Dentin Phosphophoryn-Derived Peptide Promotes Odontoblast Differentiation In Vitro and Dentin Regeneration In Vivo
Abstract
1. Introduction
2. Materials and Methods
2.1. DPP-Derived RGD-Containing Peptide Synthesis
2.2. Cell Culture
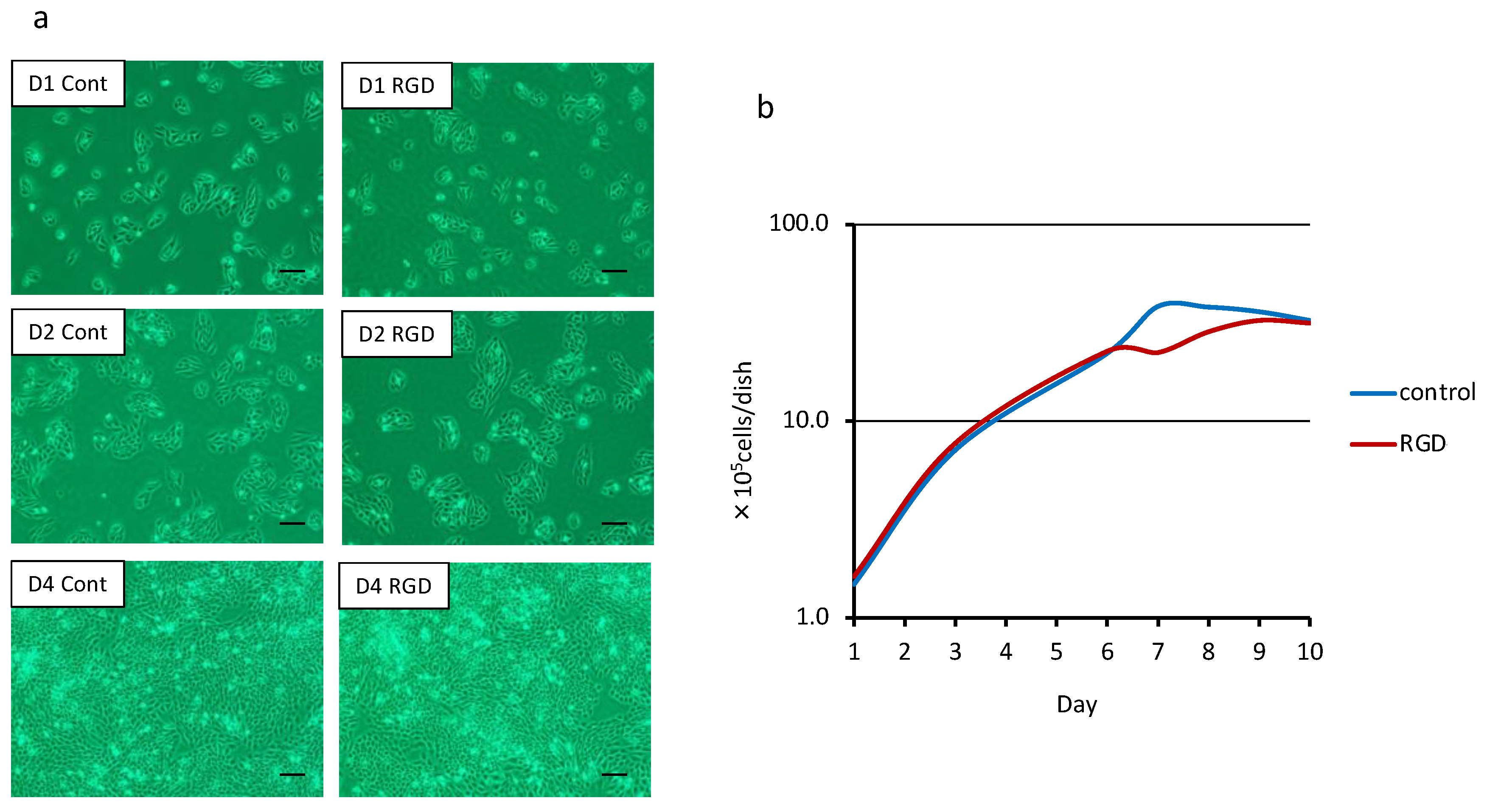
2.3. Cell Morphology Observation and Cell Number Determination
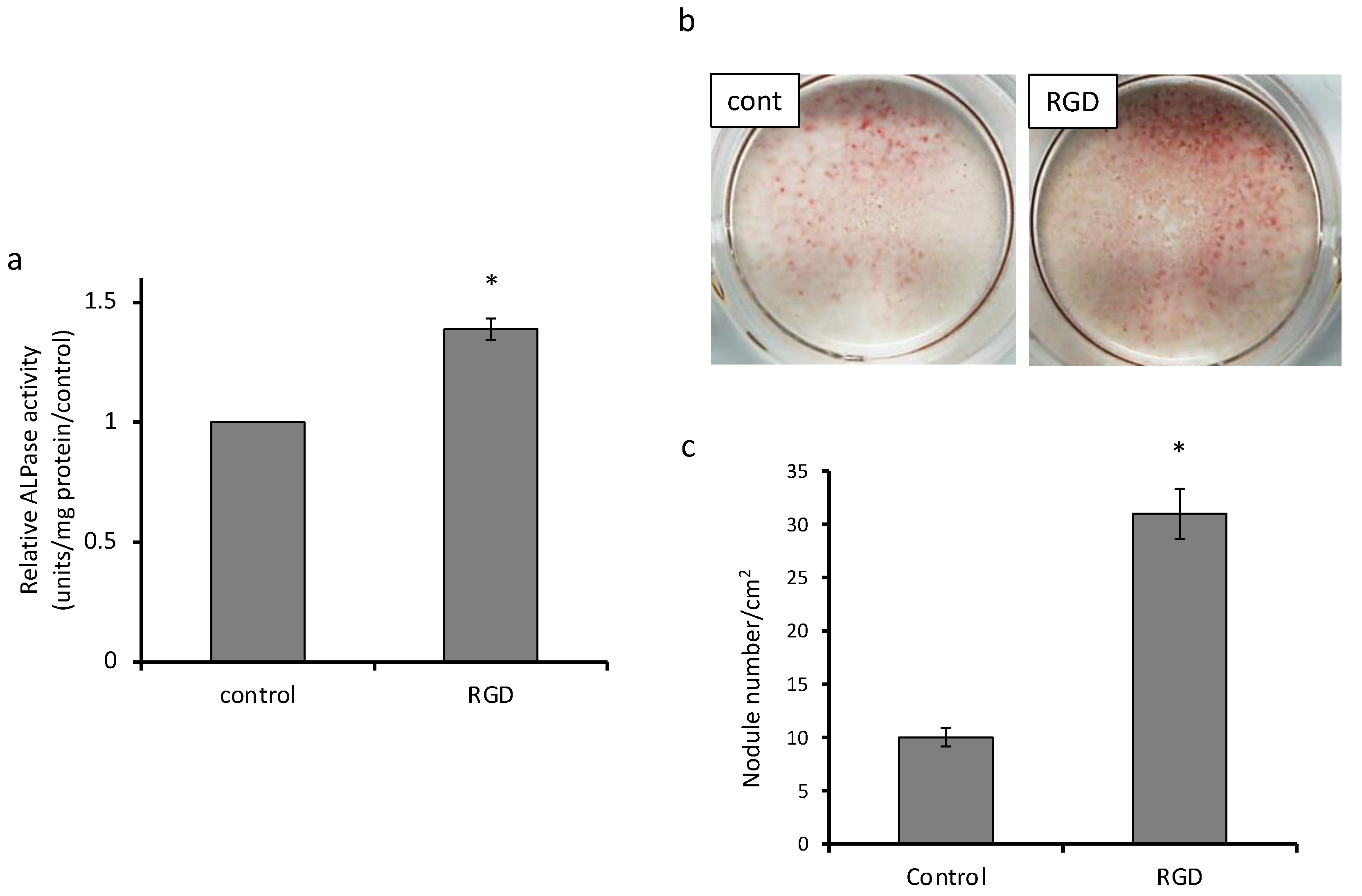
2.4. Alkaline Phosphatase (ALP) Activity Assay
2.5. Alizarin Red S Staining
2.6. Surgical Procedure in Animal Experiments
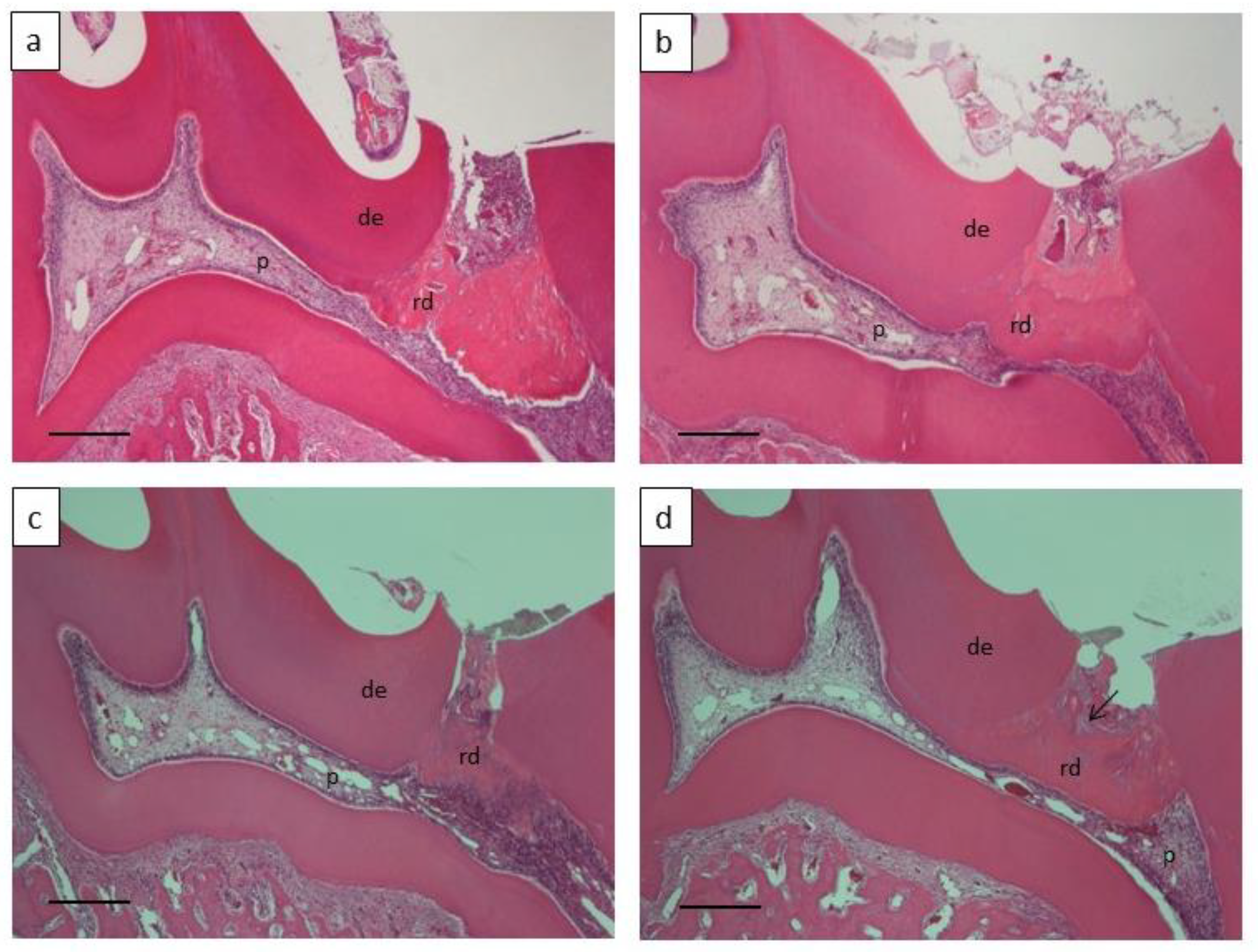
2.7. Histological Examination
2.8. Morphometrical Analysis of the Formed Reparative Dentin and Evaluation of Pulp Inflammation
2.9. Statistical Analyses
3. Results
3.1. Cell Morphology and Proliferation
3.2. Cell Differentiation and Mineralization
3.3. Dentin Regeneration Experiment in Rats
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Langer, R.S.; Vacanti, J.P. Advances in tissue engineering. J. Pediatr. Surg. 2016, 51, 8–12. [Google Scholar] [CrossRef]
- Aamodt, J.M.; Grainger, D.W. Extracellular matrix-based biomaterial scaffolds and the host response. Biomaterials 2016, 86, 68–82. [Google Scholar] [CrossRef]
- Badylak, S.F.; Freytes, D.O.; Gilbert, T.W. Extracellular matrix as a biological scaffold material: Structure and function. Acta Biomater. 2009, 5, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Badylak, S.F.; Taylor, D.; Uygun, K. Whole-Organ Tissue Engineering: Decellularization and Recellularization of Three-Dimensional Matrix Scaffolds. Annu. Rev. Biomed. Eng. 2011, 13, 27–53. [Google Scholar] [CrossRef]
- Anderson, J.M.; Rodriguez, A.; Chang, D.T. Foreign body reaction to biomaterials. Semin. Immunol. 2008, 20, 86–100. [Google Scholar] [CrossRef]
