Precipitate Evolution in 22Cr25NiWCuCo(Nb) Austenitic Heat-Resistant Stainless Steel during Heat Treatment at 1200 °C
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Suo, J.; Peng, Z.; Yang, H.; Chai, G.; Yu, M. Formation of laves phase in Sanicro 25 austenitic steel during creep-rupture test at 700 °C. Metallogr. Microstruct. Anal. 2019, 8, 281–286. [Google Scholar] [CrossRef]
- Maziasz, P.J. Development of creep-resistant and oxidation-resistant austenitic stainless steels for high temperature applications. JOM 2018, 70, 66–75. [Google Scholar] [CrossRef]
- Zieliński, A.; Dudziak, T.P.; Golański, G.; Gazdowicz, J.; Kołodziej, A. Effects of long-term ageing at high temperatures on oxide scale development and evolution of austenitic steels microstructure. Steel Res. Int. 2020, 91, 1900595. [Google Scholar] [CrossRef]
- Cempura, G.; Gil, A.; Agüero, A.; Gutiérrez, M.; Kruk, A.; Czyrska-Filemonowicz, A. Microstructural studies of the scale on Sanicro 25 after 25,000h of oxidation in steam using advanced electron microscopy techniques. Surf. Coat. Technol. 2019, 377, 124901. [Google Scholar] [CrossRef]
- Golański, G.; Zieliński, A.; Sroka, M.; Słania, J. The effect of service on microstructure and mechanical properties of HR3C heat-resistant austenitic stainless steel. Materials 2020, 13, 1297. [Google Scholar] [CrossRef]
