Sustainable Production of Arecanut Husk Ash as Potential Silica Replacement for Synthesis of Silicate-Based Glass-Ceramics Materials
Abstract
1. Introduction
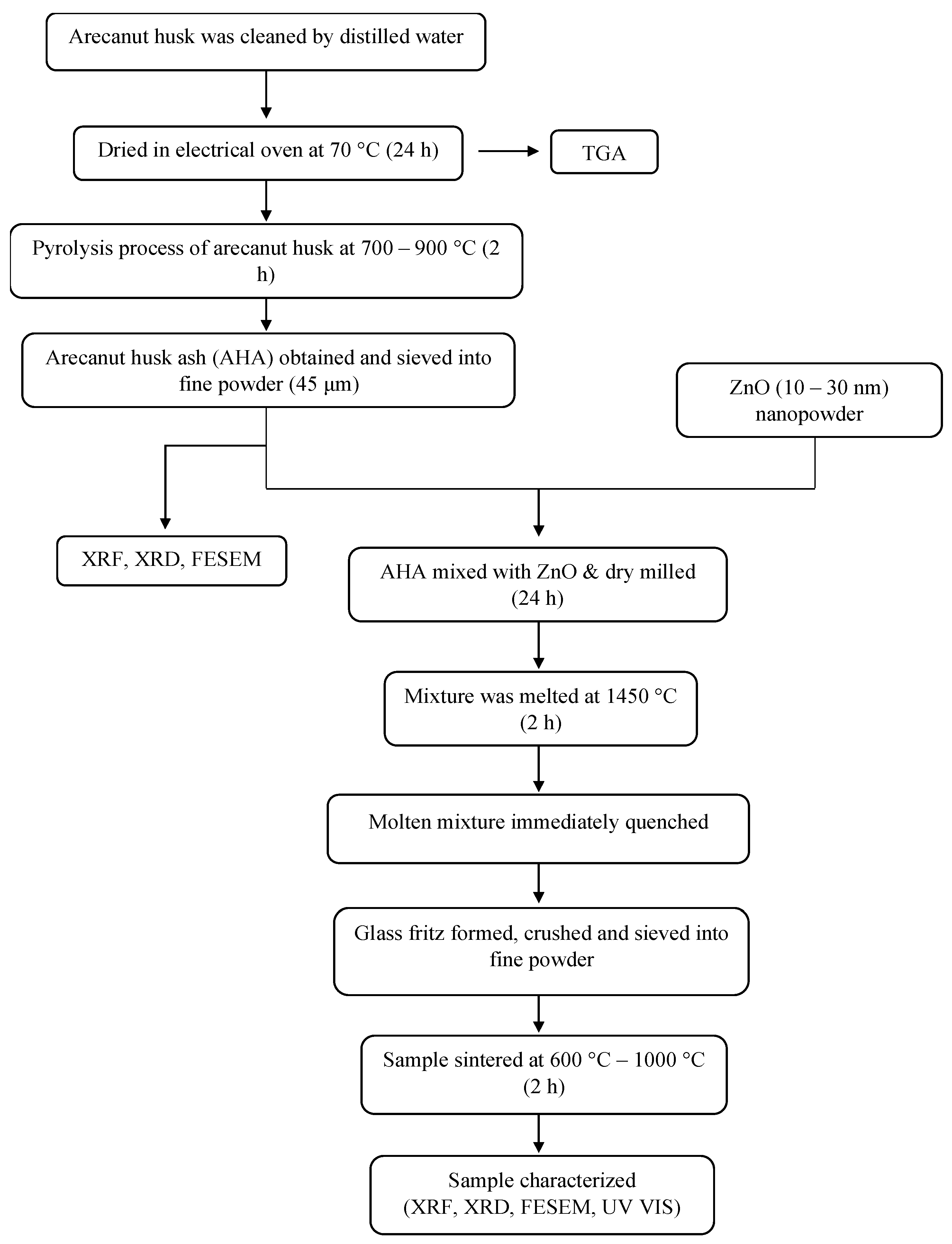
2. Experimental Procedure
2.1. Preparation of Arecanut
2.2. Synthesis of Zinc Silicate
2.3. Characterization
3. Results and Discussion
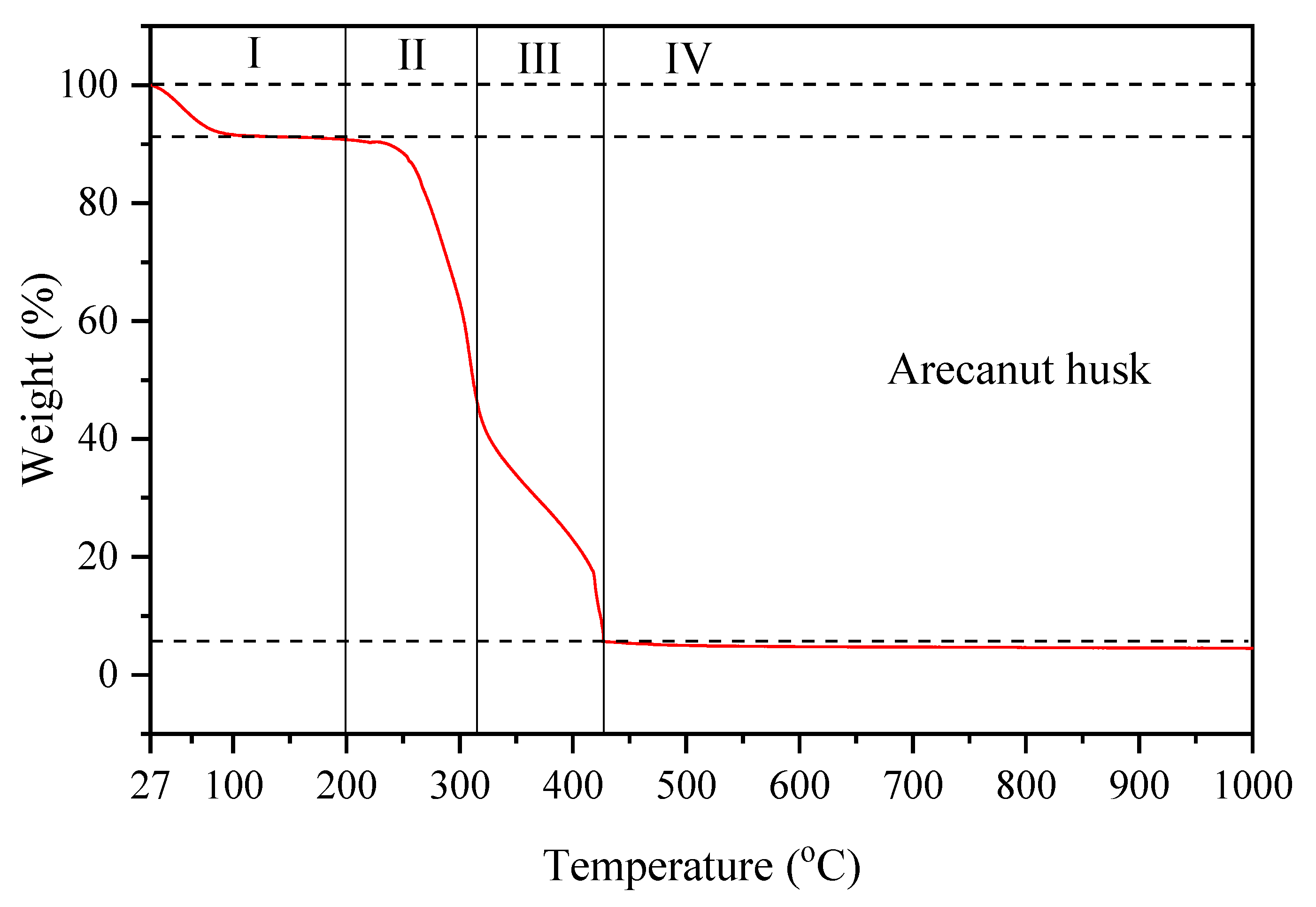
3.1. Arecanut Husk (AH)
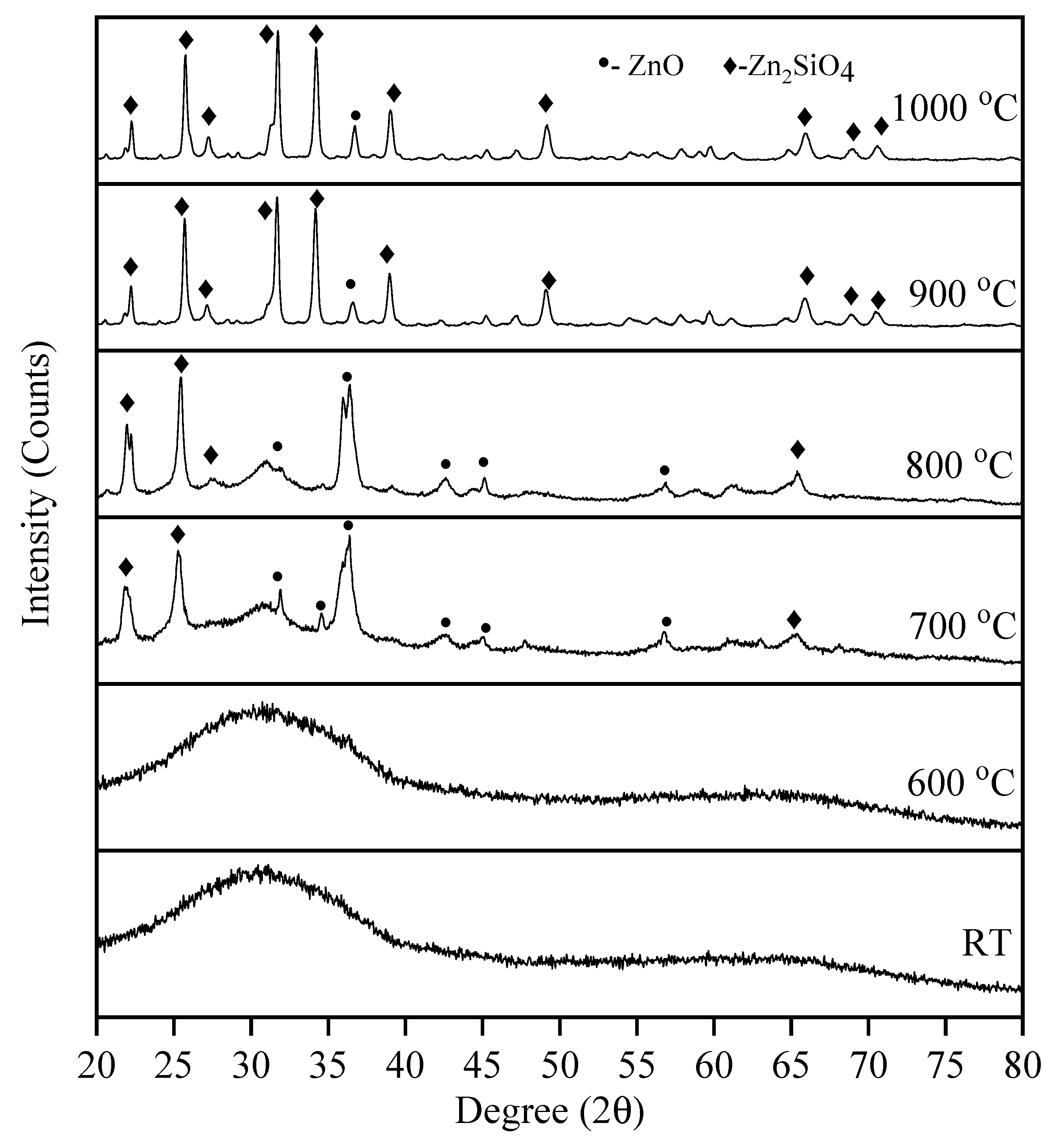
3.2. Arecanut Husk Ash
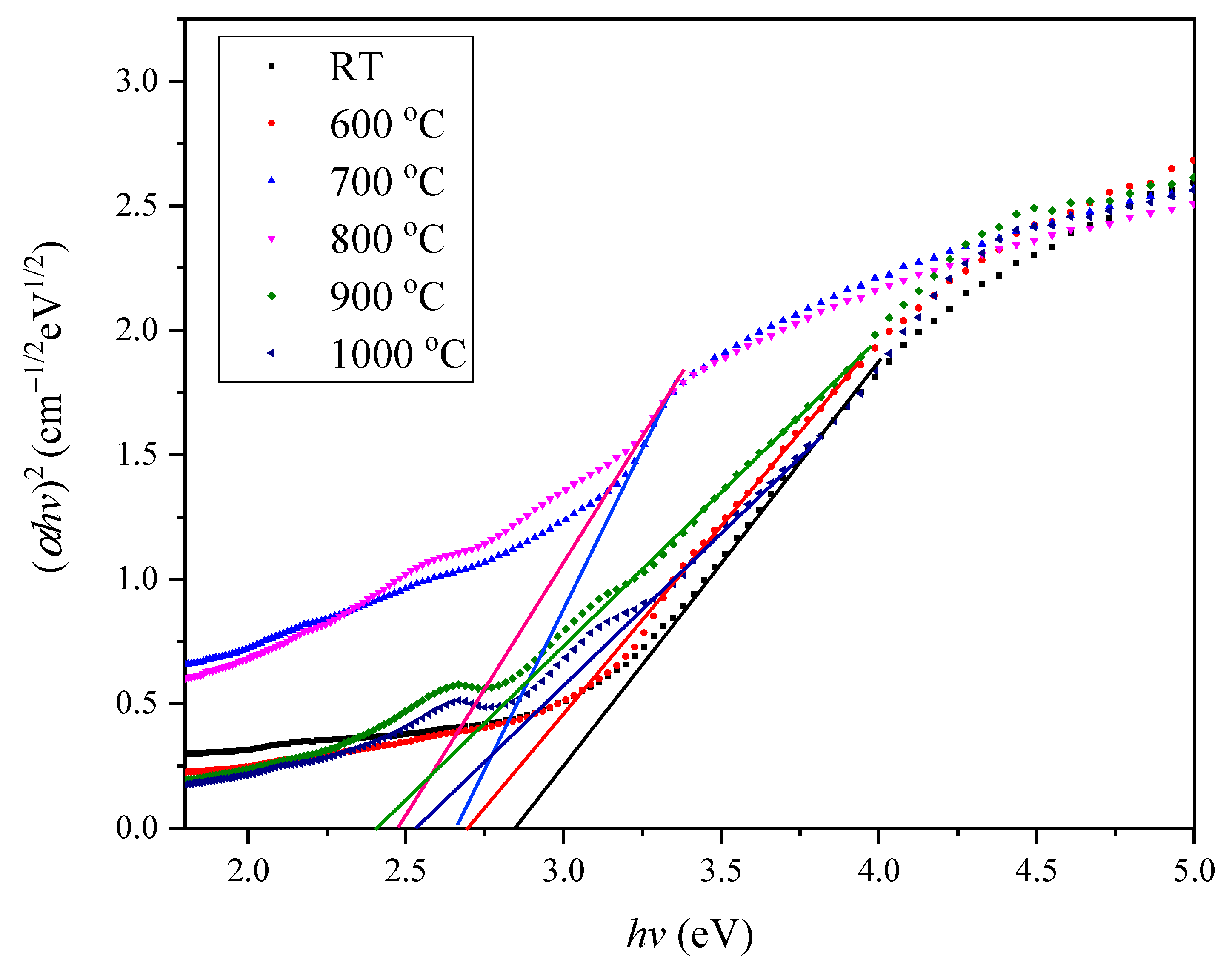
3.3. Zinc Silicate
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paramesh, V.; Arunachalam, V.; Nikkhah, A.; Das, B.; Ghnimi, S. Optimization of energy consumption and environmental impacts of arecanut production through coupled data envelopment analysis and life cycle assessment. J. Clean. Prod. 2018, 203, 674–684. [Google Scholar] [CrossRef]
- Das, N.; Singh, S. The potential of arecanut husk ash as supplementary cementitious material. Conrete Res. Lett. 2015, 6, 126–135. [Google Scholar]
- Basker, A.; Syed Shabudeen, P.S.; Daniel, S.; Vignesh Kumar, P. Adsorptive removal of malachite green from aqueous solution using areca husk carbon. Rasayan J. Chem. 2014, 7, 1–15. [Google Scholar]
- Prasad, S.; Singh, A.; Joshi, H.C. Ethanol as an alternative fuel from agricultural, industrial and urban residues. Resour. Conserv. Recycl. 2007, 50, 1–39. [Google Scholar] [CrossRef]
- Yusriah, L.; Sapuan, S.M.; Zainudin, E.S.; Mariatti, M. Exploring the potential of betel nut husk fiber as reinforcement in polymer composites: Effect of fiber maturity. Procedia Chem. 2012, 4, 87–94. [Google Scholar] [CrossRef]
