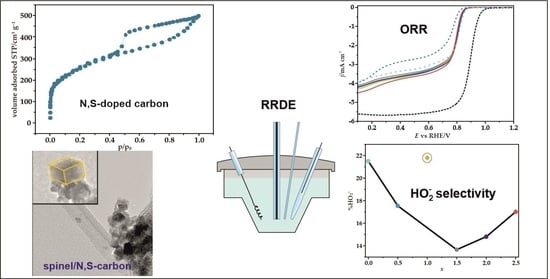
Selectivity of Mixed Iron-Cobalt Spinels Deposited on a N,S-Doped Mesoporous Carbon Support in the Oxygen Reduction Reaction in Alkaline Media
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Synthesis of Nitrogen and Sulfur Doped Mesoporous Carbon Support (N,S-MPC)
2.1.2. Synthesis of Fe-Co/N,S-MPC Catalysts
2.2. Methods
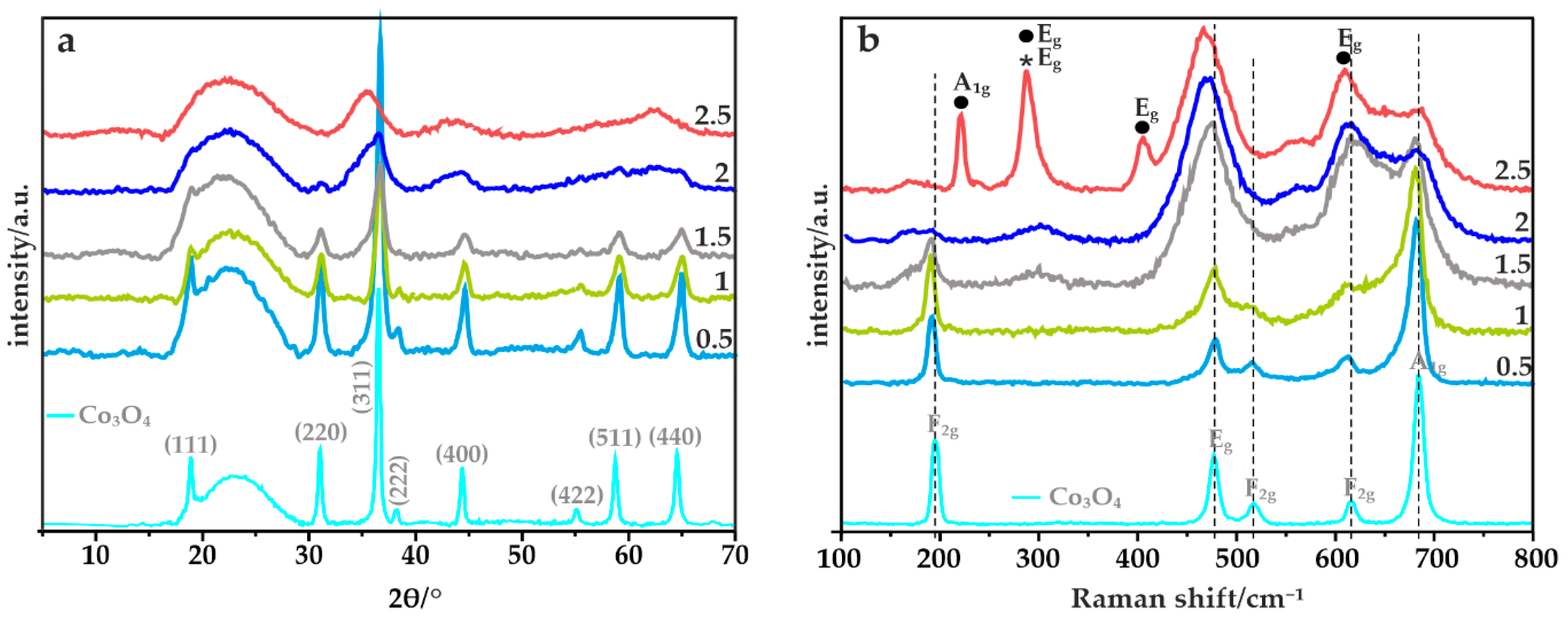
2.2.1. X-ray Powder Diffraction (XRD)
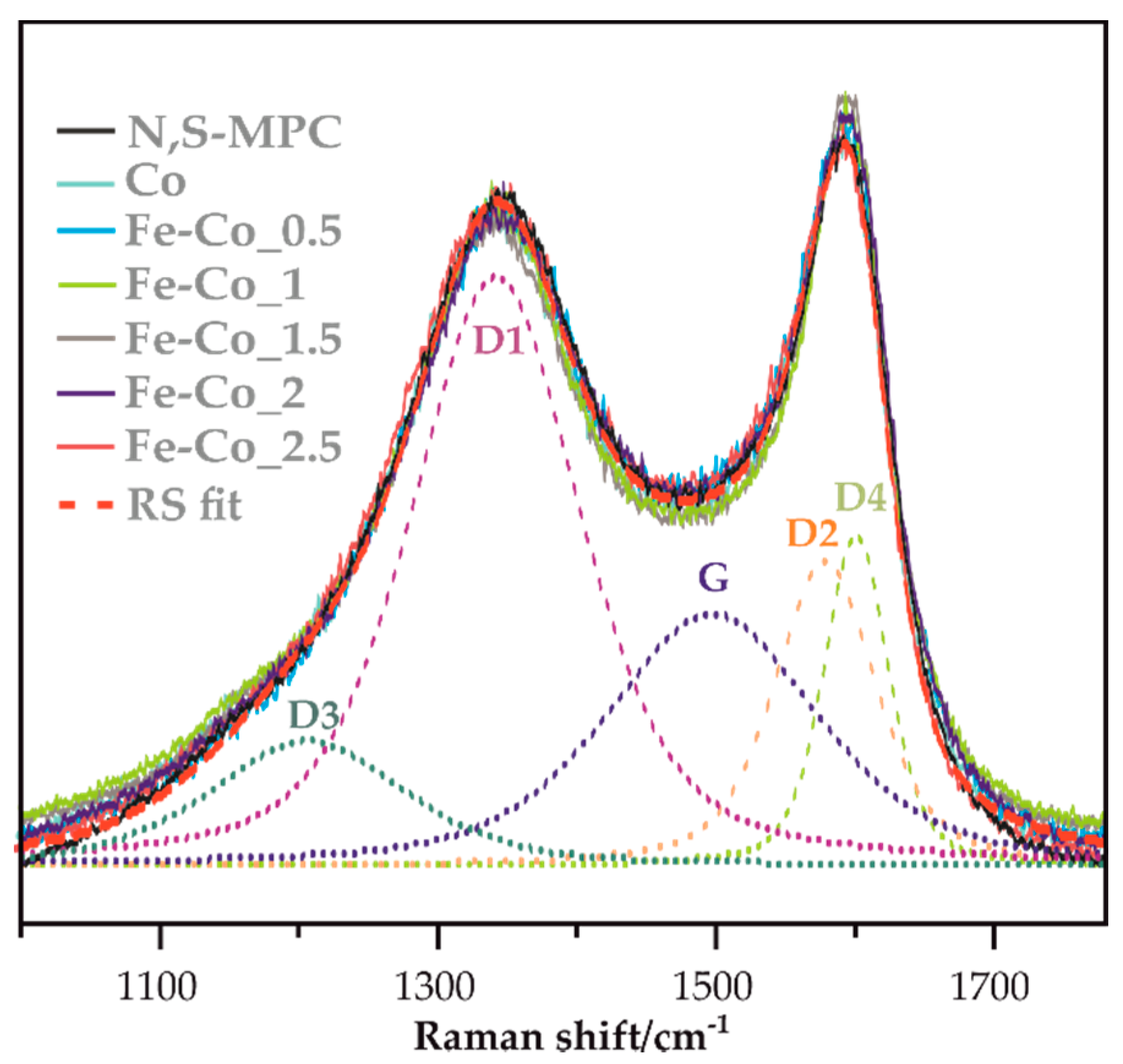
2.2.2. Raman Spectroscopy (RS)
2.2.3. Low-Temperature Nitrogen Sorption
2.2.4. X-ray Fluorescence Spectroscopy (XRF)
2.2.5. Thermogravimetric Analysis (TGA)
2.2.6. Elemental Analysis
2.2.7. X-ray Photoelectron Spectroscopy (XPS)
2.2.8. Transmission Electron Microscopy (TEM)
2.2.9. Electrode Preparation and Rotating Ring Disk Electrode (RRDE) Measurements
3. Results
3.1. Physicochemical Characterization of a Double-Doped Mesoporous Carbon Support
3.2. Physicochemical Characterization of Catalysts
3.2.1. XRF, TGA, XRD, XPS and Micro-RS Analyses
3.2.2. S/TEM/EDX Imaging
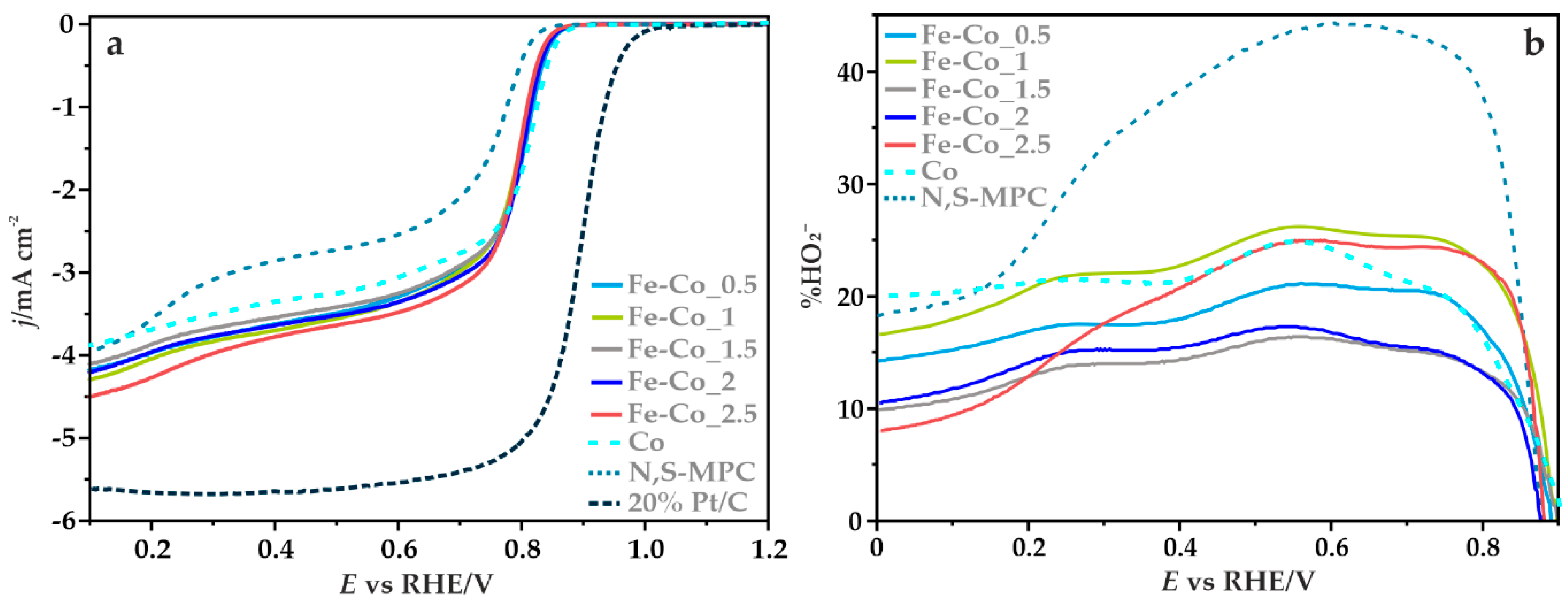
3.3. Electrochemical Reduction of Oxygen
3.3.1. Cyclic Voltammetry
3.3.2. Electrocatalytic ORR Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dai, L.; Zhi, C.; Feng, X. Bifunctional catalysts for metal-air batteries. Batter. Supercaps 2019, 2, 270–271. [Google Scholar] [CrossRef]
- Suntivich, J.; Gasteiger, H.A.; Yabuuchi, N.; Nakanishi, H.; Goodenough, J.B.; Shao-Horn, Y. Design principles for oxygen-reduction activity on perovskite oxide catalysts for fuel cells and metal–air batteries. Nat. Chem. 2011, 3, 546–550. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Zhou, Z.; Wang, H.; Huang, X.; Xu, J.; Tang, Y.; Li, J.; Chu, H.; Chen, J. N-Doped carbon supported Co3O4 nanoparticles as an advanced electrocatalyst for the oxygen reduction reaction in Al–air batteries. RSC Adv. 2016, 6, 55552–55559. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, X.; Lu, Y.; Xu, M.; Zhang, D.; Ruoff, R.S.; Stevenson, K.J.; Goodenough, J.B. CoMn2O4 spinel nanoparticles grown on graphene as bifunctional catalyst for lithium-air batteries. J. Electrochem. Soc. 2011, 158, A1379–A1382. [Google Scholar] [CrossRef]
- Gao, H.; Liu, S.; Li, Y.; Conte, E.D.; Cao, Y. A Critical Review of Spinel Structured Iron Cobalt Oxides Based Materials for Electrochemical Energy Storage and Conversion. Energies 2017, 10, 1787. [Google Scholar] [CrossRef]
- Othman, R.; Dicks, A.L.; Zhu, Z. Non precious metal catalysts for the PEM fuel cell cathode. Int. J. Hydrog. Energy 2012, 37, 357–372. [Google Scholar] [CrossRef]
