Fracture Load of Metal, Zirconia and Polyetheretherketone Posterior CAD-CAM Milled Fixed Partial Denture Frameworks
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Specimens
2.2. Mechanical Test
2.3. Statistical Analysis
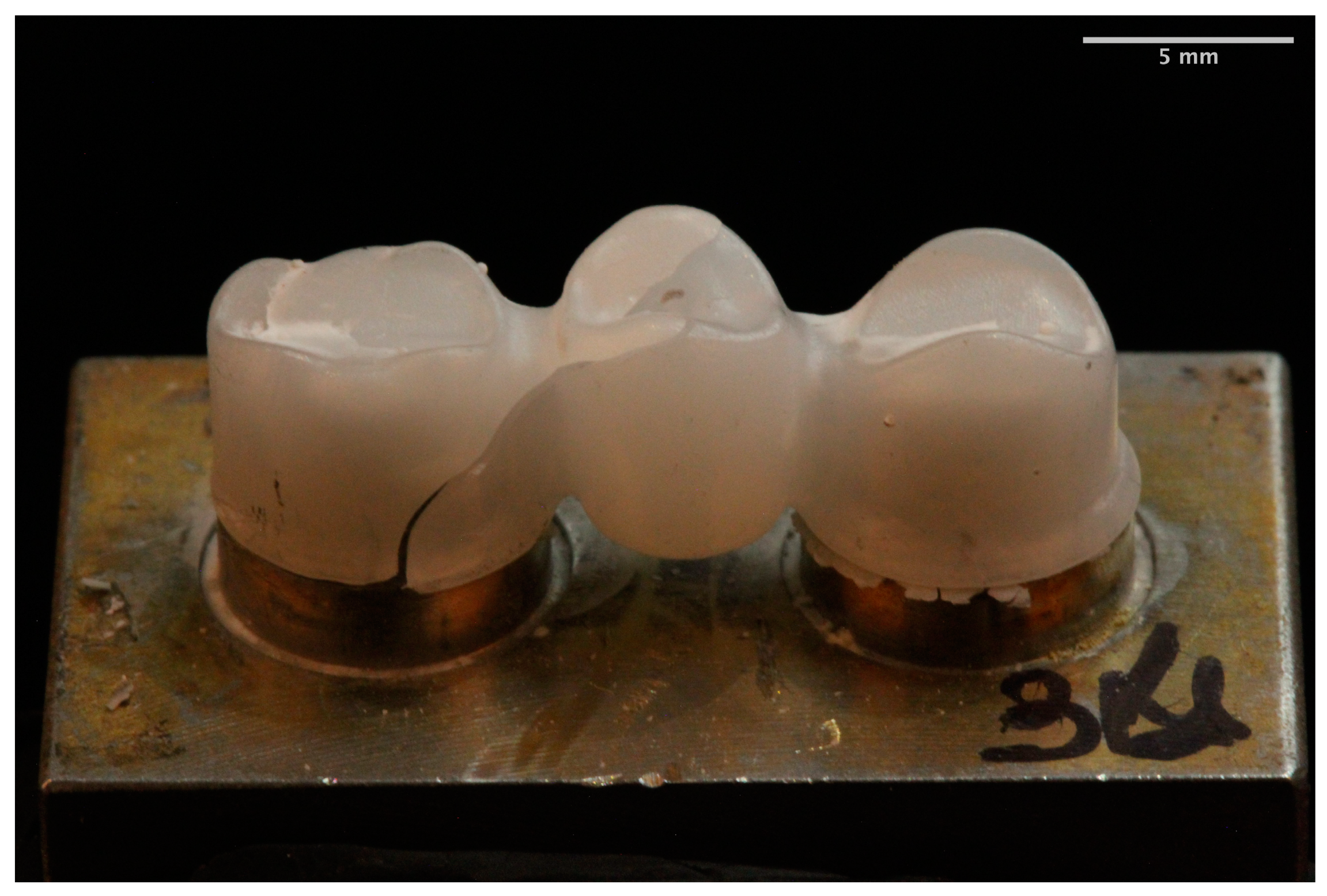
3. Results
4. Discussion
5. Conclusions
- All tested CAD-CAM milled materials demonstrated clinically acceptable fracture load values;
- The type of material influenced the load to fracture;
- Milled metal exhibited the highest load to facture values, followed by PEEK, and zirconia;
- Milled PEEK could be an alternative to metal or ceramic restorations in posterior regions;
- Different fracture patterns were observed for the analyzed materials.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lopez-Suarez, C.; Rodriguez, V.; Pelaez, J.; Agustin-Panadero, R.; Suarez, M.J. Comparative fracture behavior of monolithic and veneered zirconia posterior fixed dental prostheses. Dent. Mater. J. 2017, 36, 816–821. [Google Scholar] [CrossRef]
- Park, J.K.; Lee, W.S.; Kim, H.Y.; Kim, W.C.; Kim, J.H. Accuracy evaluation of metal copings fabricated by computer-aided milling and direct metal laser sintering systems. J. Adv. Prosthodont. 2015, 7, 122–128. [Google Scholar] [CrossRef]
- Kim, M.J.; Choi, Y.J.; Kim, S.K.; Heo, S.J.; Koak, J.Y. Marginal Accuracy and Internal Fit of 3-D Printing Laser-Sintered Co-Cr Alloy Copings. Materials 2017, 10, 93. [Google Scholar] [CrossRef] [PubMed]
- Nesse, H.; Ulstein, D.M.; Vaage, M.M.; Oilo, M. Internal and marginal fit of cobalt-chromium fixed dental prostheses fabricated with 3 different techniques. J. Prosthet. Dent. 2015, 114, 686–692. [Google Scholar] [CrossRef] [PubMed]
- Alghazzawi, T.F. Advancements in CAD/CAM technology: Options for practical implementation. J. Prosthodont. Res. 2016, 60, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Gonzalo, E.; Suarez, M.J.; Serrano, B.; Lozano, J.F. A comparison of the marginal vertical discrepancies of zirconium and metal ceramic posterior fixed dental prostheses before and after cementation. J. Prosthet. Dent. 2009, 102, 378–384. [Google Scholar] [CrossRef]
- Fischer, J.; Zbaren, C.; Stawarczyk, B.; Hammerle, C.H. The effect of thermal cycling on metal-ceramic bond strength. J. Dent. 2009, 37, 549–553. [Google Scholar] [CrossRef]
- Lopez-Suarez, C.; Tobar, C.; Sola-Ruiz, M.F.; Pelaez, J.; Suarez, M.J. Effect of Thermomechanical and Static Loading on the Load to Fracture of Metal-Ceramic, Monolithic, and Veneered Zirconia Posterior Fixed Partial Dentures. J. Prosthodont. 2019, 28, 171–178. [Google Scholar] [CrossRef]
