Electrochemical Performance Enhancement of Micro-Sized Porous Si by Integrating with Nano-Sn and Carbonaceous Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Preparation
2.2. Material Preparation
2.3. Electrochemistry Measurements
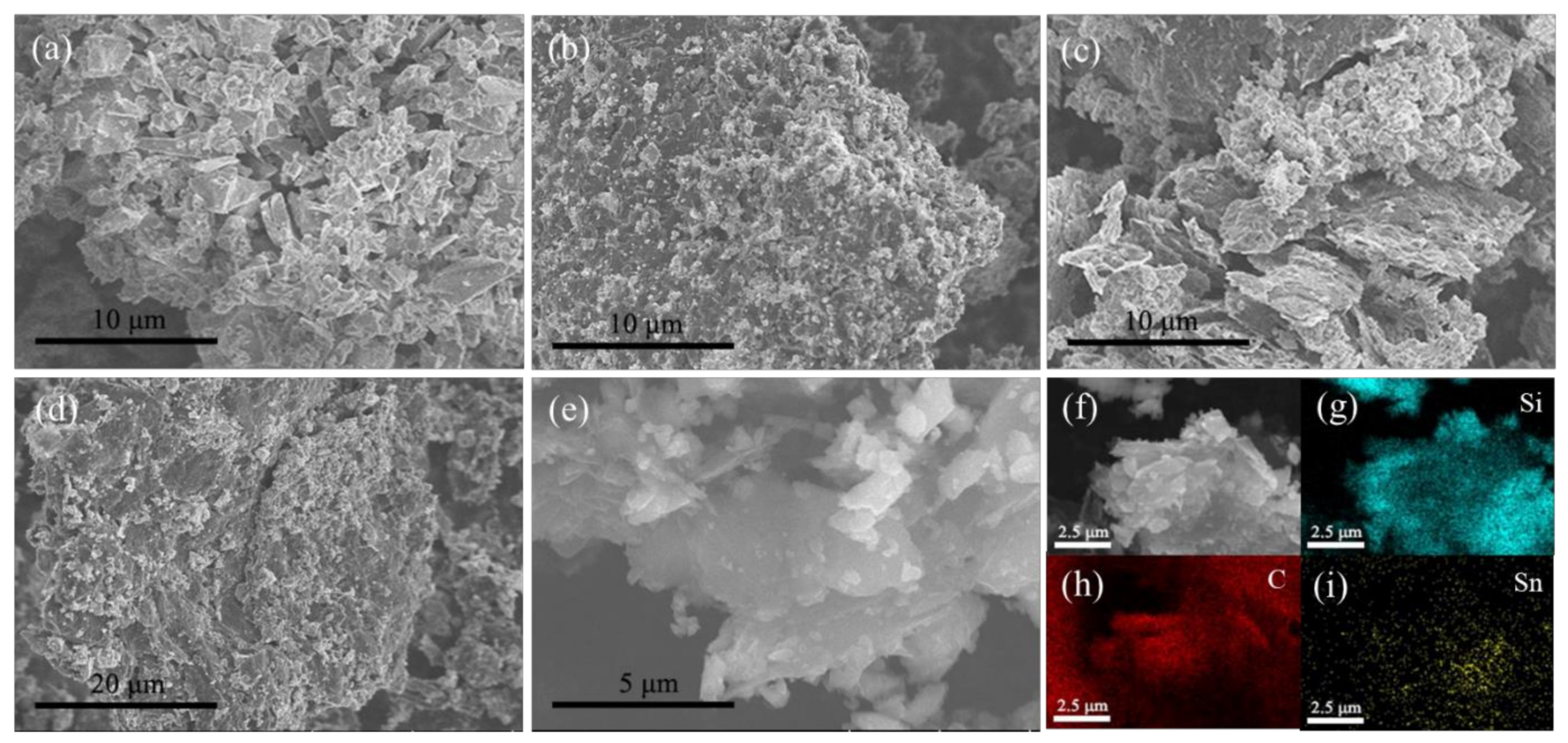
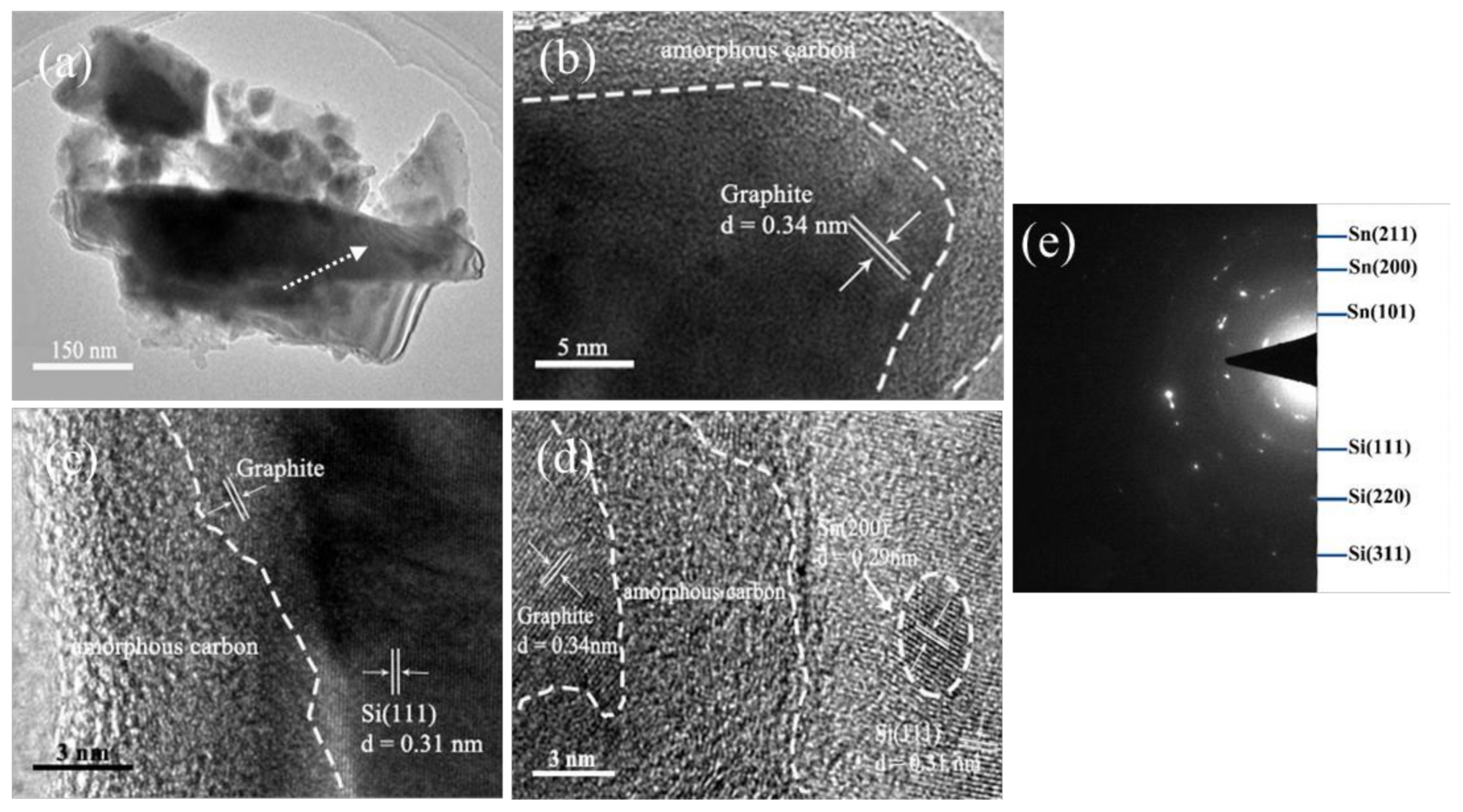
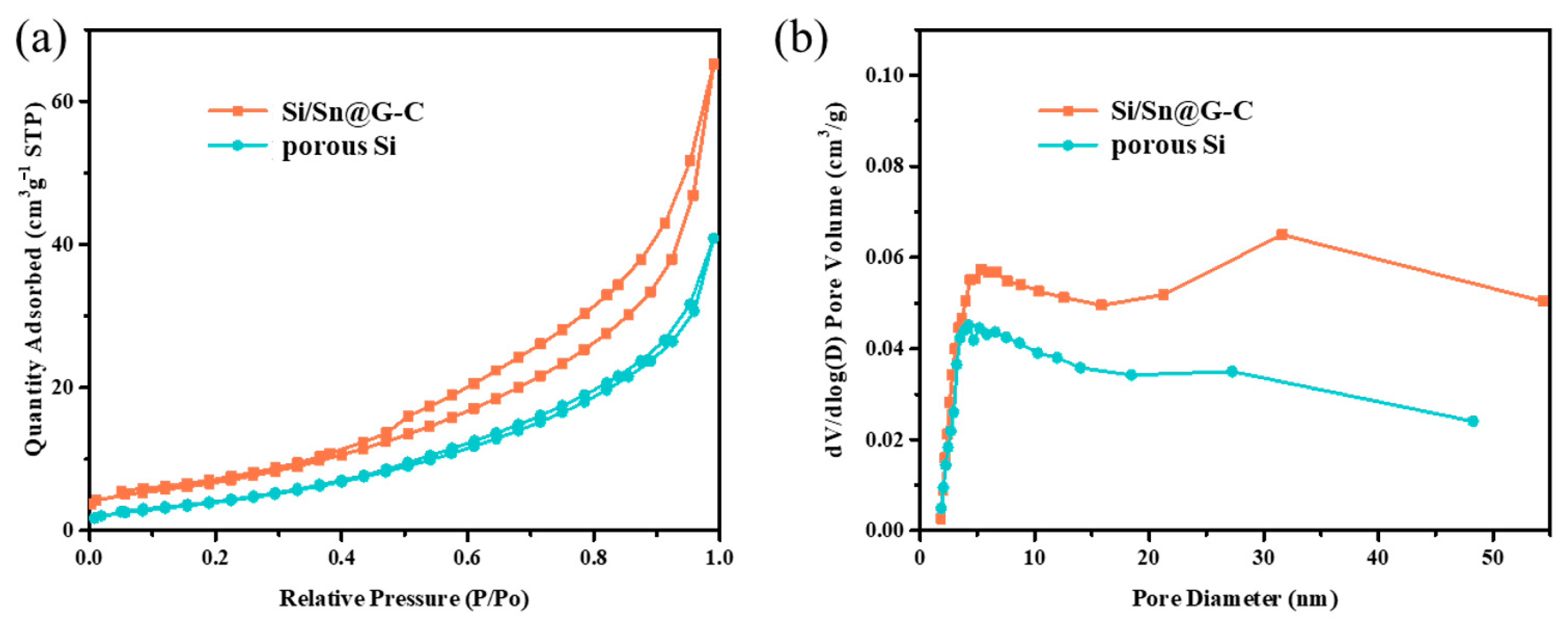
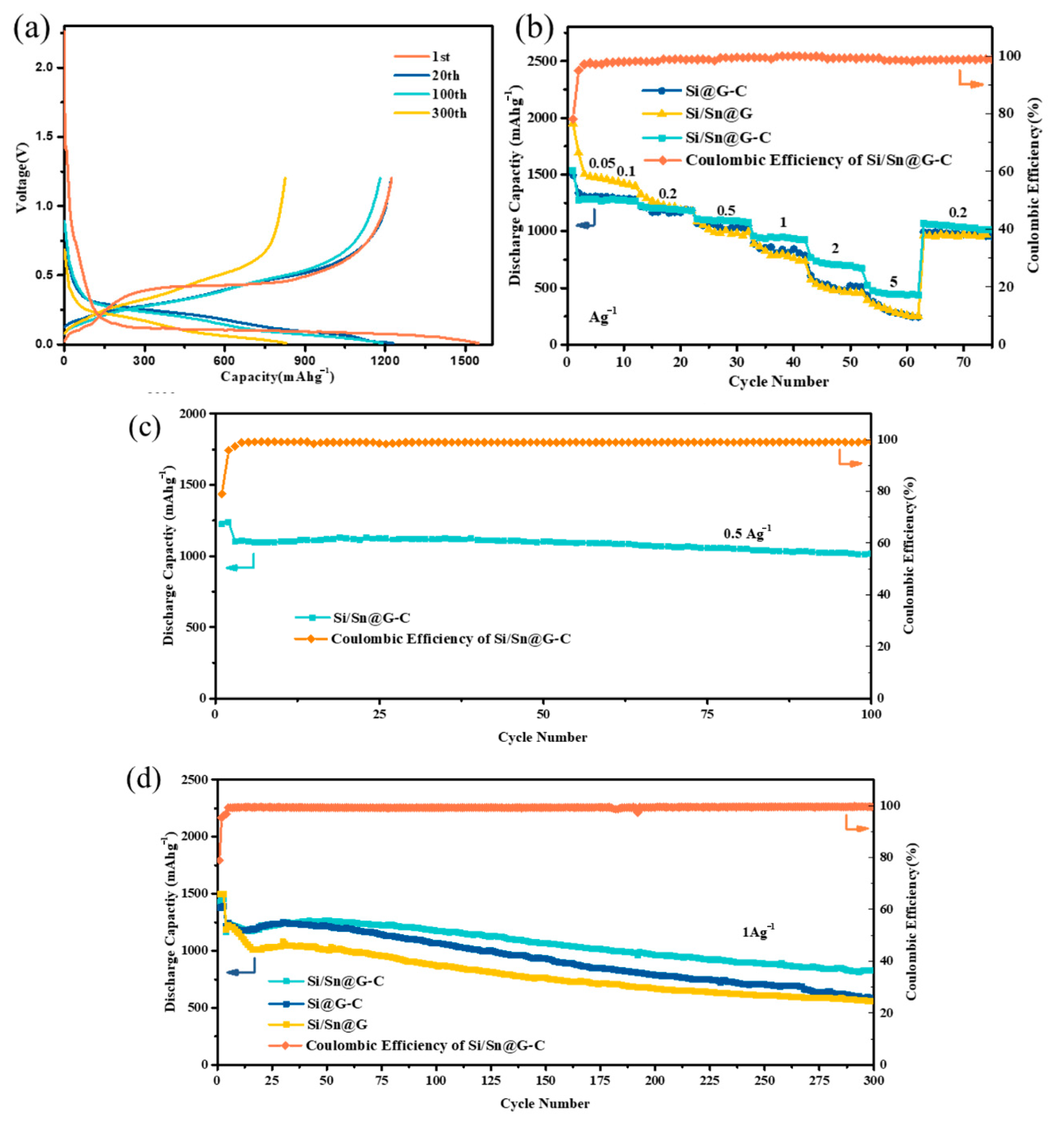
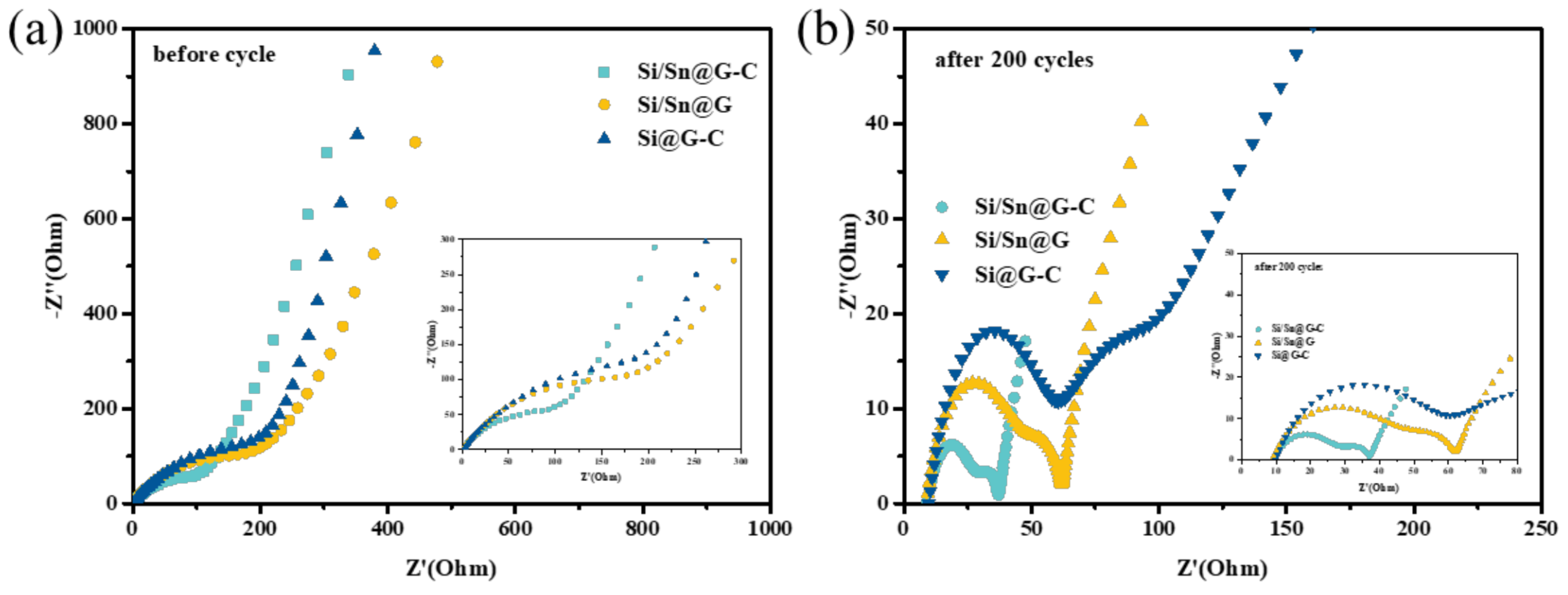
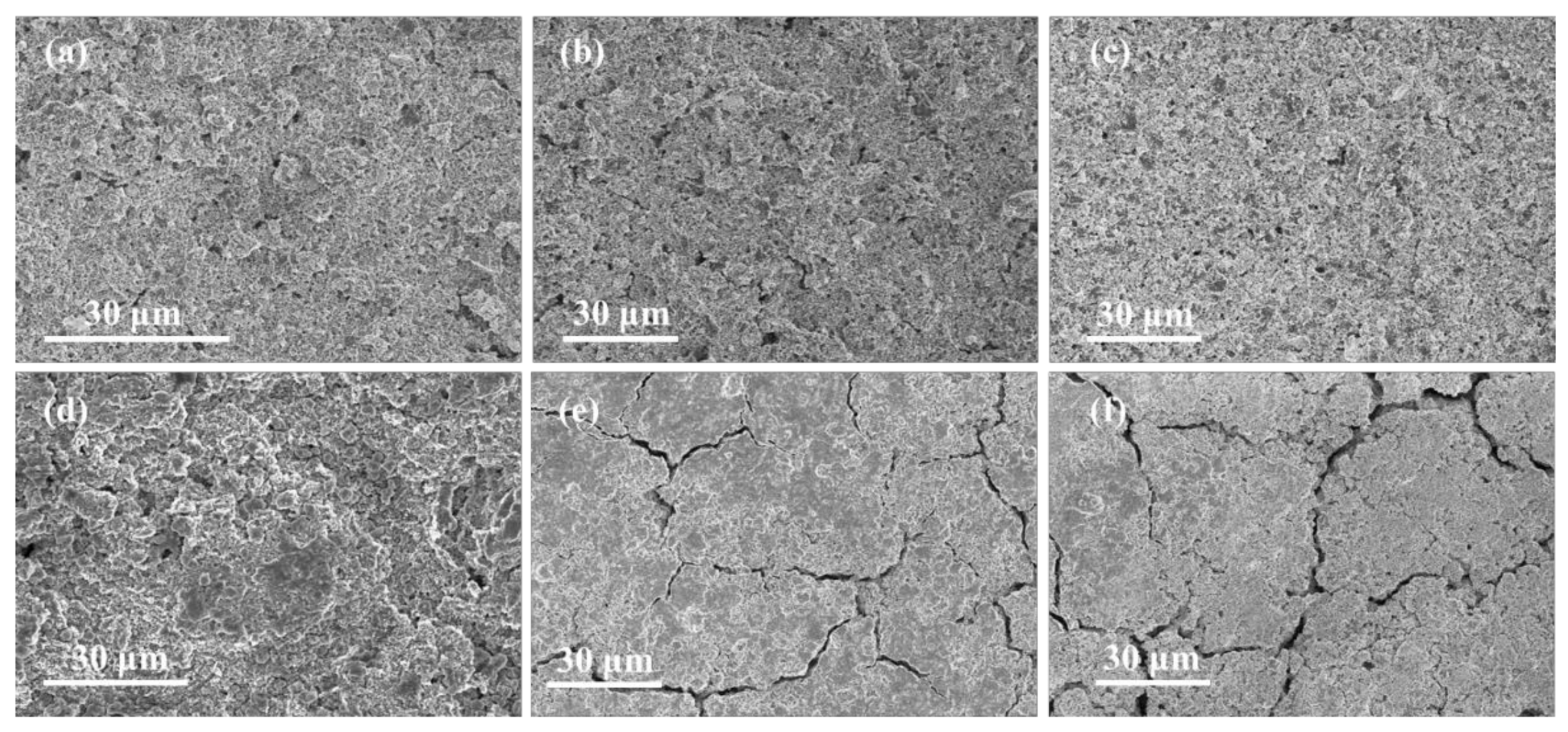
3. Result and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yu, J.; Sun, T.; Yang, Q.; Ma, J. Porous carbon networks containing Si and SnO2 as high performance anode materials for lithium-ion batteries. Mater. Lett. 2016, 184, 169–172. [Google Scholar] [CrossRef]
- He, H.; Fu, W.; Wang, H.; Wang, H.; Jin, C.; Fan, H.J.; Liu, Z. Silica-modified SnO 2 -graphene “slime” for self-enhanced li-ion battery anode. Nano Energy 2017, 34, 449–455. [Google Scholar] [CrossRef]
- Zhang, H.; Hu, R.; Liu, Y.; Cheng, X.; Liu, J.; Lu, Z.; Zeng, M.; Yang, L.; Liu, J.; Zhu, M. Highly reversible conversion reaction in Sn2Fe@SiOx nanocomposite: A high initial Coulombic efficiency and long lifetime anode for lithium storage. Energy Storage Mater. 2018, 13, 257–266. [Google Scholar] [CrossRef]
- Ma, T.; Yu, X.; Li, H.; Zhang, W.; Cheng, X.; Zhu, W.; Qiu, X. High Volumetric Capacity of Hollow Structured SnO2@Si Nanospheres for Lithium-Ion Batteries. Nano Lett. 2017, 17, 3959–3964. [Google Scholar] [CrossRef]
- Hu, R.; Zhang, H.; Lu, Z.; Liu, J.; Zeng, M.; Yang, L.; Yuan, B.; Zhu, M. Unveiling critical size of coarsened Sn nanograins for achieving high round-trip efficiency of reversible conversion reaction in lithiated SnO2 nanocrystals. Nano Energy 2018, 45, 255–265. [Google Scholar] [CrossRef]
- Zhang, S.; Ying, H.; Yuan, B.; Hu, R.; Han, W.-Q. Partial Atomic Tin Nanocomplex Pillared Few-Layered Ti3C2Tx MXenes for Superior Lithium-Ion Storage. Nano-Micro Lett. 2020, 12, 1–14. [Google Scholar] [CrossRef]
