Tuning Mg(OH)2 Structural, Physical, and Morphological Characteristics for Its Optimal Behavior in a Thermochemical Heat-Storage Application
Abstract
1. Introduction
2. Experimental Section
2.1. Synthesis of Mg(OH)2
2.2. Structural and Morphological Characterization of Mg(OH)2 Studied
2.3. Thermochemical Performance of Mg(OH)2 Studied
3. Results and Discussion
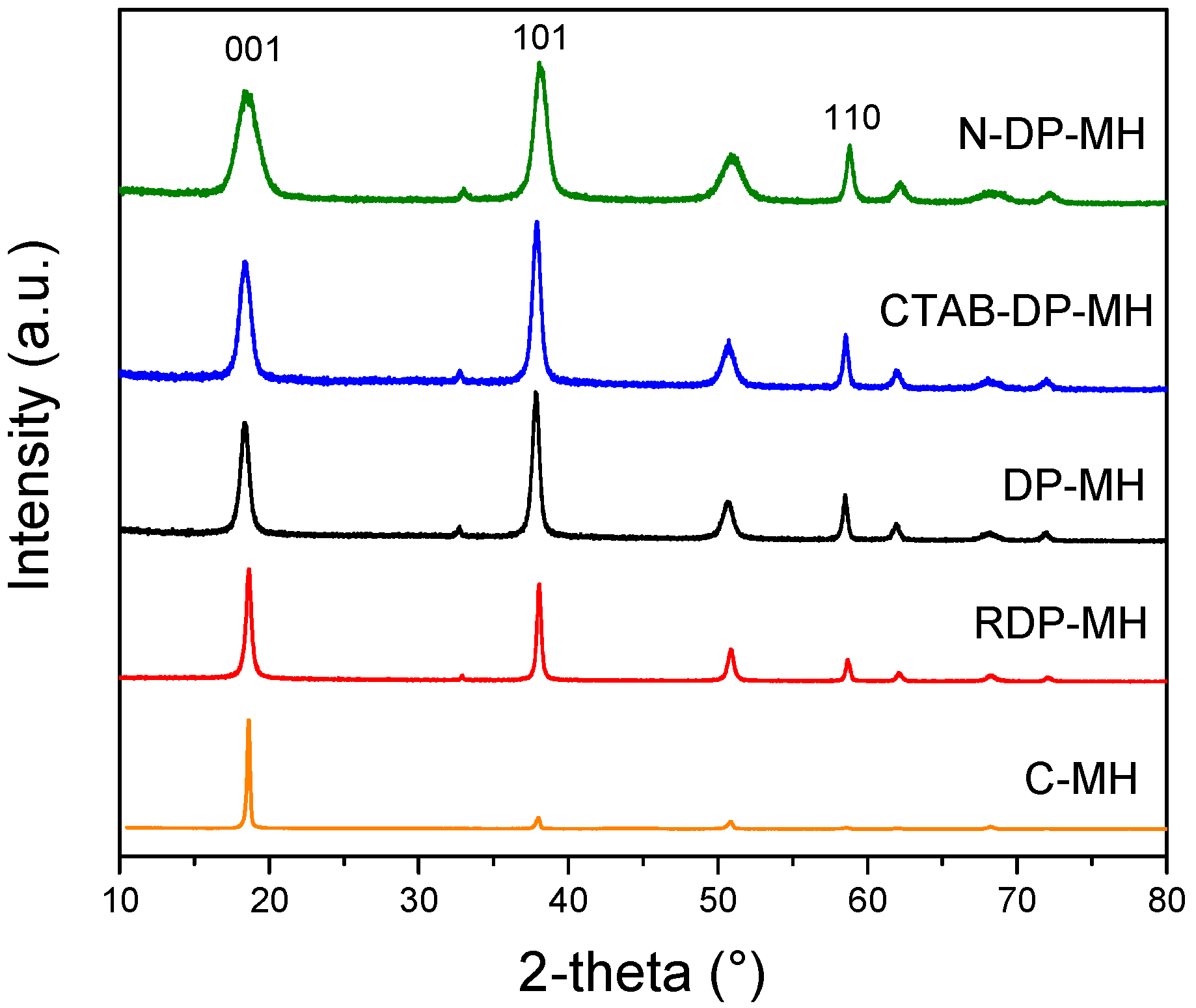
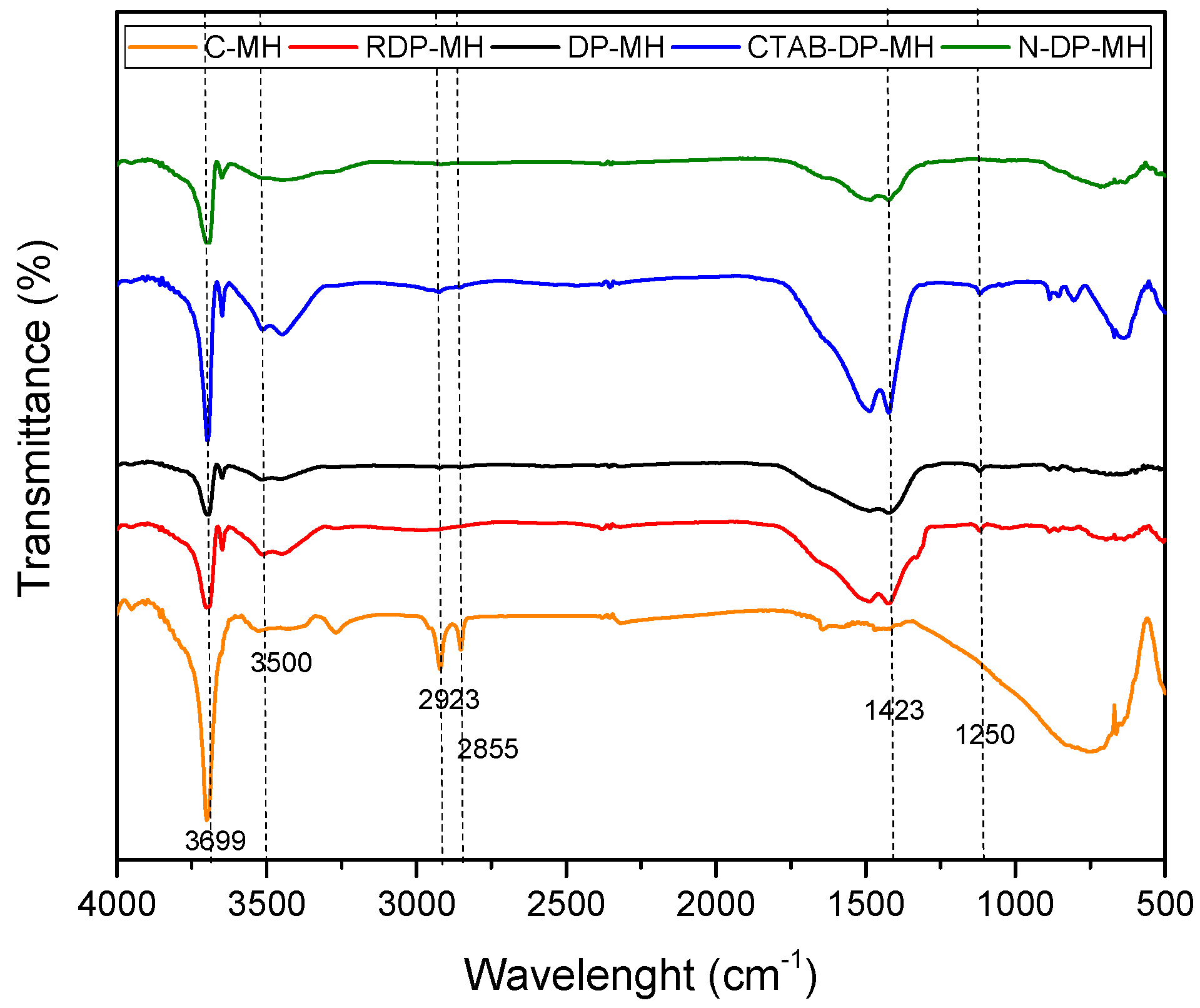
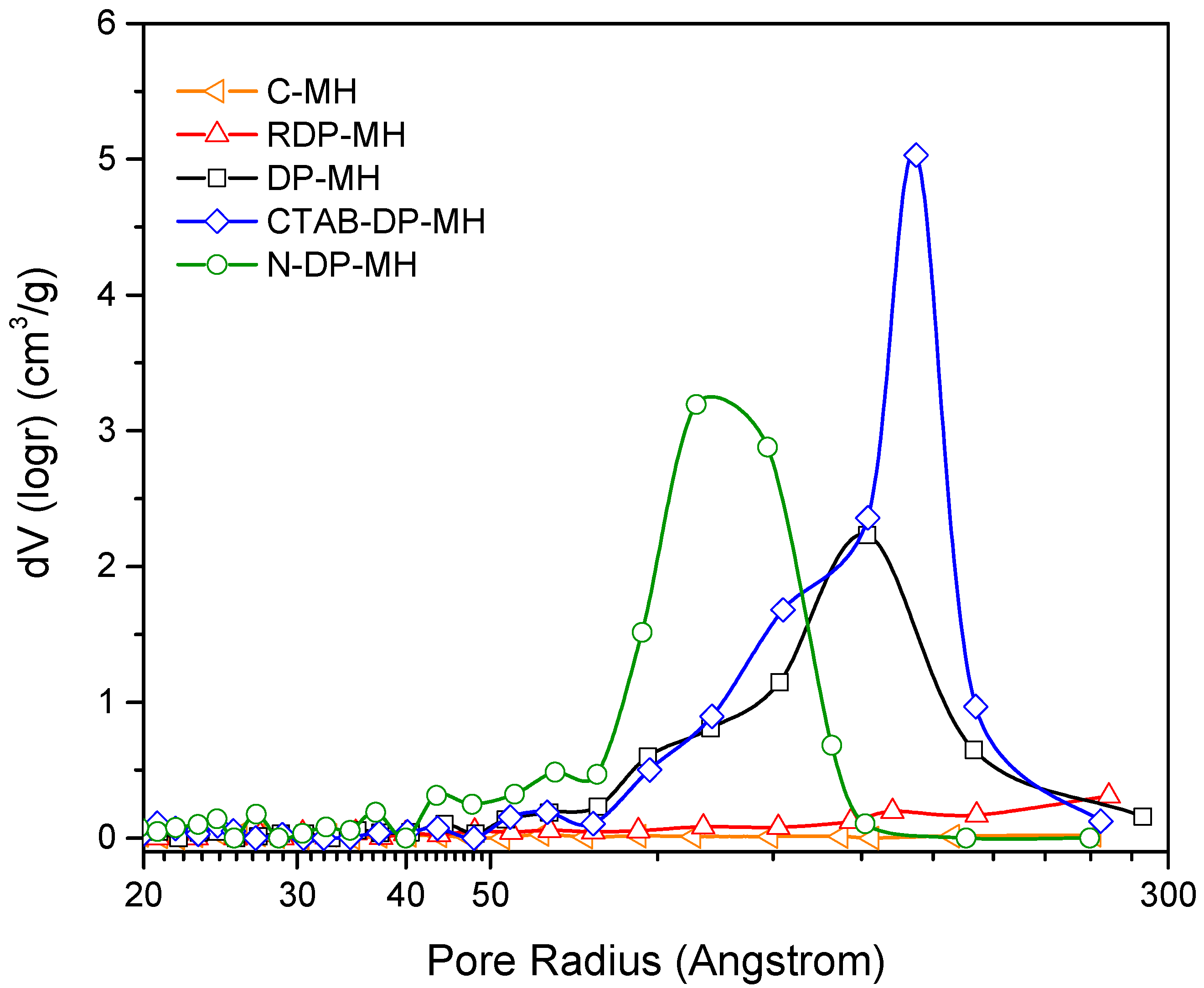
3.1. Structural, Morphological and Physical Characterization of the Samples Studied