- Shivakumaraswamy, G.R.; Mahalingegowda, R.M.; Vinod, A.R. Domestic wastewater treatment in reactors filled with areca husk fiber and pebble bed. Pollution 2013, 57, 14064–14066. [Google Scholar]
- Omar, N.A.S.; Fen, Y.W.; Matori, K.A. Photoluminescence properties of Eu3+-doped low cost zinc silicate based glass ceramics. Optik 2016, 127, 3727–3729. [Google Scholar] [CrossRef]
- Khaidir, R.E.M.; Fen, W.Y.; Zaid, M.H.M.; Matori, K.A.; Omar, N.A.S.; Anuar, M.F.; Wahab, S.A.A.; Azman, A.Z.K. Optical band gap and photoluminescence studies of Eu3+-doped zinc silicate derived from waste rice husks. Optik 2019, 182, 486–495. [Google Scholar] [CrossRef]
- Takesue, M.; Hayashi, H.; Smith, R.L. Thermal and chemical methods for producing zinc silicate (willemite): A review. Prog. Cryst. Growth Charact. Mater. 2009, 55, 98–124. [Google Scholar] [CrossRef]
- Anuar, M.F.; Fen, Y.W.; Matori, K.A.; Zaid, M.H.M. The physical and optical studies of crystalline silica derived from green synthesis of coconut husk ash. Appl. Sci. 2020, 10, 2128. [Google Scholar] [CrossRef]
- Gokul, P.V.; Singh, P.; Singh, V.P.; Sawarkar, A.N. Thermal behavior and kinetics of pyrolysis of areca nut husk. Energy Sources, Part A Recover. Util. Environ. Eff. 2019, 41, 2906–2916. [Google Scholar]
- Alves, R.H.; Reis, T.V.D.S.; Rovani, S.; Fungaro, D.A. Green synthesis and characterization of biosilica produced from sugarcane waste ash. J. Chem. 2017, 2017, 1–9. [Google Scholar] [CrossRef]