- Huang, Q.; Zhou, P.; Yang, H.; Zhu, L.; Wu, H. In situ generation of inverse spinel CoFe2O4 nanoparticles onto nitrogen-doped activated carbon for an effective cathode electrocatalyst of microbial fuel cells. Chem. Eng. J. 2017, 325, 466–473. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Thomas, I.L. Alternative energy technologies. Nature 2001, 414, 332–337. [Google Scholar] [CrossRef]
- GirishKumar, G.; McCloskey, B.; Luntz, A.C.; Swanson, S.; Wilcke, W. Lithium–air battery: Promise and challenges. J. Phys. Chem. Lett. 2010, 1, 2193–2203. [Google Scholar] [CrossRef]
- Epping Martin, K.; Kopasz, J.P.; McMurphy, K.W. Status of fuel cells and the challenges facing fuel cell technology today making further progress in eliminating cost, durability, and performance challenges that remain for fuel cell technology. In Fuel Cell Chemistry and Operation ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2010; pp. 1–13. [Google Scholar]
- Dekel, D.R.; Rasin, I.G.; Brandon, S. Predicting performance stability of anion exchange membrane fuel cells. J. Power Sources 2019, 420, 118–123. [Google Scholar] [CrossRef]
- Wierzbicki, S.; Douglin, J.C.; Kostuch, A.; Dekel, D.R.; Kruczała, K. Are radicals formed during anion-exchange membrane fuel cell operation? J. Phys. Chem. Lett. 2020, 11, 7630–7636. [Google Scholar] [CrossRef] [PubMed]
- Kostuch, A.; Gryboś, J.; Indyka, P.; Osmieri, L.; Specchia, S.; Sojka, Z.; Kruczała, K. Morphology and dispersion of nanostructured manganese–cobalt spinel on various carbon supports: The effect on the oxygen reduction reaction in alkaline media. Catal. Sci. Technol. 2018, 8, 642–655. [Google Scholar] [CrossRef]
- Bo, X.; Zhang, Y.; Li, M.; Nsabimana, A.; Guo, L. NiCo2O4 spinel/ordered mesoporous carbons as noble-metal free electrocatalysts for oxygen reduction reaction and the influence of structure of catalyst support on the electrochemical activity of NiCo2O4. J. Power Sources 2015, 288, 1–8. [Google Scholar] [CrossRef]
- Pendashteh, A.; Palma, J.; Anderson, M.; Marcilla, R. NiCoMnO4 nanoparticles on N-doped graphene: Highly efficient bifunctional electrocatalyst for oxygen reduction/evolution reactions. Appl. Catal. B Environ. 2017, 201, 241–252. [Google Scholar] [CrossRef]
- Zhao, Q.; Yan, Z.; Chen, C.; Chen, J. Spinels: Controlled preparation, oxygen reduction/evolution reaction application, and beyond. Chem. Rev. 2017, 117, 10121–10211. [Google Scholar] [CrossRef]
- Hamdani, M.; Singh, R.; Chartier, P. Co3O4 and co- based spinel oxides bifunctional oxygen electrodes. Int. J. Electrochem. Sci. 2010, 5, 556–577. [Google Scholar]
