Chemical Evaluation of Energy Dispersive X-ray Spectroscopy Analysis of Different Failing Dental Implant Surfaces: A Comparative Clinical Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Eligibility Criteria
2.2. Clinical and Radiographical Measurements
2.3. Retrieval Procedure for the Dental Implants
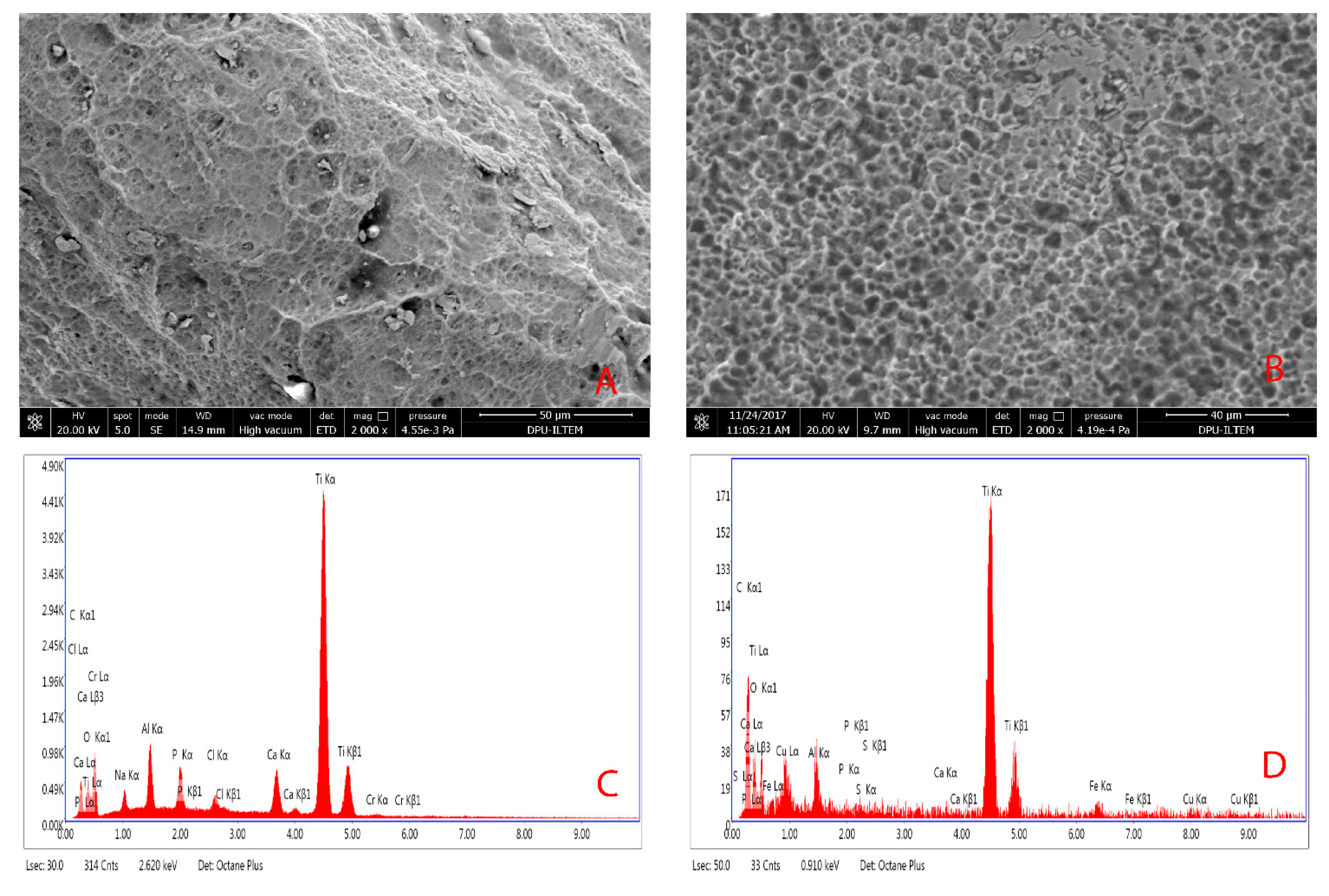
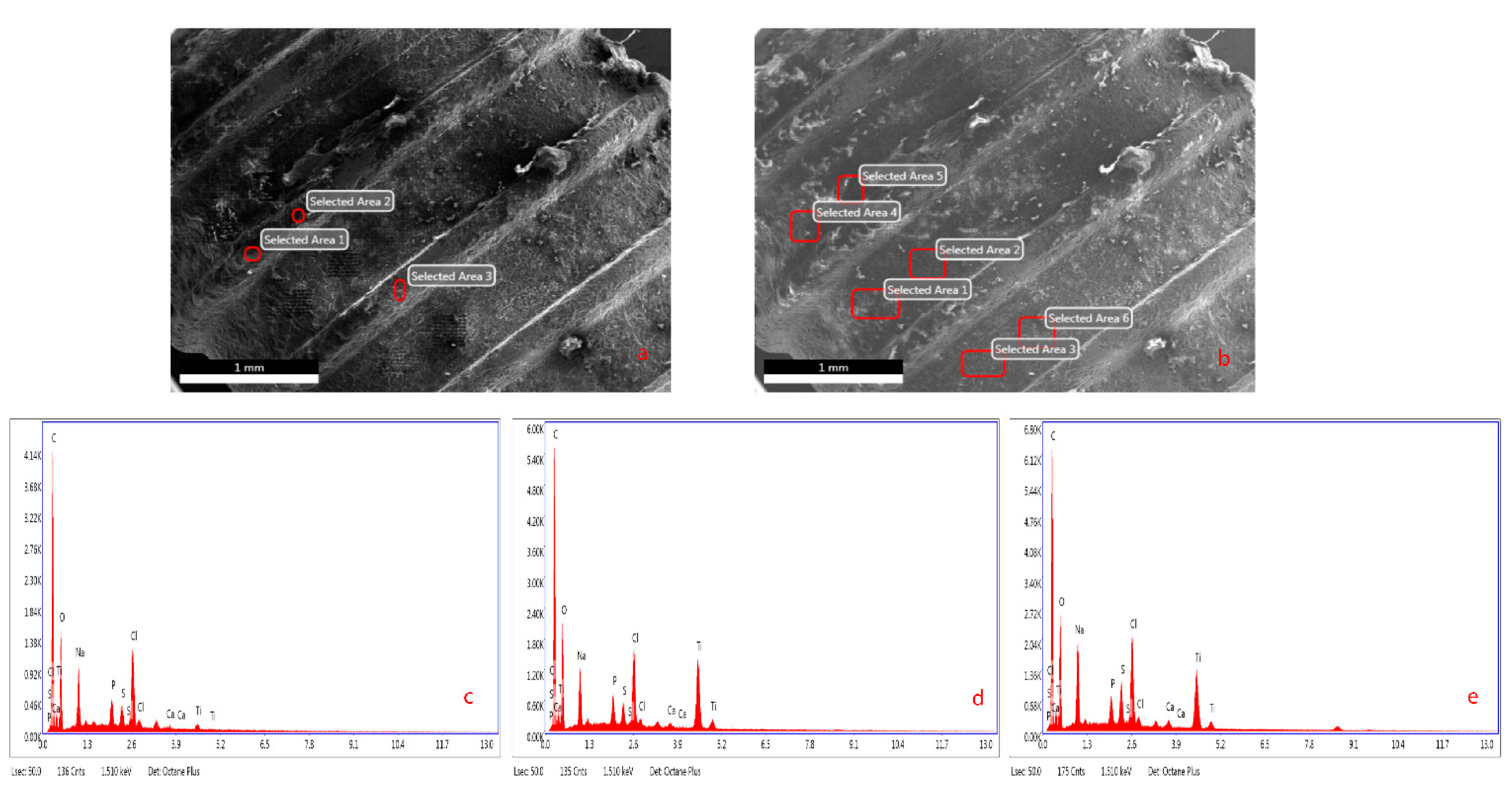
2.4. Scanning Electron Microscopy and Energy Dispersive X-ray Spectrometry Analysis
2.5. Statistical Analysis
3. Results
3.1. Demographic Data
3.2. Surface Chemistry Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Berglundh, T.; Armitage, G.; Araujo, M.G.; Avila-Ortiz, G.; Blanco, J.; Camargo, P.M.; Chen, S.; Cochran, D.; Derks, J.; Figuero, E.; et al. Peri-implant diseases and conditions: Consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89, S313–S318. [Google Scholar] [CrossRef] [PubMed]
- Albrektsson, T.C.B.; Jacobsson, M.; Wennerberg, A. Osseointegration of implants—A biological and clinical overview. JSM Dent. Surg. 2017, 2, 1022. [Google Scholar]
- De Bruyn, H.; Christiaens, V.; Doornewaard, R.; Jacobsson, M.; Cosyn, J.; Jacquet, W.; Vervaeke, S. Implant surface roughness and patient factors on long-term peri-implant bone loss. Periodontology 2017, 73, 218–227. [Google Scholar] [CrossRef]
- Ratner, B.D.; Bryant, S.J. Biomaterials: Where We Have Been and Where We Are Going. Annu. Rev. Biomed. Eng. 2004, 6, 41–75. [Google Scholar] [CrossRef]