- Zhang, S.; Huang, P.; Wang, J.; Zhuang, Z.; Zhang, Z.; Han, W.Q. Fast and Universal Solution-Phase Flocculation Strategy for Scalable Synthesis of Various Few-Layered MXene Powders. J. Phys. Chem. Lett. 2020, 11, 1247–1254. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Tan, Y.; Hu, X.; Zhu, B.; Zheng, Q.; Zhang, Z.; Zhu, G.; Yu, Q.; Jin, Z.; Zhu, J. Scalable Production of the Silicon-Tin Yin-Yang Hybrid Structure with Graphene Coating for High Performance Lithium-Ion Battery Anodes. ACS Appl. Mater. Interfaces 2017, 9, 15388–15393. [Google Scholar] [CrossRef] [PubMed]
- Vernardou, D.; Marathianou, I.; Katsarakis, N.; Koudoumas, E.; Kazadojev, I.I.; O’Brien, S.; Pemble, M.E.; Povey, I.M. Capacitive behavior of Ag doped V2O5 grown by aerosol assisted chemical vapour deposition. Electrochim. Acta 2016, 196, 294–299. [Google Scholar] [CrossRef]
- Li, X.; Zhang, M.; Yuan, S.; Lu, C. Research Progress of Silicon/Carbon Anode Materials for Lithium-Ion Batteries: Structure Design and Synthesis Method. ChemElectroChem 2020, 7, 4289–4302. [Google Scholar] [CrossRef]
- Zhao, J.; Zhou, G.; Yan, K.; Xie, J.; Li, Y.; Liao, L.; Jin, Y.; Liu, K.; Hsu, P.C.; Wang, J.; et al. Air-stable and freestanding lithium alloy/graphene foil as an alternative to lithium metal anodes. Nat. Nanotechnol. 2017, 12, 993–999. [Google Scholar] [CrossRef]
- Tian, H.; Tan, X.; Xin, F.; Wang, C.; Han, W. Micro-sized nano-porous Si/C anodes for lithium ion batteries. Nano Energy 2015, 11, 490–499. [Google Scholar] [CrossRef]
- Chae, S.; Choi, S.-H.; Kim, N.; Sung, J.; Cho, J. Integration of Graphite and Silicon Anodes for the Commercialization of High-Energy Lithium-Ion Batteries. Angew. Chem. Int. Ed. 2020, 59, 110–135. [Google Scholar] [CrossRef]
- Yi, Z.; Wang, W.; Qian, Y.; Liu, X.; Lin, N.; Qian, Y. Mechanical Pressing Route for Scalable Preparation of Microstructured/Nanostrutured Si/Graphite Composite for Lithium Ion Battery Anodes. ACS Sustain. Chem. Eng. 2018, 6, 14230–14238. [Google Scholar] [CrossRef]