- Zhou, R.; Zhu, L. Growth behavior and strengthening mechanism of Cu-rich particles in sanicro 25 austenitic heat-resistant steel after aging at 973 K. Mater. Sci. Eng. 2020, A796, 139973. [Google Scholar] [CrossRef]
- Sroka, M.; Zielinski, A.; Golański, G. Analysis of phase precipitation in Sanicro 25 Austenitic steel after ageing. Acta Phys. Pol. 2019, 135, 207–211. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, Y.; Zhou, X.; Liu, C.; Yu, J.; Huang, Y.; Li, H.; Li, W. Precipitation and hot deformation behavior of austenitic heat-resistant steels: A review. J. Mater. Sci. Technol. 2017, 33, 1448–1456. [Google Scholar] [CrossRef]
- Viswanathan, R.; Henry, J.F.; Tanzosh, J.; Stanko, G.; Shingledecker, J.; Vitalis, B.; Purgert, R.U.S. program on materials technology for ultra-supercritical coal power plants. J. Mater. Eng. Perform. 2005, 14, 281–292. [Google Scholar] [CrossRef]
- Rutkowski, B.; Gil, A.; Agüero, A.; González, V.; Czyrska-Filemonowicz, A. Microstructure, chemical- and phase composition of Sanicro 25 austenitic steel after oxidation in steam at 700 °C. Oxid. Met. 2018, 89, 183–195. [Google Scholar] [CrossRef]
- Zurek, J.; Yang, S.-M.; Lin, D.-Y.; Hüttel, T.; Singheiser, L.; Quadakkers, W.J. Microstructural stability and oxidation behavior of Sanicro 25 during long-term steam exposure in the temperature range 600–750 °C. Mater. Corros. 2015, 66, 315–327. [Google Scholar] [CrossRef]
