Properties of Biocomposites from Rapeseed Meal, Fruit Pomace and Microcrystalline Cellulose Made by Press Pressing: Mechanical and Physicochemical Characteristics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Biocomposite Production
2.3. Mechanical Properties
2.4. Water Contact Angle
2.5. Colour Analysis
2.6. Microscopic Analysis
2.7. Thermogravimetry Analysis (TGA) and Derivative Differential Thermal Analysis (DTA)
2.8. Fourier Transform Infrared Spectroscopy (FTIR)
2.9. Statistical Analysis
3. Results
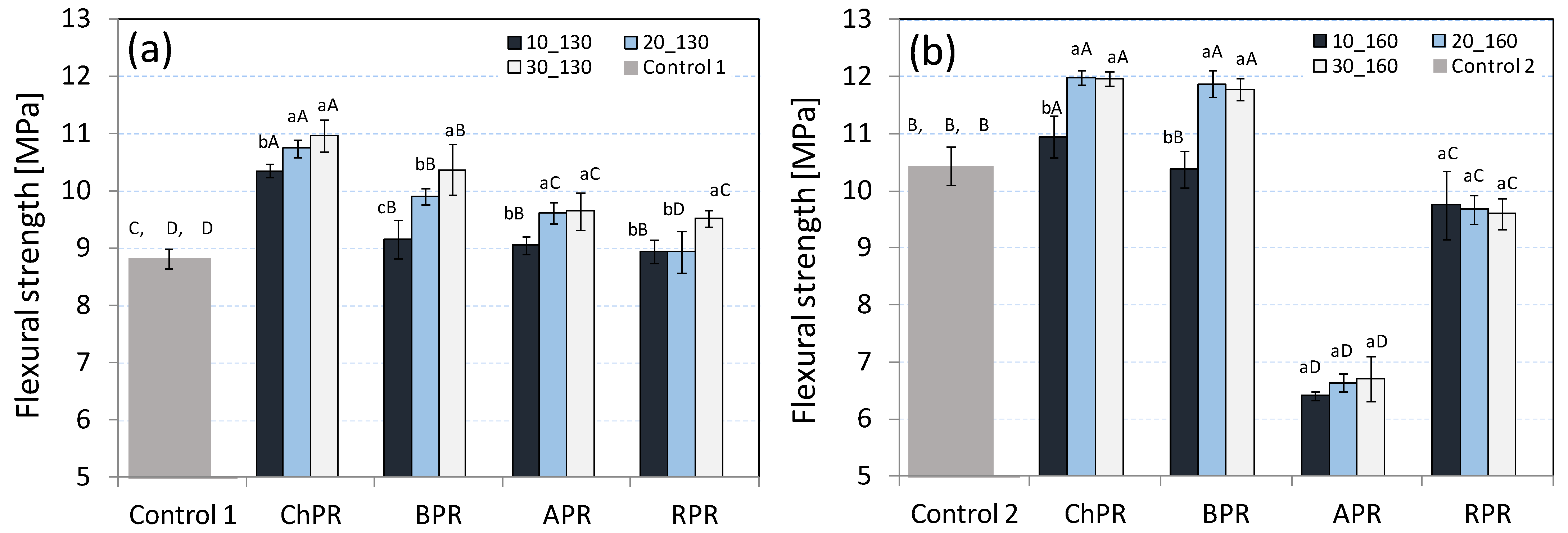
3.1. Flexural Strength and Young’s Modulus
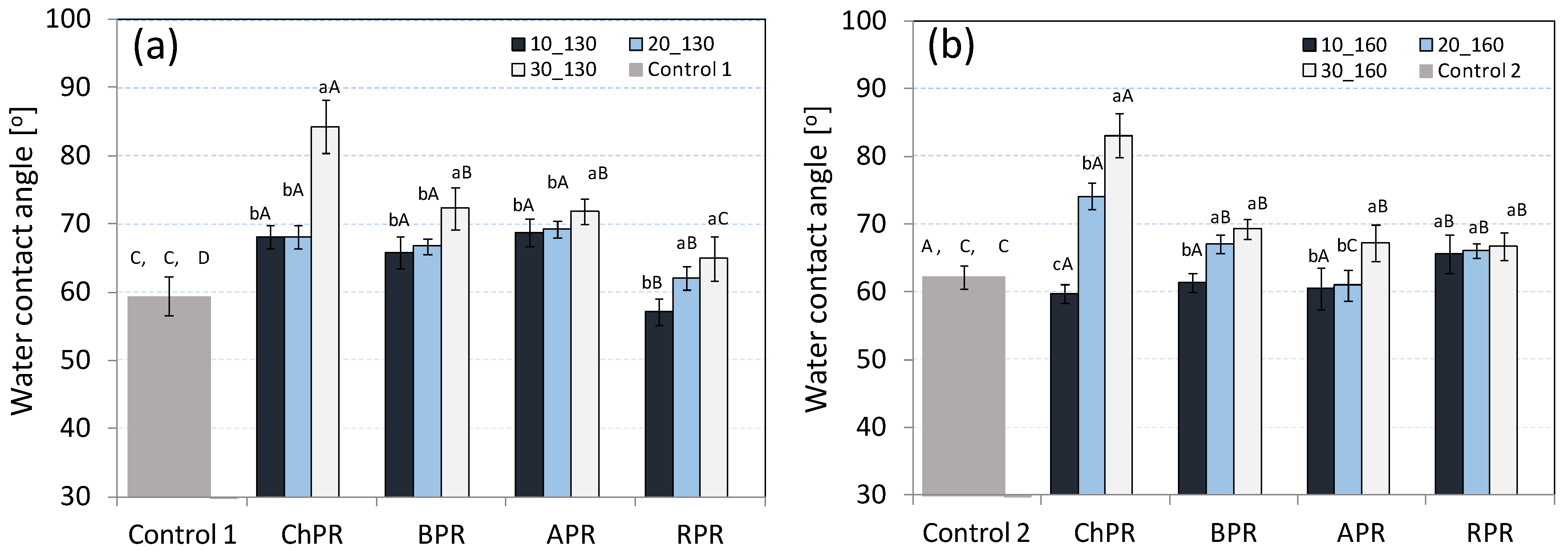
3.2. Water Contact Angle
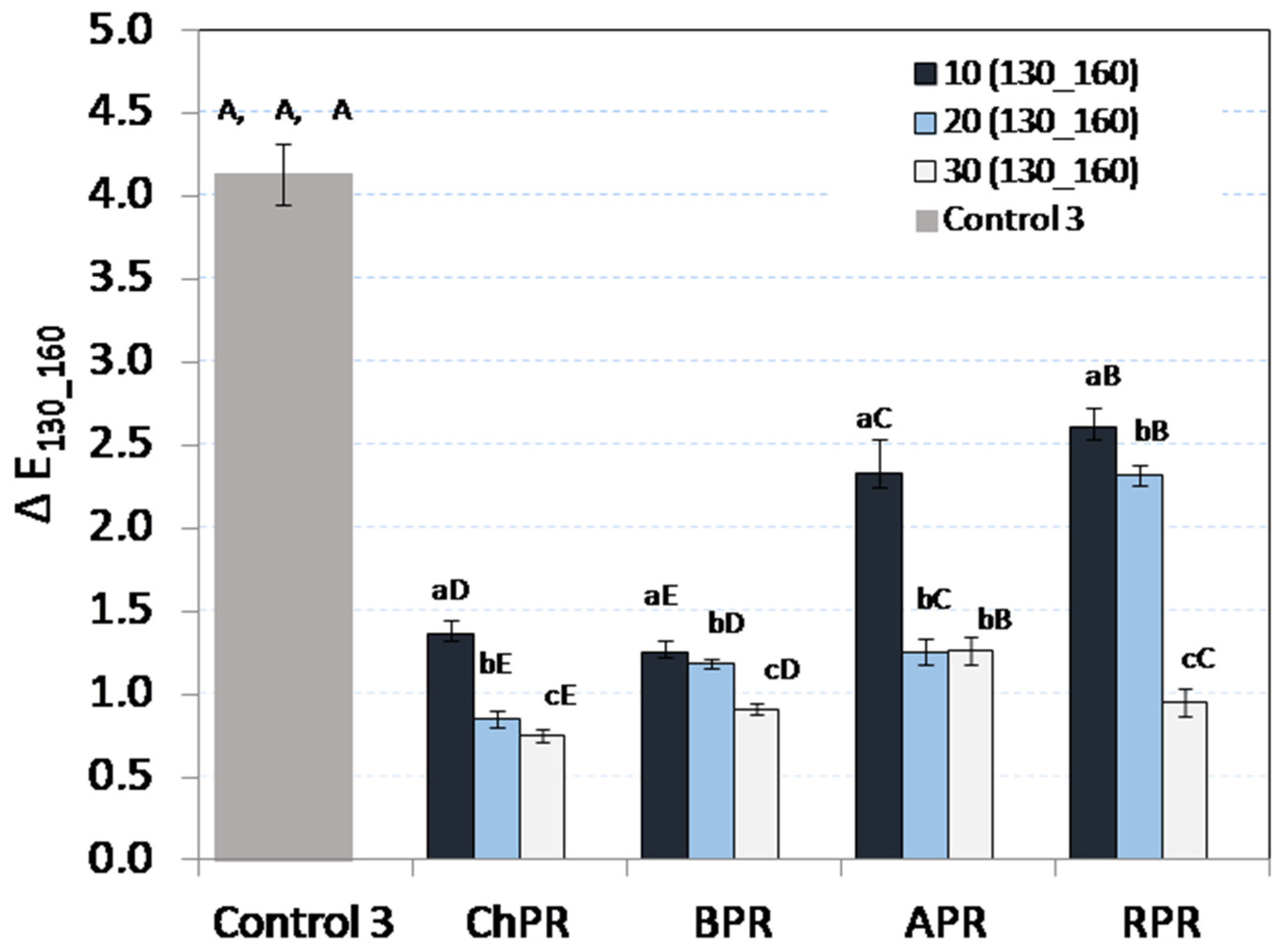
3.3. Colour Analysis
3.4. Microscopic Analysis
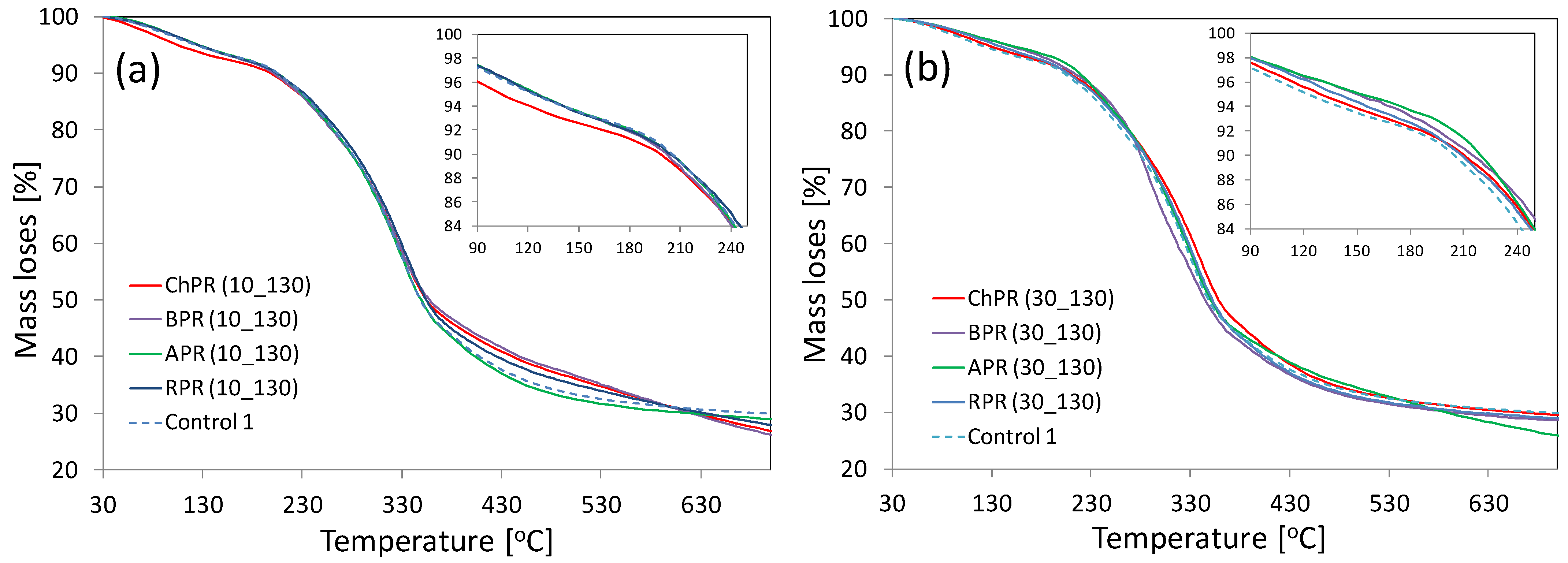
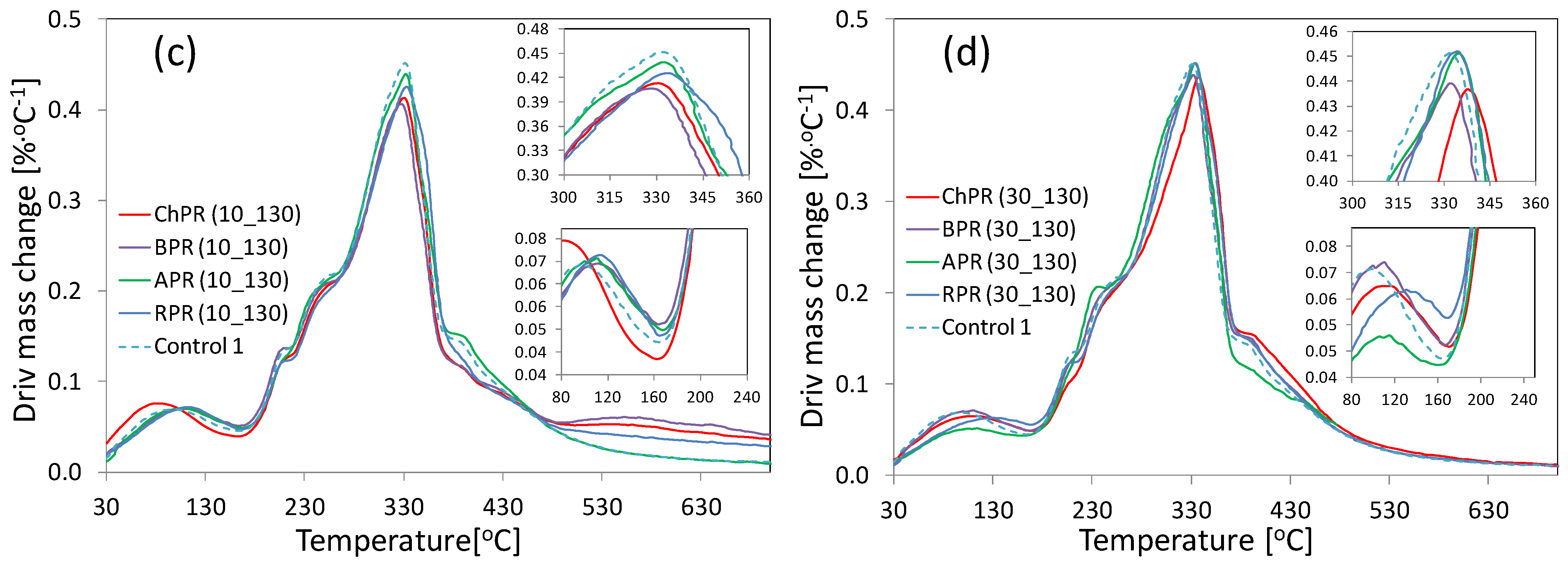
3.5. Thermogravimetry Analysis (TGA) and Derivative Differential Thermal Analysis (DTA)
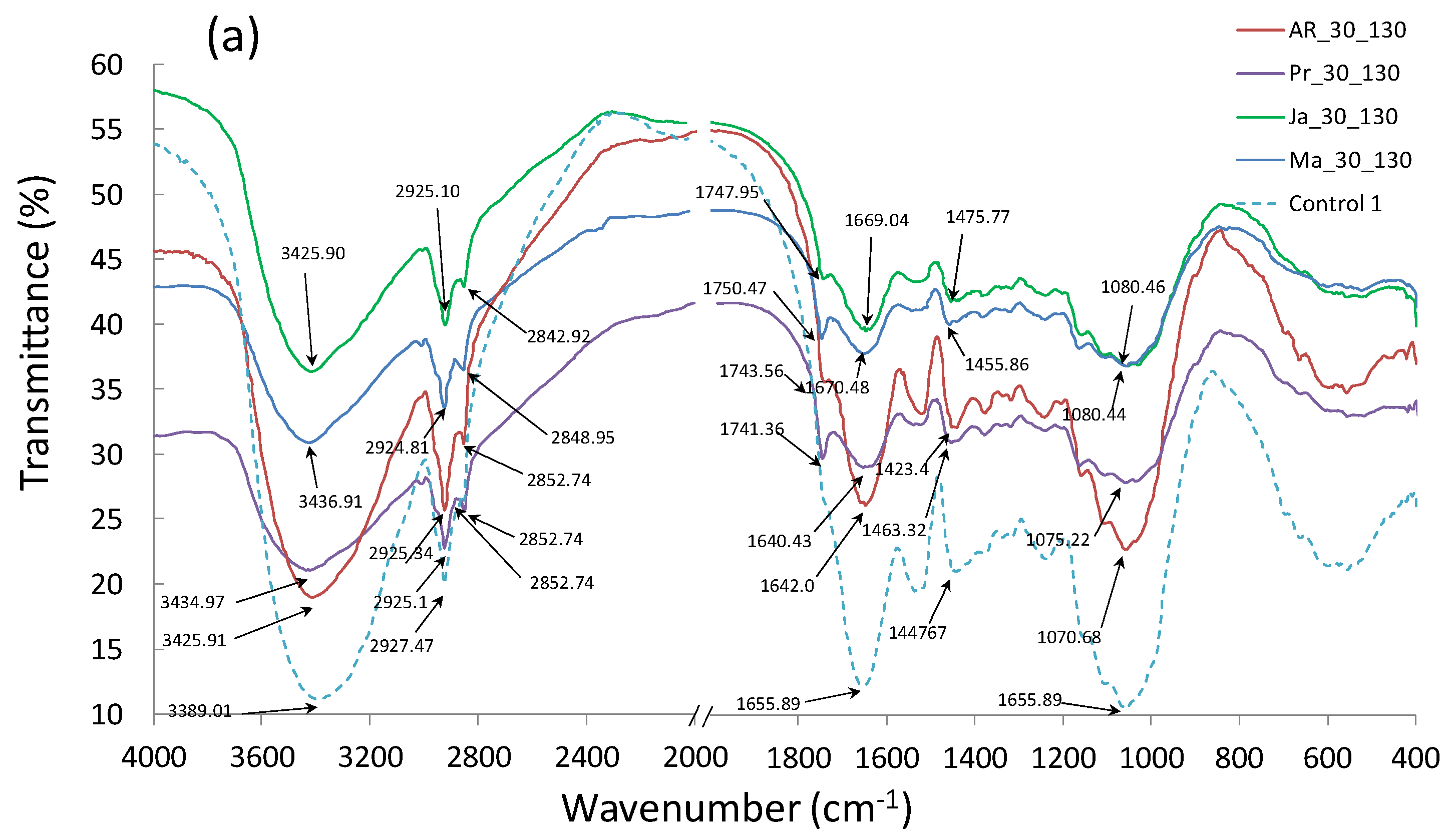
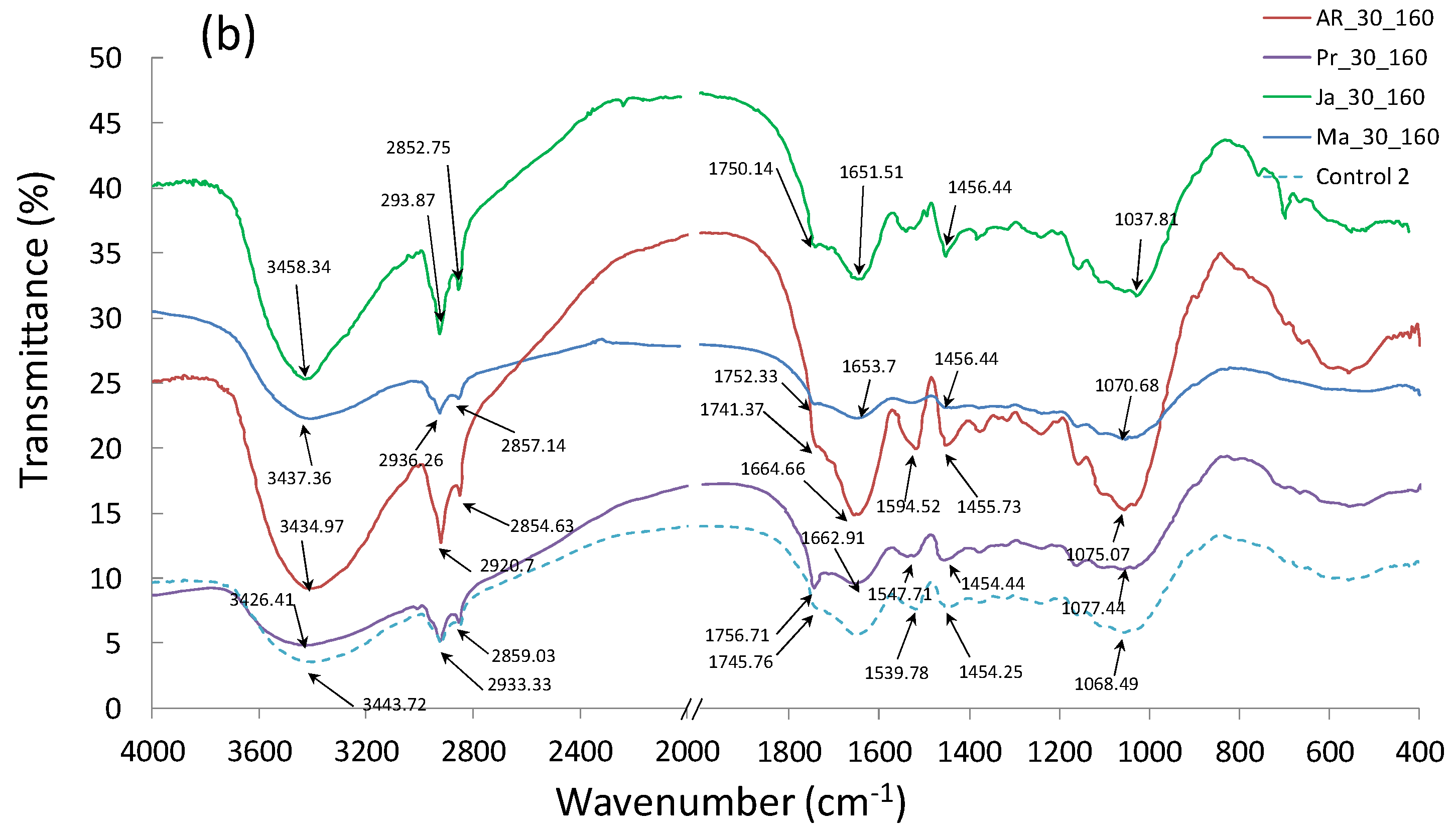
3.6. Fourier Transform Infrared Spectroscopy (FTIR)
4. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gowman, A.C.; Picard, M.C.; Lim, L.T.; Misra, M.; Mohanty, A.K. Fruit Waste Valorization for Biodegradable Biocomposite Applications: A Review. BioResources 2019, 14, 10047–10092. [Google Scholar] [CrossRef]
- Borowski, P.F. Zonal and Nodal Models of energy market in European Union. Energies 2020, 13, 4182. [Google Scholar] [CrossRef]
- Kupczyk, A.; Mączyńska-Sęczek, J.; Golisz, E.; Borowski, P.F. Renewable Energy Sources in Transport on the Example of Methyl Esters and Bioethanol. Processes 2020, 8, 1610. [Google Scholar] [CrossRef]
- Zhu, Z.; Gavahian, M.; Barba, F.J.; Roselló-Soto, E.; Kovačević, D.B.; Putnik, P.; Denoya, G.I. Valorization of waste and by-products from food industries through the use of innovative technologies. In Agri-Food Industry Strategies for Healthy Diets and Sustainability; Academic Press: Cambridge, MA, USA, 2020; pp. 249–266. [Google Scholar]
