Biomedical Radioactive Glasses for Brachytherapy
Abstract
1. Radiation Therapy: A Short Overview
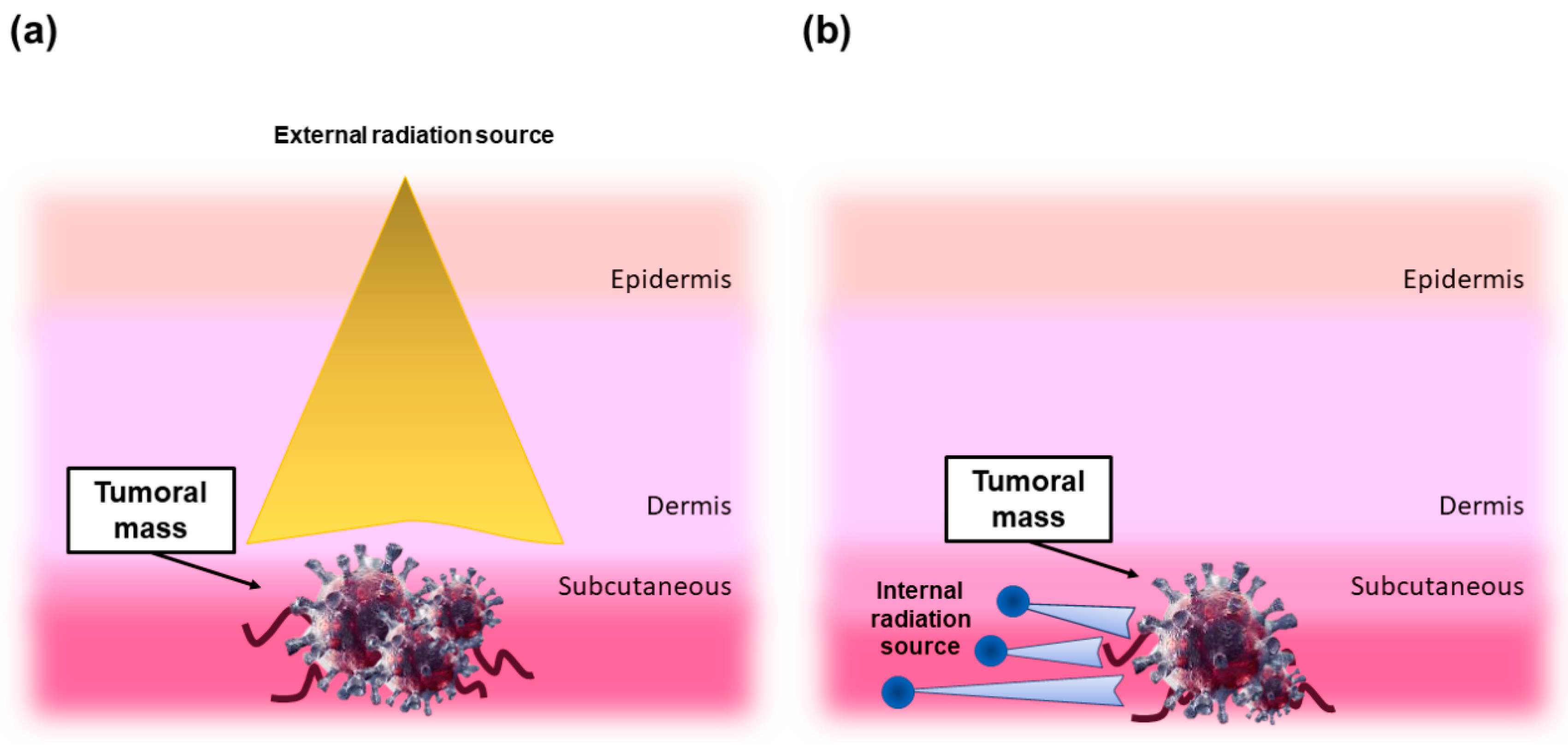
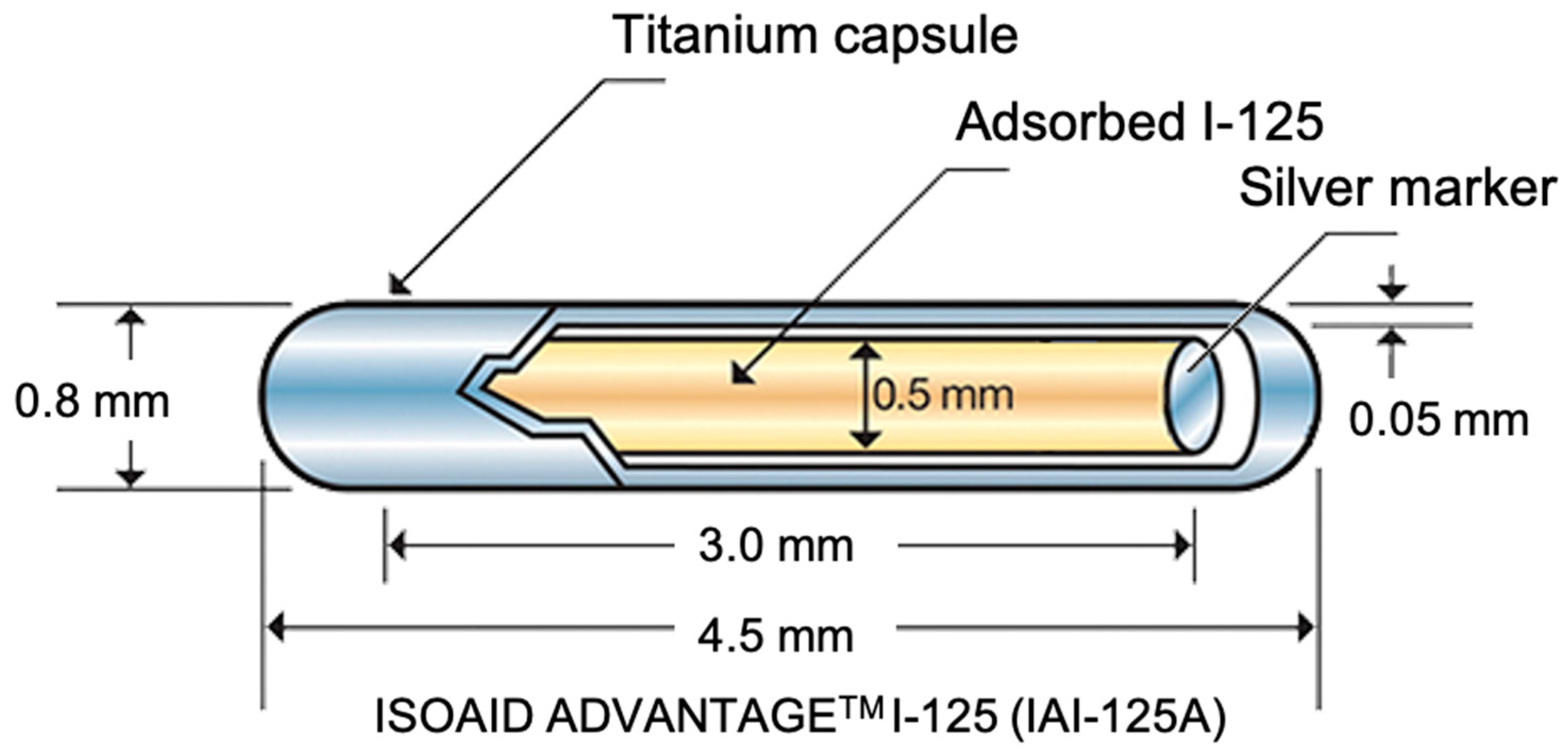
2. Why Using Radioactive Glasses in Brachytherapy?
3. Non-Degradable Glasses
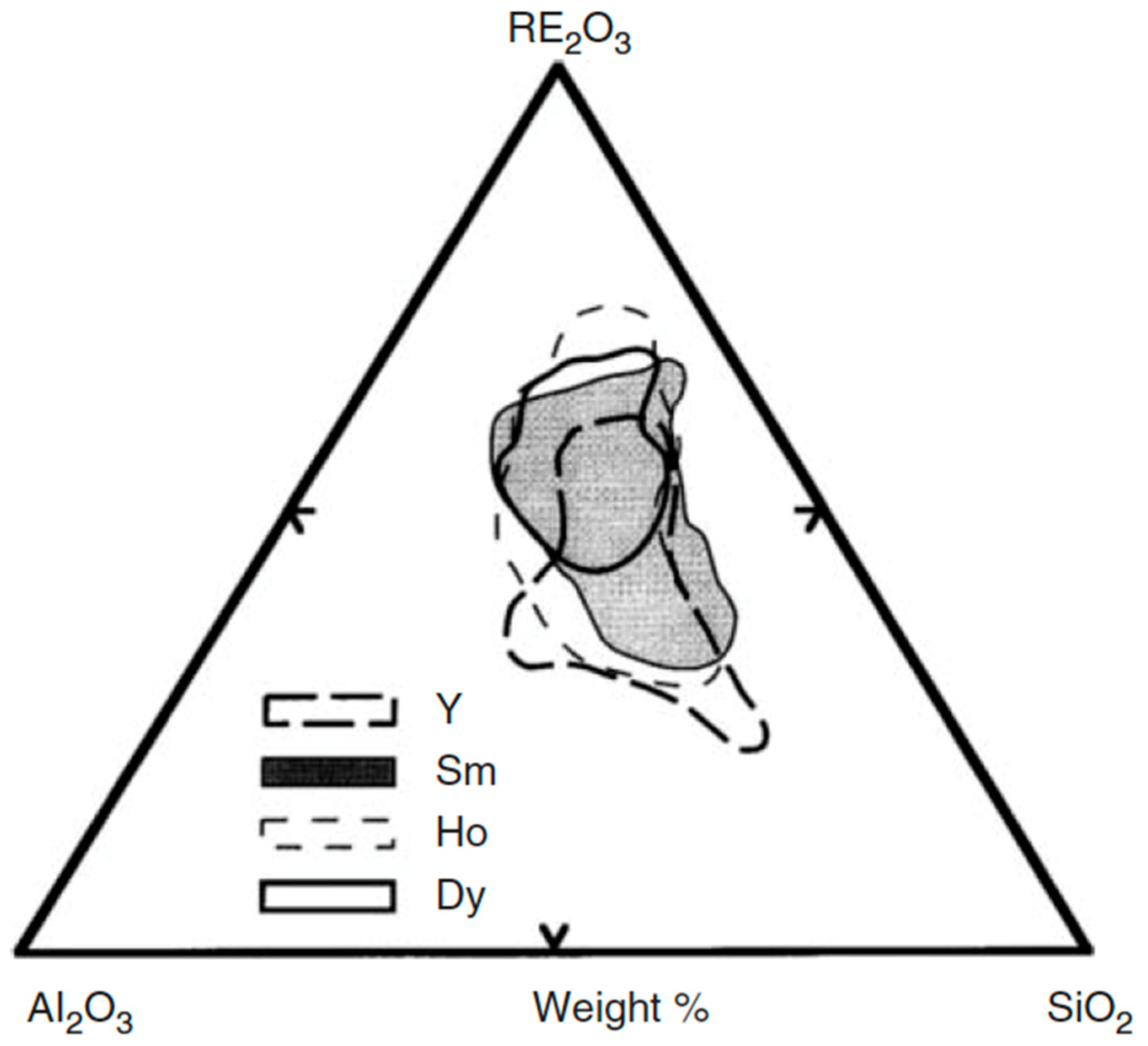
3.1. Compositions and Properties
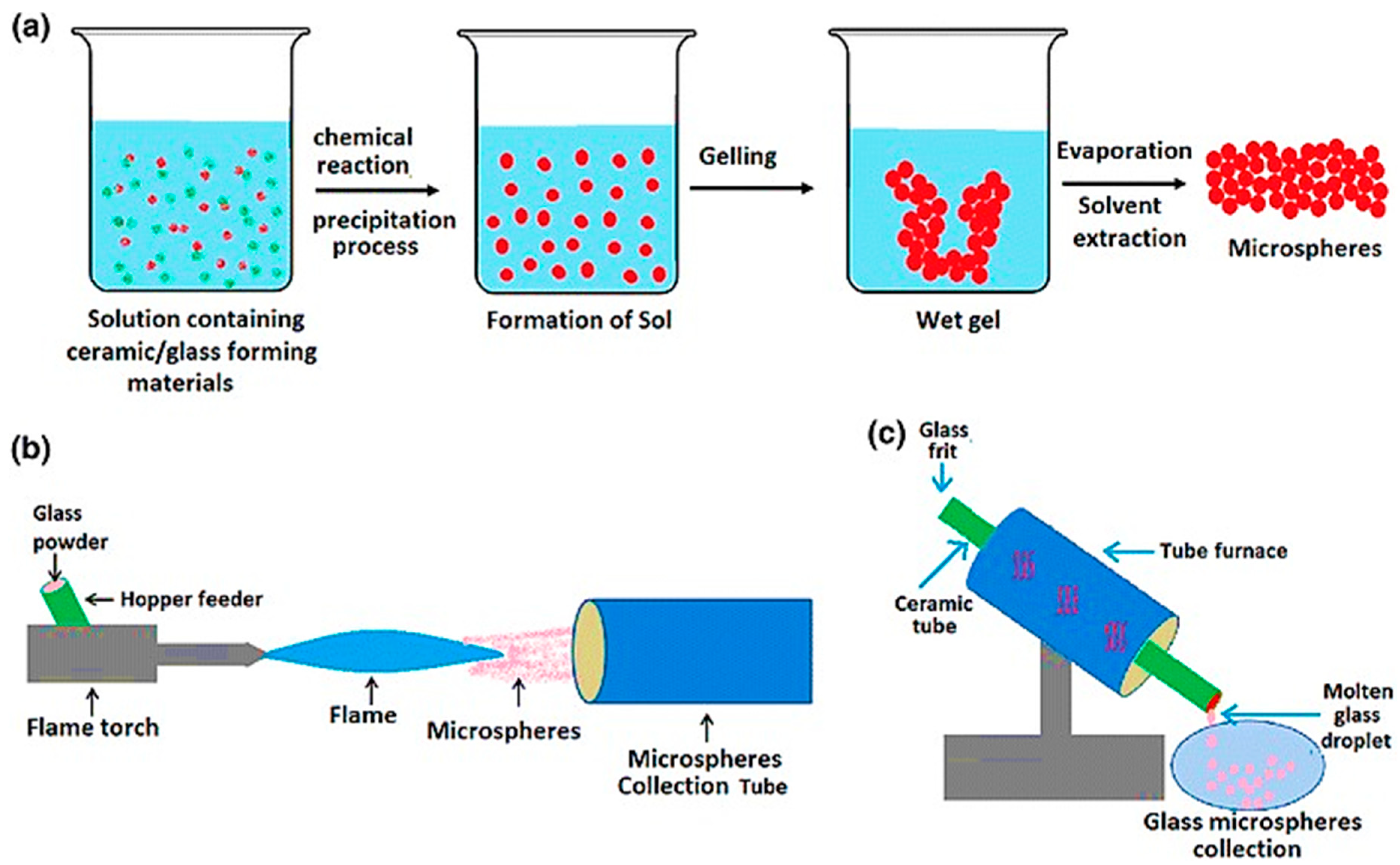
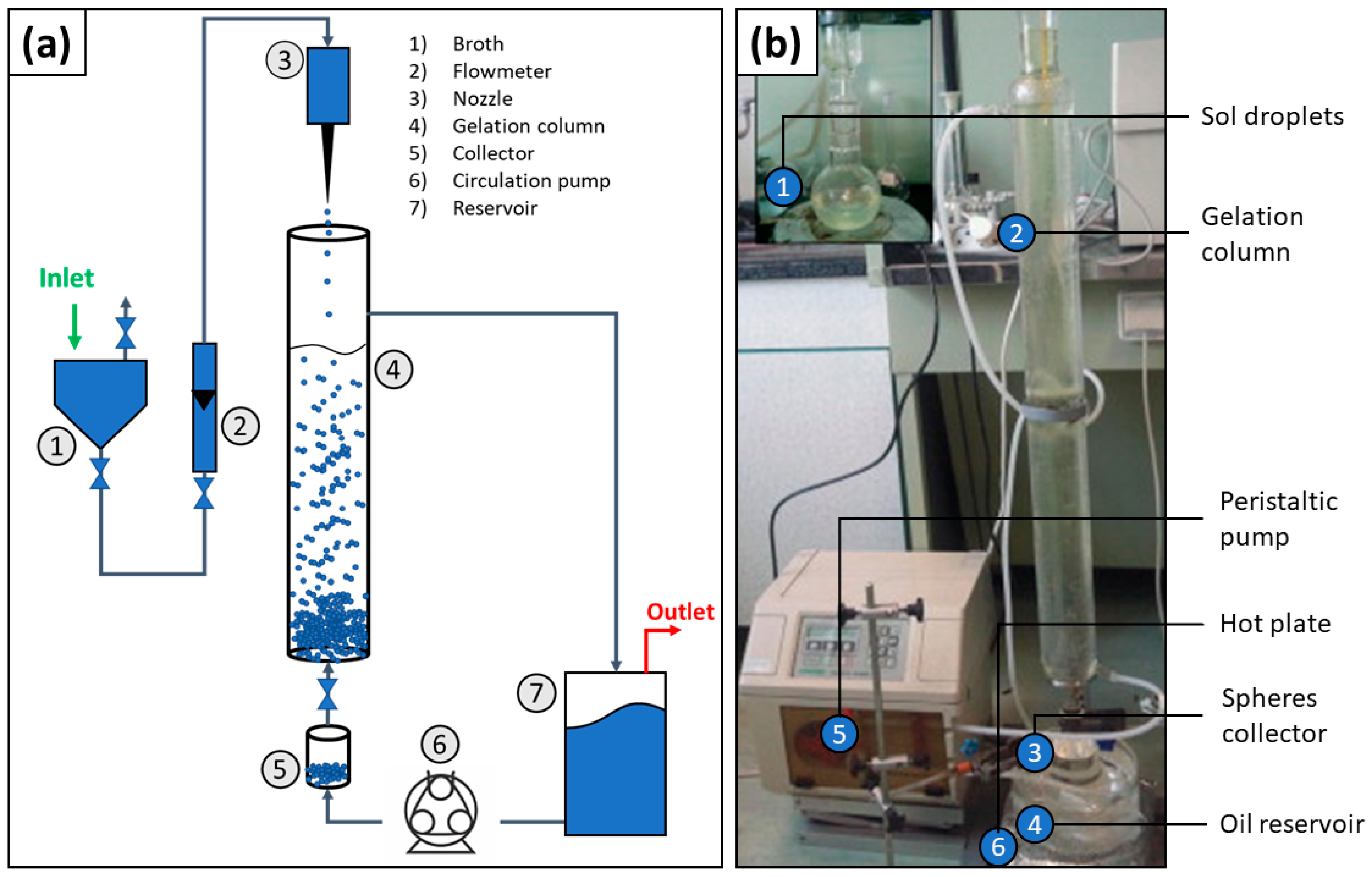
3.2. Manufacture of Glass Microspheres
4. Biodegradable Glasses
5. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pontieri, G.M.; Russo, M.A.; Frati, L. Patologia Generale, 4th ed.; Piccin Editore: Padova, Italy, 2009. [Google Scholar]
- Baskar, R.; Lee, K.A.; Yeo, R.; Yeoh, K.W. Cancer and radiation therapy: Current advances and future directions. Int. J. Med. Sci. 2012, 9, 193–199. [Google Scholar] [CrossRef]