- Shih, Y.-R.V.; Hwang, Y.; Phadke, A.; Kang, H.; Hwang, N.S.; Caro, E.J.; Nguyen, S.; Siu, M.; Theodorakis, E.A.; Gianneschi, N.C.; et al. Calcium phosphate-bearing matrices induce osteogenic differentiation of stem cells through adenosine signaling. Proc. Natl. Acad. Sci. USA 2014, 111, 990–995. [Google Scholar] [CrossRef]
- Mouhyi, J.; Ehrenfest, D.M.D.; Albrektsson, T. The Peri-Implantitis: Implant Surfaces, Microstructure, and Physicochemical Aspects. Clin. Implant. Dent. Relat. Res. 2009, 14, 170–183. [Google Scholar] [CrossRef]
- Fretwurst, T.; Nelson, K.; Tarnow, D.; Wang, H.-L.; Giannobile, W. Is Metal Particle Release Associated with Peri-implant Bone Destruction? An Emerging Concept. J. Dent. Res. 2017, 97, 259–265. [Google Scholar] [CrossRef]
- Oliveira, M.N.; Schunemann, W.V.H.; Mathew, M.T.; Henriques, B.; Magini, R.S.; Teughels, W.; Souza, J.C.M. Can degradation products released from dental implants affect peri-implant tissues? J. Periodontal Res. 2018, 53, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ellegaard, B.; Baelum, V.; Karring, T. Implant therapy in periodontally compromised patients. Clin. Oral Implant. Res. 1997, 8, 180–188. [Google Scholar] [CrossRef]
- Albrektsson, T.; Wennerberg, A. Oral implant surfaces: Part 1—Review focusing on topographic and chemical properties of different surfaces and in vivo responses to them. Int. J. Prosthodont. 2004, 17, 536–543. [Google Scholar] [PubMed]
