Correlation between Microstructure and Hydrogen Degradation of 690 MPa Grade Marine Engineering Steel
Abstract
1. Introduction
2. Experimental
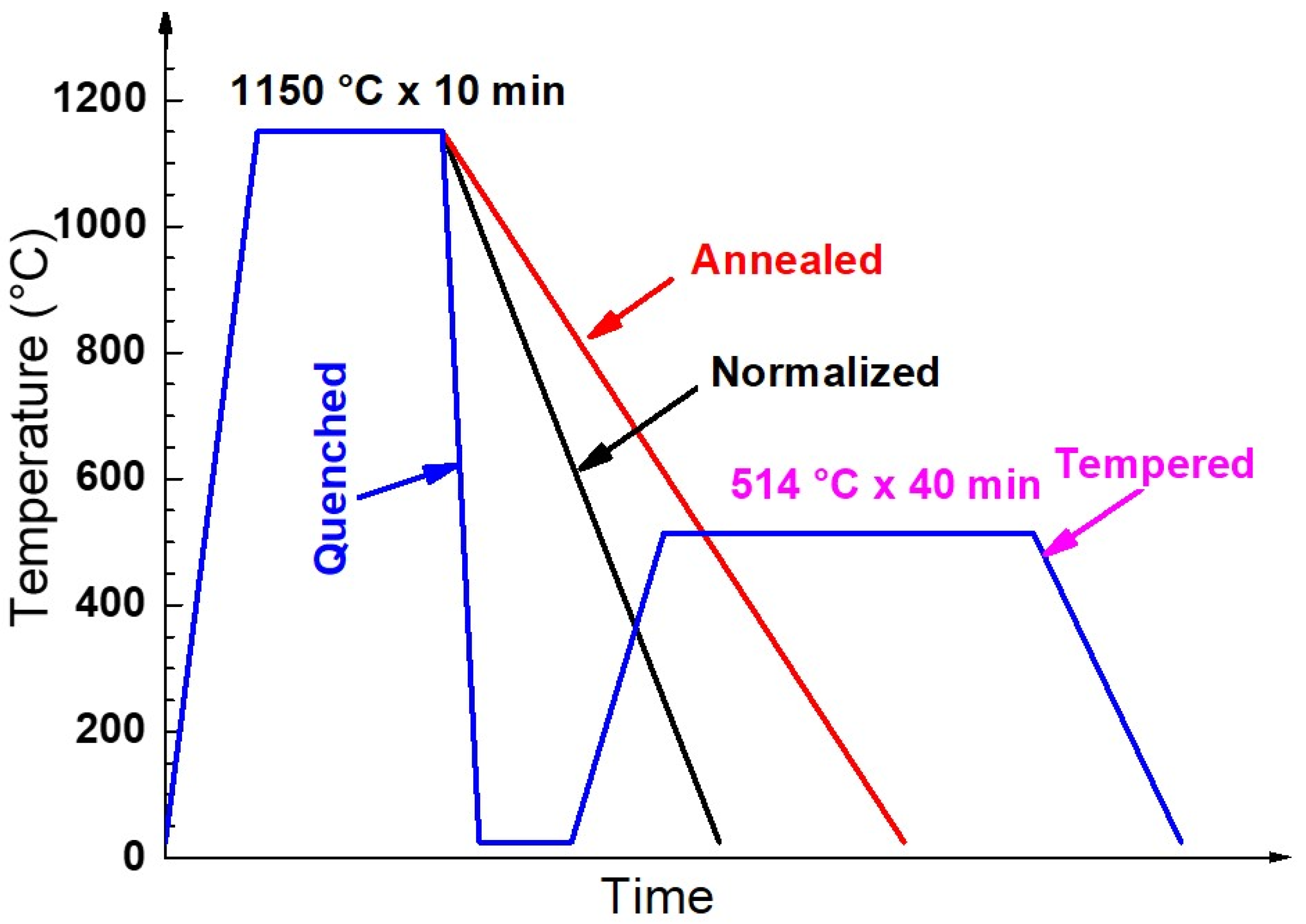
2.1. Materials
2.2. Microstructure Characterization and Microhardness Test
2.3. Electrochemical Test
2.4. Hydrogen Permeation Test
2.5. Hydrogen-Induced Cracking Test
2.6. Slow Strain Rate Tensile Test (SSRT)
3. Results and Discussion
3.1. Microstructure
3.2. Electrochemical Behavior
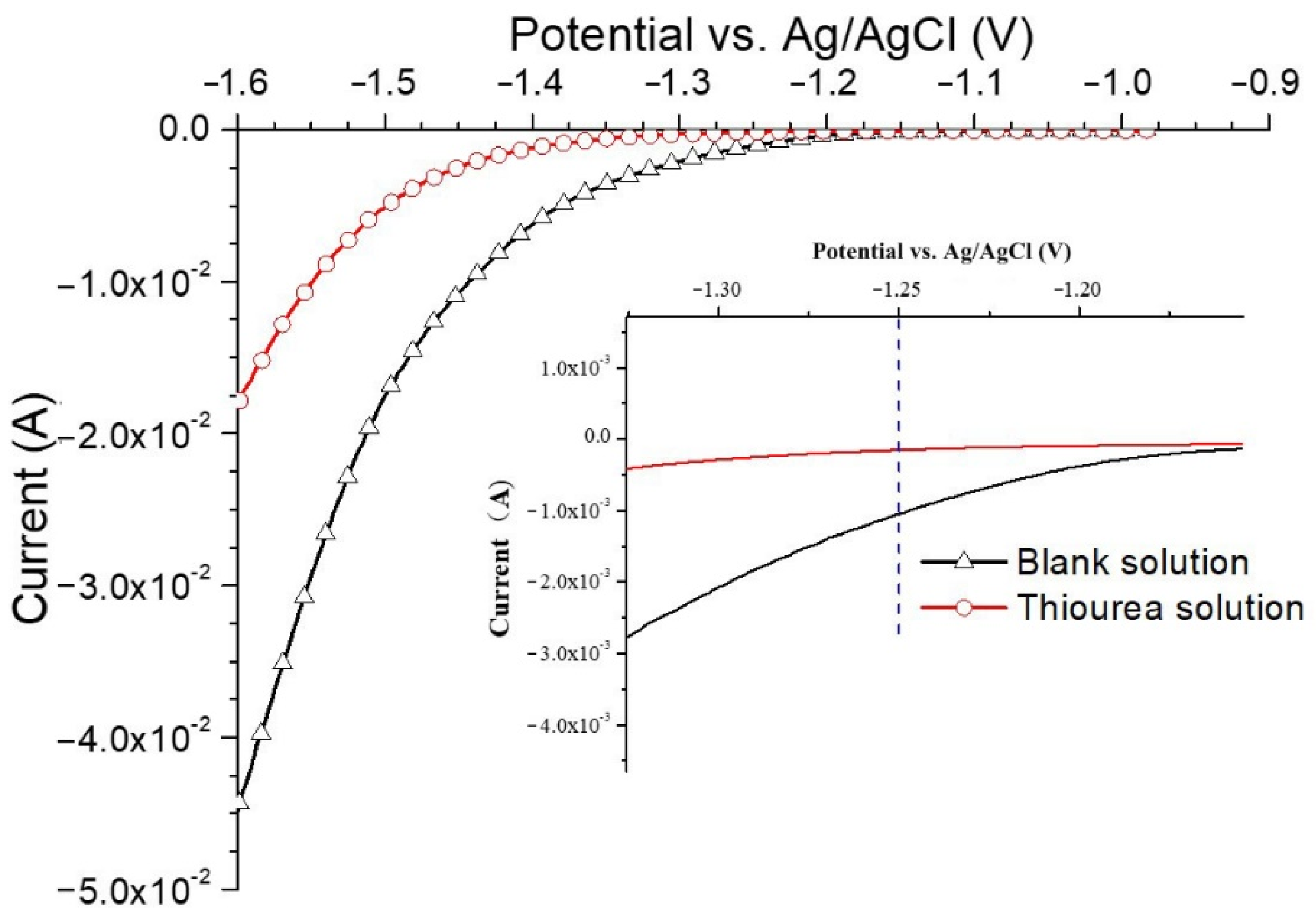
3.2.1. Effects of Thiourea on the Electrochemical Behavior
