Light and Hydrogels: A New Generation of Antimicrobial Materials
Abstract
1. Introduction
2. Light as a Promising Source of Energy
2.1. Why Use Photochemistry?
2.2. Which Photoinitiating Systems Used for Hydrogel Synthesis?
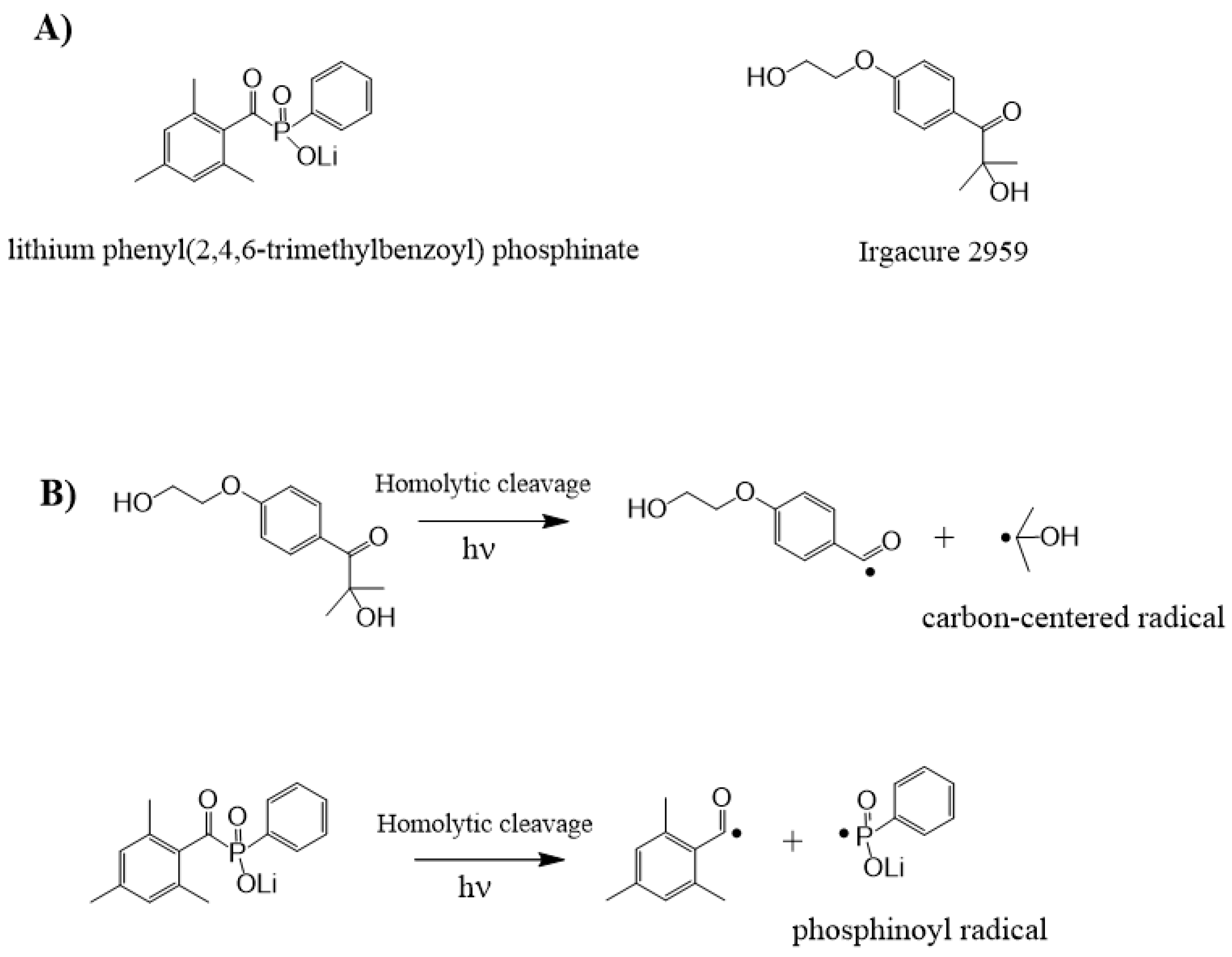
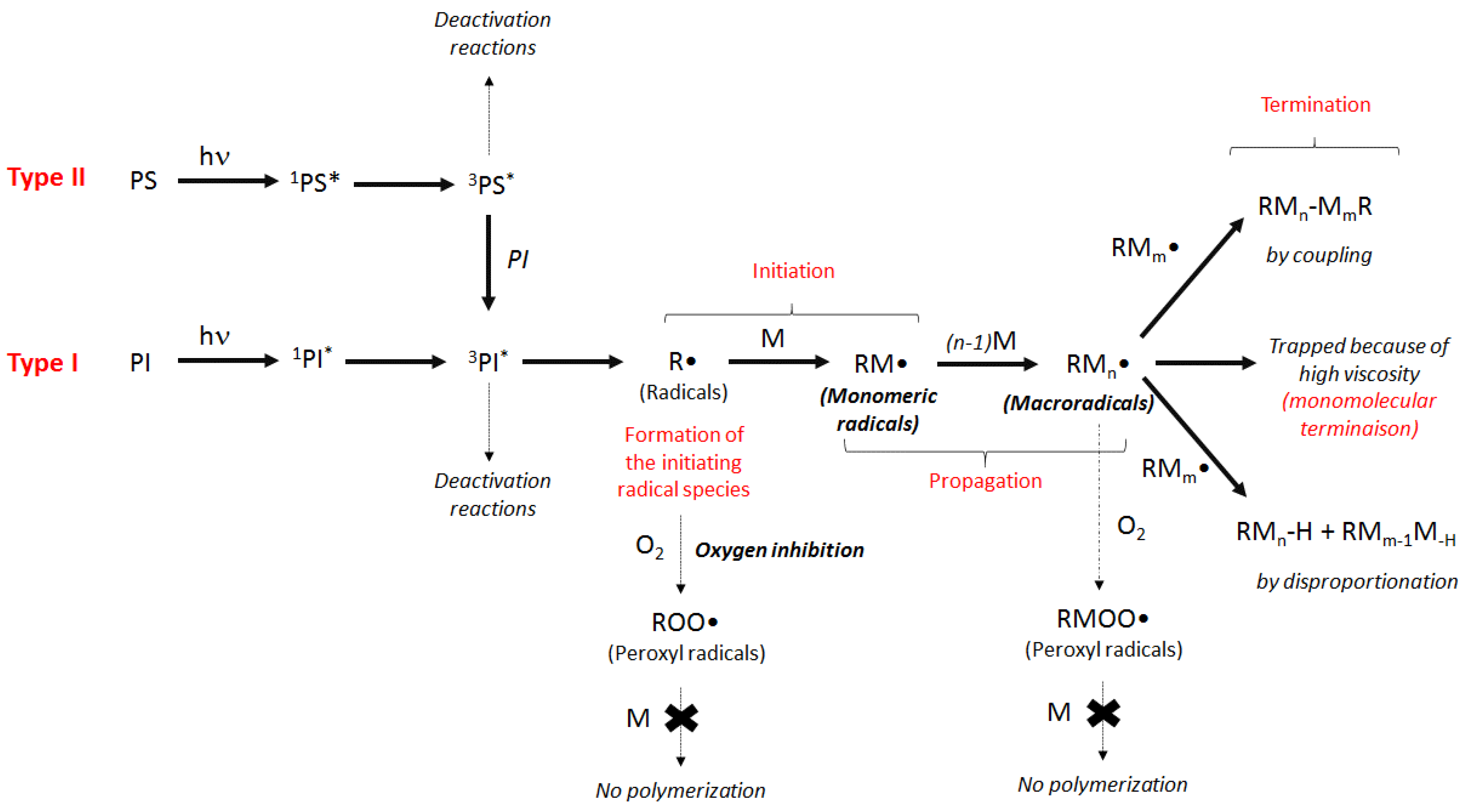
2.2.1. Norrish Type I Mechanism
2.2.2. Norrish Type II Mechanism
2.2.3. Free-Radical Photopolymerization Process
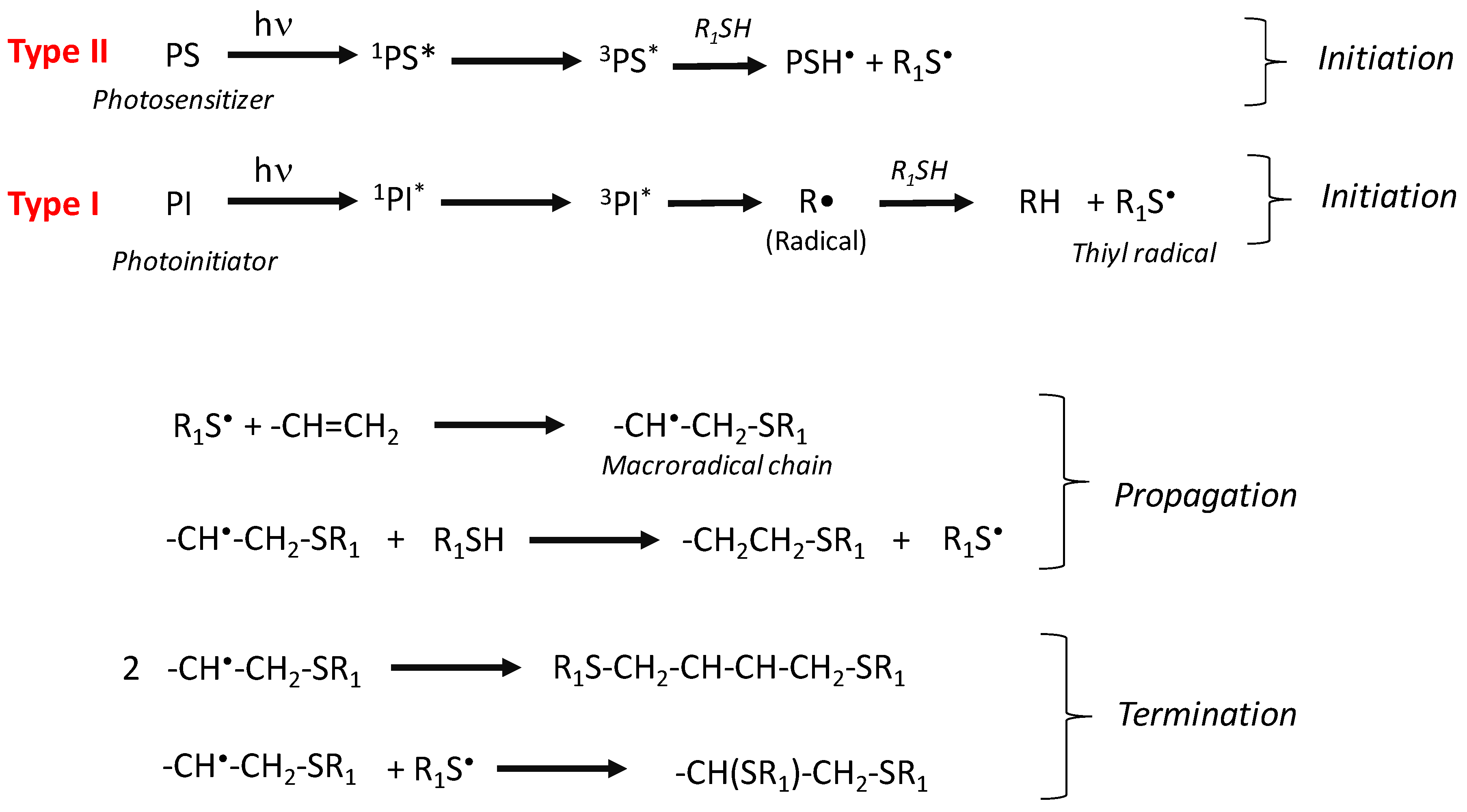
2.2.4. Initiation by the Thiol-Ene Process
3. Photochemistry and Antibacterial Hydrogels: Biological Applications
3.1. Photoinduced Antibacterial Hydrogels
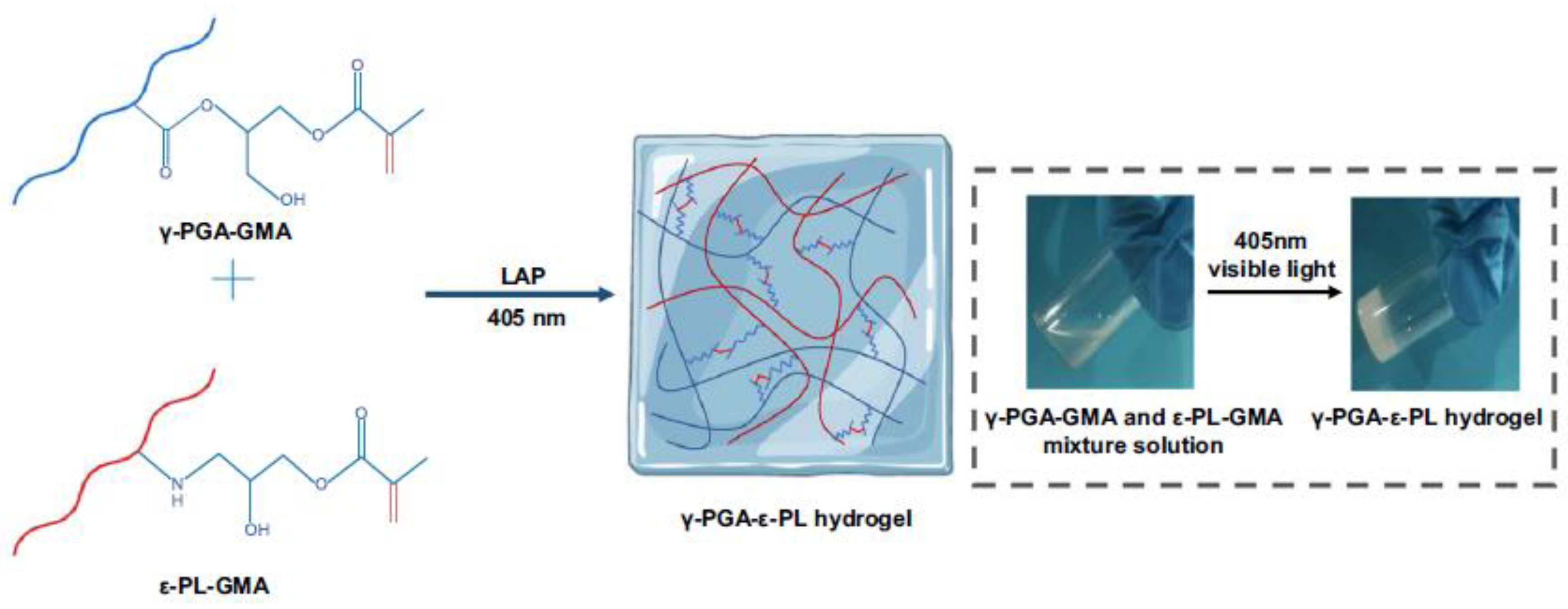
3.1.1. Organic Photoinduced Antibacterial Hydrogels
3.1.2. Hybrid Organic–Inorganic Photoinduced Antibacterial Hydrogels
3.2. Light-Triggered Antibacterial Activity of Hydrogels
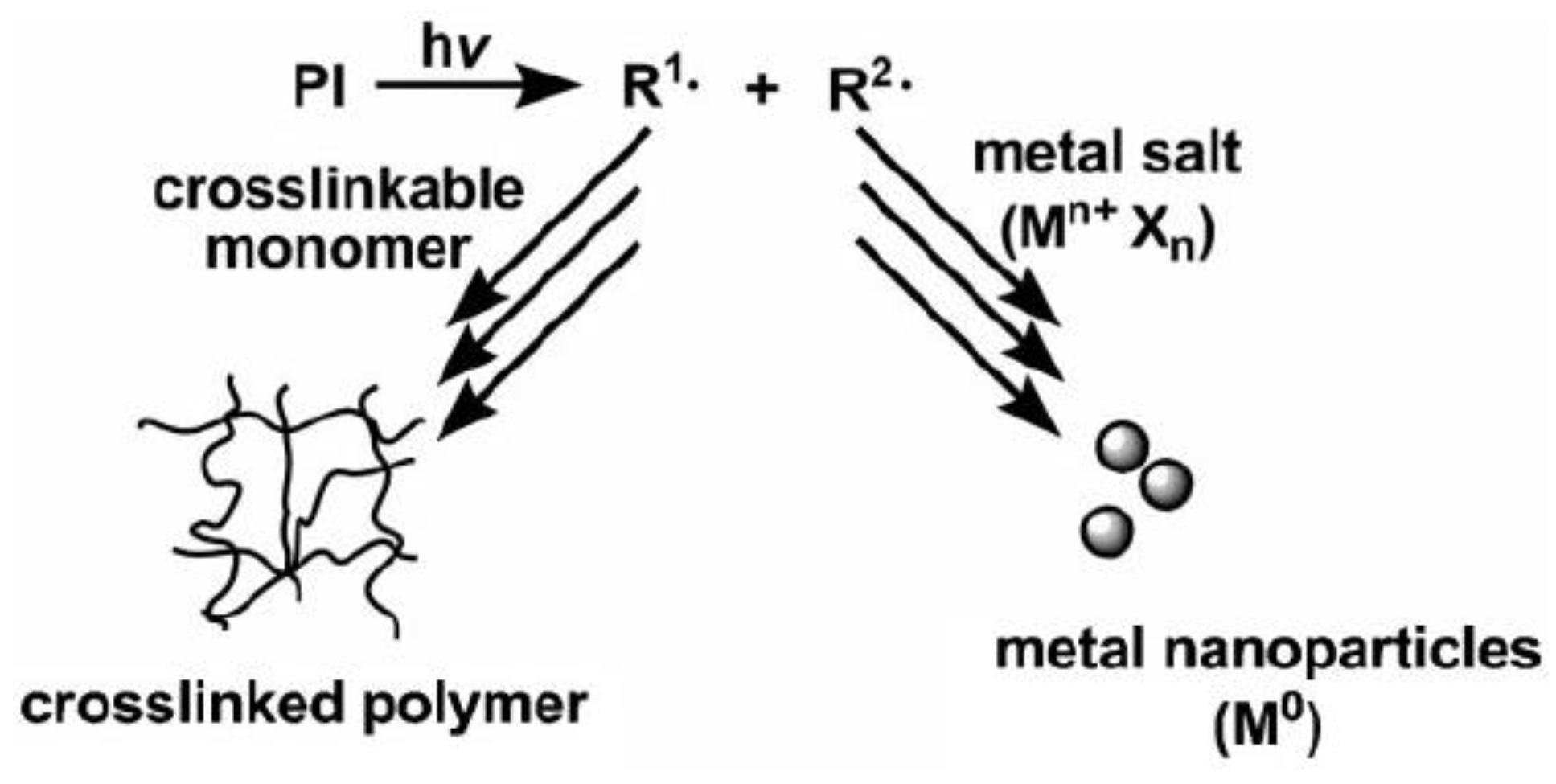
3.2.1. Light-Sensitive Antibacterial Hydrogels Loaded with Metal Nanoparticles
3.2.2. Organic Light-Sensitive Antibacterial Hydrogels
3.2.3. Hybrid Organic–Inorganic Light-Sensitive Antibacterial Hydrogels
4. Conclusions and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Klevens, R.M.; Edwards, J.R.; Richards, C.L.; Horan, T.C.; Gaynes, R.P.; Pollock, D.A.; Cardo, D.M. Estimating Health Care-Associated Infections and Deaths in U.S. Hospitals, 2002. Public Health Rep. 2007, 122, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Magill, S.S.; Edwards, J.R.; Bamberg, W.; Beldavs, Z.G.; Dumyati, G.; Kainer, M.A.; Lynfield, R.; Maloney, M.; McAllister-Hollod, L.; Nadle, J.; et al. Multistate Point-Prevalence Survey of Health Care–Associated Infections. N. Engl. J. Med. 2014, 370, 1198–1208. [Google Scholar] [CrossRef] [PubMed]
- Stone, P.W.; Pogorzelska-Maziarz, M.; Herzig, C.T.A.; Weiner, L.M.; Furuya, E.Y.; Dick, A.; Larson, E. State of Infection Prevention in US Hospitals Enrolled in the National Health and Safety Network. Am. J. Infect. Control 2014, 42, 94–99. [Google Scholar] [CrossRef]
- Graves, N.; Halton, K.; Lairson, D. Economics and Preventing Hospital-Acquired Infection: Broadening the Perspective. Infect. Control. Hosp. Epidemiol. 2007, 28, 178–184. [Google Scholar] [CrossRef]
- Stone, P.W.; Larson, E.; Kawar, L.N. A Systematic Audit of Economic Evidence Linking Nosocomial Infections and Infection Control Interventions: 1990-2000. Am. J. Infect. Control 2002, 30, 145–152. [Google Scholar] [CrossRef]
- Condat, M.; Helary, C.; Coradin, T.; Dubot, P.; Babinot, J.; Faustini, M.; Andaloussi, S.A.; Renard, E.; Langlois, V.; Versace, D.-L. Design of Cytocompatible Bacteria-Repellent Bio-Based Polyester Films via an Aqueous Photoactivated Process. J. Mater. Chem. B 2016, 4, 2842–2850. [Google Scholar] [CrossRef][Green Version]
- Sautrot-Ba, P.; Malval, J.-P.; Weiss-Maurin, M.; Paul, J.; Blacha-Grzechnik, A.; Tomane, S.; Mazeran, P.-E.; Lalevée, J.; Langlois, V.; Versace, D.-L. Paprika, Gallic Acid, and Visible Light: The Green Combination for the Synthesis of Biocide Coatings. ACS Sustain. Chem. Eng. 2018, 6, 104–109. [Google Scholar] [CrossRef]