Remark on the Role of the Synthesis Route on Mg(OH)2 Structural, Physical, and Morphological Characteristics
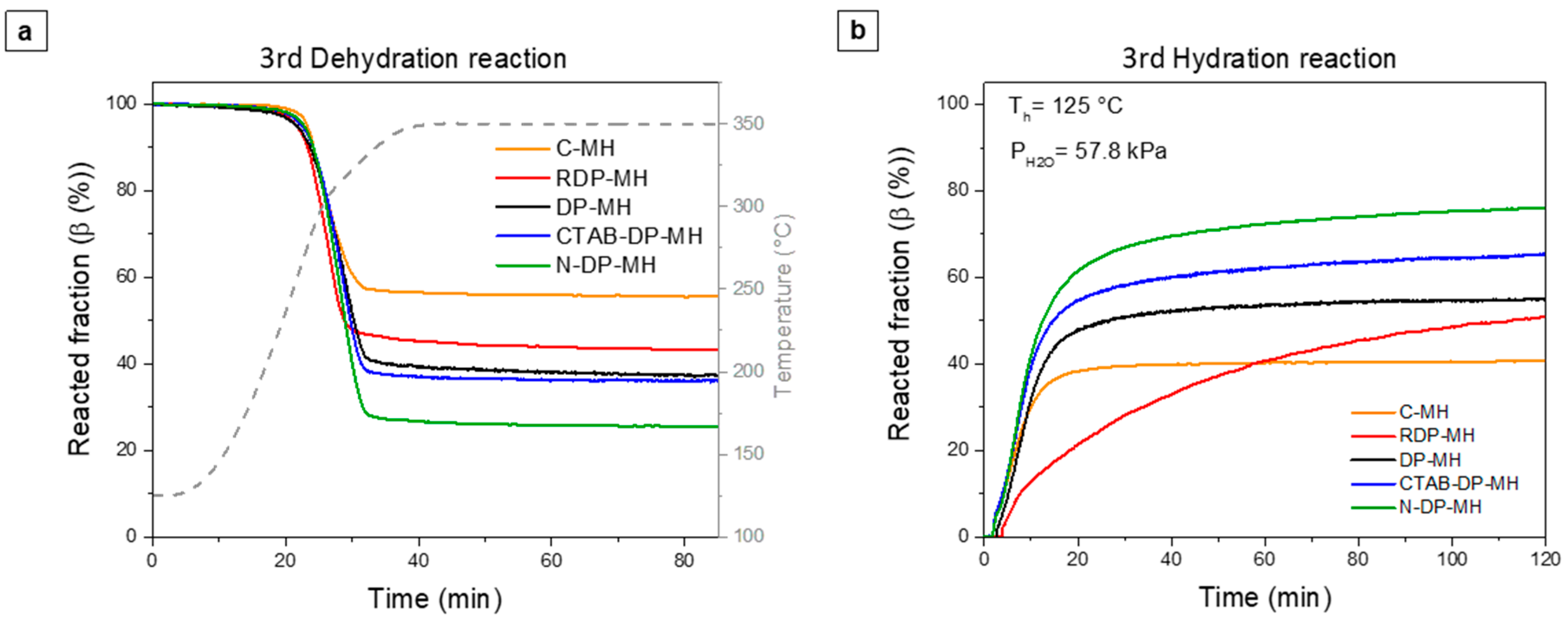
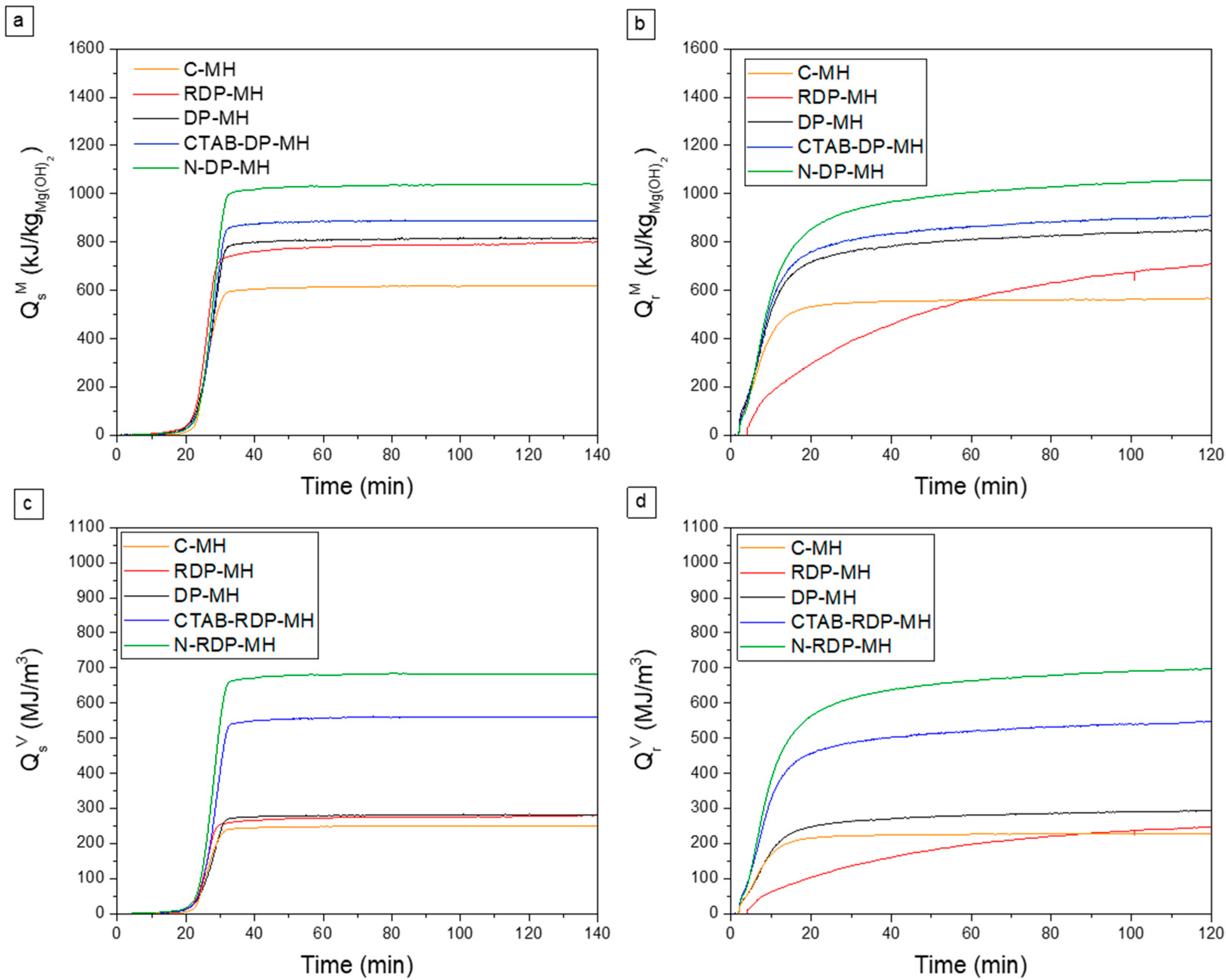
3.2. Thermochemical Performance of Mg(OH)2 Samples
3.3. Cross-Correlation between Mg(OH)2 Physical, Morphological and Thermochemical Variables
- specific surface area (SBET);
- pore volume (VPORE);
- density (ρ);
- mean particle size (MPS);
- reacted fraction (Δβ);
- volumetric heat storage capacity ().