- Liang, Y.; Li, Y.; Wang, H.; Zhou, J.; Wang, J.; Regier, T.; Dai, H. Co3O4 nanocrystals on graphene as a synergistic catalyst for oxygen reduction reaction. Nat. Mater. 2011, 10, 780–786. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, Z.; Yan, Z.; Wang, Y.; Wang, E.; Wang, S.; Wang, S.; Sun, G. Crystal-plane-dependent activity of spinel Co3O4 towards water splitting and the oxygen reduction reaction. ChemElectroChem 2018, 5, 1080–1086. [Google Scholar] [CrossRef]
- Davari, E.; Johnson, A.D.; Mittal, A.; Xiong, M.; Ivey, D.G. Manganese-cobalt mixed oxide film as a bifunctional catalyst for rechargeable zinc-air batteries. Electrochim. Acta 2016, 211, 735–743. [Google Scholar] [CrossRef]
- Mahmoud, M.; Gad-Allah, T.A.; El-Khatib, K.; El-Gohary, F.; El-Khatib, K. Power generation using spinel manganese–cobalt oxide as a cathode catalyst for microbial fuel cell applications. Bioresour. Technol. 2011, 102, 10459–10464. [Google Scholar] [CrossRef]
- Ge, X.; Liu, Y.; Goh, F.W.T.; Hor, T.S.A.; Zong, Y.; Xiao, P.; Zhang, Z.; Lim, S.H.; Li, B.; Wang, X.; et al. Dual-phase spinel MnCo2O4 and spinel MnCo2O4/nanocarbon hybrids for electrocatalytic oxygen reduction and evolution. ACS Appl. Mater. Interfaces 2014, 6, 12684–12691. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Wang, J.; Wei, Z.; Nie, Y.; Li, L.; Qi, X.; Chen, S.; Wei, Z. A strategy to promote the electrocatalytic activity of spinels for oxygen reduction by structure reversal. Angew. Chem. Int. Ed. 2016, 55, 1340–1344. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, S.; Huang, Y.-X.; Wu, L.; Sun, S. Monodisperse MxFe3−xO4 (M = Fe, Cu, Co, Mn) nanoparticles and their electrocatalysis for oxygen reduction reaction. Nano Lett. 2013, 13, 2947–2951. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Yang, Z.; Bian, W.; Yang, R. FeCo2O4/hollow graphene spheres hybrid with enhanced electrocatalytic activities for oxygen reduction and oxygen evolution reaction. Carbon 2015, 92, 74–83. [Google Scholar] [CrossRef]
- Fan, H.; Yang, L.; Wang, Y.; Zhang, X.; Wu, Q.; Che, R.; Liu, M.; Wu, Q.; Wang, X.; Hu, Z. Boosting oxygen reduction activity of spinel CoFe2O4 by strong interaction with hierarchical nitrogen-doped carbon nanocages. Sci. Bull. 2017, 62, 1365–1372. [Google Scholar] [CrossRef]
- Yan, W.; Bian, W.; Jin, C.; Tian, J.-H.; Yang, R. An Efficient Bi-functional Electrocatalyst Based on Strongly Coupled CoFe2O4/Carbon Nanotubes Hybrid for Oxygen Reduction and Oxygen Evolution. Electrochim. Acta 2015, 177, 65–72. [Google Scholar] [CrossRef]