- Yang, R.; Arola, D.; Han, Z.; Zhang, X. A comparison of the fracture resistance of three machinable ceramics after thermal and mechanical fatigue. J. Prosthet. Dent. 2014, 112, 878–885. [Google Scholar] [CrossRef]
- Li, K.C.; Prior, D.J.; Waddell, J.N.; Swain, M.V. Comparison of the microstructure and phase stability of as-cast, CAD/CAM and powder metallurgy manufactured Co-Cr dental alloys. Dent. Mater. 2015, 31, e306–e315. [Google Scholar] [CrossRef]
- Blatz, M.B.; Vonderheide, M.; Conejo, J. The Effect of Resin Bonding on Long-Term Success of High-Strength Ceramics. J. Dent. Res. 2018, 97, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Tinschert, J.; Natt, G.; Mautsch, W.; Augthun, M.; Spiekermann, H. Fracture resistance of lithium disilicate-, alumina-, and zirconia-based three-unit fixed partial dentures: A laboratory study. Int. J. Prosthodont. 2001, 14, 231–238. [Google Scholar] [PubMed]
- Rodríguez, V.; Castillo-Oyagüe, R.; López-Suárez, C.; Gonzalo, E.; Peláez, J.; Suárez-García, M.J. Fracture Load Before and After Veneering Zirconia Posterior Fixed Dental Prostheses. J. Prosthodont. 2016, 25, 550–556. [Google Scholar] [CrossRef]
- Zhang, Y.; Lawn, B.R. Novel Zirconia Materials in Dentistry. J. Dent. Res. 2018, 97, 140–147. [Google Scholar] [CrossRef]
- Lunt, A.; Salvati, E.; Baimpas, N.; Dolbnya, I.; Neo, T.K.; Korsunsky, A.M. Investigations into the interface failure of yttria partially stabilised zirconia—Porcelain dental prostheses through microscale residual stress and phase quantification. Dent. Mater. 2019, 35, 1576–1593. [Google Scholar] [CrossRef] [PubMed]
- Liebermann, A.; Wimmer, T.; Schmidlin, P.R.; Scherer, H.; Loffler, P.; Roos, M.; Stawarczyk, B. Physicomechanical characterization of polyetheretherketone and current esthetic dental CAD/CAM polymers after aging in different storage media. J. Prosthet. Dent. 2016, 115, 321–328.e322. [Google Scholar] [CrossRef]
- Schwitalla, A.D.; Spintig, T.; Kallage, I.; Muller, W.D. Flexural behavior of PEEK materials for dental application. Dent. Mater. 2015, 31, 1377–1384. [Google Scholar] [CrossRef]
- Taufall, S.; Eichberger, M.; Schmidlin, P.R.; Stawarczyk, B. Fracture load and failure types of different veneered polyetheretherketone fixed dental prostheses. Clin. Oral Investig. 2016, 20, 2493–2500. [Google Scholar] [CrossRef]
