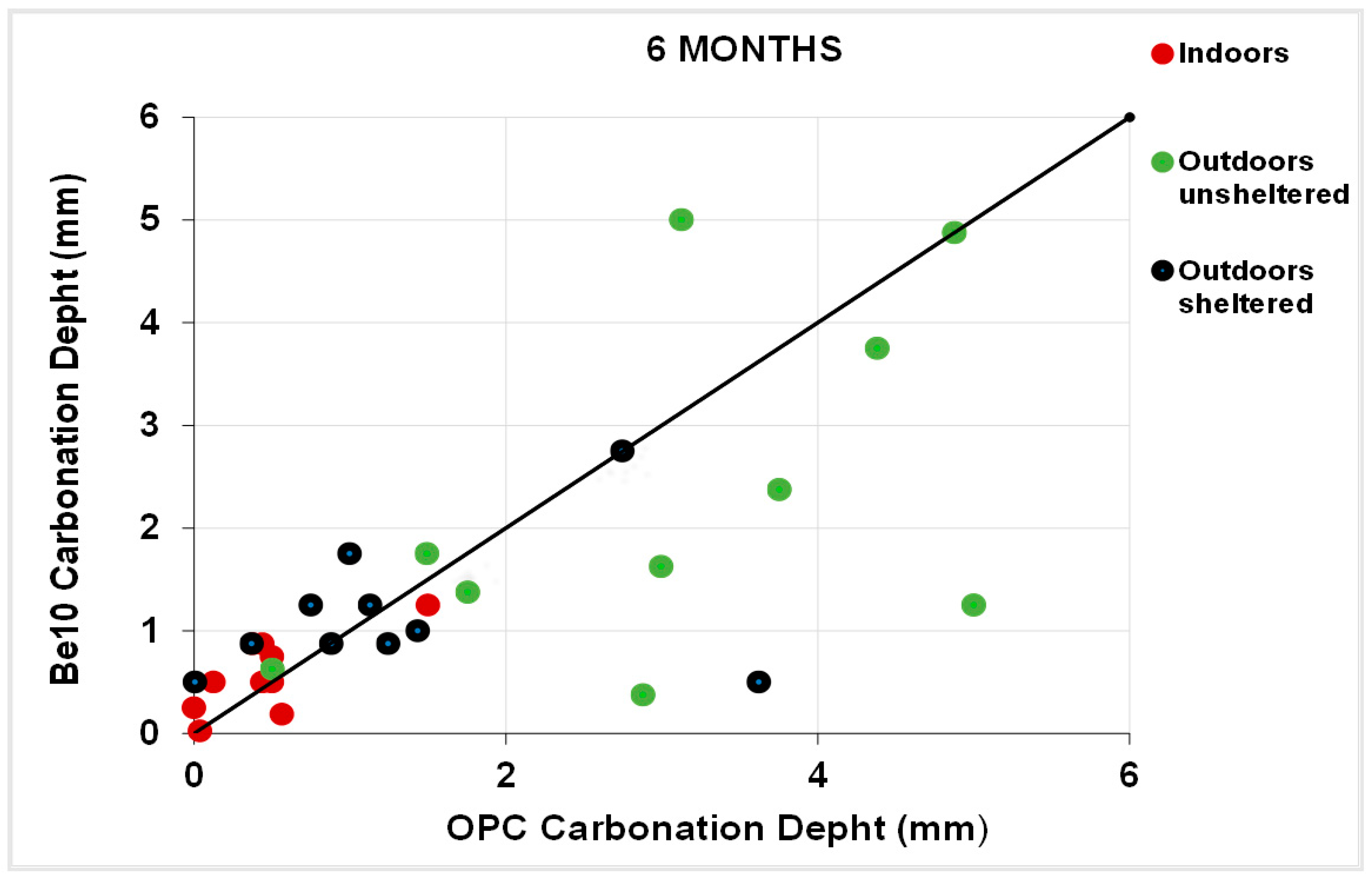
3.5. Natural Carbonation
4. Discussion
- -
- They must be inert or at least not induce expansion or degenerative reactions.