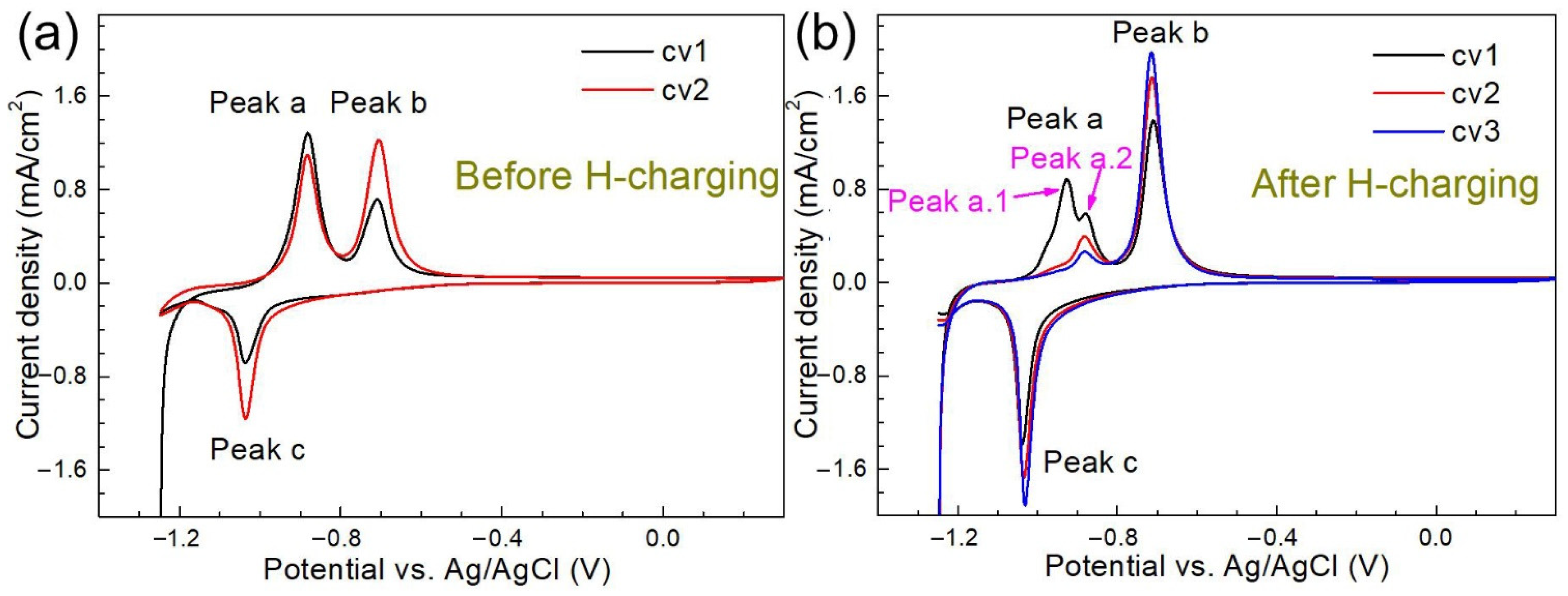
3.2.2. Effects of H charging on the Electrochemical Behavior
3.2.3. Effects of Microstructure on the Electrochemical Behavior
3.3. Hydrogen Permeation Behavior
3.4. HIC Analysis
4. Conclusions
- (1)
- The CV tests show that thiourea is an effective hydrogen permeation accelerator. The hydrogen diffusion rate in the steel base metal with uniform microstructure and fine grain is the highest, while that in the annealed steel with ferrite and pearlite microstructure is the lowest.