- Zoidis, P.; Bakiri, E.; Papathanasiou, I.; Zappi, A. Modified PEEK as an alternative crown framework material for weak abutment teeth: A case report. Gen. Dent. 2017, 65, 37–40. [Google Scholar]
- Zoidis, P.; Bakiri, E.; Polyzois, G. Using modified polyetheretherketone (PEEK) as an alternative material for endocrown restorations: A short-term clinical report. J. Prosthet. Dent. 2017, 117, 335–339. [Google Scholar] [CrossRef]
- Papathanasiou, I.; Kamposiora, P.; Papavasiliou, G.; Ferrari, M. The use of PEEK in digital prosthodontics: A narrative review. BMC Oral Health 2020, 20, 217. [Google Scholar] [CrossRef]
- Lopez-Suarez, C.; Castillo-Oyague, R.; Rodriguez-Alonso, V.; Lynch, C.D.; Suarez-Garcia, M.J. Fracture load of metal-ceramic, monolithic, and bi-layered zirconia-based posterior fixed dental prostheses after thermo-mechanical cycling. J. Dent. 2018, 73, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Freire, Y.; Gonzalo, E.; Lopez-Suarez, C.; Suarez, M.J. The Marginal Fit of CAD/CAM Monolithic Ceramic and Metal-Ceramic Crowns. J. Prosthodont. 2019, 28, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.K.; Kim, S.K.; Heo, S.J.; Koak, J.Y. Mechanical Properties and Metal-Ceramic Bond Strength of Co-Cr Alloy Manufactured by Selective Laser Melting. Materials 2020, 13, 5745. [Google Scholar] [CrossRef] [PubMed]
- Stawarczyk, B.; Eichberger, M.; Uhrenbacher, J.; Wimmer, T.; Edelhoff, D.; Schmidlin, P.R. Three-unit reinforced polyetheretherketone composite FDPs: Influence of fabrication method on load-bearing capacity and failure types. Dent. Mater. J. 2015, 34, 7–12. [Google Scholar] [CrossRef]
- Agustin-Panadero, R.; Fons-Font, A.; Roman-Rodriguez, J.L.; Granell-Ruiz, M.; del Rio-Highsmith, J.; Sola-Ruiz, M.F. Zirconia versus metal: A preliminary comparative analysis of ceramic veneer behavior. Int. J. Prosthodont. 2012, 25, 294–300. [Google Scholar]
- Xin, X.Z.; Chen, J.; Xiang, N.; Gong, Y.; Wei, B. Surface characteristics and corrosion properties of selective laser melted Co-Cr dental alloy after porcelain firing. Dent. Mater. 2014, 30, 263–270. [Google Scholar] [CrossRef]
- Rosentritt, M.; Behr, M.; Thaller, C.; Rudolph, H.; Feilzer, A. Fracture performance of computer-aided manufactured zirconia and alloy crowns. Quintessence Int. 2009, 40, 655–662. [Google Scholar]
- Flinn, B.D.; deGroot, D.A.; Mancl, L.A.; Raigrodski, A.J. Accelerated aging characteristics of three yttria-stabilized tetragonal zirconia polycrystalline dental materials. J. Prosthet. Dent. 2012, 108, 223–230. [Google Scholar] [CrossRef]