- Rana, R.; Bleck, W.; Singh, S.B.; Mohanty, O.N. Development of high strength interstitial free steel by copper precipitation hardening. Mater. Lett. 2007, 61, 2919–2922. [Google Scholar] [CrossRef]
- He, J.; Sandström, R. Application of Fundamental Models for Creep Rupture Prediction of Sanicro 25 (23Cr25NiWCoCu). Crystals 2019, 8, 638. [Google Scholar] [CrossRef]
- Zieliński, A.; Dobrzański, J.; Ppuzyńska, H.; Sikora, R.; Dziuba-Kałuża, M.; Kania, Z. Evaluation of creep strength of heterogeneous welded joint in HR6W alloy and Sanicro 25 steel Arch. Metall. Mater. 2017, 62, 2057–2064. [Google Scholar] [CrossRef]
- Calmunger, M.; Chai, G.; Eriksson, R.; Johansson, S.; Moverare, J.J. Characterization of Austenitic Stainless Steels Deformed at Elevated Temperature. Metall. Mater. Trans. 2017, A48, 4525–4538. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Liu, C.; Li, C.; Li, H. Mechanism for the formation of Z-phase in 25Cr-20Ni-Nb-N austenitic stainless steel. Mater. Lett. 2018, 233, 16–19. [Google Scholar] [CrossRef]
- Zhou, R.; Zhu, L.; Liu, Y.; Lu, Z.; Chen, L. Precipitates and precipitation strengthening of Sanicro 25 welded joint base metal crept at 973 K. Steel Res. Int. 2017, 87, 1600414. [Google Scholar] [CrossRef]
- Merda, A.; Sroka, M.; Klimaszewska, K.; Golański, G. Microstructure and mechanical properties of the Sanicro 25 steel after ageing. J. Achiev. Mater. Manuf. Eng. 2018, 91, 1–11. [Google Scholar] [CrossRef]