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| I001, I101, I110 | intensity reflections respectively of [001], [101] and [110] planes |
| min | initial sample mass (g) |
| minst | instantaneous mass (g) |
| molecular weight of Mg(OH)2 (g mol−1) | |
| MMgO | molecular weight of MgO (g mol−1) |
| stored/released heat capacity per mass unit () | |
| stored/released heat capacity per volume unit (MJ m−3) | |
| SBET | specific surface area (m2 g−1) |
| Td | dehydration temperature (°C) |
| Th | hydration temperature (°C) |
| VPORE | pore volume (cm3 g−1) |
| Greek symbols | |
| β | reacted fraction (%) |
| β fd | reacted fraction at the end of the dehydration treatment (%) |
| β id | reacted fraction at the beginning of the dehydration treatment (%) |
| βh | final reacted fraction of MgO at the point of water supply termination (%) |
| Δmreal | instantaneous real mass change (%) |
| Δmth | theoretical mass change due to the dehydration of Mg(OH)2 normalized to the total amount present in the sample (%) |
| Δβd | dehydration conversion (%) |
| Δβh | hydration conversion (%) |
| ΔH0 | dehydration/hydration reaction enthalpy (kJ mol−1) |
| ρ | bulk density (kg m−3) |
References
- Shkatulov, A.; Ryu, J.; Kato, Y.; Aristov, Y. Composite material “ Mg(OH)2/vermiculite”: A promising new candidate for storage of middle temperature heat. Energy 2012, 44, 1028–1034. [Google Scholar] [CrossRef]
- Rohde, D.; Knudsen, B.R.; Andresen, T.; Nord, N. Dynamic optimization of control setpoints for an integrated heating and cooling system with thermal energy storages. Energy 2020, 193, 116771. [Google Scholar] [CrossRef]
- Meroueh, L.; Chen, G. Thermal energy storage radiatively coupled to a supercritical Rankine cycle for electric grid support. Renew. Energy 2020, 145, 604–621. [Google Scholar] [CrossRef]
- Lu, H.; Tian, P.; He, L. Evaluating the global potential of aquifer thermal energy storage and determining the potential worldwide hotspots driven by socio-economic, geo-hydrologic and climatic conditions. Renew. Sustain. Energy Rev. 2019, 112, 788–796. [Google Scholar] [CrossRef]
- Talebi, B.; Haghighat, F.; Tuohy, P.; Mirzaei, P.A. Optimization of a hybrid community district heating system integrated with thermal energy storage system. J. Energy Storage 2019, 23, 128–137. [Google Scholar] [CrossRef]
- Gibb, D.; Johnson, M.; Romaní, J.; Gasia, J.; Cabeza, L.F.; Seitz, A. Process integration of thermal energy storage systems—Evaluation methodology and case studies. Appl. Energy 2018, 230, 750–760. [Google Scholar] [CrossRef]
- Vigneshwaran, K.; Sodhi, G.S.; Muthukumar, P.; Guha, A.; Senthilmurugan, S. Experimental and numerical investigations on high temperature cast steel based sensible heat storage system. Appl. Energy 2019, 251, 113322–113344. [Google Scholar] [CrossRef]
- Frate, G.F.; Ferrari, L.; Desideri, U. Multi-criteria investigation of a pumped thermal electricity storage (PTES) system with thermal integration and sensible heat storage. Energy Convers. Manag. 2020, 208, 112530. [Google Scholar] [CrossRef]
- Sarbu, I.; Dorca, A. Review on heat transfer analysis in thermal energy storage using latent heat storage systems and phase change materials. Int. J. Energy Res. 2019, 43, 29–64. [Google Scholar] [CrossRef]
- Kumar, A.; Jain, H.; Tripathi, B.P. Synthesis and Nanoencapsulation of Poly(ethylene glycol)-Distearates Phase Change Materials for Latent Heat Storage and Release. ACS Appl. Energy Mater. 2020, 3, 5965–5976. [Google Scholar] [CrossRef]