- Versace, D.-L.; Moran, G.; Belqat, M.; Spangenberg, A.; Méallet-Renault, R.; Abbad-Andaloussi, S.; Brezová, V.; Malval, J.-P. Highly Virulent Bactericidal Effects of Curcumin-Based μ-Cages Fabricated by Two-Photon Polymerization. ACS Appl. Mater. Interfaces 2020, 12, 5050–5057. [Google Scholar] [CrossRef] [PubMed]
- Sautrot-Ba, P.; Jockusch, S.; Malval, J.-P.; Brezová, V.; Rivard, M.; Abbad-Andaloussi, S.; Blacha-Grzechnik, A.; Versace, D.-L. Quinizarin Derivatives as Photoinitiators for Free-Radical and Cationic Photopolymerizations in the Visible Spectral Range. Macromolecules 2020, 53, 1129–1141. [Google Scholar] [CrossRef]
- Marcille, H.; Malval, J.-P.; Presset, M.; Bogliotti, N.; Blacha-Grzechnik, A.; Brezová, V.; Yagci, Y.; Versace, D.-L. Diphenyl Functional Porphyrins and Their Metal Complexes as Visible-Light Photoinitiators for Free-Radical, Cationic and Thiol–Ene Polymerizations. Polym. Chem. 2020, 11, 4237–4249. [Google Scholar] [CrossRef]
- Versace, D.-L.; Ramier, J.; Grande, D.; Andaloussi, S.A.; Dubot, P.; Hobeika, N.; Malval, J.-P.; Lalevee, J.; Renard, E.; Langlois, V. Versatile Photochemical Surface Modification of Biopolyester Microfibrous Scaffolds with Photogenerated Silver Nanoparticles for Antibacterial Activity. Adv. Healthc. Mater. 2013, 2, 1008–1018. [Google Scholar] [CrossRef] [PubMed]