- (2)
- The hydrogen-induced cracks in the steel base metal show obvious step shape and tiny cracks near the main crack. The cracks of the annealed steel are mainly distributed along pearlite. The crack propagation of martensite steel (quenched steel) is mainly transgranular, while the cracks of tempered steel along the prior austenite grain boundary.
- (3)
- HIC sensitivity of the base metal is the lowest due to its low hydrogen flux and apparent hydrogen concentration. Annealed steel exhibits higher HIC sensitivity at lower hydrogen diffusion flux and surface hydrogen concentration, due to many hydrogen traps in annealed steel. Annealed steel with a ferrite and pearlite microstructure is more susceptible to hydrogen.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ozdirik, B.; Baert, K.; Depover, T.; Vereecken, J.; Verbeken, K.; Terryn, H.; De Graeve, I. Development of an electrochemical procedure for monitoring hydrogen sorption/desorption in steel. J. Electrochem. Soc. 2017, 164, C747–C757. [Google Scholar] [CrossRef]
- Pressouyre, G.A. Classification of hydrogen traps in steel. Metall. Trans. A 1979, 10, 1571–1573. [Google Scholar] [CrossRef]
- Michler, T.; Balogh, M.P. Hydrogen environment embrittlement of an ODS RAF steel—Role of irreversible hydrogen trap sites. Int. J. Hydrogen Energy 2010, 35, 9746–9754. [Google Scholar] [CrossRef]
- Grabke, H.J.; Gehrmann, F.; Riecke, E. Hydrogen in microalloyed steels. Steel. Res. 2001, 72, 225–235. [Google Scholar] [CrossRef]
- Ćwiek, J. Prevention methods against hydrogen degradation of steel. J. Achiev. Mater. Manuf. Eng. 2010, 43, 214–221. [Google Scholar]
- Louthan, M. Hydrogen embrittlement of metals: A primer for the failure analyst. J. Fail. Anal. Prev. 2008, 8, 289–307. [Google Scholar] [CrossRef]
- Manuel, Q.L.; Noe, R.J.; Rosario, D.Y.D.; Ariadna, A.I.; Vicente, G.F.; Icoquih, Z.P. Analysis of the physicochemical, mechanical, and electrochemical parameters and their impact on the internal and external SCC of carbon steel pipelines. Materials 2020, 13, 5771. [Google Scholar]
- Wang, L.; Liang, J.; Li, H.; Cheng, L.; Cui, Z. Quantitative study of the corrosion evolution and stress corrosion cracking of high strength aluminum alloys in solution and thin electrolyte layer containing Cl. Corros. Sci. 2021, 178, 109076. [Google Scholar] [CrossRef]
- Magudeeswaran, G.; Balasubramanian, V.; Madhusudhanreddy, G. Hydrogen induced cold cracking studies on armour grade high strength, quenched and tempered steel weldments. Int. J. Hydrogen Energy 2008, 33, 1897–1908. [Google Scholar] [CrossRef]