- Lu, P.; Ghaban, R.; Duong, J.; Patel, D.; Singh, H.; Chen, W. Solvent-assisted nanochannel encapsulation of a natural phase change material in polystyrene hollow fibers for high-performance thermal energy storage. ACS Appl. Energy Mater. 2020, 3, 10089–10096. [Google Scholar] [CrossRef]
- Prasad, J.S.; Muthukumar, P.; Desai, F.; Basu, D.N.; Rahman, M.M. A critical review of high-temperature reversible thermochemical energy storage systems. Appl. Energy 2019, 254, 113733. [Google Scholar] [CrossRef]
- Maruyama, A.; Kurosawa, R.; Ryu, J. Effect of Lithium Compound Addition on the Dehydration and Hydration of Calcium Hydroxide as a Chemical Heat Storage Material. ACS Omega 2020, 5, 9820–9829. [Google Scholar] [CrossRef]
- Ervin, G. Solar heat storage using chemical reactions. J. Solid State Chem. 1977, 22, 51–61. [Google Scholar] [CrossRef]
- Mukherjee, A.; Majumdar, R.; Saha, S.K.; Subramaniam, C.; Kumar, L. Performance evaluation of an open thermochemical energy storage system integrated with flat plate solar collector. Appl. Therm. Eng. 2020, 173, 115218. [Google Scholar] [CrossRef]
- Shkatulov, A.; Joosten, R.; Fischer, H.; Huinink, H. Core-Shell Encapsulation of Salt Hydrates into Mesoporous Silica Shells for Thermochemical Energy Storage. ACS Appl. Energy Mater. 2020, 3, 6860–6869. [Google Scholar] [CrossRef]
- Liu, J.; Baeyens, J.; Deng, Y.; Wang, X.; Zhang, H. High temperature Mn2O3/Mn3O4 and Co3O4/CoO systems for thermo-chemical energy storage. J. Environ. Manag. 2020, 267, 110582. [Google Scholar] [CrossRef]
- Alonso, E.; Pérez-Rábago, C.; Licurgo, J.; Fuentealba, E.; Estrada, C.A. First experimental studies of solar redox reactions of copper oxides for thermochemical energy storage. Sol. Energy 2015, 115, 297–305. [Google Scholar] [CrossRef]
- Schrader, A.J.; Bush, H.E.; Ranjan, D.; Loutzenhiser, P.G. Aluminum-doped calcium manganite particles for solar thermochemical energy storage: Reactor design, particle characterization, and heat and mass transfer modeling. Int. J. Heat Mass Transf. 2020, 152, 119461. [Google Scholar] [CrossRef]
- Mastronardo, E.; Qian, X.; Coronado, J.M.; Haile, S.M. The favourable thermodynamic properties of Fe-doped CaMnO3 for thermochemical heat storage. J. Mater. Chem. A 2020, 8, 8503–8517. [Google Scholar] [CrossRef]
- Mastronardo, E.; Bonaccorsi, L.; Kato, Y.; Piperopoulos, E.; Milone, C. Efficiency improvement of heat storage materials for MgO/H2O/Mg(OH)2 chemical heat pumps. Appl. Energy 2016, 162, 31–39. [Google Scholar] [CrossRef]
- Mastronardo, E.; Bonaccorsi, L.; Kato, Y.; Piperopoulos, E.; Lanza, M.; Milone, C. Strategies for the enhancement of heat storage materials performances for MgO/H2O/Mg(OH)2 thermochemical storage system. Appl. Therm. Eng. 2017, 120, 626–634. [Google Scholar] [CrossRef]
- Kato, Y.; Yamashita, N.; Kobayashi, K.; Yoshio, Y. Kinetic study of the hydration of magnesium oxide for a chemical heat pump. Appl. Therm. Eng. 1996, 16, 853–862. [Google Scholar] [CrossRef]
- André, L.; Abanades, S.; Flamant, G. Screening of thermochemical systems based on solid-gas reversible reactions for high temperature solar thermal energy storage. Renew. Sustain. Energy Rev. 2016, 64, 703–715. [Google Scholar] [CrossRef]
- Kurosawa, R.; Takeuchi, M.; Ryu, J. Comparison of the Effect of Coaddition of Li Compounds and Addition of a Single Li Compound on Reactivity and Structure of Magnesium Hydroxide. ACS Omega 2019, 4, 17752–17761. [Google Scholar] [CrossRef]
- Ishitobi, H.; Hirao, N.; Ryu, J.; Kato, Y. Evaluation of heat output densities of lithium chloride-modified magnesium hydroxide for thermochemical energy storage. Ind. Eng. Chem. Res. 2013, 52, 5321–5325. [Google Scholar] [CrossRef]