- Zheng, Y.; Yang, J.; Tao, L.; Nuli, Y.-N.; Wang, J.-L. Study of nano-porous Si/Graphite/C composite anode materials for Li-ion batteries. Chin. J. Inorg. Chem. 2007, 23, 1882–1886. [Google Scholar]
- Yang, D.; Shi, J.; Shi, J.; Yang, H. Simple synthesis of Si/Sn@C-G anodes with enhanced electrochemical properties for Li-ion batteries. Electrochim. Acta 2018, 259, 1081–1088. [Google Scholar] [CrossRef]
- Pei, Y.; Wang, S.; Zhou, Q.; Xie, S.-H.; Qiao, M.-H.; Jiang, Z.-Y.; Fan, K.-N. Single crystalline SnO nanosheets as anode material for lithium secondary battery. Chin. J. Chem. 2007, 25, 1385–1388. [Google Scholar] [CrossRef]
- Song, J.-S.; Cho, G.-B.; Ahn, H.-J.; Kim, H.-S.; Ahn, J.-H.; Cho, K.-K. Electrochemical Performance of Sn/SnO Nanoparticles with Core-Shell Structure as Anode Materials for Sodium-Ion and Lithium-Ion Batteries. J. Nanosci. Nanotechnol. 2016, 16, 10735–10739. [Google Scholar] [CrossRef]
- Wang, F.; Liu, C.; Zhang, J.; Cao, L. Research status and prospect of TiO_2 anode materials for lithium-ion batteries. Chinese J. Power Sources 2018, 42, 301–303. [Google Scholar]
- Liu, Q.; Dou, Y.; Ruan, B.; Sun, Z.; Chou, S.-L.; Dou, S.X. Carbon-Coated Hierarchical SnO2 Hollow Spheres for Lithium Ion Batteries. Chem. -A Eur. J. 2016, 22, 5853–5857. [Google Scholar] [CrossRef]
- Wang, L.; Chen, Q.; Zhu, Y.; Qian, Y. Graphene-wrapped Fe2O3 nanorings for Li ion battery anodes. Chin. Sci. Bull. 2014, 59, 4271–4273. [Google Scholar] [CrossRef]
- Liu, B.; Chuang, Y.; Zhang, Y.A.J.; Chen, H.; Hayat, T.; Alsaedi, A.; Ahmad, B. The Preparation of CuO@ZnO Core-Shell Materials as High-Stability Anodes for Lithium-Ion Batteries. Int. J. Electrochem. Sci. 2019, 14, 8973–8985. [Google Scholar] [CrossRef]
- Li, H.; Huang, X.J.; Chen, L.Q. Electrochemical impedance spectroscopy study of SnO and nano-SnO anodes in lithium rechargeable batteries. J. Power Sources 1999, 81, 340–345. [Google Scholar] [CrossRef]