- Peng, X.; Kashyap, V.; Ng, B.; Kurungot, S.; Wang, L.; Varcoe, J.R.; Mustain, W.E. High-performing pgm-free aemfc cathodes from carbon-supported cobalt ferrite nanoparticles. Catalysts 2019, 9, 264. [Google Scholar] [CrossRef]
- Gong, Y.; Ding, W.; Li, Z.; Su, R.; Zhang, X.; Wang, J.; Zhou, J.; Wang, Z.; Gao, Y.; Li, S.; et al. Inverse Spinel Cobalt–Iron Oxide and N-Doped Graphene Composite as an Efficient and Durable Bifuctional Catalyst for Li–O2 Batteries. ACS Catal. 2018, 8, 4082–4090. [Google Scholar] [CrossRef]
- Chakrapani, K.; Bendt, G.; Hajiyani, H.; Lunkenbein, T.; Greiner, M.T.; Masliuk, L.; Salamon, S.; Landers, J.; Schlögl, R.; Wende, H.; et al. The Role of Composition of Uniform and Highly Dispersed Cobalt Vanadium Iron Spinel Nanocrystals for Oxygen Electrocatalysis. ACS Catal. 2018, 8, 1259–1267. [Google Scholar] [CrossRef]
- Chakrapani, K.; Bendt, G.; Hajiyani, H.; Schwarzrock, I.; Lunkenbein, T.; Salamon, S.; Landers, J.; Wende, H.; Schlögl, R.; Pentcheva, R.; et al. Role of Composition and Size of Cobalt Ferrite Nanocrystals in the Oxygen Evolution Reaction. ChemCatChem 2017, 9, 2988–2995. [Google Scholar] [CrossRef]
- Rodríguez-Reinoso, F. The role of carbon materials in heterogeneous catalysis. Carbon 1998, 36, 159–175. [Google Scholar] [CrossRef]
- Serp, P. Carbon nanotubes and nanofibers in catalysis. Appl. Catal. A Gen. 2003, 253, 337–358. [Google Scholar] [CrossRef]
- Park, J.-E.; Jang, Y.J.; Kim, Y.J.; Song, M.-S.; Yoon, S.; Kim, D.H.; Kim, S.-J. Sulfur-doped graphene as a potential alternative metal-free electrocatalyst and Pt-catalyst supporting material for oxygen reduction reaction. Phys. Chem. Chem. Phys. 2014, 16, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Al-Hakemy, A.Z.; Nassr, A.B.A.A.; Naggar, A.H.; Elnouby, M.S.; Soliman, H.M.A.E.-F.; Taher, M.A. Electrodeposited cobalt oxide nanoparticles modified carbon nanotubes as a non-precious catalyst electrode for oxygen reduction reaction. J. Appl. Electrochem. 2016, 47, 183–195. [Google Scholar] [CrossRef]
- Wei, Q.; Tong, X.; Zhang, G.; Qiao, J.; Gong, Q.; Sun, S. Nitrogen-doped carbon nanotube and graphene materials for oxygen reduction reactions. Catalysts 2015, 5, 1574–1602. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, Y.; Cheng, H.; Hu, Y.; Shi, G.; Dai, L.; Qu, L. Nitrogen-doped graphene quantum dots with oxygen-rich functional groups. J. Am. Chem. Soc. 2012, 134, 15–18. [Google Scholar] [CrossRef]