- Prithivirajan, R.; Jayabal, S.; Sundaram, S.K.; Sangeetha, V. Hybrid biocomposites from agricultural residues: Mechanical, water absorption and tribological behaviors. J. Polym. Eng. 2016, 36, 663–671. [Google Scholar] [CrossRef]
- Mishra, R.K.; Mohanty, K. Pyrolysis kinetics and thermal behavior of waste sawdust biomass using thermogravimetric analysis. Bioresour. Technol. 2018, 251, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Mansaray, K.G.; Ghaly, A.E. Determination of kinetic parameters of rice husks in oxygen using thermogravimetric analysis. Biomass Bioenerg. 1999, 17, 19–31. [Google Scholar] [CrossRef]
- Rao, D.S.; Raju, P.S.; Rao, B.S.; Murthy, K.V.R. Luminescent studies of Zn2Sio4:Mn(1.1%), Eu(1.5%) phosphor. Int. J. Lumin. Its Appl. 2014, 4, 104–106. [Google Scholar]
- Anuar, M.F.; Fen, Y.W.; Zaid, M.H.M.; Matori, K.A.; Khaidir, R.E.M. Synthesis and structural properties of coconut husk as potential silica source. Results Phys. 2018, 11, 1–4. [Google Scholar] [CrossRef]
- Khaidir, R.E.M.; Fen, Y.W.; Zaid, M.H.M.; Matori, K.A.; Omar, N.A.S.; Anuar, M.F.; Wahab, S.A.A.; Azman, A.Z.K. Exploring Eu3+-doped ZnO-SiO2 glass derived by recycling renewable source of waste rice husk for white-LEDs application. Results Phys. 2019, 15, 102596. [Google Scholar] [CrossRef]
- Zhang, Q.Y.; Pita, K.; Kam, C.H. Sol-gel derived zinc silicate phosphor films for full-color display applications. J. Phys. Chem. Solids 2003, 64, 333–338. [Google Scholar] [CrossRef]