- Shkatulov, A.; Krieger, T.; Zaikovskii, V.; Chesalov, Y.; Aristov, Y. Doping magnesium hydroxide with sodium nitrate: A new approach to tune the dehydration reactivity of heat-storage materials. ACS Appl. Mater. Interfaces 2014, 6, 19966–19977. [Google Scholar] [CrossRef]
- Mastronardo, E.; Kato, Y.; Bonaccorsi, L.; Piperopoulos, E.; Milone, C. Thermochemical storage of middle temperature wasted heat by functionalized C/Mg(OH)2 hybrid materials. Energies 2017, 10, 70. [Google Scholar] [CrossRef]
- Zamengo, M.; Ryu, J.; Kato, Y. Thermochemical performance of magnesium hydroxide-expanded graphite pellets for chemical heat pump. Appl. Therm. Eng. 2014, 64, 339–347. [Google Scholar] [CrossRef]
- Mastronardo, E.; Bonaccorsi, L.; Kato, Y.; Piperopoulos, E.; Lanza, M.; Milone, C. Thermochemical performance of carbon nanotubes based hybrid materials for MgO/H2O/Mg(OH)2 chemical heat pumps. Appl. Energy 2016, 181, 232–243. [Google Scholar] [CrossRef]
- Shkatulov, A.I.; Aristov, Y. Thermochemical Energy Storage using LiNO3-Doped Mg(OH)2: A Dehydration Study. Energy Technol. 2018, 6, 1844–1851. [Google Scholar] [CrossRef]
- Piperopoulos, E.; Fazio, M.; Mastronardo, E. Synthesis of me doped Mg(OH)2 materials for thermochemical heat storage. Nanomaterials 2018, 8, 573. [Google Scholar] [CrossRef]
- Müller, D.; Knoll, C.; Gravogl, G.; Artner, W.; Welch, J.M.; Eitenberger, E.; Friedbacher, G.; Schreiner, M.; Harasek, M.; Hradil, K.; et al. Tuning the performance of MgO for thermochemical energy storage by dehydration—From fundamentals to phase impurities. Appl. Energy 2019, 253, 113562. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, N.; Kim, T.S.; Park, G.J.; Kwon, Y.; Yu, H.K. Mg(OH)2 nano-sheet decorated MgO micro-beams by electron beam irradiation for thermochemical heat storage. Ceram. Int. 2019, 45, 18908–18913. [Google Scholar] [CrossRef]
- Piperopoulos, E.; Mastronardo, E.; Fazio, M.; Lanza, M.; Galvagno, S.; Milone, C. Enhancing the volumetric heat storage capacity of Mg(OH)2 by the addition of a cationic surfactant during its synthesis. Appl. Energy 2018, 215, 512–522. [Google Scholar] [CrossRef]
- Yan, J.; Pan, Z.H.; Zhao, C.Y. Experimental study of MgO/Mg(OH)2 thermochemical heat storage with direct heat transfer mode. Appl. Energy 2020, 275, 115356. [Google Scholar] [CrossRef]
- Piperopoulos, E.; Mastronardo, E.; Fazio, M.; Lanza, M.; Galvagno, S.; Milone, C. Synthetic strategies for the enhancement of Mg(OH)2 thermochemical performances as heat storage material. Energy Procedia 2018, 155, 269–279. [Google Scholar] [CrossRef]
- Giorgi, R.; Bozzi, C.; Dei, L.; Gabbiani, C.; Ninham, B.W.; Baglioni, P. Nanoparticles of Mg(OH)2: Synthesis and application to paper conservation. Langmuir 2005, 21, 8495–8501. [Google Scholar] [CrossRef]
- Kumari, L.; Li, W.Z.; Vannoy, C.H.; Leblanc, R.M.; Wang, D.Z. Synthesis, characterization and optical properties of Mg(OH)2 micro-/nanostructure and its conversion to MgO. Ceram Int 2009, 35, 3355–3364. [Google Scholar] [CrossRef]
- Gao, R.; Wang, J.; Zhang, X.; Yan, H.; Yang, W.; Liu, Q.; Zhang, M.; Liu, L.; Takahashi, K. Fabrication of superhydrophobic magnesium alloy through the oxidation of hydrogen peroxide. Colloids Surf. A Physicochem. Eng. Asp. 2013, 436, 906–911. [Google Scholar] [CrossRef]
- Jiang, W.; Hua, X.; Han, Q.; Yang, X.; Lu, L.; Wang, X. Preparation of lamellar magnesium hydroxide nanoparticles via precipitation method. Powder Technol. 2009, 191, 227–230. [Google Scholar] [CrossRef]