- Walton, H.W. The Influence of Residual Stresses on the Susceptibility to Hydrogen Embrittlement in Hardened Steel Components Subjected to Rolling Contact Conditions; 2002-01-1412; SAE International: Warrendale, PA, USA, 2002. [Google Scholar]
- Gangloff, R.P.; Somerday, B.P. Gaseous Hydrogen Embrittlement of Materials in Energy Technologies: The Problem, Its Characterisation and Effects on Particular Alloy Classes; Woodhead: Cambridge, UK, 2012. [Google Scholar]
- Quadrini, E. Study of the effect of heat treatment on hydrogen embrittlement of AISI 4340 steel. J. Mater. Sci. 1989, 24, 915–920. [Google Scholar] [CrossRef]
- Nagao, A.; Martin, M.L.; Dadfarnia, M.; Sofronis, P.; Robertson, I.M. The effect of nanosized (Ti, Mo) C precipitates on hydrogen embrittlement of tempered lath martensitic steel. Acta Mater. 2014, 74, 244–254. [Google Scholar] [CrossRef]
- Mohammadi, F.; Eliyan, F.F.; Alfantazi, A. Corrosion of simulated weld HAZ of API X-80 pipeline steel. Corros. Sci. 2012, 63, 323–333. [Google Scholar] [CrossRef]
- Depover, T.; Wallaert, E.; Verbeken, K. Fractographic analysis of the role of hydrogen diffusion on the hydrogen embrittlement susceptibility of DP steel. Mater. Sci. Eng. A 2016, 649, 201–208. [Google Scholar] [CrossRef]
- Venezuela, J.; Zhou, Q.; Liu, Q.; Zhang, M.; Atrens, A. Hydrogen Trapping in some automotive martensitic advanced high-strength steels. Adv. Eng. Mater. 2018, 20, 1700468. [Google Scholar] [CrossRef]
- Malina, J.; Begićhadžipašić, A.; Nižnik, Š. Electrochemical corrosion and hydrogen diffusivity in dual phase steel. Zaštita Materijala 2013, 54, 130–134. [Google Scholar]
- Avramovic-Cingara, G.; Ososkov, Y.; Jain, M.; Wilkinson, D. Effect of martensite distribution on damage behaviour in DP600 dual phase steels. Mater. Sci. Eng. A 2009, 516, 7–16. [Google Scholar] [CrossRef]
- Luppo, M.; Ovejero-Garcia, J. The influence of microstructure on the trapping and diffusion of hydrogen in a low carbon steel. Corros. Sci. 1991, 32, 1125–1136. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, H.; Zhou, Z.; Li, X.; Wu, W.; Gong, J. Effect of shot peening coverage on hydrogen embrittlement of a ferrite-pearlite steel. Int. J. Hydrogen Energy 2020, 45, 7169–7184. [Google Scholar] [CrossRef]
- Tsay, L.; Chi, M.; Wu, Y.; Wu, J.; Lin, D.-Y. Hydrogen embrittlement susceptibility and permeability of two ultra-high strength steels. Corros. Sci. 2006, 48, 1926–1938. [Google Scholar] [CrossRef]
- Capelle, J.; Dmytrakh, I.; Pluvinage, G. Comparative assessment of electrochemical hydrogen absorption by pipeline steels with different strength. Corros. Sci. 2010, 52, 1554–1559. [Google Scholar] [CrossRef]
- Escobar, D.P.; Depover, T.; Duprez, L.; Verbeken, K.; Verhaege, M. Combined thermal desorption spectroscopy, differential scanning calorimetry, scanning electron microscopy and X-ray diffraction study of hydrogen trapping in cold deformed TRIP steel. Acta Mater. 2012, 60, 2593–2605. [Google Scholar] [CrossRef]