- Jackson, S.P.; Bartek, J. The DNA-damage response in human biology and disease. Nature 2009, 461, 1071–1078. [Google Scholar] [CrossRef]
- Dewey, W.C.; Ling, C.C.; Meyn, R.E. Radiation-induced apoptosis:relevance to radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 1995, 33, 781–796. [Google Scholar] [CrossRef]
- Sato, N.; Mizumoto, K.; Nakamura, M.; Ueno, H.; Minamishima, Y.A.; Farber, J.L.; Tanaka, M. A possible role for centrosome overduplication in radiation-induced cell death. Oncogene 2000, 19, 5281–5290. [Google Scholar] [CrossRef]
- Hotchkiss, R. Cell death. N. Engl. J. Med. 2009, 9, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Begg, A.C.; Stewart, F.A.; Vens, C. Strategies to improve radiotherapy with targeted drugs. Nat. Rev. Cancer 2011, 11, 239–253. [Google Scholar] [CrossRef] [PubMed]
- Domankevich, V.; Cohen, A.; Efrati, M.; Schmidt, M.; Rammensee, H.-G.; Nair, S.S.; Tewari, A.; Kelson, I.; Keisari, Y. Combining alpha radiation-based brachytherapy with immunomodulators promotes complete tumor regression in mice via tumor-specific long-term immune response. Cancer Immunol. Immunother. 2019, 68, 1949–1958. [Google Scholar] [CrossRef]
- Arazi, L.; Cooks, T.; Schmidt, M.; Popovtzer, A.; Rosenfeld, E.; Mizrachi, A.; Ben-Hur, R.; Keisari, Y.; Kelson, I. Alpha DaRT: Revolutionary Alpha-Emitters Brachytherapy. J. Med. Imaging Rad. Sci. 2019, 50, S26–S27. [Google Scholar] [CrossRef]
- Poty, S.; Francesconi, L.C.; McDevitt, M.R.; Morris, M.J.; Lewis, J.S. α-Emitters for Radiotherapy: From Basic Radiochemistry to Clinical Studies-Part 1. J. Nucl. Med. 2018, 59, 878–884. [Google Scholar] [CrossRef]
- Brachitherapia. Available online: https://www.my-personaltrainer.it/salute-benessere/brachiterapia.html (accessed on 13 December 2020).
- Day, D.E. Glasses for Radiotherapy. In Bio-Glasses: An Introduction, 1st ed.; Jones, J.R., Clare, A.G., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2012; Volume 13, pp. 203–228. [Google Scholar]
- Radiation Therapy for Bone Cancer. Available online: https://www.cancer.org/treatment/treatments-and-side-effects/treatment-types/radiation.html (accessed on 13 December 2020).