- Kim, I.T.; Song, M.J.; Kim, Y.B.; Shin, M.W. Microwave-hydrothermal synthesis of boron/nitrogen co-doped graphene as an efficient metal-free electrocatalyst for oxygen reduction reaction. Int. J. Hydrog. Energy 2016, 41, 22026–22033. [Google Scholar] [CrossRef]
- Hao, G.-P.; Sahraie, N.R.; Zhang, Q.; Krause, S.; Oschatz, M.; Bachmatiuk, A.; Strasser, P.; Kaskel, S. Hydrophilic non-precious metal nitrogen-doped carbon electrocatalysts for enhanced efficiency in oxygen reduction reaction. Chem. Commun. 2015, 51, 17285–17288. [Google Scholar] [CrossRef]
- Jarczewski, S.; Drozdek, M.; Michorczyk, P.; Cuadrado-Collados, C.; Gandara-Loe, J.; Silvestre-Albero, J.; Kustrowski, P. Oxidative dehydrogenation of ethylbenzene over CMK-1 and CMK-3 carbon replicas with various mesopore architectures. Microporous Mesoporous Mater. 2018, 271, 262–272. [Google Scholar] [CrossRef]
- Wojdyr, M. Fityk: A general-purpose peak fitting program. J. Appl. Crystallogr. 2010, 43, 1126–1128. [Google Scholar] [CrossRef]
- Ross, J.R. Catalyst characterization. Contemp. Catal. 2019, 121–132. [Google Scholar] [CrossRef]
- CasaXPS Processing Software. Available online: http://www.casaxps.com/ (accessed on 4 February 2021).
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Sadezky, A.; Muckenhuber, H.; Grothe, H.; Niessner, R.; Pöschl, U. Raman microspectroscopy of soot and related carbonaceous materials: Spectral analysis and structural information. Carbon 2005, 43, 1731–1742. [Google Scholar] [CrossRef]
- Lezanska, M.; Pietrzyk, P.; Dudek, A.; Włoch, J. Nitration and reduction route to surface groups of mesoporous carbons obtained from sucrose and phloroglucinol/formaldehyde precursors. Mater. Chem. Phys. 2015, 149, 539–552. [Google Scholar] [CrossRef]
- Yang, Z.; Zhou, X.; Nie, H.; Yao, Z.; Huang, S. Facile construction of manganese oxide doped carbon nanotube catalysts with high activity for oxygen reduction reaction and investigations into the origin of their activity enhancement. ACS Appl. Mater. Interfaces 2011, 3, 2601–2606. [Google Scholar] [CrossRef] [PubMed]
- Hadjiev, V.G.; Iliev, M.N.; Vergilov, I.V. The Raman spectra of Co3O4. J. Phys. C Solid State Phys. 1988, 21, L199–L201. [Google Scholar] [CrossRef]
- Ercolino, G.; Stelmachowski, P.; Kotarba, A.; Specchia, S. Reactivity of mixed iron–cobalt spinels in the lean methane combustion. Top. Catal. 2017, 60, 1370–1379. [Google Scholar] [CrossRef]
- de Faria, D.L.A.; Venâncio Silva, S.; de Oliveira, M.T. Raman microspectroscopy of some iron oxides and oxyhydroxides. J. Raman Spectrosc. 1997, 28, 873–878. [Google Scholar] [CrossRef]