- Omar, N.A.S.; Fen, Y.W.; Matori, K.A. Europium doped low cost Zn2SiO4 based glass ceramics: A study on fabrication, structural, energy band gap and luminescence properties. Mater. Sci. Semicond. Process. 2017, 61, 27–34. [Google Scholar] [CrossRef]
- Rasdi, N.M.; Fen, Y.W.; Omar, N.A.S.; Azis, R.S.; Zaid, M.H.M. Effects of cobalt doping on structural, morphological, and optical properties of Zn2SiO4 nanophosphors prepared by sol-gel method. Results Phys. 2017, 7, 3820–3825. [Google Scholar] [CrossRef]
- Samsudin, N.F.; Matori, K.A.; Wahab, Z.A.; Liew, J.Y.C.; Fen, Y.W.; Aziz, S.H.A.; Zaid, M.H.M. Low cost phosphors: Structural and photoluminescence properties of Mn2+-doped willemite glass-ceramics. Optik 2016, 127, 8076–8081. [Google Scholar] [CrossRef]
- Yang, B.; Yang, Z.; Liu, Y.; Lu, F.; Li, P.; Yang, Y.; Li, X. Synthesis and photoluminescence properties of the high-brightness Eu3+-doped Sr3Y(PO4)3 red phosphors. Ceram. Int. 2012, 38, 4895–4900. [Google Scholar] [CrossRef]
- Babu, K.S.; Reddy, A.R.; Reddy, K.V.; Mallika, A.N. High thermal annealing effect on structural and optical properties of ZnO-SiO2 nanocomposite. Mater. Sci. Semicond. Process. 2014, 27, 643–648. [Google Scholar] [CrossRef]
- Omar, N.A.S.; Fen, Y.W.; Matori, K.A.; Zaid, M.H.M.; Norhafizah, M.R.; Nurzilla, M.; Zamratul, M.I.M. Synthesis and optical properties of europium doped zinc silicate prepared using low cost solid state reaction method. J. Mater. Sci. Mater. Electron. 2016, 27, 1092–1099. [Google Scholar] [CrossRef]