- Albrektsson, T.; Wennerberg, A. Oral implant surfaces: Part 2—Review focusing on clinical knowledge of different surfaces. Int. J. Prosthodont. 2004, 17, 544–564. [Google Scholar] [PubMed]
- Wennström, J.L.; Ekestubbe, A.; Gröndahl, K.; Karlsson, S.; Lindhe, J. Oral rehabilitation with implant-supported fixed partial dentures in periodontitis-susceptible subjects. A 5-year prospective study. J. Clin. Periodontol. 2004, 31, 713–724. [Google Scholar] [CrossRef]
- Shibli, J.A.; D’Avila, S.; Guastaldi, A.C.; Marcantonio, E. Analysis of Failed Commercially Pure Titanium Dental Implants: A Scanning Electron Microscopy and Energy-Dispersive Spectrometer X-ray Study. J. Periodontol. 2005, 76, 1092–1099. [Google Scholar] [CrossRef] [PubMed]
- Misch, C.E.; Perel, M.L.; Wang, H.-L.; Sammartino, G.; Galindo-Moreno, P.; Trisi, P.; Steigmann, M.; Rebaudi, A.; Palti, A.; Pikos, M.A.; et al. Implant Success, Survival, and Failure: The International Congress of Oral Implantologists (ICOI) Pisa Consensus Conference. Implant. Dent. 2008, 17, 5–15. [Google Scholar] [CrossRef]
- Miller, S.C. Textbook of Periodontia; P. Blakiston’s Son & Co., Inc.: Philadelphia, PA, USA, 1938. [Google Scholar]
- Mediadent Software V15; The Dental Imaging Company Ltd.: Shoreham by Sea, UK, 2017.