- Sourmail, T. Precipitation in creep resistant austenitic stainless steel. J. Mater. Sci. Technol. 2001, 14, 1–14. [Google Scholar] [CrossRef]
- Chai, G.; Bostrom, M.; Olaison, M.; Forsberg, U. Creep and LCF behaviours of newly developed advanced heat resistant austenitic steels for A- USC. Procedia Eng. 2013, 55, 232–239. [Google Scholar] [CrossRef]
- Peng, B.; Zhang, H.; Hong, J.; Gao, J.; Zhang, H.; Li, J.; Wang, Q. The evolution of precipitates of 22Cr-25Ni-Mo-Nb-N heat-resistant austenitic steel in long term creep. Mater. Sci. Eng. 2010, A527, 4424–4430. [Google Scholar] [CrossRef]
- Wei, L.; Hao, W.; Cheng, Y.; Tan, S. Isothermal aging embrittlement in an Fe-22Cr-25Ni alloy. Mater. Sci. Eng. 2018, A737, 40–46. [Google Scholar] [CrossRef]
- Terada, M.; Escriba, D.M.; Costa, I.; Materna-Morris, E.; Padliha, A.F. Investigation on the intergranular corrosion resistance of the AISI 316L(N) stainless steel after long time creep testing. Mater. Charact. 2008, 59, 663–668. [Google Scholar] [CrossRef]
- Kaneko, K.; Futunaga, T.; Yamada, K.; Nakada, N.; Kikuchi, M.; Saghi, Z.; Barnad, J.S.; Midgley, P.A. Formation of M23C6-Type precipitates and chromium–depleted zone in austenite stainless steel. Scr. Mater. 2011, 65, 509–512. [Google Scholar] [CrossRef]
- Raghavan, A.; Klein, C.F.; Marzinsky, C.N. Instabilities in stabilized austenitic stainless steels. Metall. Mater. Trans. 1992, 23A, 2455–2467. [Google Scholar]