- Lorenzini, C.; Haider, A.; Kang, I.-K.; Sangermano, M.; Abbad-Andalloussi, S.; Mazeran, P.-E.; Lalevée, J.; Renard, E.; Langlois, V.; Versace, D.-L. Photoinduced Development of Antibacterial Materials Derived from Isosorbide Moiety. Biomacromolecules 2015, 16, 683–694. [Google Scholar] [CrossRef]
- Condat, M.; Mazeran, P.-E.; Malval, J.-P.; Lalevée, J.; Morlet-Savary, F.; Renard, E.; Langlois, V.; Andalloussi, S.A.; Versace, D.-L. Photoinduced Curcumin Derivative-Coatings with Antibacterial Properties. RSC Adv. 2015, 5, 85214–85224. [Google Scholar] [CrossRef]
- Sautrot-Ba, P.; Al Mousawi, A.; Lalevée, J.; Mazeran, P.-E.; Park, S.J.; Kang, I.-K.; Laurent-Brocq, M.; Langlois, V.; Versace, D.-L. Copper Complex: A Key Role in the Synthesis of Biocidal Polymer Coatings. Chem. Afr. 2019, 2, 241–251. [Google Scholar] [CrossRef]
- Breloy, L.; Ouarabi, C.A.; Brosseau, A.; Dubot, P.; Brezova, V.; Abbad Andaloussi, S.; Malval, J.-P.; Versace, D.-L. β-Carotene/Limonene Derivatives/Eugenol: Green Synthesis of Antibacterial Coatings under Visible-Light Exposure. ACS Sustain. Chem. Eng. 2019, 7, 19591–19604. [Google Scholar] [CrossRef]
- Modjinou, T.; Versace, D.L.; Abbad Andaloussi, S.; Langlois, V.; Renard, E. Co-Networks Poly(Hydroxyalkanoates)-Terpenes to Enhance Antibacterial Properties. Bioengineering 2020, 7, 13. [Google Scholar] [CrossRef] [PubMed]