- Lombardi, T.; Berton, F.; Salgarello, S.; Barbalonga, E.; Rapani, A.; Piovesana, F.; Gregorio, C.; Barbati, G.; Di Lenarda, R.; Stacchi, C. Factors Influencing Early Marginal Bone Loss around Dental Implants Positioned Subcrestally: A Multicenter Prospective Clinical Study. J. Clin. Med. 2019, 8, 1168. [Google Scholar] [CrossRef]
- Statistical Package for the Social Sciences for Windows v. 20.0; IBM Statistical Package: Armonk, NY, USA, 13 April 2020.
- Wälivaara, B.; Aronsson, B.-O.; Rodahl, M.; Lausmaa, J.; Tengvall, P. Titanium with different oxides: In vitro studies of protein adsorption and contact activation. Biomaterials 1994, 15, 827–834. [Google Scholar] [CrossRef]
- Eriksson, C.; Lausmaa, J.; Nygren, H. Interactions between human whole blood and modified TiO2-surfaces: Influence of surface topography and oxide thickness on leukocyte adhesion and activation. Biomaterials 2001, 22, 1987–1996. [Google Scholar] [CrossRef]
- Chaturvedi, T.P. An overview of the corrosion aspect of dental implants (titanium and its alloys). Indian J. Dent. Res. 2009, 20, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Abbott, J.R.; Marino, V.; Bartold, P.M. Human cadaveric histomorphological and metallurgical analysis of dental implants following 12.5 years of service. Clin. Oral Implant. Res. 2014, 25, 266–271. [Google Scholar] [CrossRef]
- Insua, A.; Monje, A.; Wang, H.-L.; Miron, R.J. Basis of bone metabolism around dental implants during osseointegration and peri-implant bone loss. J. Biomed. Mater. Res. Part A 2017, 105, 2075–2089. [Google Scholar] [CrossRef]
- Medvedev, A.; Ng, H.; Lapovok, R.; Estrin, Y.; Lowe, T.; Anumalasetty, V. Effect of bulk microstructure of commercially pure titanium on surface characteristics and fatigue properties after surface modification by sand blasting and acid-etching. J. Mech. Behav. Biomed. Mater. 2016, 57, 55–68. [Google Scholar] [CrossRef]
- Revathi, A.; Borrás, A.D.; Muñoz, A.I.; Richard, C.; Manivasagam, G. Degradation mechanisms and future challenges of titanium and its alloys for dental implant applications in oral environment. Mater. Sci. Eng. C 2017, 76, 1354–1368. [Google Scholar] [CrossRef]
- Shibli, J.A.; Vitussi, T.R.C.; Garcia, R.V.; Zenóbio, E.G.; Ota-Tsuzuki, C.; Cassoni, A.; Piattelli, A.; D’Avila, S. Implant Surface Analysis and Microbiologic Evaluation of Failed Implants Retrieved From Smokers. J. Oral Implant. 2007, 33, 232–238. [Google Scholar] [CrossRef]
- Yoshida, A.; Yoshimura, M.; Ohara, N.; Yoshimura, S.; Nagashima, S.; Takehara, T.; Nakayama, K. Hydrogen Sulfide Production From Cysteine and Homocysteine by Periodontal and Oral Bacteria. J. Periodontol. 2009, 80, 1845–1851. [Google Scholar] [CrossRef] [PubMed]
- Albertini, M.; López-Cerero, L.; O’Sullivan, M.G.; Chereguini, C.F.; Ballesta, S.; Ríos, V.; Herrero-Climent, M.; Bullón, P. Assessment of periodontal and opportunistic flora in patients with peri-implantitis. Clin. Oral Implant. Res. 2015, 26, 937–941. [Google Scholar] [CrossRef]