- Lula, R.A. New Developments in Stainless Steel Technology; American Society for Metals: Metals Park, OH, USA, 1985; ISBN 978-0-871-70205-0. [Google Scholar]
- Vodárek, V. Morphology and orientation relationship of Z-phase in austenite. Scr. Metall. Mater. 1991, 25, 549–552. [Google Scholar] [CrossRef]
- Heczko, M.; Esser, B.D.; Smith, T.M.; Beran, P.; Mazánová, V.; Kruml, T.; Polák, J.; Mills, M.J. On the origin of extraordinary cyclic strengthening of the austenitic stainless steel Sanicro 25 during fatigue at 700 °C. J. Mater. Res. 2017, 32, 4342–4353. [Google Scholar] [CrossRef]
- Ţălu, Ş. Micro and Nanoscale Characterization of Three Dimensional Surfaces: Basics and Applications; Napoca Star Publishing House: Cluj-Napoca, Romania, 2015. [Google Scholar]
- Hillert, M. On the theory of normal and abnormal grain growth. Acta Metall. 1965, 13, 227–238. [Google Scholar] [CrossRef]
- Ghosh, S. Kinetic study on the coarsening behaviour of equilibrium phases in Nb alloyed ferritic stainless steels at 700 °C. Mater. Chem. Phys. 2010, 124, 13–16. [Google Scholar]
- Zhou, Y.-H.; Liu, C.-X.; Liu, Y.-C.; Guo, Q.-Y.; Li, H.-J. Coarsening behavior of MX carbonitrides in type 347H heat-resistant austenitic steel during thermal aging. Int. J. Miner. Metall. Mater. 2016, 23, 283–293. [Google Scholar] [CrossRef]