- Sangwichien, C.; Aranovich, G.L.; Donohue, M.D. Density functional theory predictions of adsorption isotherms with hysteresis loops. Colloids Surf. A Physicochem. Eng. Asp. 2002, 206, 313–320. [Google Scholar] [CrossRef]
- Xiang, L.; Jin, Y.C.; Jin, Y. Hydrothermal formation of dispersive Mg(OH)2 particles in NaOH solution. Trans. Nonferrous Met. Soc. China Engl. Ed. 2004, 14, 370–375. [Google Scholar] [CrossRef]
- Matos, C.R.S.; Xavier, M.J.; Barreto, L.S.; Costa, N.B.; Gimenez, I.F. Principal component analysis of X-ray diffraction patterns to yield morphological classification of brucite particles. Anal. Chem. 2007, 79, 2091–2095. [Google Scholar] [CrossRef]
- Henrist, C.; Mathieu, J.P.; Vogels, C.; Rulmont, A.; Cloots, R. Morphological study of magnesium hydroxide nanoparticles precipitated in dilute aqueous solution. J. Cryst. Growth 2003, 249, 321–330. [Google Scholar] [CrossRef]
- Pearson, K. Note on Regression and Inheritance in the Case of Two Parents. R. Soc. Publ. 1895, 58, 240–242. [Google Scholar]
- Lee Rodgers, J.; Alan Nice Wander, W. Thirteen ways to look at the correlation coefficient. Am. Stat. 1988, 42, 59–66. [Google Scholar] [CrossRef]





| Code | Synthesis Procedures |
|---|---|
| C-MH | Commercial Mg(OH)2 |
| RDP-MH | Reverse deposition precipitation |
| DP-MH | Deposition precipitation |
| CTAB-DP-MH | Deposition precipitation in presence of CTAB |
| N-DP-MH | Deposition precipitation using NaOH as precipitating agent |
| Code | Intensity Ratios | Physical Properties | |||
|---|---|---|---|---|---|
| I001/I101 | I001/I110 | SBET (m2/g) | VPORE (cm3/g) | (kg/m3) | |
| C-MH | 10.1 | 93.32 | 7.0 | 0.020 | 405 ± 2 |
| RDP-MH | 1.1 | 5.70 | 44.4 | 0.112 | 350 ± 5 |
| DP-MH | 0.8 | 2.52 | 64.5 | 0.618 | 345 ± 6 |
| CTAB-DP-MH | 0.8 | 2.27 | 85.6 | 0.735 | 602 ± 6 |
| N-DP-MH | 0.8 | 1.90 | 124.5 | 0.723 | 660 ± 4 |
| Code | 3rd Cycle | |||||
|---|---|---|---|---|---|---|
| Δβd (%) | Δβh (%) | (MJ/m3) | (MJ/m3) | |||
| C-MH | 44.4 ± 1.3 | 40.7 ± 1.5 | 617 ± 7.5 | 565 ± 7.7 | 250 ± 11.0 | 230 ± 10.8 |
| RDP-MH | 56.7 ± 1.5 | 50.9 ± 1.6 | 797 ± 9.8 | 707 ± 8.6 | 280 ± 12.0 | 248 ± 11.6 |
| DP-MH | 62.8 ± 1.6 | 55.5 ± 1.6 | 872.9 ± 9.6 | 771.4 ± 8.8 | 302 ± 7.5 | 254 ± 6.3 |
| CTAB-DP-MH | 66.8 ± 1.5 | 67.0 ± 1.5 | 928.5 ± 11.0 | 931.2 ± 10.3 | 559 ± 13.9 | 561 ± 14.0 |
| N-DP-MH | 74.6 ± 1.6 | 76.1 ± 1.6 | 1041 ± 10.2 | 1056 ± 9.5 | 684 ± 11.3 | 698 ± 12.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piperopoulos, E.; Fazio, M.; Mastronardo, E.; Lanza, M.; Milone, C. Tuning Mg(OH)2 Structural, Physical, and Morphological Characteristics for Its Optimal Behavior in a Thermochemical Heat-Storage Application. Materials 2021, 14, 1091. https://doi.org/10.3390/ma14051091
Piperopoulos E, Fazio M, Mastronardo E, Lanza M, Milone C. Tuning Mg(OH)2 Structural, Physical, and Morphological Characteristics for Its Optimal Behavior in a Thermochemical Heat-Storage Application. Materials. 2021; 14(5):1091. https://doi.org/10.3390/ma14051091
Chicago/Turabian StylePiperopoulos, Elpida, Marianna Fazio, Emanuela Mastronardo, Maurizio Lanza, and Candida Milone. 2021. "Tuning Mg(OH)2 Structural, Physical, and Morphological Characteristics for Its Optimal Behavior in a Thermochemical Heat-Storage Application" Materials 14, no. 5: 1091. https://doi.org/10.3390/ma14051091
APA StylePiperopoulos, E., Fazio, M., Mastronardo, E., Lanza, M., & Milone, C. (2021). Tuning Mg(OH)2 Structural, Physical, and Morphological Characteristics for Its Optimal Behavior in a Thermochemical Heat-Storage Application. Materials, 14(5), 1091. https://doi.org/10.3390/ma14051091