- Feng, S.; Wang, L.; Xiao, Z.; Maharjan, R.; Chuanxing, L.; Fujun, Z.; Jinhua, H.; Peihong, W. 125I Seed Implant Brachytherapy for Painful Bone Metastases after Failure of External Beam Radiation Therapy. Medicine 2015, 96, e1253. [Google Scholar] [CrossRef]
- Lin, l.; Guo, l.; Zhang, W.; Cai, X.; Chen, D.; Wan, X. Novel silicone-coated 125I seeds for the treatment of extrahepatic cholangiocarcinoma. PLoS ONE 2016, 11, 1. [Google Scholar] [CrossRef][Green Version]
- Awan, S.B.; Hussain, M.; Dini, S.A.; Meigooni, A.S. Historical review of interstitial prostate brachytherapy. Iran J. Radiat Res. 2008, 5, 153–168. [Google Scholar]
- I-125 Seeds. Available online: https://www.eyephysics.com/PS/PS6/UserGuide/OrderSeeds.html (accessed on 13 December 2020).
- Prodotti Medicali Brachiterapia—IsoSeeds I-125—Brumola. Available online: http://www.brumola.com/specification/isoseeds-i-125/ (accessed on 13 December 2020).
- Lin, Y.; Mauro, J.C.; Kaur, G. Bioactive Glasses for Cancer Therapy. In Biomedical, Therapeutic and Clinical Applications of Bioactive Glasses; Kaur, G., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 273–312. [Google Scholar]
- Ngwa, W.; Kumar, R.; Sridhar, S.; Korideck, H.; Zygmanski, P.; Cormack, R.A.; Berbeco, R.; Makrigiorgos, G.M. Targeted radiotherapy with gold nanoparticles: Current status and future perspectives. Nanomedicine 2014, 9, 1063–1082. [Google Scholar] [CrossRef]
- Goudreau, S.H.; Joseph, J.P.; Seiler, S.J. Preoperative radioactive seed localization for nonpalpable breast lesions: Technique, pitfalls, and solutions. Radiographics 2015, 35, 1319–1334. [Google Scholar] [CrossRef]
- Richmond, C.; Findlay, J. Half-life of Iodine-125. Heal Phys. 1966, 12, 865. [Google Scholar]
- Hench, L.L.; Day, D.E.; Höland, W.; Rheinberger, V.M. Glass and Medicine. Int. J. Appl. Glas. Sci. 2010, 1, 104–117. [Google Scholar] [CrossRef]
- Christie, J.K.; Malik, J.; Tilocca, A. Bioactive glasses as potential radioisotope vectors for in situ cancer therapy: Investigating the structural effects of yttrium. Phys. Chem. Chem. Phys. 2011, 13, 17749–17755. [Google Scholar] [CrossRef] [PubMed]
- Rahaman, M.N.; Day, D.E.; Sonny Bal, B.; Fu, Q.; Jung, S.B.; Bonewald, L.F.; Tomsia, A.P. Bioactive glass in tissue engineering. Acta Biomater. 2011, 7, 2355–2373. [Google Scholar] [CrossRef]
- Roberto, W.S.; Pereira, M.M.; Campos, T.P.R. Analysis of Bioactive Glasses Obtained by Sol-Gel Processing for Radioactive Implants. Mater. Res. 2003, 6, 123–127. [Google Scholar] [CrossRef][Green Version]
- Deliberato Aspasio, R.; Borges, R.; Marchi, J. Biocompatible glasses for cancer treatment. In Biocompatible Glasses: From Bone Regeneration to Cancer Treatment, 2nd ed.; Marchi, J., Ed.; Springer: Berlin/Heidelberg, Germany, 2016; Volume 53, pp. 249–265. [Google Scholar]
- White, J.E.; Day, D.E. Rare earth aluminosilicate glasses for in vivo radiation delivery. Key Eng. Mater. 1994, 94–95, 181–208. [Google Scholar] [CrossRef]
- Day, D.E.; Ehrhardt, G.J. Glass Microspheres US 4789501 A; University of Missouri System: Columbiam, MO, USA, 1988. [Google Scholar]
- Boos, G.; Thirwell, M.; Blanchard, R.; Cryips, C.; Herba, M.; Gonzales, L.; Rosenthall, L.; Skelton, J.; Borleau, G. Y-radioactive glasses for treatment of liver cancer. In Proceedings of the 25th Annual Meeting of the American Society Clinical Oncology, San Francisco, CA, USA, 21–23 May 1989. [Google Scholar]
- Bretcanu, O.; Evans, I. Glasses for Treatment of Liver Cancer by Radioembolization. In Biocompatible Glasses. Advanced Structured Materials; Marchi, J., Ed.; Springer: Cham, Switzerland, 2016; Volume 53, pp. 267–283. [Google Scholar]
- Abbott, A.M.; Kim, R.; Hoffe, S.E.; Arslan, B.; Biebel, B.; Choi, J.; El-Haddad, G.; Kis, B.; Sweeney, J.; Meredith, K.L.; et al. Outcomes of Therasphere Radioembolization for Colorectal Metastases. Clin. Colorectal. Cancer 2015, 14, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, N.; Mulcahy, M.F.; Salem, R.; Benson Iii, A.B.; Boucher, E.; Bukovcan, J.; Cosgrove, D.; Laframboise, C.; Lewandowski, R.J.; Master, F.; et al. TheraSphere Yttrium-90 Glass Microspheres Combined with Chemotherapy Versus Chemotherapy Alone in Second-Line Treatment of Patients with Metastatic Colorectal Carcinoma of the Liver: Protocol for the EPOCH Phase 3 Randomized Clinical Trial. JMIR Res. Protoc. 2019, 8, e11545. [Google Scholar] [CrossRef] [PubMed]
- Cerasorb® M-iRES GROUP. Available online: https://www.en.ires.dental/biomaterials/cerasorb-m/ (accessed on 18 February 2021).