- -
- They must improve or at least not alter concrete volume stability (in terms of shrinkage and creep especially).
- -
- They must improve or at least not alter mechanical performance.
- -
- They must lengthen or at least not shorten concrete or steel durability.
5. Conclusions
- Replacing up to 20% cement with bentonite enhances flexural, and up to 10%, compressive strength.
- At 10%, the addition induces no change in cement crystalline hydration products or in the components of bentonite itself (>95% montmorillonite further to supplier specifications).
- Replacing 10% of the cement with bentonite:
- a.
- In a sulfate solution for 56 d raises cement paste mechanical strength relative to the same materials stored in distilled water, attributed to greater age in the absence of expansive reactions;
- b.
- Lowers the chloride diffusion coefficient significantly;
- c.
- Reduces or maintains the carbonation depth observed in the reference material, deemed to be a very promising development.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gartner, E.; Hirao, H. A review of alternative approaches to the reduction of CO2 emissions associated with the manufacture of the binder phase in concrete. Cem. Concr. Res. 2015, 78, 126–142. [Google Scholar] [CrossRef] [Green Version]
- Gartner, E. Industrially interesting approaches to “low-CO2” cements. Cem. Concr. Res. 2004, 34, 1489–1498. [Google Scholar] [CrossRef]
- Taylor, H.F.W. Cement Chemistry, 2nd ed.; Thomas Telford: London, UK, 1997. [Google Scholar]
- Sanjuán, M.Á.; Andrade, C.; Mora, P.; Zaragoza, A. Carbon Dioxide Uptake by Cement-Based Materials: A Spanish Case Study. Appl. Sci. 2020, 10, 339. [Google Scholar] [CrossRef] [Green Version]
- Sanjuán, M.A.; Argiz, C.; Mora, P.; Zaragoza, A. Carbon Dioxide Uptake in the Roadmap 2050 of the Spanish Cement Industry. Energies 2020, 13, 3452. [Google Scholar]
- Plaza, M.G.; Martínez, S.; Rubiera, F. CO2 Capture, Use, and Storage in the Cement Industry: State of the Art and Expectations. Energies 2020, 13, 5692. [Google Scholar] [CrossRef]
- Haneklaus, N.; Zheng, Y.; Allelein, H.-J. Stop Smoking—Tube-In-Tube Helical System for Flameless Calcination of Minerals. Processes 2017, 5, 67. [Google Scholar] [CrossRef] [Green Version]
- Schneider, M.; Romer, M.; Tschudin, M.; Bolio, H. Sustainable cement production: Present and future. Cem. Concr. Res. 2011, 41, 642–650. [Google Scholar] [CrossRef]
- Secretary of State for Energy of the Ministry for the Ecological Transition (2019) Energy in Spain 2017. National State Administration Publications, Madrid, Spain. (In Spanish). Available online: https://energia.gob.es/balances/Balances/LibrosEnergia/Libro-Energia-2017.pdf (accessed on 2 February 2021).
- International Energy Agency. IEA (2018) Coal 2018. IEA Publications: Paris, France. Available online: https://www.iea.org/reports/coal-2018 (accessed on 8 February 2021).