- Syamimi, N.F.; Matori, K.A.; Lim, W.F.; Aziz, S.A.; Hafiz, M.; Zaid, M. Effect of sintering temperature on structural and morphological properties of Europium (III) oxide doped willemite. J. Spectrosc. 2014, 2014, 1–9. [Google Scholar] [CrossRef]
- Cortes, J.; Valencia, E. Phenomenological equations of the kinetics of heterogeneous adsorption with interaction between adsorbed molecules. Phys. Rev. B 1995, 51, 2621–2623. [Google Scholar] [CrossRef] [PubMed]
- Anuar, M.F.; Fen, Y.W.; Zaid, M.H.M.; Omar, N.A.S. Optical studies of crystalline ZnO–SiO2 developed from pyrolysis of coconut husk. Mater. Res. Express 2020, 7, 055901. [Google Scholar] [CrossRef]
- Tarafder, A.; Molla, A.R.; Mukhopadhyay, S.; Karmakar, B. Fabrication and enhanced photoluminescence properties of Sm3+-Doped ZnO-Al2O3-B2O3-SiO2 glass derived willemite glass-ceramic nanocomposites. Opt. Mater. 2014, 36, 1463–1470. [Google Scholar] [CrossRef]
- Anuar, M.F.; Fen, Y.W.; Hafiz, M.; Zaid, M. Sintering temperature effect on structural and optical properties of heat treated coconut husk ash. Materials 2020, 13, 2555. [Google Scholar] [CrossRef]
- Tauc, J.; Grigorovici, R.A.V. Optical properties and electronic structure of amorphous germanium. Phys. Status Solidi 1966, 15, 627–636. [Google Scholar] [CrossRef]
- Fen, Y.W.; Yunus, W.M.M.; Yusof, N.A.; Ishak, N.S.; Omar, N.A.S.; Zainudin, A.A. Preparation, characterization and optical properties of ionophore doped chitosan biopolymer thin film and its potential application for sensing metal ion. Optik 2015, 126, 4688–4692. [Google Scholar] [CrossRef]