- Yang, H.; Liang, K.; Du, J.; Tian, L.; Wen, X. Facile preparation of nanoscale silicon as anode materials for lithium ion batteries by a mild temperature metathesis route. Electron. Compon. Mater. 2017, 36, 43–47. [Google Scholar]
- Zhang, Y.; Liu, H. Research Progress on Si/ C Composite Anode Materials for Lithium-ion Battery. Bull. Chin. Ceram. Soc. 2015, 34, 989–994. [Google Scholar]
- He, X.; Pu, W.; Ren, J.; Wang, L.; Jiang, C.; Wan, C. Synthesis of nanosized Si composite anode material for Li-ion batteries. Ionics 2007, 13, 51–54. [Google Scholar] [CrossRef]
- Wang, G.X.; Ahn, J.H.; Yao, J.; Bewlay, S.; Liu, H.K. Nanostructured Si-C composite anodes for lithium-ion batteries. Electrochem. Commun. 2004, 6, 689–692. [Google Scholar] [CrossRef]
- Jia, D.; Li, X.; Huang, J. A Hierarchical, Nanofibrous, Tin-Oxide/Silicon Composite Derived from Cellulose as a High-Performance Anode Material for Lithium-Ion Batteries. ChemistrySelect 2017, 2, 5667–5676. [Google Scholar] [CrossRef]
- Wang, X.; Wen, Z.; Liu, Y.; Huang, L.; Wu, M. Study on Si–Ti alloy dispersed in a glassy matrix as an anode material for lithium-ion batteries. J. Alloys Compd. 2010, 506, 317–322. [Google Scholar] [CrossRef]
- Wang, S.; Liao, J.; Wu, M.; Xu, Z.; Gong, F.; Chen, C.; Wang, Y.; Yan, X. High Rate and Long Cycle Life of a CNT/rGO/Si Nanoparticle Composite Anode for Lithium-Ion Batteries. Part. Part. Syst. Charact. 2017, 34. [Google Scholar] [CrossRef]
- Zhou, M.; Cai, T.; Pu, F.; Chen, H.; Wang, Z.; Zhang, H.; Guan, S. Graphene/Carbon-Coated Si Nanoparticle Hybrids as High-Performance Anode Materials for Li-Ion Batteries. ACS Appl. Mater. Interfaces 2013, 5, 3449–3455. [Google Scholar] [CrossRef]
- Liu, J.L.; Cai, S.J.; Jin, G.L.; Tang, Y.S.; Wang, K.L. Gas-source MBE growth of freestanding Si nano-wires on Au/Si substrate. Superlattices Microstruct. 1999, 25, 477–479. [Google Scholar] [CrossRef]
- Hu, L.; Wu, H.; Hong, S.S.; Cui, L.; McDonough, J.R.; Bohy, S.; Cui, Y. Si nanoparticle-decorated Si nanowire networks for Li-ion battery anodes. Chem. Commun. 2011, 47, 367–369. [Google Scholar] [CrossRef]