- Badylak, S.F. Xenogeneic extracellular matrix as a scaffold for tissue reconstruction. Transpl. Immunol. 2004, 12, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Oshima, M.; Tsuji, T. Whole Tooth Regeneration as a Future Dental Treatment. Taurine 6 2015, 881, 255–269. [Google Scholar] [CrossRef]
- Ritchie, H.H. The functional significance of dentin sialoprotein-phosphophoryn and dentin sialoprotein. Int. J. Oral Sci. 2018, 10, 31. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, H.H.; Yee, C.T.; Tang, X.-N.; Dong, Z.; Fuller, R.S. DSP-PP Precursor Protein Cleavage by Tolloid-Related-1 Protein and by Bone Morphogenetic Protein-1. PLoS ONE 2012, 7, e41110. [Google Scholar] [CrossRef]
- Fisher, L.W.; Fedarko, N.S. Six genes expressed in bones and teeth encode the current members of the SIBLING family of proteins. Connect. Tissue Res. 2003, 44, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Prasad, M.; Butler, W.T.; Qin, C. Dentin sialophosphoprotein in biomineralization. Connect. Tissue Res. 2010, 51, 404–417. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Yamauchi, M.; Abiko, Y.; Matsuda, K.; Crenshaw, M.A. In Vitro Apatite Induction by Phosphophoryn Immobilized on Modified Collagen Fibrils. J. Bone Miner. Res. 2000, 15, 1615–1619. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Saito, T. Effect of dentine phosphophoryn-derived RGD peptides on odontoblast-like cells. Int. Endod. J. 2015, 49, 670–683. [Google Scholar] [CrossRef] [PubMed]
- Eapen, A.; George, A. Dentin phosphophoryn in the matrix activates AKT and mTOR signaling pathway to promote pre-odontoblast survival and differentiation. Front. Physiol. 2015, 6, 221. [Google Scholar] [CrossRef][Green Version]