- Badi, N.; Biesalski, M.; Börner, H.G.; Gattermayer, M.; Gentsch, R.; Laschewsky, A.; Lutz, J.-F.; Möhwald, H.; Petersen, S.; Polleux, J.; et al. Bioactive Surfaces; Börner, H.G., Lutz, J.-F., Badi, N., Eds.; Advances in Polymer Science; Springer: Berlin, Germany, 2011. [Google Scholar]
- Francolini, I.; Vuotto, C.; Piozzi, A.; Donelli, G. Antifouling and Antimicrobial Biomaterials: An Overview. APMIS 2017, 125, 392–417. [Google Scholar] [CrossRef]
- Koo, H.; Allan, R.N.; Howlin, R.P.; Stoodley, P.; Hall-Stoodley, L. Targeting Microbial Biofilms: Current and Prospective Therapeutic Strategies. Nat. Rev. Microbiol. 2017, 15, 740–755. [Google Scholar] [CrossRef]
- Vasilev, K.; Cavallaro, A.; Zilm, P. Special Issue: Antibacterial Materials and Coatings. Molecules 2018, 23, 585. [Google Scholar] [CrossRef] [PubMed]
- Mahira, S.; Jain, A.; Khan, W.; Domb, A.J. Chapter 1. Antimicrobial Materials—An Overview. In Biomaterials Science Series; Domb, A.J., Kunduru, K.R., Farah, S., Eds.; Royal Society of Chemistry: Cambridge, UK, 2019; pp. 1–37. [Google Scholar] [CrossRef]
- Li, X.; Wu, B.; Chen, H.; Nan, K.; Jin, Y.; Sun, L.; Wang, B. Recent Developments in Smart Antibacterial Surfaces to Inhibit Biofilm Formation and Bacterial Infections. J. Mater. Chem. B 2018, 6, 4274–4292. [Google Scholar] [CrossRef]
- Manecka, G.M.; Labrash, J.; Rouxel, O.; Dubot, P.; Lalevée, J.; Andaloussi, S.A.; Renard, E.; Langlois, V.; Versace, D.L. Green Photoinduced Modification of Natural Poly(3-Hydroxybutyrate- Co -3-Hydroxyvalerate) Surface for Antibacterial Applications. ACS Sustain. Chem. Eng. 2014, 2, 996–1006. [Google Scholar] [CrossRef]