- Mombelli, A.; Feloutzis, A.G.; Brägger, U.; Lang, N.P. Treatment of peri-implantitis by local delivery of tetracycline. Clin. Oral Implant. Res. 2001, 12, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.-S.; Shih, K.-S.; Lai, C.-H.; Takeuchi, Y.; Chen, Y.-W. Surface property alterations and osteoblast attachment to contaminated titanium surfaces after different surface treatments: An in vitro study. Clin. Implant. Dent. Relat. Res. 2018, 20, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Olefjord, I.; Hansson, S. Surface analysis of four dental implant systems. Int. J. Oral Maxillofac. Implant. 1993, 8, 32–40. [Google Scholar]
- Tastepe, C.S.; Liu, Y.; Visscher, C.M.; Wismeijer, D. Cleaning and modification of intraorally contaminated titanium discs with calcium phosphate powder abrasive treatment. Clin. Oral Implant. Res. 2012, 24, 1238–1246. [Google Scholar] [CrossRef]
- Nicolas-Silvente, A.I.; Velasco-Ortega, E.; Ortiz-Garcia, I.; Monsalve-Guil, L.; Gil, J.; Jimenez-Guerra, A. Influence of the Titanium Implant Surface Treatment on the Surface Roughness and Chemical Composition. Materials 2020, 13, 314. [Google Scholar] [CrossRef]
- Sansone, V. The effects on bone cells of metal ions released from orthopaedic implants. A review. Clin. Cases Miner. Bone Metab. 2013, 10, 34–40. [Google Scholar] [CrossRef]
- Han, C.-H.; Johansson, C.B.; Wennerberg, A.; Albrektsson, T. Quantitative and qualitative investigations of surface enlarged titanium and titanium alloy implants. Clin. Oral Implant. Res. 1998, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Stacy, A.; Abraham, N.; Jorth, P.; Whiteley, M. Microbial Community Composition Impacts Pathogen Iron Availability during Polymicrobial Infection. PLOS Pathog. 2016, 12, e1006084. [Google Scholar] [CrossRef]
- Pinto, C.M.S.d.A.; Goulart, D.R.; Asprino, L.; Olate, S.; de Moraes, M. Evaluation of Failed Implants by Metallographic and Energy Dispersive X-ray Analysis. Implant. Dent. 2018, 27, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Monje, A.; García, R.G.; Calderón, M.F.; Oliva, M.H.; Martín, M.L.G.; Del Amo, F.S.-L.; Moreno, P.G.; Wang, H.-L.; Monje, F.; Suarez, F. Surface Topographical Changes of a Failing Acid-Etched Long-Term in Function Retrieved Dental Implant. J. Oral Implant. 2016, 42, 12–16. [Google Scholar] [CrossRef]
- Annunziata, M.; Guida, L. The Effect of Titanium Surface Modifications on Dental Implant Osseointegration. Craniofacial Sutures 2015, 17, 62–77. [Google Scholar] [CrossRef]
- Ehrenfest, D.M.; Corso, M.D.; Kang, B.S.; Leclercq, P.; Mazor, Z.; Horowitz, A.R.; Russe, P.; Oh, H.K.; Zou, D.R.; Shibli, J.A.; et al. Identification Card and Codification of the Chemical and Morphological Characteristics of 62 Dental Implant Surfaces. Part 3: Sand-Blasted/Acid-Etched (SLA Type) and Related Surfaces (Group 2A, main subtractive process). POSEIDO 2014, 2, 37–55. [Google Scholar]
- Teughels, W.; Assche, N.V.; Sliepen, I.; Quirynen, M. Effect of material characteristics and/or surface topography on biofilm development. Clin. Oral Implant. Res. 2006, 17, 68–81. [Google Scholar] [CrossRef]