- Żelazinski, T.; Ekielski, A.; Tulska, E.; Vladut, V.; Durczak, K. Wood dust application for improvement of selected properties of thermoplastic starch. Inmateh-Agric. Eng. 2019, 58, 37–44. [Google Scholar]
- Oliver-Ortega, H.; Julian, F.; Espinach, F.X.; Tarrés, Q.; Ardanuy, M.; Mutjé, P. Research on the use of lignocellulosic fibers reinforced bio-polyamide 11 with composites for automotive parts: Car door handle case study. J. Clean. Prod. 2019, 226, 64–73. [Google Scholar] [CrossRef]
- Kremensas, A.; Kairytė, A.; Vaitkus, S.; Vėjelis, S.; Balčiūnas, G. Mechanical performance of biodegradable thermoplastic polymer-based biocomposite boards from hemp shivs and corn starch for the building industry. Materials 2019, 12, 845. [Google Scholar] [CrossRef] [Green Version]
- Song, Q.; Li, J.; Zeng, X. Minimizing the increasing solid waste through zero waste strategy. J. Clean. Prod. 2015, 104, 199–210. [Google Scholar] [CrossRef]
- Sánchez-Safont, E.L.; Aldureid, A.; Lagarón, J.M.; Gámez-Pérez, J.; Cabedo, L. Biocomposites of different lignocellulosic wastes for sustainable food packaging applications. Compos. Part B Eng. 2018, 145, 215–225. [Google Scholar] [CrossRef]
- Fierascu, R.C.; Sieniawska, E.; Ortan, A.; Fierascu, I.; Xiao, J. Fruits By-Products—A Source of Valuable Active Principles. A Short Review. Front. Bioeng. Biotechnol. 2020, 8, 319. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, L.J.; Peças, P.; Carvalho, H.; Orrego, C.E. A literature review on life cycle tools fostering holistic sustainability assessment: An application in biocomposite materials. J. Environ. Manag. 2020, 262, 110308. [Google Scholar] [CrossRef]
- Lisowski, A.; Pajor, M.; Świętochowski, A.; Dąbrowska, M.; Klonowski, J.; Mieszkalski, L.; Ekielski, A.; Stasiak, M.; Piątek, M. Effects of moisture content, temperature, and die thickness on the compaction process, and the density and strength of walnut shell pellets. Renew. Energy 2019, 141, 770–781. [Google Scholar] [CrossRef]
- Nanthananon, P.; Seadan, M.; Pivsa-Art, S.; Hamada, H.; Suttiruengwong, S. Reactive Compatibilization of Short-Fiber Reinforced Poly (lactic acid) Biocomposites. J. Renew. Mater. 2018, 6, 573–583. [Google Scholar] [CrossRef]