- Choi, Y.S.; Kim, S.H.; Lee, J.B.; Han, J.S.; Yeo, I.S. In vitro evaluation of fracture strength of zirconia restoration veneered with various ceramic materials. J. Adv. Prosthodont. 2012, 4, 162–169. [Google Scholar] [CrossRef]
- Yildiz, C.; Vanlioğlu, B.A.; Evren, B.; Uludamar, A.; Ozkan, Y.K. Marginal-internal adaptation and fracture resistance of CAD/CAM crown restorations. Dent. Mater. J. 2013, 32, 42–47. [Google Scholar] [CrossRef][Green Version]
- Silva, N.R.; Bonfante, E.A.; Rafferty, B.T.; Zavanelli, R.A.; Rekow, E.D.; Thompson, V.P.; Coelho, P.G. Modified Y-TZP core design improves all-ceramic crown reliability. J. Dent. Res. 2011, 90, 104–108. [Google Scholar] [CrossRef]
- Ambré, M.J.; Aschan, F.; Vult von Steyern, P. Fracture strength of yttria-stabilized zirconium-dioxide (Y-TZP) fixed dental prostheses (FDPs) with different abutment core thicknesses and connector dimensions. J. Prosthodont. 2013, 22, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Senyilmaz, D.P.; Canay, S.; Heydecke, G.; Strub, J.R. Influence of thermomechanical fatigue loading on the fracture resistance of all-ceramic posterior crowns. Eur. J. Prosthodont. Restor. Dent. 2010, 18, 50–54. [Google Scholar] [PubMed]
- Vult von Steyern, P.; Ebbesson, S.; Holmgren, J.; Haag, P.; Nilner, K. Fracture strength of two oxide ceramic crown systems after cyclic pre-loading and thermocycling. J. Oral Rehabil. 2006, 33, 682–689. [Google Scholar] [CrossRef] [PubMed]
- Rosentritt, M.; Hmaidouch, R.; Behr, M.; Handel, G.; Schneider-Feyrer, S. Fracture resistance of zirconia FPDs with adhesive bonding versus conventional cementation. Int. J. Prosthodont. 2011, 24, 168–171. [Google Scholar]
- Pallis, K.; Griggs, J.A.; Woody, R.D.; Guillen, G.E.; Miller, A.W. Fracture resistance of three all-ceramic restorative systems for posterior applications. J. Prosthet. Dent. 2004, 91, 561–569. [Google Scholar] [CrossRef]
- López-Suárez, C.; Gonzalo, E.; Peláez, J.; Rodríguez, V.; Suárez, M.J. Fracture resistance and failure mode of posterior fixed dental prostheses fabricated with two zirconia CAD/CAM systems. J. Clin. Exp. Dent. 2015, 7, e250–e253. [Google Scholar] [CrossRef]
- Abdullah, A.O.; Tsitrou, E.A.; Pollington, S. Comparative in vitro evaluation of CAD/CAM vs conventional provisional crowns. J. Appl. Oral Sci. 2016, 24, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Stawarczyk, B.; Beuer, F.; Wimmer, T.; Jahn, D.; Sener, B.; Roos, M.; Schmidlin, P.R. Polyetheretherketone-a suitable material for fixed dental prostheses? J. Biomed. Mater. Res. B Appl. Biomater. 2013, 101, 1209–1216. [Google Scholar] [CrossRef]