- Van Olphen, H. An Introduction to Clay Colloid Chemistry; Interscience Publishers: New York, NY, USA, 1963. [Google Scholar]
- Fernandez, R.; Martirena, F.; Scrivener, K.L.B. The origin of the pozzolanic activity of calcined clay minerals: A comparison between kaolinite, illite and montmorillonite. Cem. Concr. Res. 2011, 41, 113–122. [Google Scholar] [CrossRef]
- Courard, L.; Darimont, A.; Schouterden, M.; Ferauche, F.; Willem, X.; Degeimbre, R. Durability of mortars modified with metakaolin. Cem. Concr. Res. 2003, 33, 1473–1479. [Google Scholar] [CrossRef]
- Lima Souza, P.S.; Dal Molin, D.C.C. Viability of using calcined clays from industrial by-products. as pozzolans of high reactivity. Cem. Concr. Res. 2005, 35, 1993–1998. [Google Scholar] [CrossRef]
- Pillai, R.G.; Gettu, R.; Santhanam, M.; Rengaraju, S.; Dhandapani, Y.; Rathnarajan, S.; Basavaraj, A.S. Service life and life cycle assessment of reinforced concrete systems with limestone calcined clay cement (LC3). Cem. Concr. Res. 2019, 118, 111–119. [Google Scholar] [CrossRef]
- Memon, S.A.; Arsalan, R.; Khan, S.; Lo, T.Y. Utilization of Pakistani bentonite as partial replacement of cement in concrete. Constr. Build. Mater. 2012, 30, 237–242. [Google Scholar] [CrossRef]
- Liu, M.; Hu, Y.; Lai, Z.; Yan, T.; He, X.; Wu, J.; Lu, Z.; Lv, S. Influence of various bentonites on the mechanical properties and impermeability of cement mortars. Cem. Concr. Res. 2020, 241, 118015. [Google Scholar] [CrossRef]
- Huang, W.-H. Properties of cement-fly ash grout admixed with bentonite. Silica fume and organic fiber. Cem. Concr. Res. 1997, 27, 395–406. [Google Scholar] [CrossRef]
- Wei, J.; Gencturk, B. Hydration of ternary Portland cement blends containing metakaolin and sodium bentonite. Cem. Concr. Res. 2019, 123, 105772. [Google Scholar] [CrossRef]
- Masood, B.; Elahi, A.; Barbhuiya, S.; Ali, B. Mechanical and durability performance of recycled aggregate concrete incorporating low calcium bentonite. Constr. Build. Mater. 2020, 237, 117760. [Google Scholar] [CrossRef]
- Rehman, S.-U.; Kiani, U.A.; Yaqub, M.; Ali, T. Controlling natural resources depletion through Montmorillonite replacement for cement-low cost construction. Constr. Build. Mater. 2020, 232, 117188. [Google Scholar] [CrossRef]
- Yang, H.; Long, D.; Lai, Z.; He, Y.; Yan, T.; He, X.; Wu, J.; Lu, Z. Effects of bentonite on pore structure and permeability of cement mortar. Constr. Build. Mater. 2019, 224, 276–283. [Google Scholar] [CrossRef]
- Zhao, N.; Wang, S.; Wang, C.; Quan, X.; Yan, Q.; Li, B. Study on the durability of engineered cementitious composites (ECCs) containing high-volume fly ash and bentonite against the combined attack of sulfate and freezing-thawing (F-T). Constr. Build. Mater. 2020, 233, 117313. [Google Scholar] [CrossRef]
- Terzic, A.; Pezo, L.; Mijatovic, N.; Stojanovic, J.; Kragovic, M.; Milicic, L.; Andric, L. The effect of alternations in mineral additives (zeolite. bentonite. fly ash) on physico-chemical behavior of Portland cement based binders. Constr. Build. Mater. 2018, 180, 199–210. [Google Scholar] [CrossRef]
- Sha, F.; Li, S.; Liu, R.; Li, Z.; Zhang, Q. Experimental study on performance of cement-based grouts admixed with fly ash, bentonite, superplasticizer and water glass. Constr. Build. Mater. 2018, 161, 282–291. [Google Scholar] [CrossRef]
- Kalpokaité-Dickuviené, R.; Lukosiuté, I.; Cesniené, J.; Brinkiené, K.; Baltusnikas, A. Cement substitution by organoclay. The role of organoclay type. Cem. Concr. Compos. 2015, 62, 90–96. [Google Scholar] [CrossRef]
- Ata, A.A.; Salem, T.N.; Elkhawas, N.M. Properties of soil bentonite-cement bypass mixture for cutoff walls. Constr. Build. Mater. 2015, 93, 950–956. [Google Scholar] [CrossRef]
- Garvinô, S.L.; Hayles, C.S. The chemical compatibility of cement-bentonite cut-off wall material. Constr. Build. Mater. 1999, 13, 329–341. [Google Scholar] [CrossRef]