- Friesenbichler, J.; Maurer-Ertl, W.; Bergovec, M.; Holzer, L.A.; Ogris, K.; Leitner, L.; Leithner, A. Clinical experience with the artificial bone graft substitute Calcibon used following curettage of benign and low-grade malignant bone tumors. Sci. Rep. 2017, 7, 1736. [Google Scholar] [CrossRef] [PubMed]
- Tas, A.C. Preparation of porous apatite granules from calcium phosphate cement. J. Mater. Sci. Mater. Med. 2008, 19, 2231–2239. [Google Scholar] [CrossRef]
- Lupron Depot® (Leuprolide Acetate for Depot Suspension). Available online: https://www.luprongyn.com/ (accessed on 18 February 2021).
- Hirota, K.; Doty, A.C.; Ackermann, R.; Zhou, J.; Olsen, K.F.; Feng, M.R.; Wang, Y.; Choi, S.; Qu, W.; Schwendeman, A.S.; et al. Characterizing release mechanisms of leuprolide acetate-loaded PLGA microspheres for IVIVC development I: In vitro evaluation. JCR 2016, 244, 302–313. [Google Scholar] [CrossRef]
- Nutropin Depot—FDA. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2004/21075s008lbl.pdf (accessed on 18 February 2021).
- Cleland, J.L.; Geething, N.C.; Moore, J.A.; Rogers, B.C.; Spink, B.J.; Wang, C.W.; Alters, S.E.; Stemmer, W.P.C.; Schellenberger, V. A novel long-acting human growth hormone fusion protein (vrs-317): Enhanced in vivo potency and half-life. J. Pharm. Sci. 2012, 101, 2744–2754. [Google Scholar] [CrossRef]
- Zhou, J.; Walker, J.; Ackermann, R.; Olsen, K.; Hong, J.K.Y.; Wang, Y.; Schwendeman, S.P. Effect of Manufacturing Variables and Raw Materials on the Composition-Equivalent PLGA Microspheres for 1-Month Controlled Release of Leuprolide. Mol. Pharm. 2020, 17, 1502–1515. [Google Scholar] [CrossRef]
- Foglio illustrativo: Informazioni per il Paziente. ENANTONE 3.75 mg/mL polvere e Solvente per Sospensione Iniettabile a Rilascio Prolungato per uso Intramuscolare Sottocutaneo. Available online: https://www.takeda.com/siteassets/it-it/home/products/gine/enantone-mgml-1123all2-pil.pdf (accessed on 18 February 2021).
- Wang, S.; Wu, M.; Li, D.; Jiao, M.; Wang, L.; Zhang, H.; Liu, H.Y.; Wang, D.; Han, B. Preparation, characterization and related in vivo release, safety and toxicity studies of long acting lanreotide microspheres. Biol. Pharm. Bull. 2012, 35, 1898–1906. [Google Scholar] [CrossRef]
- Ipsen to Present Improvements to Somatuline® Autogel® Pre-filled Syringe at the 16th European Neuroendocrine Tumor Society (ENETS) Annual Conference. Available online: https://www.ipsen.com/press-releases/ipsen-to-present-improvements-to-somatuline-autogel-pre-filled-syringe-at-the-16th-european-neuroendocrine-tumor-society-enets-annual-conference/ (accessed on 18 February 2021).
- Wang, T.; Xue, P.; Wang, A.; Yin, M.; Han, J.; Tang, S.; Liang, R. Pore change during degradation of octreotide acetate-loaded PLGA microspheres: The effect of polymer blends. Eur. J. Pharm. Sci. 2019, 139, 104990. [Google Scholar] [CrossRef]
- Sandostatina LAR-Novartis Farma S.p.A. Available online: https://www.codifa.it/farmaci/s/sandostatina-lar-octreotide-acetato-ormoni-anticrescita#indicazioni (accessed on 18 February 2021).
- TheraGlass®—Helping the Body to Heal Naturally. Available online: http://theraglass.co.uk/ (accessed on 18 February 2021).
- TheraSphere™—Y-90 Glass Microspheres. Available online: https://www.bostonscientific.com/en-US/products/cancer-therapies/therasphere-y90-glass-microspheres.html (accessed on 18 February 2021).
- Hossain, K.M.Z.; Patel, U.; Ahmed, I. Development of microspheres for biomedical applications: A review. Prog. Biomater. 2015, 4, 1–19. [Google Scholar] [CrossRef]
- Fu, H.; Rahaman, M.N.; Day, D.E. Effect of process variables on the microstructure of hollow hydroxyapatite microspheres prepared by a glass conversion method. J. Am. Ceram. Soc. 2010, 93, 3116–3123. [Google Scholar] [CrossRef]
- Day, D.E.; White, J.E.; Brown, R.F.; McMenamin, K.D. Transformation of borate glasses into biologically useful materials. Glass. Technol. 2003, 44, 75–81. [Google Scholar]
- Conzone, S.D.; Brown, R.F.; Day, D.E.; Ehrhardt, G.J. In vitro and in vivo dissolution behavior of a dysprosium lithium borate glass designed for the radiation synovectomy treatment of rheumatoid arthritis. J. Biomed. Mater. Res. 2002, 60, 260–268. [Google Scholar] [CrossRef]
- Martinelli, J.R.; Sene, F.F.; Kamikawachi, C.N.; Partiti, C.S.D.; Cornejo, D.R. Synthesis and characterization of glass-ceramic microspheres for thermotherapy. J. Cryst. Solids 2010, 356, 2683–2688. [Google Scholar] [CrossRef]
- Lakhkar, N.J.; Park, J.-H.; Mordan, N.J.; Salih, V.; Wall, I.B.; Kinm, H.-W.; King, S.P.; Hanna, J.V.; Martin, R.A.; Addison, O.; et al. Titanium phosphate glass microspheres for bone tissue engineering. Acta Biomater. 2012, 8, 4181–4190. [Google Scholar] [CrossRef] [PubMed]