- Xia, Z.X.; Zhang, C.; Yang, Z.G. Control of precipitation behavior in reduced activation steels by intermediate heat treatment. Mater. Sci. Eng. 2011, A528, 6764–6768. [Google Scholar]
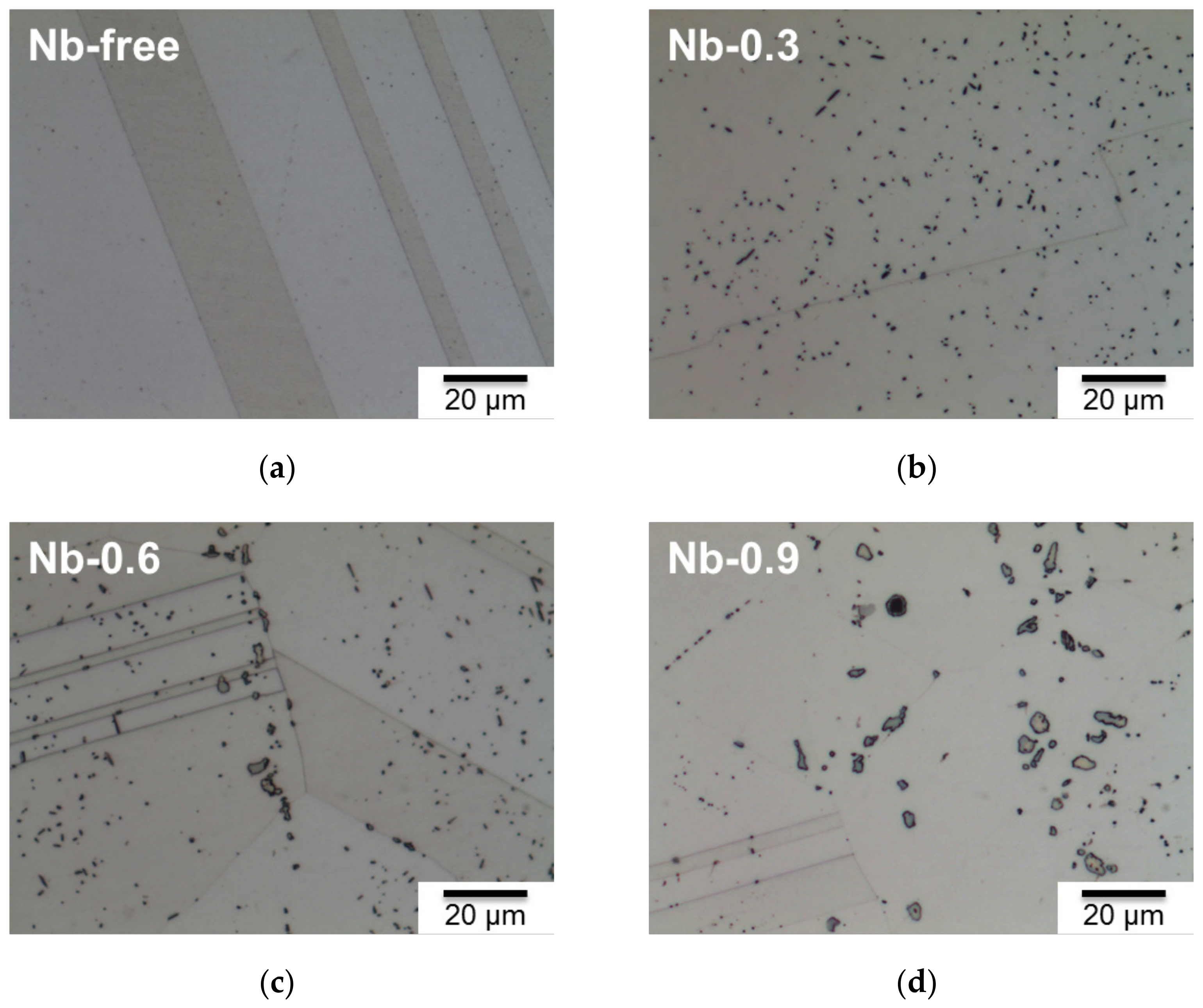

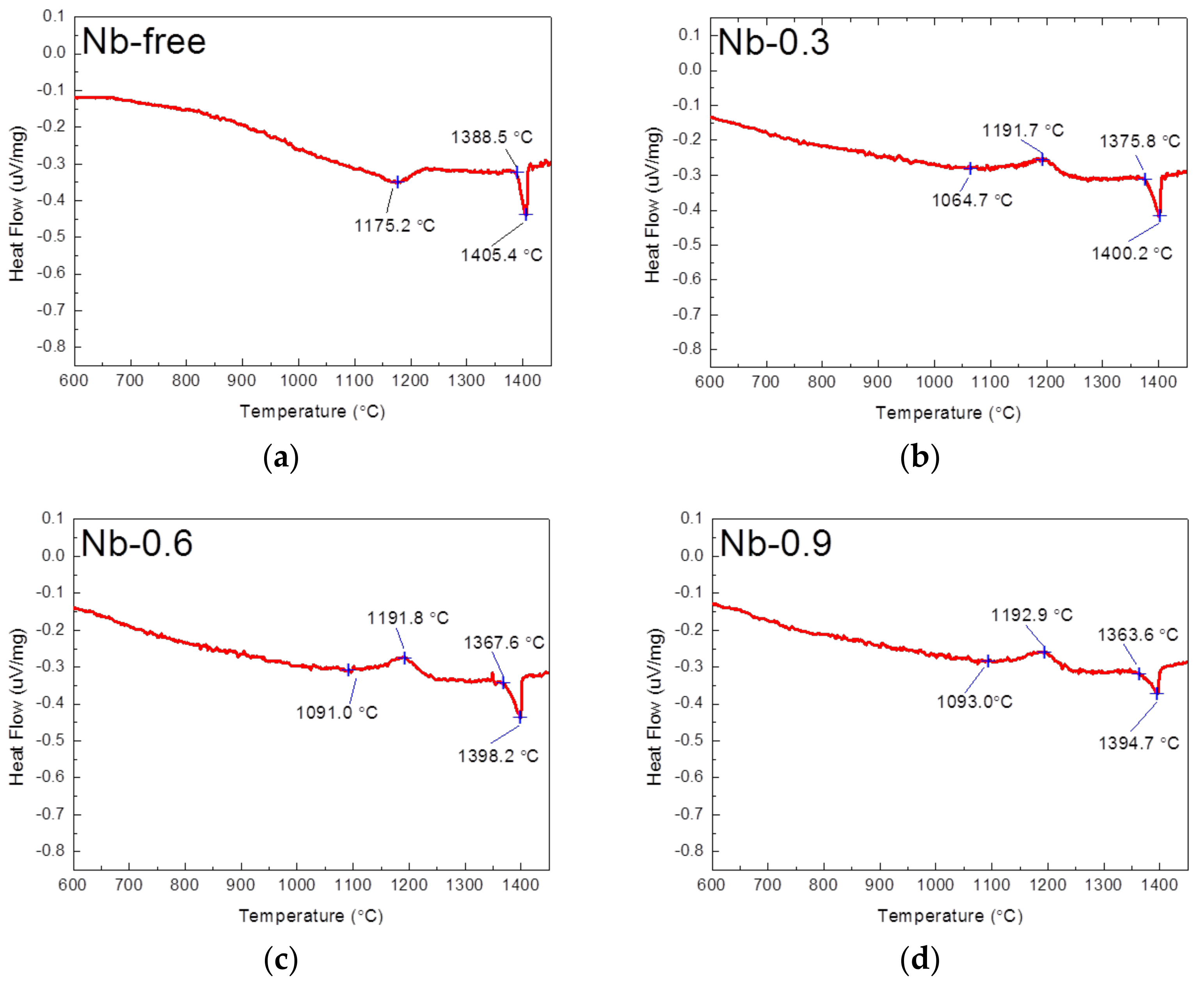
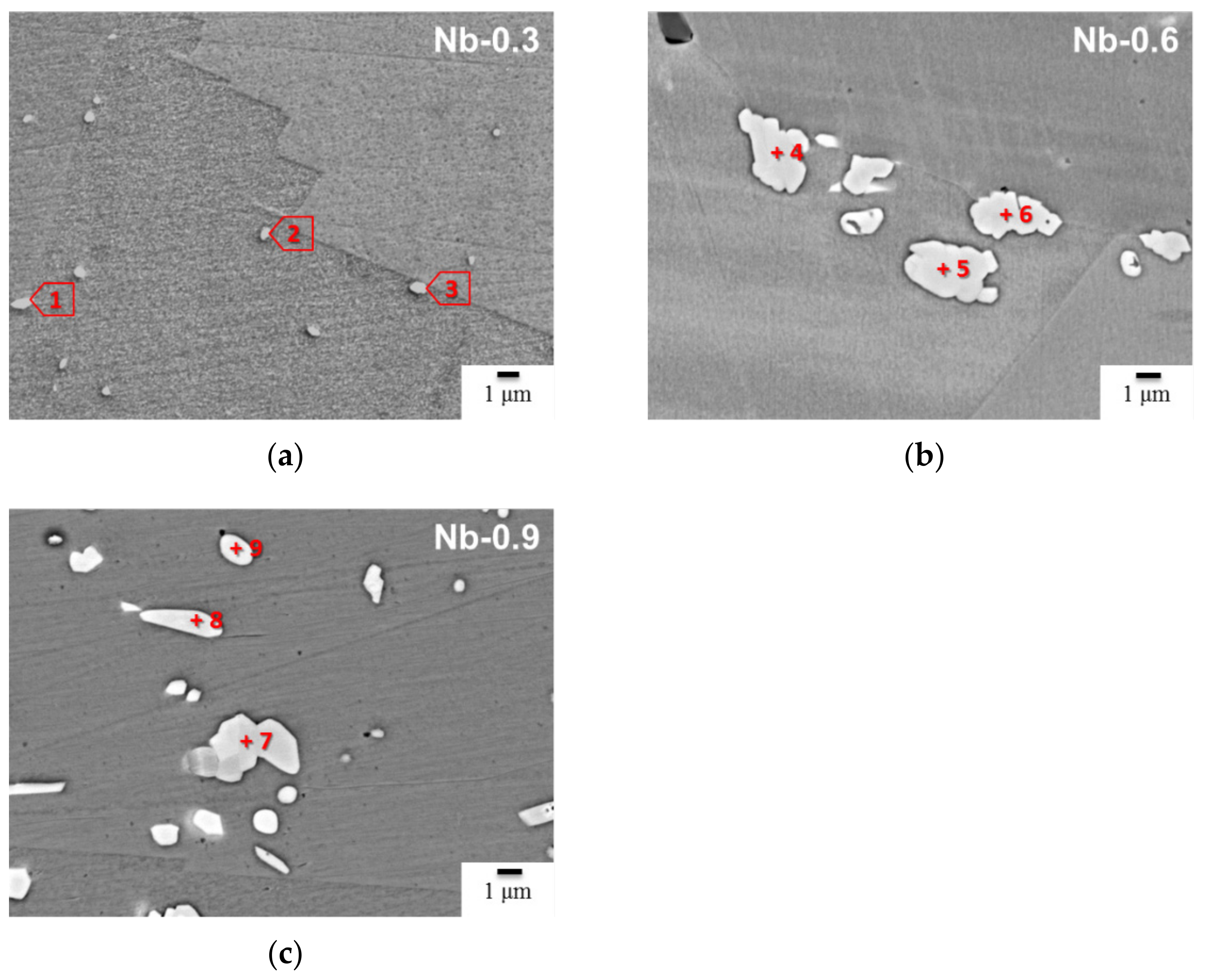



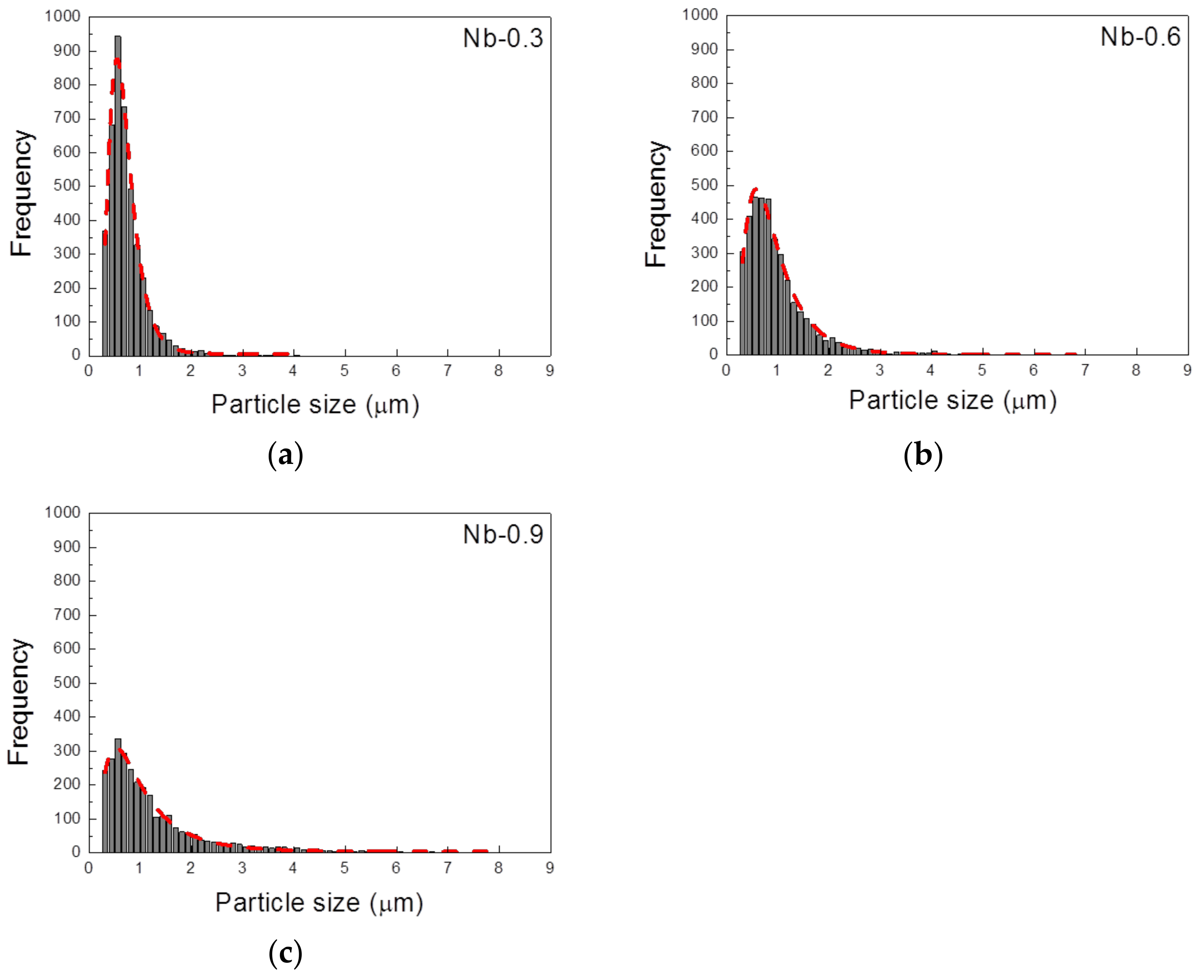

| Cr | Ni | W | Cu | Co | N | C | Si | Mn | P | S | Nb | Fe | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nb-free | 22.20 | 24.40 | 3.39 | 2.90 | 1.47 | 0.23 | 0.07 | 0.19 | 0.49 | 0.009 | 0.005 | - | Bal. |
| Nb-0.3 | 22.10 | 24.30 | 3.40 | 2.90 | 1.49 | 0.23 | 0.07 | 0.19 | 0.49 | 0.010 | 0.005 | 0.29 | Bal. |
| Nb-0.6 | 21.90 | 24.20 | 3.36 | 2.90 | 1.45 | 0.23 | 0.07 | 0.20 | 0.49 | 0.010 | 0.005 | 0.58 | Bal. |
| Nb-0.9 | 21.80 | 24.10 | 3.52 | 2.90 | 1.54 | 0.23 | 0.07 | 0.20 | 0.49 | 0.010 | 0.005 | 0.86 | Bal. |
| Cr at.% | Fe at.% | Nb at.% | W at.% | C at.% | N at.% | Si at.% | Phase | |
|---|---|---|---|---|---|---|---|---|
| 1 (Nb-0.3) | 28.5 ± 0.9 | 5.5 ± 0.3 | 31.9 ± 1.6 | 2.5 ± 0.8 | 16.0 ± 0.8 | 15.6 ± 0.5 | - | CrNb(C, N) |
| 2 (Nb-0.3) | 26.2 ± 0.9 | 4.9 ± 0.3 | 29.1 ± 1.6 | 1.9 ± 0.9 | 20.8 ± 0.9 | 17.1 ± 0.6 | - | CrNb(C, N) |
| 3 (Nb-0.3) | 5.6 ± 0.3 | 3.4 ± 0.2 | 47.8 ± 1.4 | - | 27.0 ± 1.0 | 16.2 ± 0.5 | - | MX |
| 4 (Nb-0.3) | 30.5 ± 1.0 | 4.0 ± 0.3 | 33.6 ± 1.6 | 1.3 ± 0.9 | - | 30.6 ± 0.5 | - | CrNbN |