- Depover, T.; Wallaert, E.; Verbeken, K. On the synergy of diffusible hydrogen content and hydrogen diffusivity in the mechanical degradation of laboratory cast Fe-C alloys. Mater. Sci. Eng. A 2016, 664, 195–205. [Google Scholar] [CrossRef]
- Padhy, G.; Ramasubbu, V.; Murugesan, N.; Ramesh, C.; Parvathavarthini, N.; Albert, S. Determination of apparent diffusivity of hydrogen in 9Cr-1MoVNbN steel using hot extraction-PEMHS technique. Int. J. Hydrogen Energy 2013, 38, 10683–10693. [Google Scholar] [CrossRef]
- Cao, F.; Wu, H.; Yang, Y.; Cao, J.; Zhang, T.; Wang, F. Study on chemical bonding between epoxy coating and metal substrate using γ-aminopropyltrimethoxysilaneto modify epoxy resin molecule. Acta Metall. Sin. 2019, 55, 238–248. [Google Scholar]
- Uluc, A.V.; Tichelaar, F.D.; Terryn, H.; Böttger, A.J. The role of heat treatment and alloying elements on hydrogen uptake in Aermet 100 ultrahigh-strength steel. J. Electroanal. Chem. 2015, 739, 130–136. [Google Scholar] [CrossRef]
- Graś, M.; Wojciechowski, J.; Lota, K.; Buchwald, T.; Ryl, J.; Lota, G. Correlation between partial inhibition of hydrogen evolution using thiourea and catalytic activity of AB5-type hydrogen storage alloy towards borohydride electrooxidation. J. Alloys Compd. 2020, 829, 154553. [Google Scholar] [CrossRef]
- Cui, Z.; Liu, Z.; Wang, L.; Li, X.; Du, C.; Wang, X. Effect of plastic deformation on the electrochemical and stress corrosion cracking behavior of X70 steel in near-neutral pH environment. Mater. Sci. Eng. A 2016, 677, 259–273. [Google Scholar] [CrossRef]
- Ma, H.; Liu, Z.; Du, C.; Li, X.; Cui, Z. Comparative study of the SCC behavior of E690 steel and simulated HAZ microstructures in a SO2-polluted marine atmosphere. Mater. Sci. Eng. A 2016, 650, 93–101. [Google Scholar] [CrossRef]
- Ming, L.; Wang, Q.; Wang, H.; Zhang, C.; Wei, Z.; Guo, A. A remarkable role of niobium precipitation in refining microstructure and improving toughness of A QT-treated 20CrMo47NbV steel with ultrahigh strength. Mater. Sci. Eng. A 2014, 613, 240–249. [Google Scholar] [CrossRef]
- Janusz, Ć. Hydrogen-degradation-of-high-strength-steels. J. Achiev. Mater. Manuf. Eng. 2009, 37, 193–212. [Google Scholar]
- Jerkiewicz, G.; Borodzinski, J.J.; Chrzanowski, W. Examination of factors influencing promotion of H absorption into metals by site-blocking elements. J. Electrochem. Soc. 1995, 142, 3755–3763. [Google Scholar] [CrossRef]
- MacDonald, D.; Roberts, B. The cyclic voltammetry of carbon steel in concentrated sodium hydroxide solution. Electrochim. Acta 1978, 23, 781–786. [Google Scholar] [CrossRef]