- Koike, T.; Polan, M.A.A.; Izumikawa, M.; Saito, T. Induction of Reparative Dentin Formation on Exposed Dental Pulp by Dentin Phosphophoryn/Collagen Composite. BioMed Res. Int. 2014, 2014, 745139. [Google Scholar] [CrossRef]
- Jadlowiec, J.; Koch, H.; Zhang, X.; Campbell, P.G.; Seyedain, M.; Sfeir, C. Phosphophoryn Regulates the Gene Expression and Differentiation of NIH3T3, MC3T3-E1, and Human Mesenchymal Stem Cells via the Integrin/MAPK Signaling Pathway. J. Biol. Chem. 2004, 279, 53323–53330. [Google Scholar] [CrossRef]
- Linde, A.; Lussi, A.; Crenshaw, M.A. Mineral induction by immobilized polyanionic proteins. Calcif. Tissue Int. 1989, 44, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Crenshaw, M.A.; Matsuda, K. Role of Phosphophoryn Free in Solution in Biomineralization In Vitro. J. Hard Tissue Biol. 2003, 12, 6–10. [Google Scholar] [CrossRef][Green Version]
- Marsh, M.E. Self-association of calcium and magnesium complexes of dentin phosphophoryn. Biochemisty 1989, 28, 339–345. [Google Scholar] [CrossRef]
- Hayashi, K.; Handa, K.; Koike, T.; Saito, T. The possibility of genistein as a new direct pulp capping agent. Dent. Mater. J. 2013, 32, 976–985. [Google Scholar] [CrossRef]
- Scribante, A.; Dermenaki-Farahani, M.R.; Marino, G.; Matera, C.; Rodriguez, Y.; Baena, R.; Lanteri, V.; Butera, A. Biomimetic effect of nano-hydroxyapatite in demineralized enamel before orthodontic bonding of brackets and attachments: Visual, adhesion strength, and hardness in in vitro tests. BioMed Res. Int. 2020, 2020, 6747498. [Google Scholar] [CrossRef] [PubMed]
- Bächli, K.; Schmidlin, P.R.; Wegehaupt, F.J.; Paqué, F.; Ramenzoni, L.L.; Botter, S.M. Remineralization of Artificial Dentin Caries Using Dentin and Enamel Matrix Proteins. Materials 2019, 12, 2116. [Google Scholar] [CrossRef] [PubMed]