| 5 (Nb-0.6) | 29.8 ± 1.0 | 6.7 ± 0.3 | 30.2 ± 1.5 | 1.6 ± 0.8 | 8.9 ± 0.8 | 22.8 ± 0.6 | - | CrNb(C, N) |
| 6 (Nb-0.6) | 29.3 ± 0.9 | 3.9 ± 0.2 | 30.7 ± 1.4 | 1.7 ± 0.6 | 4.4 ± 0.4 | 30.0 ± 0.5 | - | CrNb(C, N) |
| 7 (Nb-0.6) | 27.1 ± 0.8 | 5.1 ± 0.3 | 34.1 ± 1.6 | 1.8 ± 0.9 | 4.9 ± 0.6 | 27.0 ± 0.5 | - | CrNb(C, N) |
| 8 (Nb-0.9) | 30.1 ± 0.9 | 5.3 ± 0.3 | 30.5 ± 1.6 | 1.3 ± 0.9 | 15.2 ± 0.8 | 17.6 ± 0.6 | - | CrNb(C, N) |
| 9 (Nb-0.9) | 24.9 ± 0.8 | 4.4 ± 0.3 | 30.3 ± 1.5 | 1.4 ± 0.8 | 30.1 ± 1.0 | 8.9 ± 0.5 | CrNb(C, N) | |
| 10 (Nb-0.9) | 28.4 ± 0.9 | 4.5 ± 0.3 | 31.1 ± 1.6 | 1.4 ± 0.9 | 25.6 ± 0.9 | 7.3 ± 0.5 | 1.7 ± 0.6 | CrNb(C, N) |
| 11 (Nb-0.9) | 36.1 ± 1.2 | 17.5 ± 0.6 | 3.0 ± 0.6 | 1.3 ± 0.5 | 22.7 ± 1.0 | 19.4 ± 0.5 | MX | |
| 12 (Nb-0.9) | 33.3 ± 1.1 | 5.3 ± 0.2 | 29.4 ± 1.4 | 1.9 ± 1.0 | 12.6 ± 0.7 | 16.3 ± 0.6 | 1.2 ± 0.6 | CrNb(C, N) |
| 13 (Nb-0.9) | 3.0 ± 0.2 | 1.6 ± 0.3 | 46.7 ± 1.8 | - | 41.9 ± 1.2 | 4.0 ± 0.3 | 2.8 ± 0.8 | MX |
| Cr at.% | Fe at.% | Nb at.% | W at.% | C at.% | N at.% | Cu at.% | Ni at.% | Co at.% | Mn at.% | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 (Nb-0.3) | 29.3 | 27.6 | 14.9 | 1.3 | 3.3 | 7.5 | 1.2 | 13.7 | 0.8 | 0.4 |
| 2 (Nb-0.3) | 25.8 | 36.6 | 5.4 | 1.2 | 1.6 | 6.5 | 1.8 | 19.4 | 1.2 | 0.5 |
| 3 (Nb-0.3) | 23.8 | 34.9 | 5.6 | 1.0 | 2.1 | 11.1 | 1.6 | 18.2 | 1.2 | 0.5 |
| 4 (Nb-0.6) | 29.1 | 4.5 | 29.5 | 1.3 | 4.2 | 30.3 | 0.1 | 0.8 | 0.1 | 0.1 |