- Trivedi, D.P.; Holmes, R.G.G.; Brown, D. Monitoring the in-situ performance of a cement/bentonite cut-off wall at a low level waste disposal site. Cem. Concr. Res. 1992, 22, 339–349. [Google Scholar] [CrossRef]
- Lei, L.; Plank, J. A study on the impact of different clay minerals on the dispersing force of conventional and modified vinyl ether based polycarboxylate superplasticizers. Cem. Concr. Res. 2014, 60, 1–10. [Google Scholar] [CrossRef]
- Lei, L.; Plank, J. A concept for a polycarboxylate superplasticizer possessing enhanced clay tolerance. Cem. Concr. Res. 2012, 42, 1299–1306. [Google Scholar] [CrossRef]
- Sahmaran, M.; Ozkan, N.; Keskin, S.B.; Uzal, B.; Yaman, I.O.; Erdem, T.K. Evaluation of natural zeolite as a viscosity-modifying agent for cement-based grouts. Cem. Concr. Res. 2008, 38, 930–937. [Google Scholar] [CrossRef] [Green Version]
- Kaci, A.; Chaouche, M.; Andreani, P.-A. Influence of bentonite clay on the rheological behaviour of fresh mortars. Cem. Concr. Res. 2011, 41, 373–379. [Google Scholar] [CrossRef]
- Mohammed, M.H.; Pusch, R.; Knutsson, S.; Warr, L.N. Hydrothermal alteration of clay and low pH concrete applicable to deep borehole disposal of high-level radioactive waste—A pilot study. Constr. Build. Mater. 2016, 104, 1–8. [Google Scholar] [CrossRef]
- Dauzeres, A.; Le Bescop, P.; Sardini, P.; Cau Dit Coumes, C. Physico-chemical investigation of clayey/cement-based materials interaction in the context of geological waste disposal: Experimental approach and results. Cem. Concr. Res. 2010, 40, 1327–1340. [Google Scholar] [CrossRef]
- Fernandez, R.; Cuevas, J.; Mader, U.K. Modeling experimental results of diffusion of alkaline solutions through a compacted bentonite barrier. Cem. Concr. Res. 2010, 40, 1255–1264. [Google Scholar] [CrossRef]
- Hidalgo, A.; Llorente, I.; Alonso, C.; Andrade, C. Study of Concrete/Bentonite Interaction Using Accelerated and Natural Leaching Tests; RILEM Publications SARL: Paris, France, 2014. [Google Scholar]
- Page, C.L.; Short, N.R.; El Tarras, A. Diffusion of chloride ions in hardened cement pastes. Cem. Concr. Res. 1981, 11, 395–406. [Google Scholar] [CrossRef]
- Andrade, C.; d’Andrea, R.; Rebolledo, N. Chloride ion penetration in concrete: The reaction factor in the electrical resistivity model. Cem. Concr. Compos. 2014, 47, 41–46. [Google Scholar] [CrossRef]
- Sanjuán, M.Á.; Estévez, E.; Argiz, C. Carbon Dioxide Absorption by Blast-Furnace Slag Mortars in Function of the Curing Intensity. Energies 2019, 12, 2346. [Google Scholar] [CrossRef] [Green Version]
- González, J.A.; Algaba, S.; Andrade, C. Corrosion of reinforcing bars in carbonated concrete. Br. Corros. J. 1980, 3, 135–139. [Google Scholar] [CrossRef]
- Alonso, C.; Andrade, C. Corrosion behavior of steel during accelerated carbonation of solutions which simulate the pore concrete solution. Mater. Construcción 1987, 37, 5–16. [Google Scholar]
- Sanjuán, M.A.; Andrade, C.; Cheyrezy, M. Concrete carbonation tests in natural and accelerated conditions. Advances in. Cem. Res. 2003, 15, 171–180. [Google Scholar] [CrossRef]
- Muggler, C.C.; Pape, T.H.; Buurman, P. Laser grain-size determination in soil genetic studies 2. Clay content, clay formation and aggregation in some brazilian oxisols. Soil Sci. 1997, 162, 219–228. [Google Scholar] [CrossRef]
- Naswir, M.; Arita, S.; Salni, M. Characterization of bentonite by XRD and SEM-EDS and use to increase pH and color removal. Fe and organic substances in peat water. J. Clean Energy Technol. 2013, 1, 313–317. [Google Scholar] [CrossRef] [Green Version]
- CEN-CENELEC. European Standard EN 196-1. Methods of Resting Cement—Part 1: Determination of Strenght; Cement and Building Limes: Brussels, Belgium, 2005. [Google Scholar]
- Tests on Concrete Durability. Test of Chloride Penetration and Reinforcement Corrosion: Accelerated Integral Test; PrUNE 83992-2; Materials: Madrid, Spain, 2019.