- Jiang, Y.; Simonsen, J.; Zhao, Y. Compression-molded biocomposite boards from red and white wine grape pomaces. J. Appl. Polym. Sci. 2011, 119, 2834–2846. [Google Scholar] [CrossRef]
- Kim, J.T.; Netravali, A.N. Mechanical, thermal, and interfacial properties of green composites with ramie fiber and soy resins. J. Agric. Food Chem. 2010, 58, 5400–5407. [Google Scholar] [CrossRef] [PubMed]
- Prochoń, M.; Ntumba, Y.H.T. Effects of Biopolymer Keratin Waste Sources in XNBR Compounds. Rubber Chem. Technol. 2015, 88, 258–275. [Google Scholar] [CrossRef]
- Kim, J.R.; Netravali, A.N. Self-healing green composites based on soy protein and microfibrillated cellulose. Compos. Sci. Technol. 2017, 143, 22–30. [Google Scholar] [CrossRef] [Green Version]
- Cibulkova, Z.; Cerna, A.; Simon, P.; Uhlar, J.; Kosar, K.; Lehocky, P. Stabilization effect of potential antioxidants on the thermooxidative stability of styrene–butadiene rubber. J. Therm. Anal. Calorim. 2011, 105, 607–613. [Google Scholar] [CrossRef]
- Prochon, M.; Janowska, G.; Przepiorkowska, A.; Kucharska-Jastrzabek, A. Thermal stability and flammability of biodecomposable elastomer materials. Polimery 2013, 58, 413–420. [Google Scholar] [CrossRef]
- Goudarzi, T.; Spring, D.W.; Paulino, G.H.; Lopez-Pamies, O. Filled elastomers: A theory of filler reinforcement based on hydrodynamic and interphasial effects. J. Mech. Phys. Solids 2015, 80, 37–67. [Google Scholar] [CrossRef] [Green Version]
- Pichandi, S.; Rana, S.; Parveen, S.; Fangueiro, R. A green approach of improving interface and performance of plant fibre composites using microcrystalline cellulose. Carbohydr. Polym. 2018, 197, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Trache, D.; Hussin, M.H.; Chuin, C.T.H.; Sabar, S.; Fazita, M.N.; Taiwo, O.F.A.; Hassan, T.M.; Haafiz, M.K.M. Microcrystalline cellulose: Isolation, characterization and bio-composites application—A review. Int. J. Biol. Macromol. 2016, 93, 789–804. [Google Scholar] [CrossRef] [PubMed]