- Engku Ali, E.A.G.; Matori, K.A.; Saion, E.; Aziz, S.H.A.; Zaid, M.H.M.; Alibe, I.M. Structural and optical properties of heat treated Zn2SiO4 composite prepared by impregnation of ZnO on SiO2 amorphous nanoparticles. Asm Sci. J. Spec. Issue 2018, 1, 75–85. [Google Scholar]
- Lee, C.S.; Matori, K.A.; Ab Aziz, S.H.; Kamari, H.M.; Ismail, I.; Zaid, M.H.M. Fabrication and characterization of glass and glass-ceramic from rice husk ash as a potent material for opto-electronic applications. J. Mater. Sci. Mater. Electron. 2017, 28, 17611–17621. [Google Scholar] [CrossRef]
- Khaidir, R.E.M.; Fen, Y.W.; Mohd Zaid, M.H.; Matori, K.A.; Omar, N.A.S.; Anuar, M.F.; Abdul Wahab, S.A.; Khirel Azman, A.Z. Addition of ZnO nanoparticles on waste rice husk as potential host material for red-emitting phosphor. Mater. Sci. Semicond. Process. 2020, 106, 104774. [Google Scholar] [CrossRef]
- Sahu, I.P.; Bisen, D.P.; Brahme, N.; Tamrakar, R.K. Photoluminescence properties of europium doped di-strontium magnesium di-silicate phosphor by solid state reaction method. J. Radiat. Res. Appl. Sci. 2015, 8, 104–109. [Google Scholar] [CrossRef]
- Shaker, A.; Zekry, A. A new and simple model for plasma and doping and doping induced band gap narrowing. J. Electron. Devices 2010, 8, 293–299. [Google Scholar]





| Elements | Percentages of Compositions (%) | ||
|---|---|---|---|
| 700 °C | 800 °C | 900 °C | |
| Al2O3 | 0.64 | 2.00 | 0.00 |