- Ward, J.M.; Khalfi, K.; Chormaic, S.N. Short vertical tube furnace for the fabrication of doped glass microsphere lasers. Rev. Sci. Instr. 2010, 81, 073106. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.Y.; Tan, J.; Heng, J.Y.Y.; Cheeseman, C. A comparative study of production of glass microspheres by using thermal process. Mater. Sci. Eng. 2017, 205, 012022. [Google Scholar] [CrossRef]
- Sakka, S. The Outline of Applications of the Sol-Gel Method. In Handbook of Sol-Gel Science and Technology; Klein, L., Aparicio, M., Jitianu, A., Eds.; Springer: Cham, Switzerland, 2018. [Google Scholar]
- Todea, M.; Frentiu, B.; Turcu, R.F.V.; Simon, S. Structural properties of yttrium aluminosilicates microspheres. J. Phys. Chem. Solids 2012, 72, 164–168. [Google Scholar] [CrossRef]
- Poorbaygi, H.; Aghamiri, S.M.R.; Sheibani, S.; Kamali-asl, A.; Mohagheghpoor, E. Production of glass microspheres comprising 90Y and 177Lu for treating of hepatic tumors with SPECT imaging capabilities. Appl. Radiat. Isotop. 2011, 69, 1407–1414. [Google Scholar] [CrossRef] [PubMed]
- Ghahramani, M.R.; Garibov, A.A.; Agayev, T.N.; Mohammadi, M.A. A novel way to production yttrium glass microspheres for medical applications. Glass Phys. Chem. 2014, 40, 283–287. [Google Scholar] [CrossRef]
- Arranja, A.G.; Hennink, W.E.; Denkova, A.G.; Hendrikx, R.W.A.; Nijsen, J.F.W. Radioactive holmium phosphate microspheres for cancer treatment. Int. J. Pharm. 2018, 548, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Saxena, S.K.; Kumar, Y.; Jagadeesan, K.C.; Nuwad, J.; Bamankar, Y.R.; Dash, A. Studies on the development of 169Yb-brachytherapy seeds: New generation brachytherapy sources for the management of cancer. Appl. Rad. Isotop. 2015, 101, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Todea, M.; Vanea, E.; Bran, S.; Berce, P.; Simon, S. XPS analysis of aluminosilicate microspheres bioactivity tested in vitro. Appl. Surf. Sci. 2013, 270, 777–783. [Google Scholar] [CrossRef]
- Sosnik, A.; Seremeta, K.P. Advantages and challenges of the spray-drying technology for the production of pure drug particles and drug-loaded polymeric carriers. Adv. Coll. Inter. Sci. 2015, 223, 40–54. [Google Scholar] [CrossRef] [PubMed]
- Baino, F.; Fiume, E.; Miola, M.; Leone, F.; Onida, B.; Laviano, F.; Gerbaldo, R.; Verné, E. Fe-doped sol-gel glasses and glass-ceramics for magnetic hyperthermia. Materials 2018, 11, 173. [Google Scholar] [CrossRef] [PubMed]
- Baino, F.; Fiume, E.; Miola, M.; Leone, F.; Onida, B.; Verné, E. Fe-doped bioactive glass-derived scaffolds produced by sol-gel foaming. Mater. Lett. 2019, 235, 207–211. [Google Scholar] [CrossRef]
- Borges, R.; Mendonça-Ferreira, L.; Rettori, C.; Pereira, I.S.O.; Baino, F.; Marchi, J. New sol-gel-derived magnetic bioactive glass-ceramics containing superparamagnetic hematite nanocrystals for hyperthermia application. Mater Sci Eng C 2021, 120, 111692. [Google Scholar] [CrossRef]
- Kargozar, S.; Mozafari, M.; Hamzehlou, S.; Kim, H.-W.; Baino, F. Mesoporous bioactive glasses (MBGs) in cancer therapy: Full of hope and promise. Mater. Lett. 2019, 251, 241–246. [Google Scholar] [CrossRef]
- Campos, T.P.R.; Andrade, J.P.L.; Costa, I.T.; Silva, C.H.T. Study of the Sm-153 seeds degradation and evaluation of the absorbed dose in rabbit’s liver implants. Prog. Nucl. Energy 2008, 50, 757–766. [Google Scholar] [CrossRef]
- Cacaina, D.; Ylänen, H.; Hupa, M.; Simon, S. Study of yttrium containing bioactive glasses behaviour in simulated body fluid. J. Mater. Sci. Mater. Med. 2006, 17, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Cacaina, D.; Ylänen, H.; Simon, S.; Hupa, M. The behavior of selected yttrium containing bioactive glass microspheres in simulated body environments. J. Mater. Sci. Mater. Med. 2008, 19, 1225–1233. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, L.B.; Campos, T.P.R. Nuclear Characterization of Radioactive Bioglass Seed for Brachytherapy Studies. In Proceedings of the International Conference on Mathematics and Computational Methods Applied to Nuclear Science and Engineering, Rio de Janeiro, Brazil, 8–12 May 2011. [Google Scholar]
- Nogueira, L.B.; Campos, T.P.R. Synthesis, chemical characterization and radiological response of Ho and HoZr bioglass seeds. J. Sol-Gel. Sci. Technol. 2016, 77, 688–698. [Google Scholar] [CrossRef]
- Piagentini Delpino, G.; Borges, R.; Zambanini, T.; Joca, J.F.S.; Gaubeur, I.; Santos de Souza, A.C.; Marchi, J. Sol-gel-derived 58S bioactive glass containing holmium aiming brachytherapy applications: A dissolution, bioactivity and cytotoxicity study. Mater. Sci. Eng. C 2021, 119, 111595. [Google Scholar] [CrossRef]