- Ren, W.; Wang, C.; Lu, L.; Li, D.; Cheng, C.; Liu, J. SnO2@Si core-shell nanowire arrays on carbon cloth as a flexible anode for Li ion batteries. J. Mater. Chem. A 2013, 1, 13433–13438. [Google Scholar] [CrossRef]
- Hao, Q.; Hou, J.; Ye, J.; Yang, H.; Du, J.; Xu, C. Hierarchical macroporous Si/Sn composite: Easy preparation and optimized performances towards lithium storage. Electrochim. Acta 2019, 306, 427–436. [Google Scholar] [CrossRef]
- Kim, M.G.; Yun, Z.; Lyou, J.; Cho, S.; Park, Y.J.; Kim, E.K. Visible photoluminescence from porous poly-Si/Si and amorphous-Si/Si structures. J. Korean Phys. Soc. 2001, 38, 750–753. [Google Scholar]
- Huang, T.; Sun, D.; Yang, W.; Wu, Q.; Xiao, R. The Fabrication of Porous Si with Interconnected Micro-Sized Dendrites and Tunable Morphology through the Dealloying of a Laser Remelted Al-Si Alloy. Materials 2017, 10, 357. [Google Scholar] [CrossRef]
- Fang, R.; Xiao, W.; Miao, C.; Mei, P.; Yan, X.; Zhang, Y.; Jiang, Y. Improved lithium storage performance of pomegranate-like Si@NC/rGO composite anodes by facile in-situ nitrogen doped carbon coating and freeze drying processes. J. Alloys Compd. 2020, 834, 155230. [Google Scholar] [CrossRef]
- Yang, W.; Ying, H.; Zhang, S.; Guo, R.; Wang, J.; Han, W.-Q. Electrochemical performance enhancement of porous Si lithium-ion battery anode by integrating with optimized carbonaceous materials. Electrochim. Acta 2020, 337, 135687. [Google Scholar] [CrossRef]
- Autthawong, T.; Namsar, O.; Yu, A.; Sarakonsri, T. Cost-effective production of SiO2/C and Si/C composites derived from rice husk for advanced lithium-ion battery anodes. J. Mater. Sci. Mater. Electron. 2020, 31, 9126–9132. [Google Scholar] [CrossRef]
- Xia, K.; Qu, L.; Liu, X.; Han, H.; Hou, Z.; Li, Y.; Deng, S. Effect of SnCl2 addition on the structure and lithium storage performance of SiOC anodes. Appl. Surf. Sci. 2020, 506, 144775. [Google Scholar] [CrossRef]