- Park, S.I.; Jiang, Y.; Simonsen, J.; Zhao, Y. Feasibility of creating compression-molded biocomposite boards from berry fruit pomaces. J. Appl. Polym. Sci. 2010, 115, 127–136. [Google Scholar] [CrossRef]
- Picard, M.C.; Rodriguez-Uribe, A.; Thimmanagari, M.; Misra, M.; Mohanty, A.K. Sustainable biocomposites from poly (butylene succinate) and apple pomace: A study on compatibilization performance. Waste Biomass Valorization 2020, 11, 3775–3787. [Google Scholar] [CrossRef]
- Górecka, D.; Pachołek, B.; Dziedzic, K.; Górecka, M. Raspberry pomace as a potential fiber source for cookies enrichment. Acta Sci. Pol. Technol. Aliment. 2010, 9, 451–461. [Google Scholar]
- Nawirska, A.; Kwaśniewska, M. Dietary fibre fractions from fruit and vegetable processing waste. Food Chem. 2005, 91, 221–225. [Google Scholar] [CrossRef]
- DIN ISO 3310-1: 2017-11. Test Sieves—Technical Requirements and Testing—Part 1: Test Sieves of Metal Wire Cloth; German Institute for Standardization: Berlin, Germany, 2017. [Google Scholar]
- PN-EN ISO 178:2011. Plastics—Determination of Flexural Properties (Tworzywa Sztuczne—Oznaczanie Właściwości Przy Zginaniu); Polish Standardization Committee: Warsaw, Poland, 2011. [Google Scholar]
- Giri, J.; Lach, R.; Grellmann, W.; Susan, M.A.; Saiter, J.M.; Henning, S.; Katiyar, V.; Adhikari, R. Compostable composites of wheat stalk micro-and nanocrystalline cellulose and poly (butylene adipate-co-terephthalate): Surface properties and degradation behavior. J. Appl. Polym. Sci. 2019, 136, 48149. [Google Scholar] [CrossRef]
- Robin, F.; Dubois, C.; Pineau, N.; Schuchmann, H.P.; Palzer, S. Expansion mechanism of extruded foams supplemented with wheat bran. J. Food Eng. 2011, 107, 80–89. [Google Scholar] [CrossRef]
- Sarasini, F.; Fiore, V. A systematic literature review on less common natural fibres and their biocomposites. J. Clean. Prod. 2018, 195, 240–267. [Google Scholar] [CrossRef]
- Ramesh, M.; Palanikumar, K.; Reddy, K.H. Plant fibre based bio-composites: Sustainable and renewable green materials. Renew. Sustain. Energy Rev. 2017, 79, 558–584. [Google Scholar] [CrossRef]