- Zetterqvist, L.; Feldman, S.; Rotter, B.; Vincenzi, G.; Wennström, J.L.; Chierico, A.; Stach, R.M.; Kenealy, J.N. A Prospective, Multicenter, Randomized-Controlled 5-Year Study of Hybrid and Fully Etched Implants for the Incidence of Peri-Implantitis. J. Periodontol. 2010, 81, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Shibli, J.A.; Martins, M.C.; Lotufo, R.F.M.; Marcantonio, E. Microbiologic and radiographic analysis of ligature-induced peri-implantitis with different dental implant surfaces. Int. J. Oral Maxillofac. Implant. 2003, 18, 383–390. [Google Scholar]
- Martins, M.C.; Abi-Rached, R.S.G.; Shibli, J.A.; Araujo, M.W.B.; Marcantonio, E. Experimental peri-implant tissue breakdown around different dental implant surfaces: Clinical and radiographic evaluation in dogs. Int. J. Oral Maxillofac. Implant. 2004, 19, 839–848. [Google Scholar]
- Albouy, J.-P.; Abrahamsson, I.; Persson, L.G.; Berglundh, T. Spontaneous progression of ligatured induced peri-implantitis at implants with different surface characteristics. An experimental study in dogs II: Histological observations. Clin. Oral Implant. Res. 2009, 20, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Duddeck, D.U.; Albrektsson, T.; Wennerberg, A.; Larsson, C.; Beuer, F. On the Cleanliness of Different Oral Implant Systems: A Pilot Study. J. Clin. Med. 2019, 8, 1280. [Google Scholar] [CrossRef] [PubMed]
| Dental Implant Location | (n = 34 Dental Implants) |
|---|---|
| Right-left maxillary molar | 12 (35.29%) |
| Right-left mandibulary molar | 18 (52.94%) |
| Right-left maxillary incisors | 1 (2.94%) |
| Right-left mandibulary incisors | 3 (8.82%) |
| Implant survival year (mean ± SD; min-max year) | 4.94 ± 3.38 (1.5–14) |
| Implant commercial name and surface structure | |
| Xive S (Dentsply Sirona Implants, Mannheim, Germany) | 2 |
| Neo Implants (Alpha Biotec Implantology, Washington, DC, USA) | 7 |
| Legacy II (ImplantDirect, Thousand Oaks, CA, USA) | 7 |
| DTI (TUBITAK, Gebze, Kocaeli, Turkey) | 4 |
| AstraTech (Denstply Sirona, Mölndal, Sweden) | 3 |
| Bicon Integra CP (Bicon systems, Boston, MA, USA) | 2 |
| MIS C1 (Divident, Yehudah, Israel) | 7 |
| Straumann BL Tapered (Straumann Holding, Basel, Switzerland) | 2 |
| Clinical Measurements | Group I (n = 9) Mean ± SD | Group II (n = 25) Mean ± SD | p-Values |
|---|---|---|---|
| PD | 5.39 ± 2.25 | 6.12 ± 1.76 | 0.280 |
| CAL | 5.73 ± 2.7 | 6.3 ± 1.65 | 0.216 |
| Implant Length (mm) | 7.89 ± 2.24 | 9.32 ± 1.43 | 0.037 |
| Pus | 55.5% (5/9) | 76.0% (19/25) | 0.355 |
| Functional Pain | 44.4% (4/9) | 72.0% (18/25) | 0.237 |
| Radiographic Bone Loss/Implant Length | 0.55 ± 0.39 | 0.47 ± 0.33 | 0.584 |
| Occlusal Trauma % | 44.4% (4/9) | 24% (6/25) | 0.092 |
| Mobility (Miller Class 2) | 0 | 8.0% (2/25) | - |
| Group I | Group II | p-Values | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Coronal * | Middle ** | Apical *** | p-Values | Coronal * | Middle ** | Apical *** | p-Values | ||
| Ti | 6.17 ± 6.48 | 20.22 ± 15.69 | 23.26 ± 19.1 | 0.049 | 3.59 ± 4.25 | 33.96 ± 13.62 | 33.36 ± 17.47 | 0.000 | * 0.22 ** 0.02 *** 0.23 |