- Su, L.; Zhou, Z.; Ren, M. Core double-shell Si@SiO2@C nanocomposites as anode materials for Li-ion batteries. Chem. Commun. (Camb) 2010, 46, 2590–2592. [Google Scholar] [CrossRef]
- Wu, W.; Wang, M.; Wang, J.; Wang, C.; Deng, Y. Green Design of Si/SiO2/C Composites as High-Performance Anodes for Lithium-Ion Batteries. ACS Appl. Energy Mater. 2020, 3, 3884–3892. [Google Scholar] [CrossRef]
- Zhong, L.; Beaudette, C.; Guo, J.; Bozhilov, K.; Mangolini, L. Tin nanoparticles as an effective conductive additive in silicon anodes. Sci. Rep. 2016, 6, 30952. [Google Scholar] [CrossRef]
- Hassoun, J.; Panero, S.; Mulas, G.; Scrosati, B. An electrochemical investigation of a Sn–Co–C ternary alloy as a negative electrode in Li-ion batteries. J. Power Sources 2007, 171, 928–931. [Google Scholar] [CrossRef]
- Zhang, J.; Liang, Y.; Zhou, Q.; Peng, Y.; Yang, H. Enhancing electrochemical properties of silicon-graphite anodes by the introduction of cobalt for lithium-ion batteries. J. Power Sources 2015, 290, 71–79. [Google Scholar] [CrossRef]
- Hao, Q.; Zhao, D.; Duan, H.; Zhou, Q.; Xu, C. Si/Ag composite with bimodal micro-nano porous structure as a high-performance anode for Li-ion batteries. Nanoscale 2015, 7, 5320–5327. [Google Scholar] [CrossRef]
- Wu, X.D.; Wang, Z.X.; Chen, L.Q.; Huang, X.J. Ag-enhanced SEI formation on Si particles for lithium batteries. Electrochem. Commun. 2003, 5, 935–939. [Google Scholar] [CrossRef]
- Guojun, Z.; Naigen, H. Synthesis and Properties of Si/Ag and Si/Ag/CMS Composites as Anode Materials for Li-ion Batteries. Silicon 2019, 11, 2517–2520. [Google Scholar] [CrossRef]
- Park, M.-S.; Rajendran, S.; Kang, Y.-M.; Han, K.-S.; Han, Y.-S.; Lee, J.-Y. Si–Ni alloy–graphite composite synthesized by arc-melting and high-energy mechanical milling for use as an anode in lithium-ion batteries. J. Power Sources 2006, 158, 650–653. [Google Scholar] [CrossRef]
- Kim, B.C.; Uono, H.; Satou, T.; Fuse, T.; Ishihara, T.; Ue, M.; Senna, M. Cyclic properties of Si-Cu/carbon nanocomposite anodes for Li-ion secondary batteries. J. Electrochem. Soc. 2005, 152, A523–A526. [Google Scholar] [CrossRef]