- Manshor, M.R.; Anuar, H.; Aimi, M.N.; Fitrie, M.A.; Nazri, W.W.; Sapuan, S.M.; El-Shekeil, Y.A.; Wahit, M.U. Mechanical, thermal and morphological properties of durian skin fibre reinforced PLA biocomposites. Mater. Des. 2014, 59, 279–286. [Google Scholar] [CrossRef]
- Su, J.F.; Huang, Z.; Yuan, X.Y.; Wang, X.Y.; Li, M. Structure and properties of carboxymethyl cellulose/soy protein isolate blend edible films crosslinked by Maillard reactions. Carbohydr. Polym. 2010, 79, 145–153. [Google Scholar] [CrossRef]
- Mishra, P.K.; Ekielski, A. A simple method to synthesize lignin nanoparticles. Colloids Interfaces 2019, 3, 52. [Google Scholar] [CrossRef] [Green Version]
- Raj, S.A.; Muthukumaran, E.; Jayakrishna, K. A case study of 3D printed PLA and its mechanical properties. Mater. Today Proc. 2018, 5, 11219–11226. [Google Scholar] [CrossRef]
- Grylewicz, A.; Spychaj, T.; Zdanowicz, M. Thermoplastic starch/wood biocomposites processed with deep eutectic solvents. Compos. Part A Appl. Sci. Manuf. 2019, 121, 517–524. [Google Scholar] [CrossRef]
- Nourbakhsh, A.; Baghlani, F.F.; Ashori, A. Nano-SiO2 filled rice husk/polypropylene composites: Physico-mechanical properties. Ind. Crop. Prod. 2011, 33, 183–187. [Google Scholar] [CrossRef]
- Aydin, I.; Colakoglu, G. Variation in surface roughness, wettability and some plywood properties after preservative treatment with boron compounds. Build. Environ. 2007, 42, 3837–3840. [Google Scholar] [CrossRef]
- Rodríguez-Gutiérrez, G.; Rubio-Senent, F.; Lama-Muñoz, A.; Garcia, A.; Fernández-Bolaños, J. Properties of lignin, cellulose, and hemicelluloses isolated from olive cake and olive stones: Binding of water, oil, bile acids, and glucose. J. Agric. Food Chem. 2014, 62, 8973–8981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamdem, D.P.; Shen, Z.; Nabinejad, O.; Shu, Z. Development of biodegradable composite chitosan-based films incorporated with xylan and carvacrol for food packaging application. Food Packag. Shelf Life 2019, 21, 100344. [Google Scholar] [CrossRef]
- Waldron, K.W.; Parker, M.L.; Smith, A.C. Plant cell walls and food quality. Compr. Rev. Food Sci. Food Saf. 2003, 2, 128–146. [Google Scholar] [CrossRef]