- Lawson, N.C.; Jurado, C.A.; Huang, C.T.; Morris, G.P.; Burgess, J.O.; Liu, P.R.; Kinderknecht, K.E.; Lin, C.P.; Givan, D.A. Effect of Surface Treatment and Cement on Fracture Load of Traditional Zirconia (3Y), Translucent Zirconia (5Y), and Lithium Disilicate Crowns. J. Prosthodont. 2019, 28, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Ortega, R.; Gonzalo, E.; Gomez-Polo, M.; Lopez-Suarez, C.; Suarez, M.J. SEM evaluation of the precision of fit of CAD/CAM zirconia and metal-ceramic posterior crowns. Dent. Mater. J. 2017, 36, 387–393. [Google Scholar] [CrossRef]
- Krug, K.P.; Knauber, A.W.; Nothdurft, F.P. Fracture behavior of metal-ceramic fixed dental prostheses with frameworks from cast or a newly developed sintered cobalt-chromium alloy. Clin. Oral Investig. 2015, 19, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Eroğlu, Z.; Gurbulak, A.G. Fatigue behavior of zirconia-ceramic, galvano-ceramic, and porcelain-fused-to-metal fixed partial dentures. J. Prosthodont. 2013, 22, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhu, H.; Gai, X.; Wang, Y. Evaluation of the mechanical properties and porcelain bond strength of cobalt-chromium dental alloy fabricated by selective laser melting. J. Prosthet. Dent. 2014, 111, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Park, J.H.; Park, Y.B.; Moon, H.S. Fracture load of zirconia crowns according to the thickness and marginal design of coping. J. Prosthet. Dent. 2012, 108, 96–101. [Google Scholar] [CrossRef]
- Fardin, V.P.; de Paula, V.G.; Bonfante, E.A.; Coelho, P.G.; Bonfante, G. Lifetime prediction of zirconia and metal ceramic crowns loaded on marginal ridges. Dent. Mater. 2016, 32, 1543–1554. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, M.; Giovannetti, A.; Carrabba, M.; Bonadeo, G.; Rengo, C.; Monticelli, F.; Vichi, A. Fracture resistance of three porcelain-layered CAD/CAM zirconia frame designs. Dent. Mater. 2014, 30, e163–e168. [Google Scholar] [CrossRef] [PubMed]
- Turk, A.G.; Ulusoy, M.; Yuce, M.; Akin, H. Effect of different veneering techniques on the fracture strength of metal and zirconia frameworks. J. Adv. Prosthodont. 2015, 7, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Rosentritt, M.; Behr, M.; Gebhard, R.; Handel, G. Influence of stress simulation parameters on the fracture strength of all-ceramic fixed-partial dentures. Dent. Mater. 2006, 22, 176–182. [Google Scholar] [CrossRef]