| SiO2 | 29.17 | 44.14 | 45.43 |
| P2O5 | 26.91 | 14.64 | 18.62 |
| Cl | 0.75 | 0.33 | 0.30 |
| K2O | 16.65 | 7.28 | 10.35 |
| CaO | 20.07 | 13.99 | 14.16 |
| Fe2O3 | 4.02 | 13.57 | 9.62 |
| ZnO | 0.57 | 0.66 | 0.54 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anuar, M.F.; Fen, Y.W.; Azizan, M.Z.; Rahmat, F.; Zaid, M.H.M.; Khaidir, R.E.M.; Omar, N.A.S. Sustainable Production of Arecanut Husk Ash as Potential Silica Replacement for Synthesis of Silicate-Based Glass-Ceramics Materials. Materials 2021, 14, 1141. https://doi.org/10.3390/ma14051141
Anuar MF, Fen YW, Azizan MZ, Rahmat F, Zaid MHM, Khaidir REM, Omar NAS. Sustainable Production of Arecanut Husk Ash as Potential Silica Replacement for Synthesis of Silicate-Based Glass-Ceramics Materials. Materials. 2021; 14(5):1141. https://doi.org/10.3390/ma14051141
Chicago/Turabian StyleAnuar, Muhammad Fahmi, Yap Wing Fen, Muhammad Zakwan Azizan, Fida’i Rahmat, Mohd Hafiz Mohd Zaid, Rahayu Emilia Mohamed Khaidir, and Nur Alia Sheh Omar. 2021. "Sustainable Production of Arecanut Husk Ash as Potential Silica Replacement for Synthesis of Silicate-Based Glass-Ceramics Materials" Materials 14, no. 5: 1141. https://doi.org/10.3390/ma14051141
APA StyleAnuar, M. F., Fen, Y. W., Azizan, M. Z., Rahmat, F., Zaid, M. H. M., Khaidir, R. E. M., & Omar, N. A. S. (2021). Sustainable Production of Arecanut Husk Ash as Potential Silica Replacement for Synthesis of Silicate-Based Glass-Ceramics Materials. Materials, 14(5), 1141. https://doi.org/10.3390/ma14051141