- Wang, X.; Pan, Y.; Yuan, H.; Su, M.; Shao, C.; Liu, C.; Guo, Z.; Shen, C.; Liu, X. Simple fabrication of superhydrophobic PLA with honeycomb-like structures for high-efficiency oil-water separation. Chin. Chem. Lett. 2020, 31, 365–368. [Google Scholar] [CrossRef]
- Bhasney, S.M.; Patwa, R.; Kumar, A.; Katiyar, V. Plasticizing effect of coconut oil on morphological, mechanical, thermal, rheological, barrier, and optical properties of poly(lactic acid): A promising candidate for food packaging. J. Appl. Polym. Sci. 2017, 134, 45390. [Google Scholar] [CrossRef]
- Roda-Serrat, M.C.; Andrade, T.A.; Rindom, J.; Lund, P.B.; Norddahl, B.; Errico, M. Optimization of the recovery of anthocyanins from chokeberry juice pomace by homogenization in acidified water. Waste Biomass Valorization 2020, 1–13. [Google Scholar] [CrossRef]
- Murata, M. Browning and pigmentation in food through the Maillard reaction. Glycoconj. J. 2020, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mofokeng, J.P.; Luyt, A.S.; Tábi, T.; Kovács, J. Comparison of injection moulded, natural fibre-reinforced composites with PP and PLA as matrices. J. Thermoplast. Compos. Mater. 2012, 25, 927–948. [Google Scholar] [CrossRef]
- Muthuraj, R.; Misra, M.; Mohanty, A.K. Biodegradable biocomposites from poly (butylene adipate-co-terephthalate) and miscanthus: Preparation, compatibilization, and performance evaluation. J. Appl. Polym. Sci. 2017, 134, 45448. [Google Scholar] [CrossRef]
- Cichosz, S.; Masek, A. Thermal Behavior of Green Cellulose-Filled Thermoplastic Elastomer Polymer Blends. Molecules 2020, 25, 1279. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.S.; Yang, H.S.; Kim, H.J.; Lee, B.J.; Hwang, T.S. Thermal properties of agro-flour-filled biodegradable polymer bio-composites. J. Therm. Anal. Calorim. 2005, 81, 299–306. [Google Scholar] [CrossRef]
- Yang, S.; Tang, Y.; Wang, J.; Kong, F.; Zhang, J. Surface treatment of cellulosic paper with starch-based composites reinforced with nanocrystalline cellulose. Ind. Eng. Chem. Res. 2014, 53, 13980–13988. [Google Scholar] [CrossRef]