- Sadeghi, M.; Taghdiri, F.; Hosseini, S.H.; Tenreiro, C. Monte Carlo calculated TG-60 dosimetry parameters for the β-Emitter 153Sm brachytherapy source. Med. Phys. 2010, 37, 5370–5375. [Google Scholar] [CrossRef]
- Hosseini, S.H.; Enferadi, M.; Sadeghi, M. Dosimetric aspects of 166Ho brachytherapy biodegradable glass seed. Appl. Radiat. Isot. 2013, 73, 109–115. [Google Scholar] [CrossRef]
- Khorshidi, A.; Ahmadinejad, M.; Hosseini, S.H. Evaluation of a proposed biodegradable 188Re source for brachytherapy application: A review of dosimetric parameters. Medicine 2015, 94, 1–7. [Google Scholar] [CrossRef]
- Christie, J.K.; Tilocca, A. Integrating biological activity into radioisotope vectors: Molecular dynamics models of yttrium-doped bioactive glasses. J. Mater. Chem. 2012, 22, 12023–12031. [Google Scholar] [CrossRef]




| Radionuclide | Half-Life | Decay Energy/MeV | Decay Product |
|---|---|---|---|
| 90Y | 64.0 h | 2.284 | 90Zr |
| 32P | 14.3 days | 1.710 | 32S |
| 186Re | 90.6 h | 1.076 | 186Os |
| 188Re | 17.0 h | 2.119 | 188Os |
| 166Ho | 26.7 h | 1.854 | 166Er |
| 153Sm | 46.7 h | 0.817 | 153Eu |
| 45Ca | 162.7 days | 0.26 | 45Sc |
| 40K | 1.25 × 109 years | 1.31 | 40Ca |
| Material/Loaded Drugs/Biological Compounds | Clinical Application | Manufacturing Technology | Commercial Products | Ref. |
|---|---|---|---|---|
| Pure β-Tricalcium Phosphate(TCP) (≥99%) | Filler of oral and maxillofacial surgery bone defects | Sintering + crushing and sieving | Cerasorb® (Curasan, Kleinostheim, Germany) | [34] |
| 62.5% α-Tricalcium Phosphate (TCP), 26.8% dicalcium phosphate dihydrate, 8.9% calcium carbonate and 1.8% precipitated hydroxyapatite | Orthopedics: Bone graft substitute | Sintering + crushing and sieving | Calcibon (Biomet Deutschland GmbH, Berlin, Germany)) | [35,36] |
| Poly(lactic-co-glycolic acid) (PLGA) loaded with Leuprolide acetate | Drug delivery: Endometrisis or Anemia prior to Uterine Fibroid surgery | Double emulsion-solvent evaporation method/self-healing encapsulation method | Lupron Depot (TAP Pharmaceutical Products Inc., Deerfield, IL, USA; Nihonbashi, Chuo, Tokyo, Japan) | [37,38] |
| Poly(lactic-co-glycolic acid) (PLGA) microspheres loaded with rhGH (recombinant human Growth Hormone) | Growth hormone regulator acting on skeletal and cell growth, protein, carbohydrate, lipid, mineral, and connective tissue metabolism | Spray freeze drying | Nutropin Depot® (Genetech Inc., San Francisco, CA, USA; no longer available) | [39,40] |
| Poly(lactic-co-glycolic acid) (PLGA) loaded with Leuprorelin | Prostate cancer treatment, endometriosis treatment, breast cancer treatment, uterine fibroid treatment | Solvent evaporation encapsulation method | Enantone LP (Takeda Pharmaceutical Company Limited, Tokyo, Japan) | [41,42] |
| Poly(lactic-co-glycolic acid) (PLGA) loaded with Lanreotide | Neuroendocrine tumors, acromegaly treatment, carcinoid syndrome | Multiple-emulsion solvent evaporation method | Somatuline® (IPSEN Pharma, Paris, France) | [43,44] |
| Poly(lactic-co-glycolic acid) (PLGA) loaded with Ocreotide | Acromegaly treatment, carcinoid syndrome, Neuroendocrine tumors, treatment of pituitary adenoma secreting TSH | O/W emulsion solvent evaporation technique | Sandostatin LAR (Novartis Farma, Origgio (VA), Italy) | [45,46] |
| 70SiO2-30CaO glass (mol.%) | Dentistry/orthopedics | Sol-gel method | TheraGlass ® (Imperial College, London, UK) | [47] |
| 40Y2O3-20Al2O3-40SiO2 glass (wt.%) | Targeted HCC therapy (Intent or palliative treatment of liver cancer) | Flame spheroidization method | TheraSphere® (Boston Scientific Corporation, Watertown, MA, USA) | [23,24,28,48] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baino, F.; Fiume, E.; Ciavattini, S.; Kargozar, S.; Borges, R.; Genova, L.A.; Marchi, J.; Verné, E. Biomedical Radioactive Glasses for Brachytherapy. Materials 2021, 14, 1131. https://doi.org/10.3390/ma14051131
Baino F, Fiume E, Ciavattini S, Kargozar S, Borges R, Genova LA, Marchi J, Verné E. Biomedical Radioactive Glasses for Brachytherapy. Materials. 2021; 14(5):1131. https://doi.org/10.3390/ma14051131
Chicago/Turabian StyleBaino, Francesco, Elisa Fiume, Sara Ciavattini, Saeid Kargozar, Roger Borges, Luis A. Genova, Juliana Marchi, and Enrica Verné. 2021. "Biomedical Radioactive Glasses for Brachytherapy" Materials 14, no. 5: 1131. https://doi.org/10.3390/ma14051131
APA StyleBaino, F., Fiume, E., Ciavattini, S., Kargozar, S., Borges, R., Genova, L. A., Marchi, J., & Verné, E. (2021). Biomedical Radioactive Glasses for Brachytherapy. Materials, 14(5), 1131. https://doi.org/10.3390/ma14051131