- Tian, Z.-Y.; Kouotou, P.M.; El Kasmi, A.; Ngamou, P.H.T.; Kohse-Höinghaus, K.; Vieker, H.; Beyer, A.; Gölzhäuser, A. Low-temperature deep oxidation of olefins and DME over cobalt ferrite. Proc. Combust. Inst. 2015, 35, 2207–2214. [Google Scholar] [CrossRef]
- Ortiz-Quiñonez, J.-L.; Pal, U.; Villanueva, M.S. Structural, Magnetic, and Catalytic Evaluation of Spinel Co, Ni, and Co–Ni Ferrite Nanoparticles Fabricated by Low-Temperature Solution Combustion Process. ACS Omega 2018, 3, 14986–15001. [Google Scholar] [CrossRef]
- Grzybek, G.; Ciura, K.; Gryboś, J.; Indyka, P.; Davó-Quiñonero, A.; Lozano-Castelló, D.; Bueno-López, A.; Kotarba, A.; Sojka, Z. CO-PROX Reaction over Co3O4|Al2O3 Catalysts—Impact of the Spinel Active Phase Faceting on the Catalytic Performance. J. Phys. Chem. C 2019, 123, 20221–20232. [Google Scholar] [CrossRef]
- Xu, J.; Gao, P.; Zhao, T.S. Non-precious Co3O4 nano-rod electrocatalyst for oxygenreduction reaction in anion-exchange membranefuelcells. Energy Environ. Sci. 2011, 5, 5333–5339. [Google Scholar] [CrossRef]
- Alegreab, C.; Busacca, C.; Di Blasi, A.; Aricò, A.; Antonucci, V.; Baglio, V. Toward more efficient and stable bifunctional electrocatalysts for oxygen electrodes using FeCo2O4/carbon nanofiber prepared by electrospinning. Mater. Today Energy 2020, 18, 100508. [Google Scholar] [CrossRef]
- Lee, E.; Jang, J.-H.; Kwon, Y.-U. Composition effects of spinel MnxCo3−xO4 nanoparticles on their electrocatalytic properties in oxygen reduction reaction in alkaline media. J. Power Sources 2015, 273, 735–741. [Google Scholar] [CrossRef]
- Naveen, A.N.; Selladurai, S. Investigation on physiochemical properties of Mn substituted spinel cobalt oxide for supercapacitor applications. Electrochim. Acta 2014, 125, 404–414. [Google Scholar] [CrossRef]
- Osmieri, L.; Escudero-Cid, R.; Armandi, M.; Videla, A.H.M.; Fierro, J.L.G.; Ocón, P.; Specchia, S. Fe-N/C catalysts for oxygen reduction reaction supported on different carbonaceous materials. Performance in acidic and alkaline direct alcohol fuel cells. Appl. Catal. B Environ. 2017, 205, 637–653. [Google Scholar] [CrossRef]
- Barrera, D.A.; Florent, M.; Sapag, K.; Bandosz, T.J. Insight into the mechanism of oxygen reduction reaction on micro/mesoporous carbons: Ultramicropores versus nitrogen-containing catalytic centers in ordered pore structure. ACS Appl. Energy Mater. 2019, 2, 7412–7424. [Google Scholar] [CrossRef]
- Li, R.; Wang, Y.; Zhou, C.; Wang, C.; Ba, X.; Li, Y.; Huang, X.; Liu, J. Carbon-stabilized high-capacity ferroferric oxide nanorod array for flexible solid-state alkaline battery-supercapacitor hybrid device with high environmental suitability. Adv. Funct. Mater. 2015, 25, 5384–5394. [Google Scholar] [CrossRef]