- Guzman, R.S.; Vilche, J.; Arvia, A. The potentiodynamic behaviour of iron in alkaline solutions. Electrochim. Acta 1979, 24, 395–403. [Google Scholar] [CrossRef]
- Hugot-Le Goff, A.; Flis, J.; Boucherit, N.; Joiret, S.; Wilinski, J. Use of Raman spectroscopy and rotating split ring disk electrode for identification of surface layers on iron in 1M NaOH. J. Electrochem. Soc. 1990, 137, 2684. [Google Scholar] [CrossRef]
- Geana, D.; El Miligy, A.; Lorenz, W. Electrochemical behaviour of iron in alkaline sulphate solutions. J. Appl. Electrochem. 1974, 4, 337–345. [Google Scholar] [CrossRef]
- Wu, W.; Liu, Z.; Li, X.; Du, C.; Cui, Z. Influence of different heat-affected zone microstructures on the stress corrosion behavior and mechanism of high-strength low-alloy steel in a sulfurated marine atmosphere. Mater. Sci. Eng. A 2019, 759, 124–141. [Google Scholar] [CrossRef]
- Zhang, T.; Zhao, W.; Deng, Q.; Jiang, W.; Wang, Y.; Wang, Y.; Jiang, W. Effect of microstructure inhomogeneity on hydrogen embrittlement susceptibility of X80 welding HAZ under pressurized gaseous hydrogen. Int. J. Hydrogen Energy 2017, 42, 25102–25113. [Google Scholar] [CrossRef]
- Tian, H.; Wang, X.; Cui, Z.; Lu, Q.; Wang, L.; Lei, L.; Li, Y.; Zhang, D. Electrochemical corrosion, hydrogen permeation and stress corrosion cracking behavior of E690 steel in thiosulfate-containing artificial seawater. Corros. Sci. 2018, 144, 145–162. [Google Scholar] [CrossRef]
- Tian, H.; Xin, J.; Li, Y.; Wang, X.; Cui, Z. Combined effect of cathodic potential and sulfur species on calcareous deposition, hydrogen permeation, and hydrogen embrittlement of a low carbon bainite steel in artificial seawater. Corros. Sci. 2019, 158, 108089. [Google Scholar] [CrossRef]
- Djukic, M.; Zeravcic, V.S.; Bakic, G.; Sedmak, A.; Rajicic, B. Hydrogen embrittlement of low carbon structural steel. Procedia Mater. Sci. 2014, 3, 1167–1172. [Google Scholar] [CrossRef]
- Arafin, M.A.; Szpunar, J.A. Effect of bainitic microstructure on the susceptibility of pipeline steels to hydrogen induced cracking. Mater. Sci. Eng. A 2011, 528, 4927–4940. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Li, X.G.; Cheng, Y.F. Mechanistic aspect of near-neutral pH stress corrosion cracking of pipelines under cathodic polarization. Corros. Sci. 2012, 55, 54–60. [Google Scholar] [CrossRef]
- Nagao, A.; Smith, C.D.; Dadfarnia, M.; Sofronis, P.; Robertson, I.M. The role of hydrogen in hydrogen embrittlement fracture of lath martensitic steel. Acta Mater. 2012, 60, 5182–5189. [Google Scholar] [CrossRef]