- El Habnouni, S.; Darcos, V.; Garric, X.; Lavigne, J.-P.; Nottelet, B.; Coudane, J. Mild Methodology for the Versatile Chemical Modification of Polylactide Surfaces: Original Combination of Anionic and Click Chemistry for Biomedical Applications. Adv. Funct. Mater. 2011, 21, 3321–3330. [Google Scholar] [CrossRef]
- Schmitt, M.A.; Weisblum, B.; Gellman, S.H. Unexpected Relationships between Structure and Function in α,β-Peptides: Antimicrobial Foldamers with Heterogeneous Backbones. J. Am. Chem. Soc. 2004, 126, 6848–6849. [Google Scholar] [CrossRef]
- Schmitt, M.A.; Weisblum, B.; Gellman, S.H. Interplay among Folding, Sequence, and Lipophilicity in the Antibacterial and Hemolytic Activities of α/β-Peptides. J. Am. Chem. Soc. 2007, 129, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Kenawy, E. Biologically Active Polymers: Synthesis and Antimicrobial Activity of Modified Glycidyl Methacrylate Polymers Having a Quaternary Ammonium and Phosphonium Groups. J. Control. Release 1998, 50, 145–152. [Google Scholar] [CrossRef]
- Chang, Y.; Yandi, W.; Chen, W.-Y.; Shih, Y.-J.; Yang, C.-C.; Chang, Y.; Ling, Q.-D.; Higuchi, A. Tunable Bioadhesive Copolymer Hydrogels of Thermoresponsive Poly( N -Isopropyl Acrylamide) Containing Zwitterionic Polysulfobetaine. Biomacromolecules 2010, 11, 1101–1110. [Google Scholar] [CrossRef] [PubMed]