- Mahmood, D.J.; Linderoth, E.H.; Vult Von Steyern, P. The influence of support properties and complexity on fracture strength and fracture mode of all-ceramic fixed dental prostheses. Acta Odontol. Scand. 2011, 69, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Larsson, C.; El Madhoun, S.; Wennerberg, A.; Vult von Steyern, P. Fracture strength of yttria-stabilized tetragonal zirconia polycrystals crowns with different design: An in vitro study. Clin. Oral Implants Res. 2012, 23, 820–826. [Google Scholar] [CrossRef]
- Kohorst, P.; Dittmer, M.P.; Borchers, L.; Stiesch-Scholz, M. Influence of cyclic fatigue in water on the load-bearing capacity of dental bridges made of zirconia. Acta Biomater. 2008, 4, 1440–1447. [Google Scholar] [CrossRef] [PubMed]
- Beuer, F.; Steff, B.; Naumann, M.; Sorensen, J.A. Load-bearing capacity of all-ceramic three-unit fixed partial dentures with different computer-aided design (CAD)/computer-aided manufacturing (CAM) fabricated framework materials. Eur. J. Oral Sci. 2008, 116, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Baladhandayutham, B.; Lawson, N.C.; Burgess, J.O. Fracture load of ceramic restorations after fatigue loading. J. Prosthet. Dent. 2015, 114, 266–271. [Google Scholar] [CrossRef]
- Niem, T.; Youssef, N.; Wostmann, B. Influence of accelerated ageing on the physical properties of CAD/CAM restorative materials. Clin. Oral Investig. 2020, 24, 2415–2425. [Google Scholar] [CrossRef]
- Kim, S.Y.; Choi, J.W.; Ju, S.W.; Ahn, J.S.; Yoon, M.J.; Huh, J.B. Fracture Strength After Fatigue Loading of Lithium Disilicate Pressed Zirconia Crowns. Int. J. Prosthodont. 2016, 29, 369–371. [Google Scholar] [CrossRef]
- Rosentritt, M.; Steiger, D.; Behr, M.; Handel, G.; Kolbeck, C. Influence of substructure design and spacer settings on the in vitro performance of molar zirconia crowns. J. Dent. 2009, 37, 978–983. [Google Scholar] [CrossRef]
- Dejak, B.; Mlotkowski, A.; Langot, C. Three-dimensional finite element analysis of molars with thin-walled prosthetic crowns made of various materials. Dent. Mater. 2012, 28, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Oilo, M.; Nesse, H.; Lundberg, O.J.; Gjerdet, N.R. Mechanical properties of cobalt-chromium 3-unit fixed dental prostheses fabricated by casting, milling, and additive manufacturing. J. Prosthet. Dent. 2018, 120, 156.e1–156.e7. [Google Scholar] [CrossRef]
- Miller, L.L. Framework design in ceramo-metal restorations. Dent. Clin. N. Am. 1977, 21, 699–716. [Google Scholar]