- Neves, A.C.C.; Rohen, L.A.; Mantovani, D.P.; Carvalho, J.P.; Vieira, C.M.F.; Lopes, F.P.; Simonassi, N.T.; da Luz, F.S.; Monteiro, S.N. Comparative mechanical properties between biocomposites of Epoxy and polyester matrices reinforced by hemp fiber. J. Mater. Res. Technol. 2020, 9, 1296–1304. [Google Scholar] [CrossRef]



| Sample Acronym | Composition | Process Temperature (°C) |
|---|---|---|
| ChPR | (10, 20, 30 wt%) Chokeberry Pomace + (83, 73, 63 wt%) Rapeseed meal +7 wt% microcrystalline cellulose | 130; 160 |
| BPR | (10, 20, 30 wt%) Blackcurrant Pomace + (83, 73, 63 wt%) Rapeseed meal + 7 wt% microcrystalline cellulose | 130; 160 |
| APR | (10, 20, 30 wt%) Apple Pomace + (83, 73, 63 wt%) Rapeseed meal +7 wt% microcrystalline cellulose | 130; 160 |
| RPR | (10, 20, 30 wt%) Raspberry Pomace + (83, 73, 63 wt%) Rapeseed meal +7 wt% microcrystalline cellulose | 130; 160 |
| Control 1 | 93 wt% Rapeseed meal + 7 wt% microcrystalline cellulose | 130 |
| Control 2 | 93 wt% Rapeseed meal + 7 wt% microcrystalline cellulose | 160 |
| Control 3 * | 93 wt% Rapeseed meal + 7 wt% microcrystalline cellulose | ΔE colour 130_160 °C |
| Size Range | Mass Percent (wt%) | ||||
|---|---|---|---|---|---|
| Rapeseed Meal | Chokeberry Pomace | Blackcurrant Pomace | Apple Pomace | Raspberry Pomace | |
| 1.6–1.0 mm | 1.94 | 2.45 | 3.08 | 3.57 | 4.60 |
| 1.0–0.8 mm | 10.35 | 12.29 | 10.94 | 11.19 | 9.13 |
| 0.8–0.71 mm | 5.49 | 6.58 | 7.51 | 7.55 | 6.51 |
| 0.71–0.5 mm | 22.61 | 21.84 | 21.57 | 22.64 | 25.60 |
| 0.5–0.25 mm | 51.73 | 47.88 | 50.11 | 47.76 | 46.92 |
| <0.25 mm | 7.01 | 8.19 | 6.61 | 6.90 | 6.49 |
| Total mass accounted for | 99.1 | 99.2 | 99.8 | 99.6 | 99.2 |
| Samples | Process Temperature 130 °C | Process Temperature 160 °C | ||||
|---|---|---|---|---|---|---|
| L* | a* | b* | L* | a* | b* | |
| ChPR (10_130) | 10.80 (±0.51) | −0.08 (±0.0052) | 1.78 (±0.085) | 9.49 (±0.81) | −0.06 (±0.0072) | 1.36 (±0.095) |
| ChPR (20_130) | 9.33 (±0.82) | −0.12 (±0.0096) | 1.44 (±0.073) | 8.55 (±0.53) | 0.01(±0.0042) | 1.13(±0.083) |