- Podsiadlo, P.; Paternel, S.; Rouillard, J.-M.; Zhang, Z.; Lee, J.; Lee, J.-W.; Gulari, E.; Kotov, N.A. Layer-by-Layer Assembly of Nacre-like Nanostructured Composites with Antimicrobial Properties. Langmuir 2005, 21, 11915–11921. [Google Scholar] [CrossRef]
- Modjinou, T.; Rodriguez-Tobias, H.; Morales, G.; Versace, D.-L.; Langlois, V.; Grande, D.; Renard, E. UV-Cured Thiol–Ene Eugenol/ZnO Composite Materials with Antibacterial Properties. RSC Adv. 2016, 6, 88135–88142. [Google Scholar] [CrossRef]
- Tang, Z.; Kotov, N.A.; Magonov, S.; Ozturk, B. Nanostructured Artificial Nacre. Nature Mater 2003, 2, 413–418. [Google Scholar] [CrossRef]
- Park, K.D.; Kim, Y.S.; Han, D.K.; Kim, Y.H.; Lee, E.H.B.; Suh, H.; Choi, K.S. Bacterial Adhesion on PEG Modified Polyurethane Surfaces. Biomaterials 1998, 19, 851–859. [Google Scholar] [CrossRef]
- Genzer, J.; Efimenko, K. Recent Developments in Superhydrophobic Surfaces and Their Relevance to Marine Fouling: A Review. Biofouling 2006, 22, 339–360. [Google Scholar] [CrossRef]
- Khurana, B.; Gierlich, P.; Meindl, A.; Gomes-da-Silva, L.C.; Senge, M.O. Hydrogels: Soft Matters in Photomedicine. Photochem. Photobiol. Sci. 2019, 18, 2613–2656. [Google Scholar] [CrossRef]
- Varaprasad, K.; Raghavendra, G.M.; Jayaramudu, T.; Yallapu, M.M.; Sadiku, R. A Mini Review on Hydrogels Classification and Recent Developments in Miscellaneous Applications. Mater. Sci. Eng. C 2017, 79, 958–971. [Google Scholar] [CrossRef] [PubMed]