- Kang, Y.-M.; Park, M.-S.; Lee, J.-Y.; Liu, H.-K. Si-Cu/carbon composites with a core-shell structure for Li-ion secondary battery. Carbon 2007, 45, 1928–1933. [Google Scholar] [CrossRef]
- Yoon, S.; Lee, S.-I.; Kim, H.; Sohn, H.-J. Enhancement of capacity of carbon-coated Si-Cu3Si composite anode using metal-organic compound for lithium-ion batteries. J. Power Sources 2006, 161, 1319–1323. [Google Scholar] [CrossRef]
- Zhao, W.; Du, N.; Xiao, C.; Wu, H.; Zhang, H.; Yang, D. Large-scale synthesis of Ag–Si core–shell nanowall arrays as high-performance anode materials of Li-ion batteries. J. Mater. Chem. A 2014, 2, 13949–13954. [Google Scholar] [CrossRef]
- Li, C.; Zhang, P.; Jiang, Z. Effect of nano Cu coating on porous Si prepared by acid etching Al-Si alloy powder. Electrochim. Acta 2015, 161, 408–412. [Google Scholar] [CrossRef]
- Liu, X.; Gao, Y.; Jin, R.; Luo, H.; Peng, P.; Liu, Y. Scalable synthesis of Si nanostructures by low-temperature magnesiothermic reduction of silica for application in lithium ion batteries. Nano Energy 2014, 4, 31–38. [Google Scholar] [CrossRef]
- Kim, H.; Seo, M.; Park, M.H.; Cho, J. A critical size of silicon nano-anodes for lithium rechargeable batteries. Angew. Chem. Int. Ed. Engl. 2010, 49, 2146–2149. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Xin, F.; Wang, X.; He, W.; Han, W. High capacity group-IV elements (Si, Ge, Sn) based anodes for lithium-ion batteries. J. Mater. 2015, 1, 153–169. [Google Scholar] [CrossRef]
- Liu, D.; Liu, Z.J.; Li, X.; Xie, W.; Wang, Q.; Liu, Q.; Fu, Y.; He, D. Group IVA Element (Si, Ge, Sn)-Based Alloying/Dealloying Anodes as Negative Electrodes for Full-Cell Lithium-Ion Batteries. Small 2017, 13, 13. [Google Scholar] [CrossRef]
- He, W.; Tian, H.; Xin, F.; Han, W. Scalable fabrication of micro-sized bulk porous Si from Fe–Si alloy as a high performance anode for lithium-ion batteries. J. Mater. Chem. A 2015, 3, 17956–17962. [Google Scholar] [CrossRef]