| O | 39.36 ± 19.4 | 3.8 ± 2.5 | 26.29 ± 3.0 | 0.000 | 30.6 ± 11.17 | 3.67 ± 4.31 | 26.29 ± 9.7 | 0.000 | * 0.15 ** 0.69 *** 0.84 |
| C | 19.77 ± 5.01 | 21.3 ± 9.79 | 40.9 ± 22.08 | 0.007 | 24.9 ± 9.7 | 22.9 ± 8.5 | 28.7 ± 16.65 | 0.247 | * 0.14 ** 1.00 *** 0.15 |
| N | 27.86 ± 21.24 | 0 | 0 | 0.661 | 36.85 ± 18.93 | 7.32 ± 3.29 | 0 | 0.000 | * 0.20 ** - *** 0.13 |
| Al | 0.03 ± 0.06 | 0 | 4.51 ± 2.65 | 0.000 | 0.15 ± 0.25 | 0.16 ± 0.27 | 4.94 ± 3.8 | 0.000 | * 0.20 ** 0.02 *** 0.70 |
| Ca | 0.02 ± 0.07 | 6.6 ± 16.01 | 2.09 ± 2.57 | 0.001 | 0.04 ± 0.17 | 2.64 ± 2.32 | 3.6 ± 3.2 | 0.000 | * 0.84 ** 0.14 *** 0.16 |
| S | 1.3 ± 2.87 | 0.26 ± 0.44 | 0.12 | 0.264 | 1.32 ± 0.24 | 0.11 ± 0.15 | 0.21 ± 0.18 | 0.001 | * 0.33 ** 0.81 *** 0.16 |
| K | 3.03 ± 3.76 | 0 | 0.4 | 0.853 | 3.99 ± 3.91 | 0.4 | 0.27 | 0.955 | * 0.59 ** -*** 0.32 |
| Fe | 0.1 ± 0.16 | 1.38 | 0.42 | 0.066 | 0.04 ± 0.08 | 0.7 ± 0.18 | 0.36 ± 0.06 | 0.001 | * 0.24 ** 0.22 *** 0.22 |
| Cr | 0.5 ± 0.98 | 0.34 | 0 | 0.590 | 0.62 ± 1.08 | 0.14 ± 0.13 | 0.27 ± 0.07 | 0.537 | * 0.66 ** 0.31 *** - |
| P | 0 | 0.69 ± 0.93 | 1.7 ± 1.56 | 0.000 | 0.17 ± 0.08 | 1.3 ± 1.45 | 1.77 ± 1.65 | 0.000 | * 0.55 ** 0.39 *** 0.96 |
| Mg | 0 | 0 | 0.3 | 0.003 | 0.1 ± 0.42 | 0 | 0.55 | 0.001 | * 0.39 ** - *** 0.32 |
| Cu | 0 | 0 | 0 | - | 0.46 ± 0.23 | 0.57 ± 0.68 | 1.17 ± 0.5 | 0.000 | * 0.55 ** - *** - |
| Peri-Implant PD Elements | B | SE | β | t | p-Value |
|---|---|---|---|---|---|
| Peri-implant PD-Coronal * | |||||
| Mg | 12.678 | 3.734 | 0.49 | 3.395 | 0.002 |
| N | 0.197 | 0.071 | 0.397 | 2.777 | 0.009 |
| Al | 0.138 | 0.055 | 0.361 | 2.536 | 0.017 |
| Peri-implant PD-Middle ** | |||||
| Al | 0.201 | 0.079 | 0.411 | 2.548 | 0.016 |
| Peri-implant PD-Apical *** | |||||
| S | 6.065 | 1.31 | 0.617 | 4.629 | 0.000 |
| Al | 0.345 | 0.08 | 0.604 | 4.307 | 0.000 |
| P | 0.14 | 0.051 | 0.355 | 2.725 | 0.011 |
| N | 0.367 | 0.173 | 0.311 | 2.122 | 0.042 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guler, B.; Uraz, A.; Hatipoğlu, H.; Yalım, M. Chemical Evaluation of Energy Dispersive X-ray Spectroscopy Analysis of Different Failing Dental Implant Surfaces: A Comparative Clinical Trial. Materials 2021, 14, 986. https://doi.org/10.3390/ma14040986
Guler B, Uraz A, Hatipoğlu H, Yalım M. Chemical Evaluation of Energy Dispersive X-ray Spectroscopy Analysis of Different Failing Dental Implant Surfaces: A Comparative Clinical Trial. Materials. 2021; 14(4):986. https://doi.org/10.3390/ma14040986
Chicago/Turabian StyleGuler, Berceste, Ahu Uraz, Hasan Hatipoğlu, and Mehmet Yalım. 2021. "Chemical Evaluation of Energy Dispersive X-ray Spectroscopy Analysis of Different Failing Dental Implant Surfaces: A Comparative Clinical Trial" Materials 14, no. 4: 986. https://doi.org/10.3390/ma14040986
APA StyleGuler, B., Uraz, A., Hatipoğlu, H., & Yalım, M. (2021). Chemical Evaluation of Energy Dispersive X-ray Spectroscopy Analysis of Different Failing Dental Implant Surfaces: A Comparative Clinical Trial. Materials, 14(4), 986. https://doi.org/10.3390/ma14040986