- Ono, M.; Oshima, M.; Ogawa, M.; Sonoyama, W.; Hara, E.S.; Oida, Y.; Shinkawa, S.; Nakajima, R.; Mine, A.; Hayano, S.; et al. Practical whole-tooth restoration utilizing autologous bioengineered tooth germ transplantation in a postnatal canine model. Sci. Rep. 2017, 7, srep44522. [Google Scholar] [CrossRef] [PubMed]
- Yelick, P.; Sharpe, P. Tooth Bioengineering and Regenerative Dentistry. J. Dent. Res. 2019, 98, 1173–1182. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Ren, J.; Li, R.; Guan, C.; Feng, Z.; Bao, B.; Wang, W.; Zhou, C. Tooth regeneration: Insights from tooth devel-opment and spatial-temporal control of bioactive drug release. Stem Cell Rev. Rep. 2020, 16, 41–55. [Google Scholar] [CrossRef]
- Suzuki, S.; Kobuke, S.; Haruyama, N.; Hoshino, H.; Kulkarni, A.B.; Nishimura, F. Adhesive and migratory effects of phos-phophoryn are modulated by flanking peptides of the integrin binding motif. PLoS ONE 2014, 9, e112490. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Tabata, Y. Experimental tissue regeneration by DDS technology of bio-signaling molecules. J. Dermatol. Sci. 2007, 47, 189–199. [Google Scholar] [CrossRef]
- Meyer, M. Processing of collagen based biomaterials and the resulting materials properties. Biomed. Eng. Online 2019, 18, 1–74. [Google Scholar] [CrossRef]
- Trivedi, S.; Srivastava, K.; Saluja, T.S.; Shyam, H.; Kumar, S.; Singh, A.; Saxena, S.K.; Mehrotra, D.; Singh, S.K. Hydroxyap-atite–collagen augments osteogenic differentiation of dental pulp stem cells. Odontology 2019, 108, 251–259. [Google Scholar] [CrossRef]
- Ramesh, N.; Moratti, S.C.; Dias, G.J. Hydroxyapatite-polymer biocomposites for bone regeneration: A review of current trends. J. Biomed. Mater. Res. Part. B Appl. Biomater. 2018, 106, 2046–2057. [Google Scholar] [CrossRef]

| Degrees of Pulp Inflammation | Score |