- Duschek, K.; Uhlemann, M.; Schlörb, H.; Nielsch, K.; Leistner, K. Electrochemical and in situ magnetic study of iron/iron oxide films oxidized and reduced in KOH solution for magneto-ionic switching. Electrochem. Commun. 2016, 72, 153–156. [Google Scholar] [CrossRef]
- Wu, G.; More, K.L.; Johnston, C.M.; Zelenay, P. High-performance electrocatalysts for oxygen reduction derived from polyaniline, iron, and cobalt. Science 2011, 332, 443–447. [Google Scholar] [CrossRef]
- Ramaswamy, N.; Mukerjee, S. Fundamental Mechanistic Understanding of Electrocatalysis of Oxygen Reduction on Pt and Non-Pt Surfaces: Acid versus Alkaline Media. Adv. Phys. Chem. 2012, 2012, 1–17. [Google Scholar] [CrossRef]
- Osmieri, L.; Videla, A.H.M.; Armandi, M.; Specchia, S. Influence of different transition metals on the properties of Me–N–C (Me = Fe, Co, Cu, Zn) catalysts synthesized using SBA-15 as tubular nano-silica reactor for oxygen reduction reaction. Int. J. Hydrog. Energy 2016, 41, 22570–22588. [Google Scholar] [CrossRef]
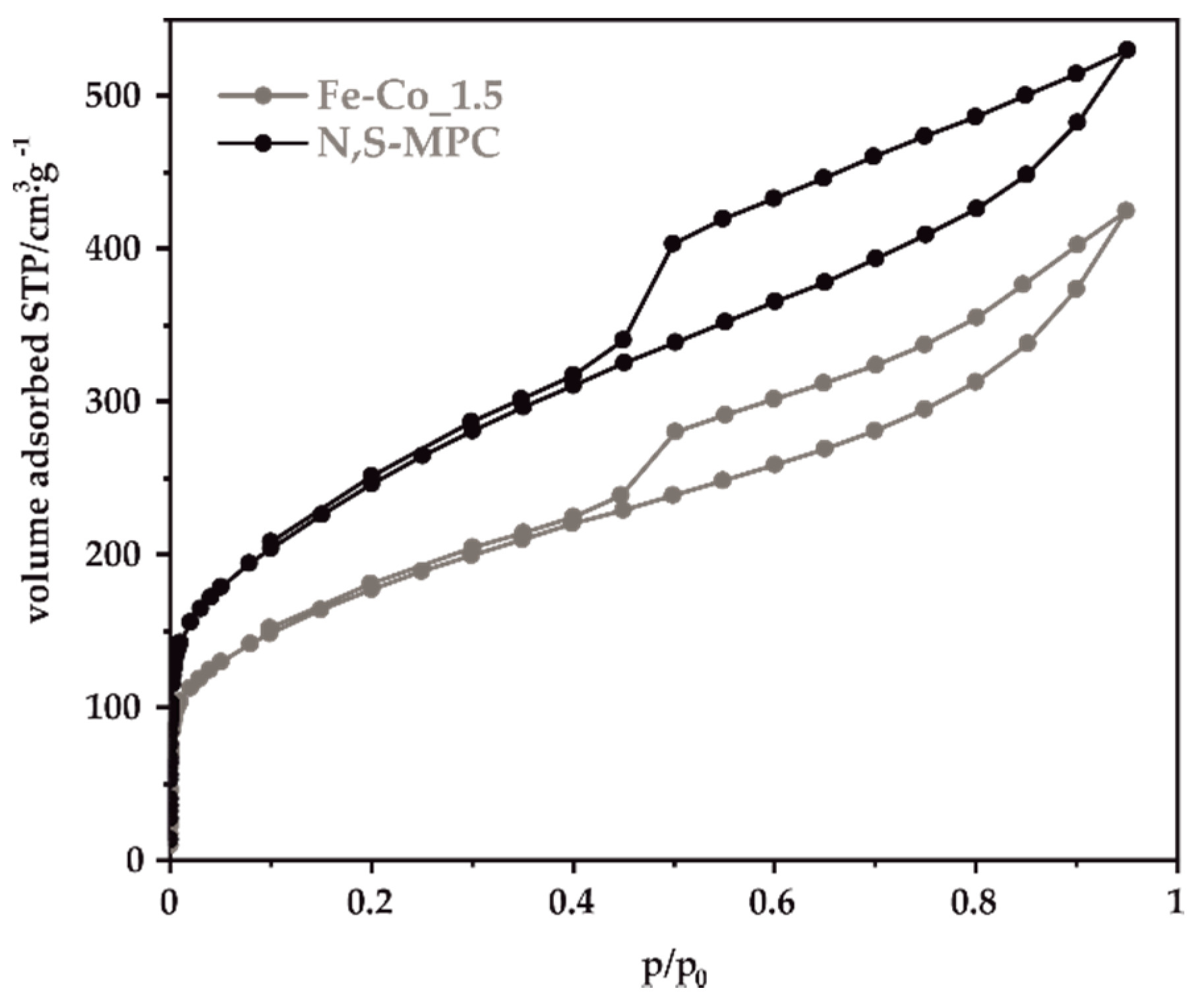
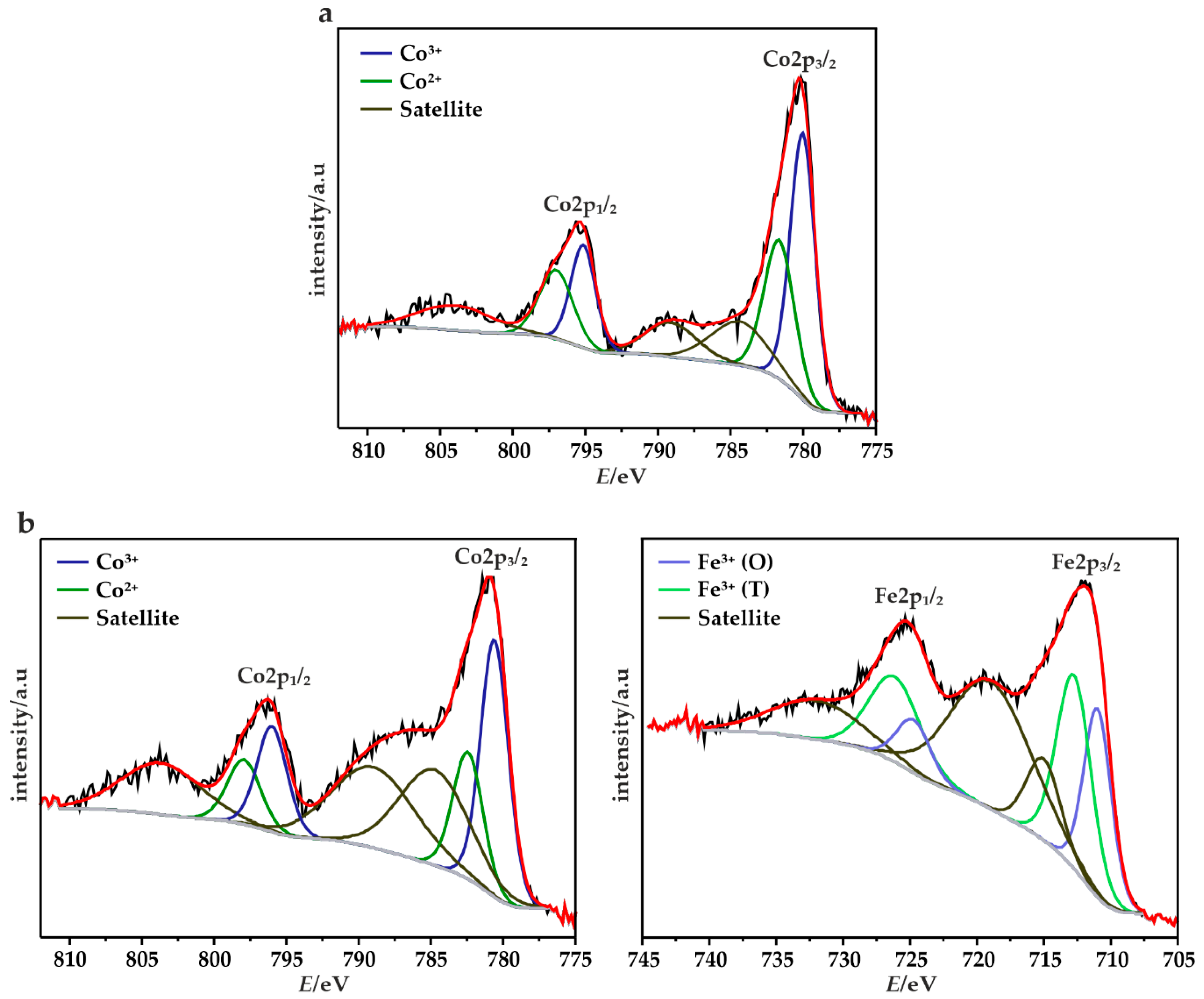
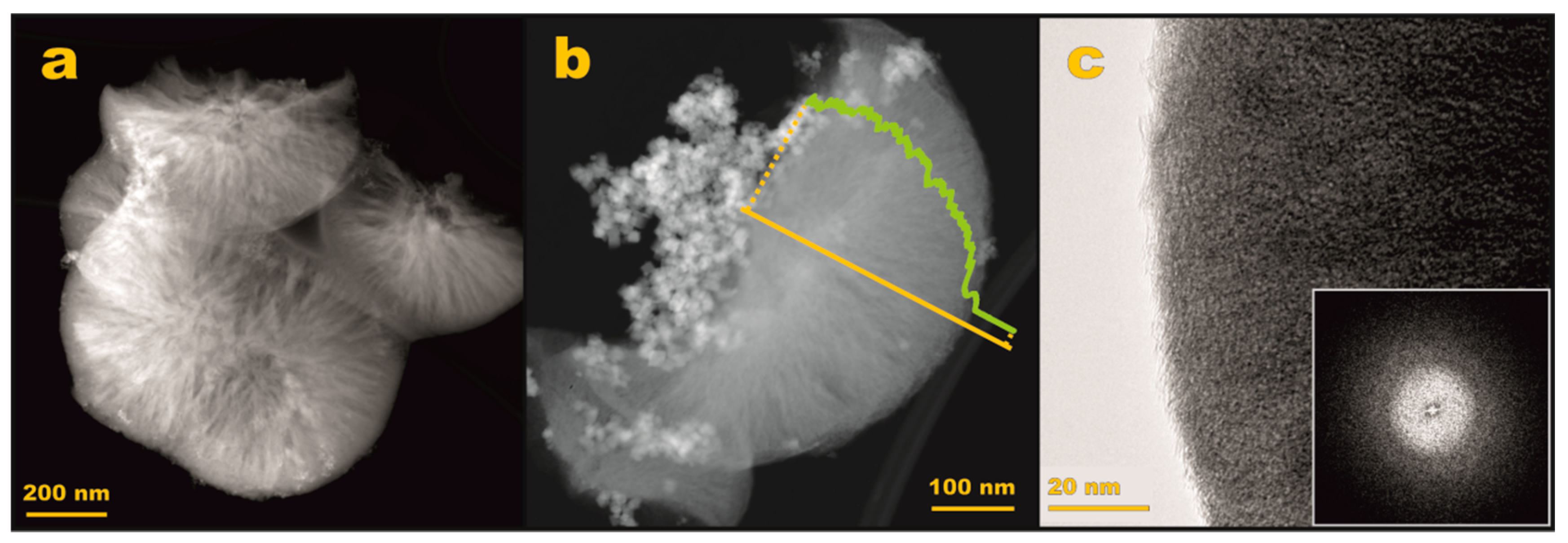
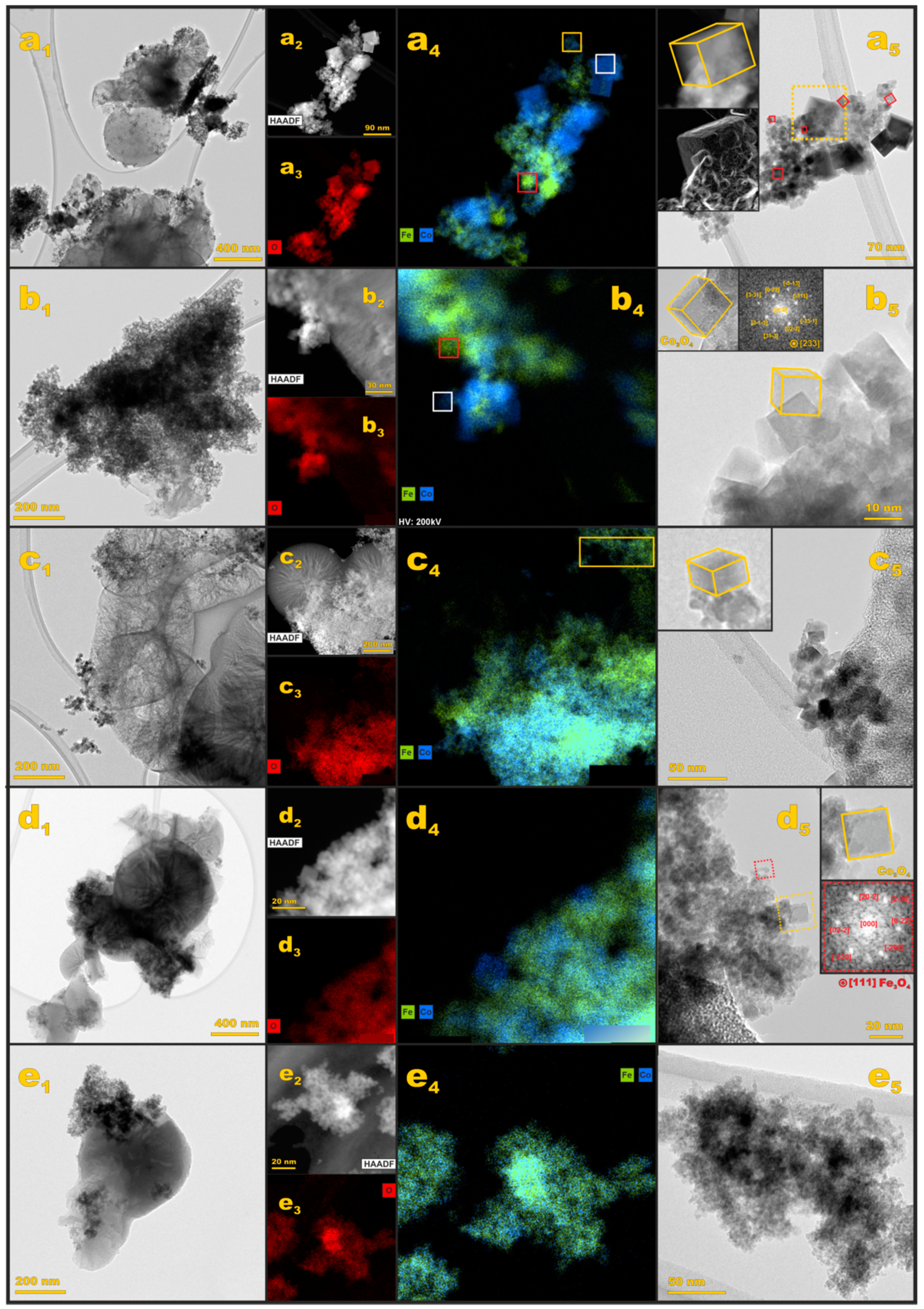
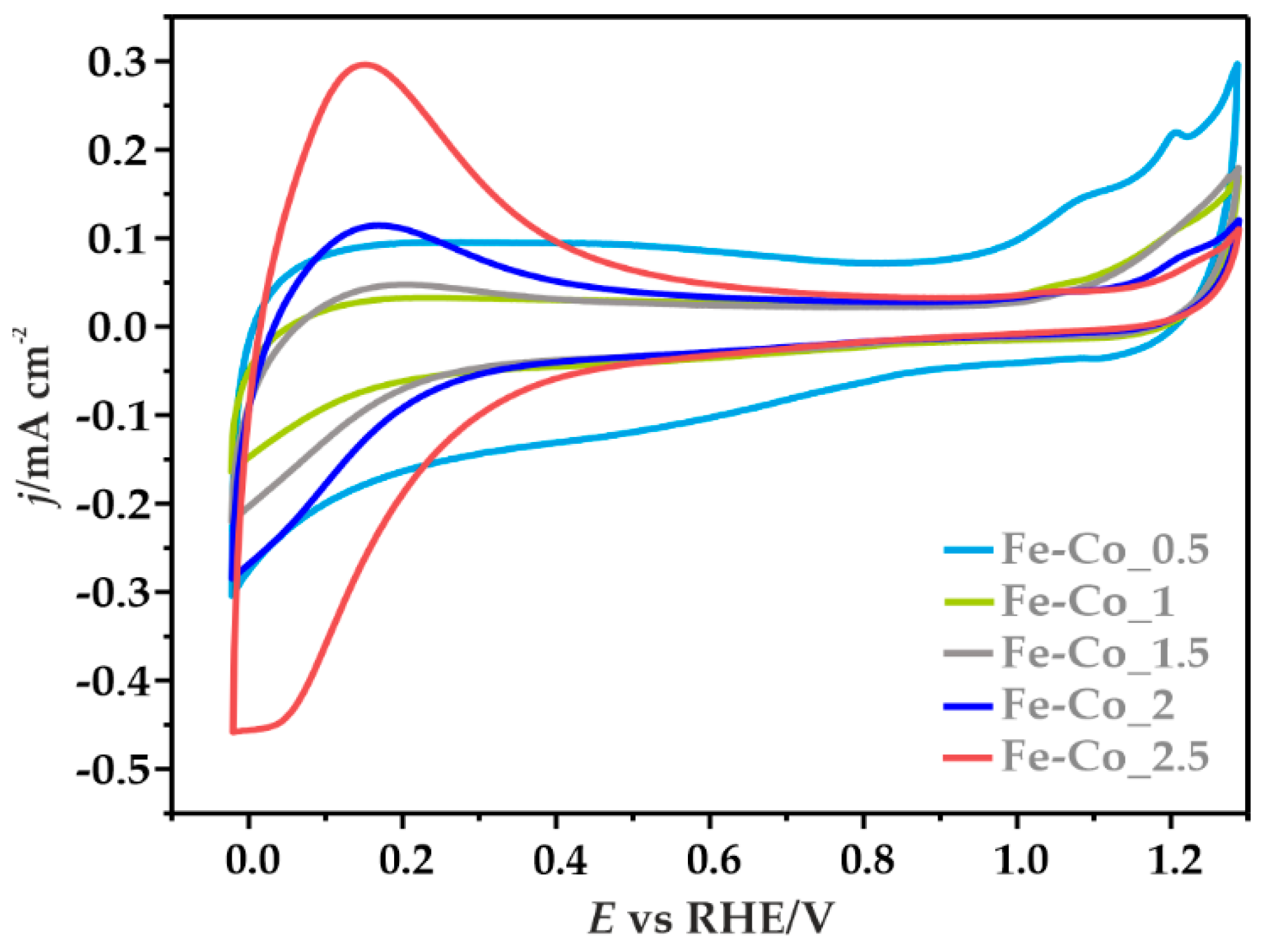
| Sample | Textural Parameters | |||
|---|---|---|---|---|
| SBET/m2 g−1 | Vtotal/cm3 g−1 | Vmicro/cm3 g−1 | Vmeso/cm3 g−1 | |
| N,S-MPC | 728 | 0.822 | 0.055 | 0.767 |
| Fe-Co_0.5 | 531 | 0.591 | 0.063 | 0.528 |
| Fe-Co_1 | 691 | 0.802 | 0.084 | 0.718 |
| Fe-Co_1.5 | 524 | 0.658 | 0.049 | 0.609 |
| Fe-Co_2 | 523 | 0.678 | 0.131 | 0.547 |
| Fe-Co_2.5 | 533 | 0.639 | 0.067 | 0.572 |
| Sample | DScherrer/nm | Elemental Composition/at.% 1 | Oxide Phase Loading on Carbon Support/wt.% 2 | |
|---|---|---|---|---|
| Fe | Co | |||
| Fe-Co_0.5 | 15 | 16 | 84 | 24 |
| Fe-Co_1 | 11 | 32 | 68 | 31 |
| Fe-Co_1.5 | 9 | 47 | 53 | 31 |
| Fe-Co_2 | n.d. | 63 | 37 | 28 |
| Fe-Co_2.5 | n.d. | 80 | 20 | 28 |
| E/eV | Chemical Composition/at.% | |||||||
|---|---|---|---|---|---|---|---|---|
| Co | Fe-Co_0.5 | Fe-Co_1 | Fe-Co_1.5 | Fe-Co_2 | Fe-Co_2.5 | |||
| 296.0–281.5 | C 1s | 83.27 | 71.57 | 65.28 | 62.3 | 64.06 | 66.34 | |
| 408.3–394.9 | N 1s | 3.45 | 2.06 | 2.42 | 2.42 | 2.16 | 2.71 | |
| 172.4–161.2 | S 2p | 1.47 | 1.05 | 1.35 | 1.43 | 1.33 | 1.23 | |
| 537.0–527.5 | O 1s | 8.82 | 15.86 | 19.98 | 20.85 | 20.84 | 19.33 | |
| Co 2p | ||||||||
| 780.1 ± 0.6 | Co3+ | 2p3/2 | 0.91 | 0.97 | 1.21 | 1.26 | 0.7 | 0.19 |
| 781.7 ± 0.7 | Co2+ | 0.58 | 0.88 | 0.89 | 0.67 | 0.53 | 0.42 | |