- Reales, O.A.M.; Duda, P.; Silva, E.C.; Paiva, M.D.; Toledo Filho, R.D. Nanosilica particles as structural buildup agents for 3D printing with Portland cement pastes. Constr. Build. Mater. 2019, 219, 91–100. [Google Scholar] [CrossRef]
- Afzal, S.; Shahzada, K.; Fahad, M.; Saeed, S.; Ashraf, M. Assessment of early-age autogenous shrinkage strains in concrete using bentonite clay as internal curing technique. Constr. Build. Mater. 2014, 66, 403–409. [Google Scholar] [CrossRef]
- EN 197-1:2011. Cement—Part 1: Composition, Specifications and Conformity Criteria for Common Cement; European Committee for Standardization (CEN): Brussels, Belgium, 2011.









| Cement | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | SO3 | Na2O | K2O | LOI | IR | Cl− |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CEM I 42.5 R | 20.24 | 3.99 | 2.92 | 62.88 | 1.41 | 3.47 | 0.08 | 0.86 | 2.78 | 0.10 | 0.02 |
| CEM I 52.5R–SR 3 | 21.73 | 3.67 | 4.31 | 66.12 | 1.32 | 3.00 | 0.49 | 0.57 | 1.12 | 0.19 | 0.01 |
| CEM II/A-P 42.5 R | 29.09 | 5.29 | 2.98 | 51.60 | 1.78 | 2.82 | 0.39 | 0.53 | 2.94 | - | 0.06 |
| CEM II/A-P 42.5 R | 28.17 | 6.20 | 3.13 | 52.41 | 1.32 | 3.11 | 0.38 | 0.67 | 2.34 | -- | 0.05 |
| CEM II/A-S 42.5 N | 23.24 | 5.74 | 2.46 | 61.82 | 2.29 | 2.83 | 0.46 | 0.59 | - | - | 0.05 |
| CEM II/A- V 42.5 R | 23.00 | 6.30 | 3.50 | 58.00 | 1.42 | 3.22 | 0.49 | 0.80 | 2.30 | 2.10 | 0.06 |
| CEM III/A 42.5 N | 24.55 | 6.42 | 2.14 | 57.14 | 3.00 | 2.80 | 0.40 | 0.50 | 0.91 | 0.21 | 0.05 |
| CEM IV/A (V) 42.5 R-SR | 27.14 | 5.25 | 3.20 | 53.10 | 1.58 | 2.82 | 0.37 | 0.49 | 2.70 | - | 0.06 |
| IV/A(P) 42.5 R/MR | 28.36 | 4.72 | 3.17 | 52.81 | 2.16 | 2.59 | 0.33 | 0.51 | 2.43 | - | 0.04 |
| BL II/B-LL 42.5 R | 18.70 | 3.83 | 2.64 | 61.70 | 1.29 | 2.97 | 0.06 | 0.81 | 10.86 | 0.33 | 0.02 |
| Cement | K | V | L/LL | S | P | Addition |
|---|---|---|---|---|---|---|
| CEM I 42.5 R | 95 | 5 | ||||
| CEM I 52.5R–SR 3 | 95 | 5 | ||||
| CEM II/A-P 42.5 R | 83 | 11 | 6 | |||
| CEM II/A-P 42.5 R | 80 | 16 | 4 | |||
| CEM II/A-S 42.5 N | 83 | 12 | 5 | |||
| CEM II/A-V 42.5 R | 80 | 15 | 5 | |||
| CEM III/A 42.5 N | 59 | 39 | 2 | |||
| CEM IV/A (V) 42.5 R-SR | 74 | 23 | 3 | |||
| IV/A(P) 42.5 R/MR | 87 | 13 | 0 | |||
| BL II/B-LL 42.5 R | 74 | 26 | 0 |
| Sample | Test Time (h) | Maximum Penetration (mm) | Mean Penetration (mm) | Dns Diffusion Coefficient (cm2/s) |