- Fang, G. A Hybrid Si@C@CNT@C Anode by Anchoring Silicon Nanoparticles onto CNT for Enhancing Performance in Lithium Ion Battery. Int. J. Electrochem. Sci. 2019, 10, 1580–1590. [Google Scholar] [CrossRef]
- Han, W.Q.; Zettl, A. Coating single-walled carbon nanotubes with tin oxide. Nano Lett. 2003, 3, 681–683. [Google Scholar] [CrossRef]
- Zhao, D.; Li, P. Penetrating needling from Qiuxu (GB 40) to Zhaohai (KI 6) for 30 cases of palpitation. Zhongguo zhen jiu = Chin. Acupunct. Moxibustion 2014, 34. [Google Scholar]
- Vogl, U.S.; Lux, S.F.; Das, P.; Weber, A.; Placke, T.; Kostecki, R.; Winter, M. The Mechanism of SEI Formation on Single Crystal Si(100), Si(110) and Si(111) Electrodes. J. Electrochem. Soc. 2015, 162, A2281–A2288. [Google Scholar] [CrossRef]
- Li, P.; Hwang, J.Y.; Sun, Y.K. Nano/Microstructured Silicon-Graphite Composite Anode for High-Energy-Density Li-Ion Battery. ACS Nano 2019, 13, 2624–2633. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, T.; Ying, H.; Zhang, S.; Wang, J.; Zhang, Z.; Han, W.-Q. Electrochemical Performance Enhancement of Micro-Sized Porous Si by Integrating with Nano-Sn and Carbonaceous Materials. Materials 2021, 14, 920. https://doi.org/10.3390/ma14040920
Yang T, Ying H, Zhang S, Wang J, Zhang Z, Han W-Q. Electrochemical Performance Enhancement of Micro-Sized Porous Si by Integrating with Nano-Sn and Carbonaceous Materials. Materials. 2021; 14(4):920. https://doi.org/10.3390/ma14040920
Chicago/Turabian StyleYang, Tiantian, Hangjun Ying, Shunlong Zhang, Jianli Wang, Zhao Zhang, and Wei-Qiang Han. 2021. "Electrochemical Performance Enhancement of Micro-Sized Porous Si by Integrating with Nano-Sn and Carbonaceous Materials" Materials 14, no. 4: 920. https://doi.org/10.3390/ma14040920
APA StyleYang, T., Ying, H., Zhang, S., Wang, J., Zhang, Z., & Han, W.-Q. (2021). Electrochemical Performance Enhancement of Micro-Sized Porous Si by Integrating with Nano-Sn and Carbonaceous Materials. Materials, 14(4), 920. https://doi.org/10.3390/ma14040920