| 783.9 ± 0.9 | Satellite-1 /Fe LMM | 0.34 | 1.27 | 1.26 | 1.16 | 1.13 | 0.96 | |
| 788.6 ± 0.5 | Satellite-2 /Fe LMM | 0.25 | 0.87 | 0.82 | 1.17 | 1.03 | 0.92 | |
| 795.4 ± 0.6 | Co3+ | 2p1/2 | 0.35 | 0.45 | 0.53 | 0.55 | 0.26 | 0.06 |
| 797.3 ± 0.7 | Co2+ | 0.31 | 0.46 | 0.57 | 0.37 | 0.26 | 0.2 | |
| 803.5 ± 0.6 | Satellite-3 | 0.25 | 0.68 | 0.45 | 0.71 | 0.44 | 0.26 | |
| 2.99 | 5.58 | 5.73 | 5.89 | 4.35 | 3.01 | |||
| Fe 2p | ||||||||
| 710.5 ± 0.4 | Fe3+ (O) | 2p3/2 | 0.24 | 0.72 | 1.07 | 1.05 | 1.18 | |
| 712.4 ± 0.4 | Fe3+ (T) | 0.98 | 1.20 | 1.37 | 1.58 | 1.43 | ||
| 715.1 ± 0.3 | Satellite-1 /Co LMM | 0.89 | 0.9 | 0.57 | 0.69 | 0.65 | ||
| 719.2 ± 0.2 | Satellite-2 /Co LMM | 1.03 | 1.13 | 2.12 | 1.62 | 1.83 | ||
| 724.2 ± 0.6 | Fe3+ (O) | 2p1/2 | 0.13 | 0.29 | 0.37 | 0.64 | 0.51 | |
| 726.0 ± 0.4 | Fe3+ (T) | 0.44 | 0.6 | 0.89 | 0.88 | 0.9 | ||
| 731.7 ± 0.5 | Satellite-3 | 0.17 | 0.4 | 0.71 | 0.82 | 0.88 | ||
| 0 | 3.88 | 5.24 | 7.10 | 7.28 | 7.38 | |||
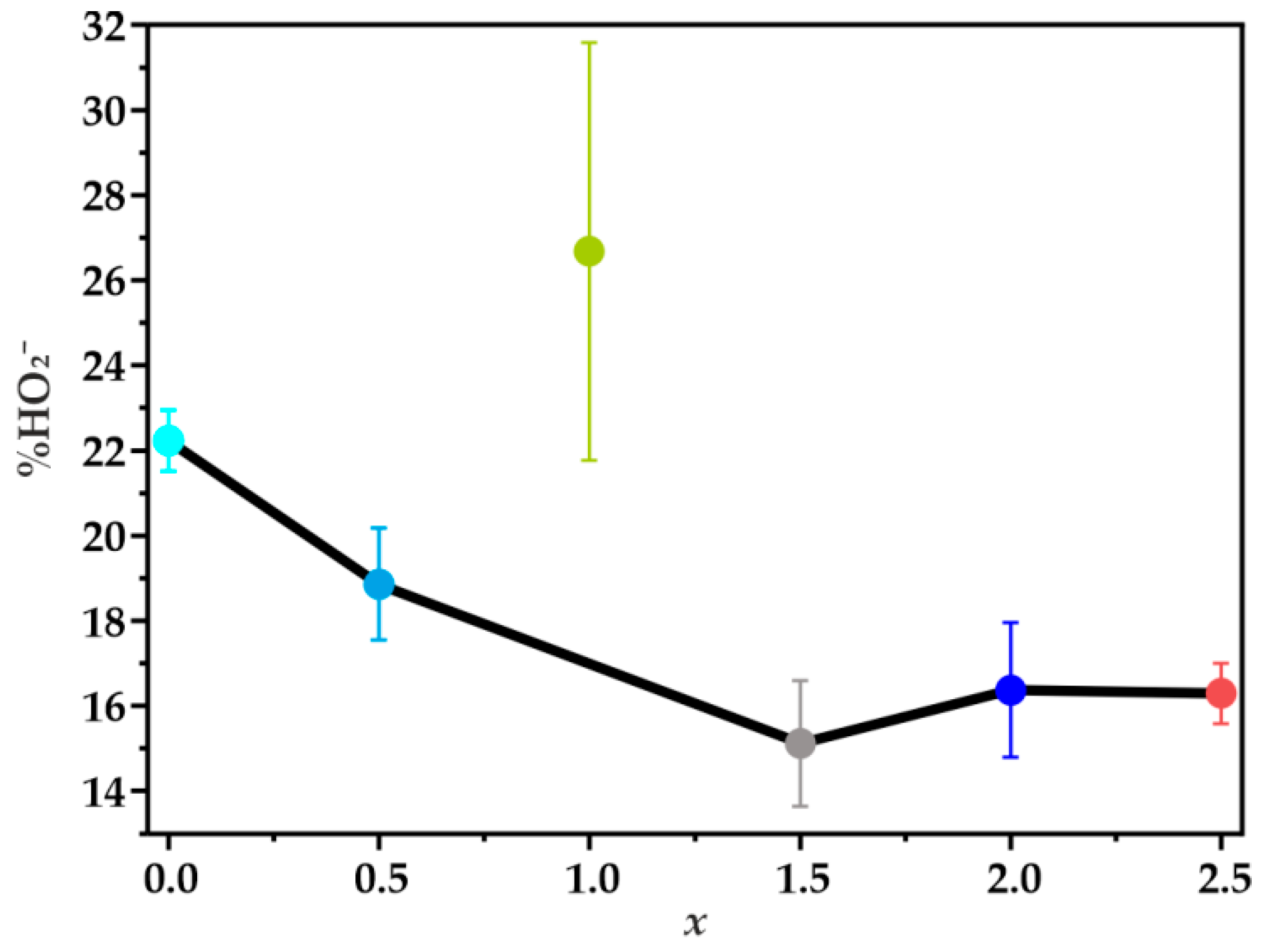
| Sample | Eonset/V | E1/2/V | n | |
|---|---|---|---|---|
| Co | 0.87 | 0.79 | 3.56 ± 0.01 | 22.24 ± 0.72 |
| Fe-Co_0.5 | 0.86 | 0.78 | 3.63 ± 0.02 | 18.86 ± 1.32 |
| Fe-Co_1 | 0.86 | 0.78 | 3.47 ± 0.09 | 26.68 ± 4.90 |
| Fe-Co_1.5 | 0.86 | 0.78 | 3.70 ± 0.03 | 15.12 ± 1.48 |
| Fe-Co_2 | 0.86 | 0.78 | 3.67 ± 0.03 | 16.38 ± 1.58 |
| Fe-Co_2.5 | 0.85 | 0.78 | 3.67 ± 0.01 | 16.30 ± 0.71 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostuch, A.; Gryboś, J.; Wierzbicki, S.; Sojka, Z.; Kruczała, K. Selectivity of Mixed Iron-Cobalt Spinels Deposited on a N,S-Doped Mesoporous Carbon Support in the Oxygen Reduction Reaction in Alkaline Media. Materials 2021, 14, 820. https://doi.org/10.3390/ma14040820
Kostuch A, Gryboś J, Wierzbicki S, Sojka Z, Kruczała K. Selectivity of Mixed Iron-Cobalt Spinels Deposited on a N,S-Doped Mesoporous Carbon Support in the Oxygen Reduction Reaction in Alkaline Media. Materials. 2021; 14(4):820. https://doi.org/10.3390/ma14040820
Chicago/Turabian StyleKostuch, Aldona, Joanna Gryboś, Szymon Wierzbicki, Zbigniew Sojka, and Krzysztof Kruczała. 2021. "Selectivity of Mixed Iron-Cobalt Spinels Deposited on a N,S-Doped Mesoporous Carbon Support in the Oxygen Reduction Reaction in Alkaline Media" Materials 14, no. 4: 820. https://doi.org/10.3390/ma14040820
APA StyleKostuch, A., Gryboś, J., Wierzbicki, S., Sojka, Z., & Kruczała, K. (2021). Selectivity of Mixed Iron-Cobalt Spinels Deposited on a N,S-Doped Mesoporous Carbon Support in the Oxygen Reduction Reaction in Alkaline Media. Materials, 14(4), 820. https://doi.org/10.3390/ma14040820