| 5 (Nb-0.6) | 28.7 | 4.5 | 30.2 | 1.4 | 4.0 | 30.2 | - | 0.8 | 0.1 | 0.1 |
| 6 (Nb-0.6) | 28.8 | 4.3 | 32.0 | 1.5 | 4.5 | 28.1 | - | 0.6 | 0.1 | 0.1 |
| 7 (Nb-0.9) | 27.3 | 4.5 | 28.6 | 1.3 | 2.9 | 34.2 | - | 0.9 | 0.2 | 0.1 |
| 8 (Nb-0.9) | 28.6 | 5.2 | 29.1 | 1.4 | 2.9 | 31.4 | - | 1.1 | 0.1 | 0.2 |
| 9 (Nb-0.9) | 27.0 | 12.4 | 22.9 | 1.3 | 2.9 | 27.8 | 0.4 | 4.9 | 0.3 | 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.-M.; Wu, J.-L.; Pan, Y.-T.; Lin, D.-Y. Precipitate Evolution in 22Cr25NiWCuCo(Nb) Austenitic Heat-Resistant Stainless Steel during Heat Treatment at 1200 °C. Materials 2021, 14, 1104. https://doi.org/10.3390/ma14051104
Yang S-M, Wu J-L, Pan Y-T, Lin D-Y. Precipitate Evolution in 22Cr25NiWCuCo(Nb) Austenitic Heat-Resistant Stainless Steel during Heat Treatment at 1200 °C. Materials. 2021; 14(5):1104. https://doi.org/10.3390/ma14051104
Chicago/Turabian StyleYang, Sheng-Min, Jing-Lin Wu, Yeong-Tsuen Pan, and Dong-Yih Lin. 2021. "Precipitate Evolution in 22Cr25NiWCuCo(Nb) Austenitic Heat-Resistant Stainless Steel during Heat Treatment at 1200 °C" Materials 14, no. 5: 1104. https://doi.org/10.3390/ma14051104
APA StyleYang, S.-M., Wu, J.-L., Pan, Y.-T., & Lin, D.-Y. (2021). Precipitate Evolution in 22Cr25NiWCuCo(Nb) Austenitic Heat-Resistant Stainless Steel during Heat Treatment at 1200 °C. Materials, 14(5), 1104. https://doi.org/10.3390/ma14051104