|---|---|---|---|---|
| OPC-1 | 110 | 34.04–33.03 | 33.535 | 17 × 10−12 |
| OPC-2 | 110 | 33.94–33.14 | 33.54 | 15 × 10−12 |
| Be10-1 | 90 | 34.12–34.06 | 21.015 | 4.75·× 10−12 |
| Be10-2 | 90 | 33.92–33.89 | 33.91 | 4.6·× 10−12 |
| Bar in Reference | Bar in Be10 | |
|---|---|---|
| Chloride Surface concentration | 1.10 | 1.64 |
| Chloride threshold (% mass mortar) | 0.35 | 0.13 |
| CEMENT | 3 MONTHS | 6 MONTHS | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| INDOORS | OUTDOORS UNSHELTERD | OUTDOORS SHELTERED | INDOORS | OUTDOORS UNSHELTERD | OUTDOORS SHELTERED | |||||||
| OPC | 10% Be | OPC | 10% Be | OPC | 10% Be | OPC | 10% Be | OPC | 10% Be | OPC | 10% Be | |
| CEM I 42.5R |  |  |  |  |  |  |  |  |  |  |  |  |
| CEM I 52.5R-SR3 |  |  |  |  |  |  |  |  |  |  |  |  |
| CEMII/AS42.5N |  |  |  |  |  |  |  |  |  |  |  |  |
| CEMII/A-P(16) 42.5R |  |  |  |  |  |  |  |  |  |  |  |  |
| CEMII/A-P(13) 42.5R |  |  |  |  |  |  |  |  |  |  |  |  |
| CEMII/AV42.5R |  |  |  |  |  |  |  |  |  |  |  |  |
| CEMIII/A42.5N |  |  |  |  |  |  |  |  |  |  |  |  |
| CEMIV/A(V) 42.5R SR |  |  |  |  |  |  |  |  |  |  |  |  |
| IV/A(P) 42.5R/MR |  |  |  |  |  |  |  |  |  |  |  |  |
| BLII/B-LL42.5R |  |  |  |  |  |  |  |  |  |  |  |  |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrade, C.; Martínez-Serrano, A.; Sanjuán, M.Á.; Tenorio Ríos, J.A. Reduced Carbonation, Sulfate and Chloride Ingress Due to the Substitution of Cement by 10% Non-Precalcined Bentonite. Materials 2021, 14, 1300. https://doi.org/10.3390/ma14051300
Andrade C, Martínez-Serrano A, Sanjuán MÁ, Tenorio Ríos JA. Reduced Carbonation, Sulfate and Chloride Ingress Due to the Substitution of Cement by 10% Non-Precalcined Bentonite. Materials. 2021; 14(5):1300. https://doi.org/10.3390/ma14051300
Chicago/Turabian StyleAndrade, Carmen, Ana Martínez-Serrano, Miguel Ángel Sanjuán, and José Antonio Tenorio Ríos. 2021. "Reduced Carbonation, Sulfate and Chloride Ingress Due to the Substitution of Cement by 10% Non-Precalcined Bentonite" Materials 14, no. 5: 1300. https://doi.org/10.3390/ma14051300
APA StyleAndrade, C., Martínez-Serrano, A., Sanjuán, M. Á., & Tenorio Ríos, J. A. (2021). Reduced Carbonation, Sulfate and Chloride Ingress Due to the Substitution of Cement by 10% Non-Precalcined Bentonite. Materials, 14(5), 1300. https://doi.org/10.3390/ma14051300