- Larsson, C.; Holm, L.; Lövgren, N.; Kokubo, Y.; Vult von Steyern, P. Fracture strength of four-unit Y-TZP FPD cores designed with varying connector diameter. An in-vitro study. J. Oral Rehabil. 2007, 34, 702–709. [Google Scholar] [CrossRef] [PubMed]
- Dal Piva, A.M.O.; Tribst, J.P.M.; Borges, A.L.S.; Souza, R.; Bottino, M.A. CAD-FEA modeling and analysis of different full crown monolithic restorations. Dent. Mater. 2018, 34, 1342–1350. [Google Scholar] [CrossRef] [PubMed]
- Skirbutis, G.; Dzingute, A.; Masiliunaite, V.; Sulcaite, G.; Zilinskas, J. PEEK polymer′s properties and its use in prosthodontics. A review. Stomatologija 2018, 20, 54–58. [Google Scholar] [PubMed]
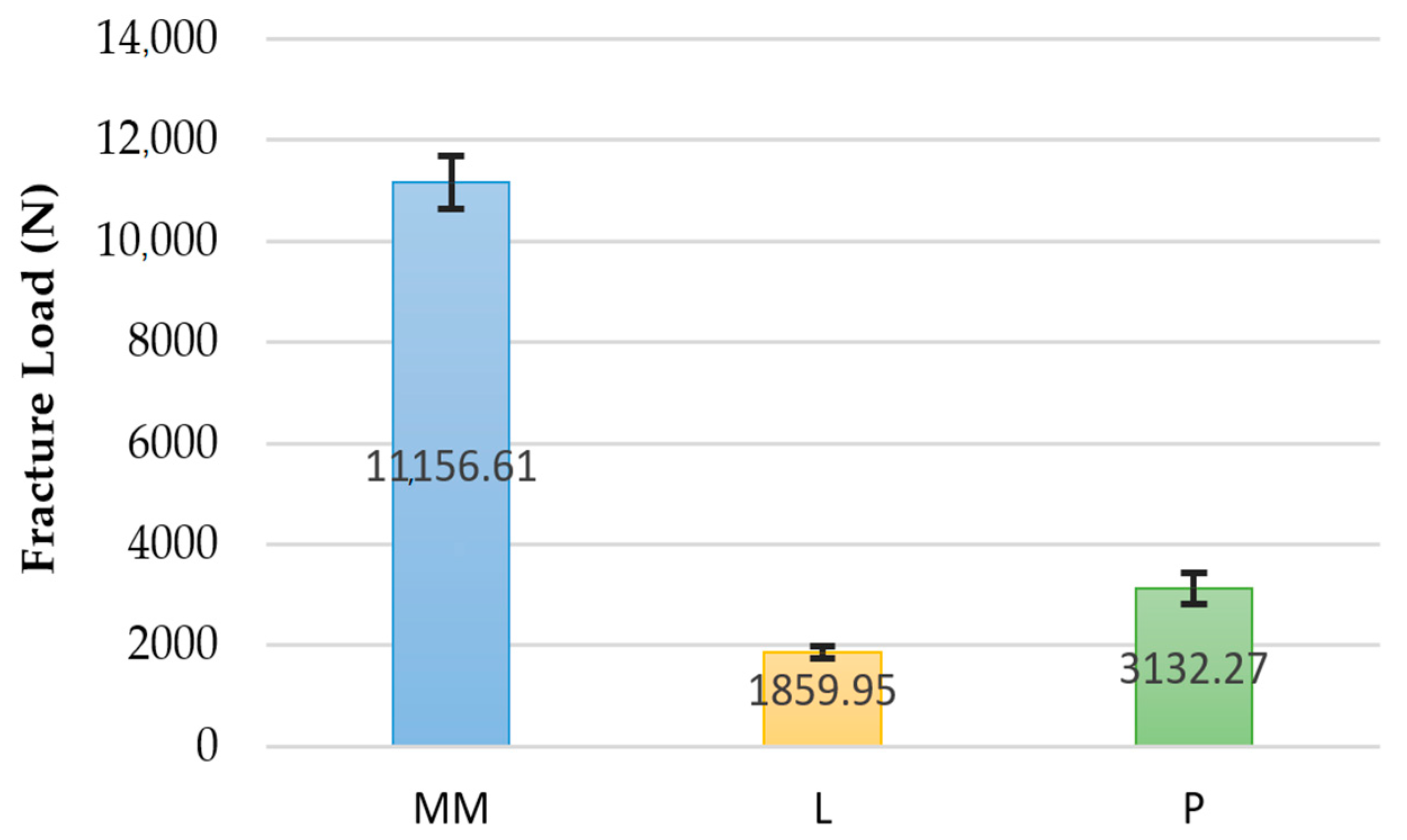



| Group | N | Mean | SD | Min | Max |
|---|---|---|---|---|---|
| Milled Metal | 10 | 11,156.61 | 530.69 | 10,430.14 | 11,860.94 |
| Zirconia | 10 | 1859.95 | 128.53 | 1687.68 | 2016.84 |
| PEEK | 10 | 3132.27 | 307.15 | 2730.28 | 3729.88 |
| (I) Group | (J) Group | Mean Difference (I-J) | Deviation Error | Sig. | Superior Limit | Inferior Limit | |
|---|---|---|---|---|---|---|---|
| Tamhane | MM | Z | 9296.66 | 172.67 | 0.000 | 8803.28 | 9790.03 |
| P | 8024.33 | 193.90 | 0.000 | 7501.07 | 8547.59 | ||
| Z | MM | −9296.66 | 172.67 | 0.000 | −9790.03 | −8803.28 | |
| P | −1272.32 | 105.29 | 0.000 | −1563.79 | −980.85 | ||
| P | MM | −8024.33 | 193.90 | 0.000 | −8547.59 | −7501.07 | |
| Z | 1272.32 | 172.67 | 0.000 | 980.85 | 1563.79 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez, V.; Tobar, C.; López-Suárez, C.; Peláez, J.; Suárez, M.J. Fracture Load of Metal, Zirconia and Polyetheretherketone Posterior CAD-CAM Milled Fixed Partial Denture Frameworks. Materials 2021, 14, 959. https://doi.org/10.3390/ma14040959
Rodríguez V, Tobar C, López-Suárez C, Peláez J, Suárez MJ. Fracture Load of Metal, Zirconia and Polyetheretherketone Posterior CAD-CAM Milled Fixed Partial Denture Frameworks. Materials. 2021; 14(4):959. https://doi.org/10.3390/ma14040959
Chicago/Turabian StyleRodríguez, Verónica, Celia Tobar, Carlos López-Suárez, Jesús Peláez, and María J. Suárez. 2021. "Fracture Load of Metal, Zirconia and Polyetheretherketone Posterior CAD-CAM Milled Fixed Partial Denture Frameworks" Materials 14, no. 4: 959. https://doi.org/10.3390/ma14040959
APA StyleRodríguez, V., Tobar, C., López-Suárez, C., Peláez, J., & Suárez, M. J. (2021). Fracture Load of Metal, Zirconia and Polyetheretherketone Posterior CAD-CAM Milled Fixed Partial Denture Frameworks. Materials, 14(4), 959. https://doi.org/10.3390/ma14040959