|---|---|
| Minimal inflammation (i.e., no or few scattered inflammatory cells are present in the pulp, similar to the normal dental pulp) | 4 |
| Mild inflammation (i.e., a little vasodilation is present, indicating mild hyperemia) | 3 |
| Moderate inflammation (i.e., weak vasodilation is present without infiltration of blood cells into the dental pulp, but some inflammatory cells—such as polymorphonuclear leukocytes and neutrophils—can be observed) | 2 |
| Severe inflammation (i.e., intense vasodilation is present, appearing as an abscess, and significant inflammatory infiltration of polymorphonuclear leukocytes and neutrophils can be seen throughout the crown) | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altankhishig, B.; Polan, M.A.A.; Qiu, Y.; Hasan, M.R.; Saito, T. Dentin Phosphophoryn-Derived Peptide Promotes Odontoblast Differentiation In Vitro and Dentin Regeneration In Vivo. Materials 2021, 14, 874. https://doi.org/10.3390/ma14040874
Altankhishig B, Polan MAA, Qiu Y, Hasan MR, Saito T. Dentin Phosphophoryn-Derived Peptide Promotes Odontoblast Differentiation In Vitro and Dentin Regeneration In Vivo. Materials. 2021; 14(4):874. https://doi.org/10.3390/ma14040874
Chicago/Turabian StyleAltankhishig, Bayarchimeg, Mohammad Ali Akbor Polan, Youjing Qiu, Md Riasat Hasan, and Takashi Saito. 2021. "Dentin Phosphophoryn-Derived Peptide Promotes Odontoblast Differentiation In Vitro and Dentin Regeneration In Vivo" Materials 14, no. 4: 874. https://doi.org/10.3390/ma14040874
APA StyleAltankhishig, B., Polan, M. A. A., Qiu, Y., Hasan, M. R., & Saito, T. (2021). Dentin Phosphophoryn-Derived Peptide Promotes Odontoblast Differentiation In Vitro and Dentin Regeneration In Vivo. Materials, 14(4), 874. https://doi.org/10.3390/ma14040874