| ChPR (30_130) | 9.04 (±0.88) | −0.04 (±0.0062) | 1.38 (±0.43) | 9.80 (±0.72) | −0.01(±0.0082) | 1.35(±0.095) |
| APR (10_130) | 12.09 (±0.51) | −0.08 (±0.0048) | 2.06 (±0.051) | 9.77(±0.63) | −0.26(±0.0078) | 1.66(±0.055) |
| APR (20_130) | 11.39 (±1.25) | 0.13 (±0.0062) | 2.12(±0.047) | 10.24 (±0.25) | −0.13 (±0.0082) | 1.67(±0.021) |
| APR (30_130) | 11.19 (±0.42) | 0.10 (±0.0092) | 2.05 (±0.045) | 10.07(±0.91) | −0.13 (±0.012) | 1.52(±0.085) |
| RPR (10_130) | 11.72 (±0.33) | −0.07 (±0.0031) | 2.11(±0.40) | 9.20 (±0.74) | −0.09(±0.0033) | 1.45 (±0.11) |
| RPR (20_130) | 11.67 (±1.12) | −0.16 (±0.0102) | 2.00(±0.14) | 9.40 (±0.66) | −0.08(±0.0093) | 1.63(±0.091) |
| RPR (30_130) | 10.72 (±0.91) | 0.08 (±0.0022) | 2.49 (±0.094) | 10.28 (±0.81) | −0.18 (±0.0079) | 1.68(±0.099) |
| BPR (10_130) | 10.65 (±0.38) | 0.02 (±0.001) | 1.61(±0.075) | 9.40 (±0.28) | −0.09 (±0.0092) | 1.42(±0.088) |
| BPR (20_130) | 9.83 (±0.43) | −0.03(±0.0012) | 1.48(±0.11) | 8.65(±0.64) | −0.02 (±0.002) | 1.40(±0.094) |
| BPR (30_130) | 9.63 (±0.30) | −0.04(±0.0043) | 1.29(±0.025) | 8.72(±0.49) | −0.06 (±0.0031) | 1.28(±0.10) |
| Control 1 | 15.47(±0.13) | −0.32 (±0.0093) | 2.87(±0.014) | - | - | - |
| Control 2 | - | - | - | 9.33 (±0.11) | −0.03 (±0.001) | 1.31(±0.014) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Żelaziński, T. Properties of Biocomposites from Rapeseed Meal, Fruit Pomace and Microcrystalline Cellulose Made by Press Pressing: Mechanical and Physicochemical Characteristics. Materials 2021, 14, 890. https://doi.org/10.3390/ma14040890
Żelaziński T. Properties of Biocomposites from Rapeseed Meal, Fruit Pomace and Microcrystalline Cellulose Made by Press Pressing: Mechanical and Physicochemical Characteristics. Materials. 2021; 14(4):890. https://doi.org/10.3390/ma14040890
Chicago/Turabian StyleŻelaziński, Tomasz. 2021. "Properties of Biocomposites from Rapeseed Meal, Fruit Pomace and Microcrystalline Cellulose Made by Press Pressing: Mechanical and Physicochemical Characteristics" Materials 14, no. 4: 890. https://doi.org/10.3390/ma14040890