- Lu, Q.; Wang, L.; Xin, J.; Tian, H.; Wang, X.; Cui, Z. Corrosion evolution and stress corrosion cracking of E690 steel for marine construction in artificial seawater under potentiostatic anodic polarization. Constr. Build. Mater. 2020, 238, 117763. [Google Scholar] [CrossRef]
- Depover, T.; Verbeken, K. Hydrogen trapping and hydrogen induced mechanical degradation in lab cast Fe-C-Cr alloys. Mater. Sci. Eng. A 2016, 669, 134–149. [Google Scholar] [CrossRef]
- Galindo-Nava, E.; Basha, B.; Rivera-Díaz-del-Castillo, P. Hydrogen transport in metals: Integration of permeation, thermal desorption and degassing. J. Mater. Sci. Technol. 2017, 33, 1433–1447. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, C.; Wang, D.; Liu, J.; Li, Y.; Di, X. The mutual effect of hydrogen and cyclic plastic deformation on ductility degradation of X65 reeled-pipeline welded joint. Mater. Sci. Eng. A 2020, 791, 139739. [Google Scholar] [CrossRef]
- Pan, H.; Wang, L.; Lin, Y.; Ge, F.; Zhao, K.; Wang, X.; Cui, Z. Mechanistic study of ammonium-induced corrosion of AZ31 magnesium alloy in sulfate solution. J. Mater. Sci. Technol. 2020, 54, 1–13. [Google Scholar] [CrossRef]
- Li, G.; Wang, L.; Wu, H.; Liu, C.; Wang, X.; Cui, Z. Dissolution kinetics of the sulfide-oxide complex inclusion and resulting localized corrosion mechanism of X70 steel in deaerated acidic environment. Corros. Sci. 2020, 174, 108815. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Z.; Fan, E.; Cui, Z.; Zhao, J. The effect of crack tip environment on crack growth behaviour of a low alloy steel at cathodic potentials in artificial seawater. J. Mater. Sci. Technol. 2020, 54, 119–131. [Google Scholar] [CrossRef]
- Huang, F.; Liu, J.; Deng, Z.J.; Cheng, J.H.; Lu, Z.H.; Li, X.G. Effect of microstructure and inclusions on hydrogen induced cracking susceptibility and hydrogen trapping efficiency of X120 pipeline steel. Mater. Sci. Eng. A 2010, 527, 6997–7001. [Google Scholar] [CrossRef]
- Chatzidouros, E.V.; Papazoglou, V.J.; Tsiourva, T.E.; Pantelis, D.I. Hydrogen effect on fracture toughness of pipeline steel welds, with in situ hydrogen charging. Int. J. Hydrogen Energy 2011, 36, 12626–12643. [Google Scholar] [CrossRef]
- Mohtadi-Bonab, M.A.; Eskandari, M. A focus on different factors affecting hydrogen induced cracking in oil and natural gas pipeline steel. Eng. Fail. Anal. 2017, 79, 351–360. [Google Scholar] [CrossRef]
- Ghosh, G.; Rostron, P.; Garg, R.; Panday, A. Hydrogen induced cracking of pipeline and pressure vessel steels: A review. Eng. Fract. Mech. 2018, 199, 609–618. [Google Scholar] [CrossRef]
- Wang, S.; Martin, M.L.; Robertson, I.M.; Sofronis, P. Effect of hydrogen environment on the separation of Fe grain boundaries. Acta Mater. 2016, 107, 279–288. [Google Scholar] [CrossRef]
- Martin, M.; Somerday, B.; Ritchie, R.; Sofronis, P.; Robertson, I. Hydrogen-induced intergranular failure in nickel revisited. Acta Mater. 2012, 60, 2739–2745. [Google Scholar] [CrossRef]
- McMahon, C., Jr. Hydrogen-induced intergranular fracture of steels. Eng. Fract. Mech. 2001, 68, 773–788. [Google Scholar] [CrossRef]
- Alvaro, A.; Olden, V.; Akselsen, O.M. 3D cohesive modelling of hydrogen embrittlement in the heat affected zone of an X70 pipeline steel—Part II. Int. J. Hydrogen Energy 2014, 39, 3528–3541. [Google Scholar] [CrossRef]
- Laurent, J.; Lapasset, G.; Aucouturier, M.; Lacombe, P. Use of electron high resolution autoradiography in studying hydrogen embrittlement. In Hydrogen in Metals; Berlin/Heidelberg, Germany, 1974. [Google Scholar]


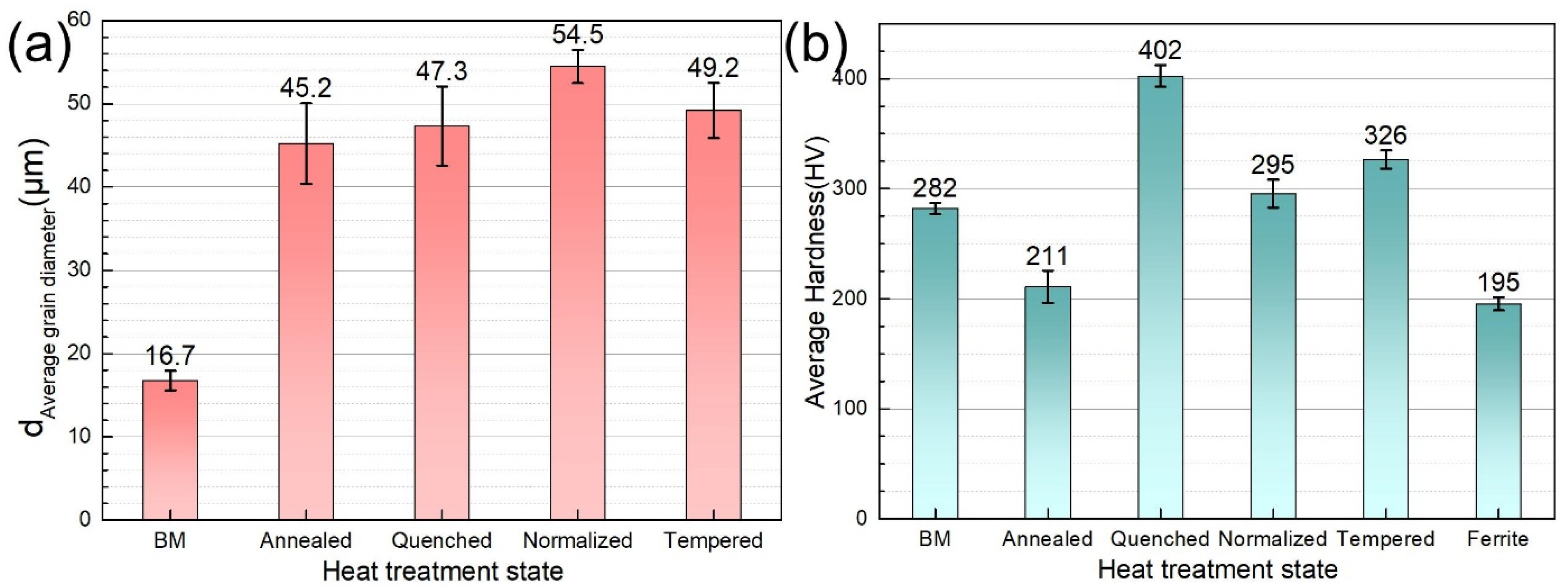


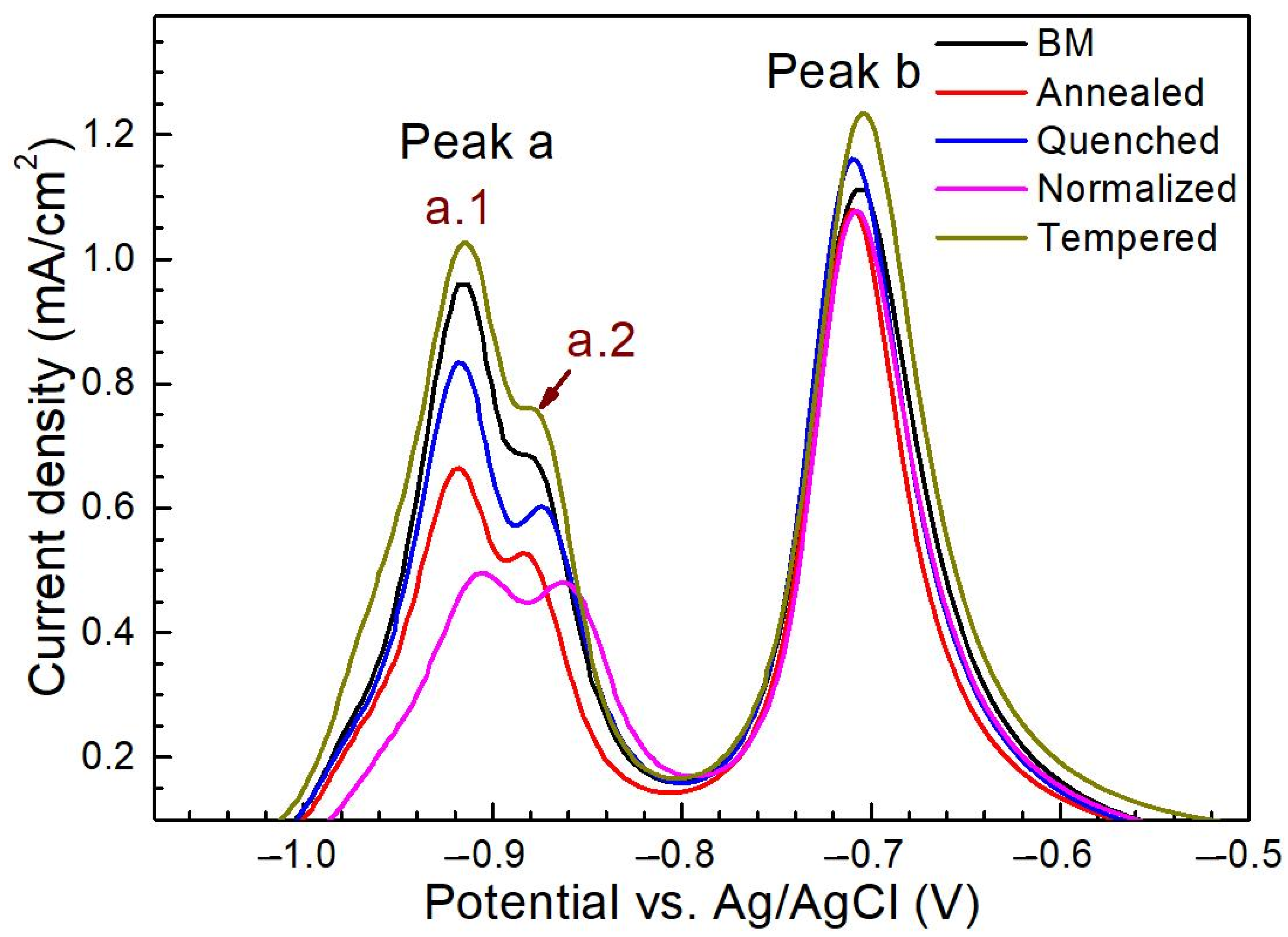
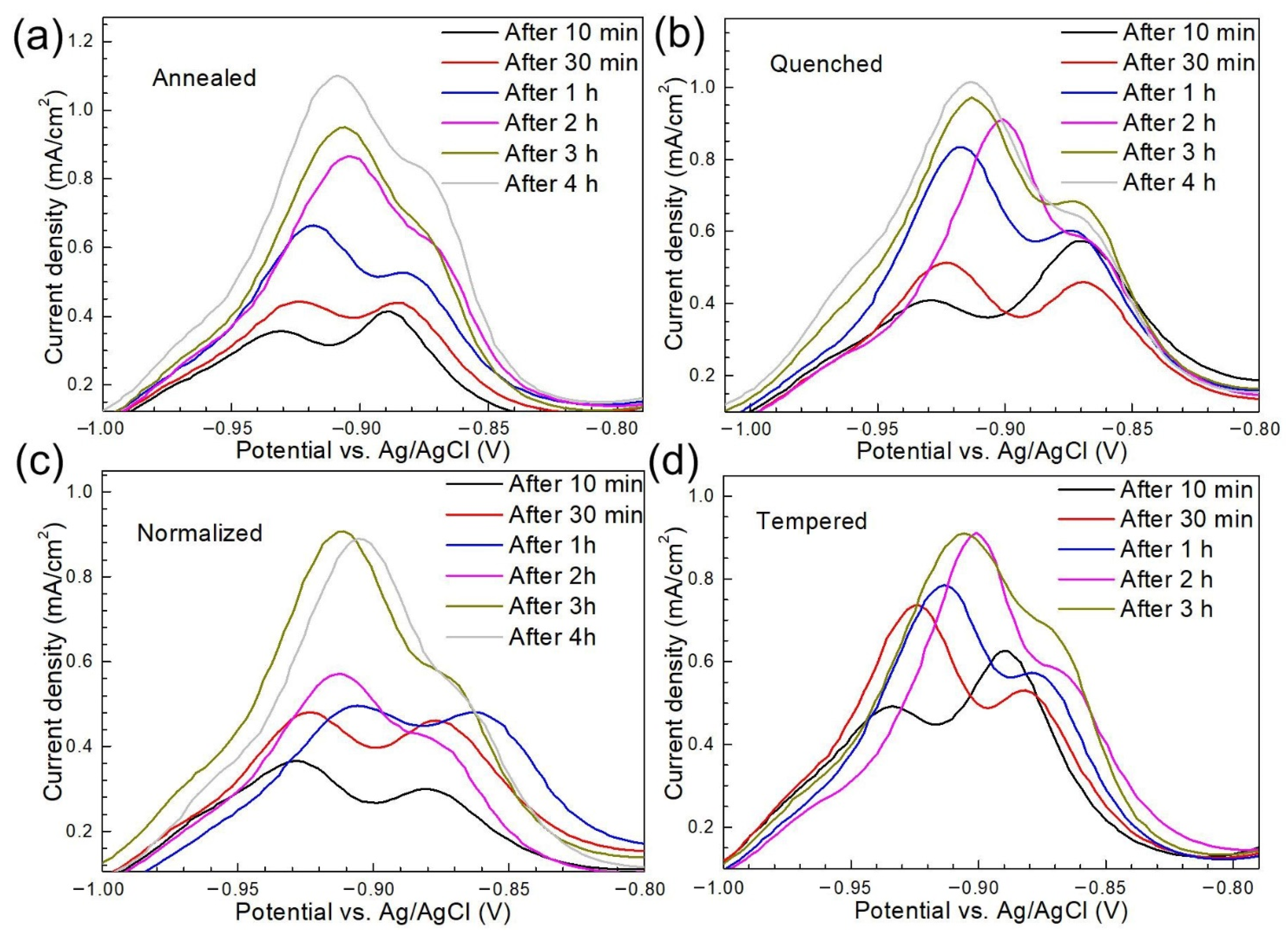




| Material | Microstructure Morphology | Average Grain Diameter (μm) | The Absorbed H-Concentration (×10−6 mol cm−3) | J (mol cm2·s−1) | Capp (mol cm−1) | Crack Patterns | HIC Sensitivity |
|---|---|---|---|---|---|---|---|
| BM | Lath Bainite | 15.9 ± 0.8 | 0.235 ± 0.012 | 0.429 ± 0.021 | 0.405 ± 0.020 | Intergranular cracking | 12.67 ± 0.61 |
| Annealed steel | Ferrite and Pearlite | 31.8 ± 1.6 | 0.448 ± 0.022 | 0.679 ± 0.034 | 0.935 ± 0.046 | Distribution along the pearlite | 45.34 ± 3.13 |
| Quenched steel | Lath Martensite | 63.5 ± 3.2 | 0.39 ± 0.020 | 0.859 ± 0.043 | 1.168 ± 0.058 | Transgranular cracking | 30.16 ± 0.91 |
| Normalized steel | Low-Carbon Bainite | 44.9 ± 2.2 | 0.559 ± 0.028 | 2.19 ± 0.110 | 5.537 ± 0.280 | Transgranular cracking | 42.87 ± 1.86 |
| Tempered steel | Tempered Sorbite and Acicular Martensite | 63.5 ± 3.2 | 0.541 ± 0.027 | 1.794 ± 0.090 | 1.808 ± 0.091 | Intergranular cracking | 29.79 ± 0.75 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, H.; Tian, H.; Xin, J.; Cui, Z. Correlation between Microstructure and Hydrogen Degradation of 690 MPa Grade Marine Engineering Steel. Materials 2021, 14, 851. https://doi.org/10.3390/ma14040851
Ma H, Tian H, Xin J, Cui Z. Correlation between Microstructure and Hydrogen Degradation of 690 MPa Grade Marine Engineering Steel. Materials. 2021; 14(4):851. https://doi.org/10.3390/ma14040851
Chicago/Turabian StyleMa, Heng, Huiyun Tian, Juncheng Xin, and Zhongyu Cui. 2021. "Correlation between Microstructure and Hydrogen Degradation of 690 MPa Grade Marine Engineering Steel" Materials 14, no. 4: 851. https://doi.org/10.3390/ma14040851
APA StyleMa, H., Tian, H., Xin, J., & Cui, Z. (2021). Correlation between Microstructure and Hydrogen Degradation of 690 MPa Grade Marine Engineering Steel. Materials, 14(4), 851. https://doi.org/10.3390/ma14040851