- Bashir, S.; Hina, M.; Iqbal, J.; Rajpar, A.H.; Mujtaba, M.A.; Alghamdi, N.A.; Wageh, S.; Ramesh, K.; Ramesh, S. Fundamental Concepts of Hydrogels: Synthesis, Properties, and Their Applications. Polymers 2020, 12, 2702. [Google Scholar] [CrossRef] [PubMed]
- Ghobril, C.; Grinstaff, M.W. The Chemistry and Engineering of Polymeric Hydrogel Adhesives for Wound Closure: A Tutorial. Chem. Soc. Rev. 2015, 44, 1820–1835. [Google Scholar] [CrossRef] [PubMed]
- Ng, V.W.L.; Chan, J.M.W.; Sardon, H.; Ono, R.J.; García, J.M.; Yang, Y.Y.; Hedrick, J.L. Antimicrobial Hydrogels: A New Weapon in the Arsenal against Multidrug-Resistant Infections. Adv. Drug Deliv. Rev. 2014, 78, 46–62. [Google Scholar] [CrossRef] [PubMed]
- Mehrotra, D.; Dwivedi, R.; Nandana, D.; Singh, R.K. From Injectable to 3D Printed Hydrogels in Maxillofacial Tissue Engineering: A Review. J. Oral Biol. Craniofac. Res. 2020, 10, 680–689. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Xu, H.; Che, L.; Sha, D.; Huang, C.; Meng, T.; Song, D. Application of Inorganic Nanocomposite Hydrogels in Bone Tissue Engineering. iScience 2020, 23, 101845. [Google Scholar] [CrossRef]
- Sun, A.; He, X.; Li, L.; Li, T.; Liu, Q.; Zhou, X.; Ji, X.; Li, W.; Qian, Z. An Injectable Photopolymerized Hydrogel with Antimicrobial and Biocompatible Properties for Infected Skin Regeneration. NPG Asia Mater. 2020, 12, 25. [Google Scholar] [CrossRef]
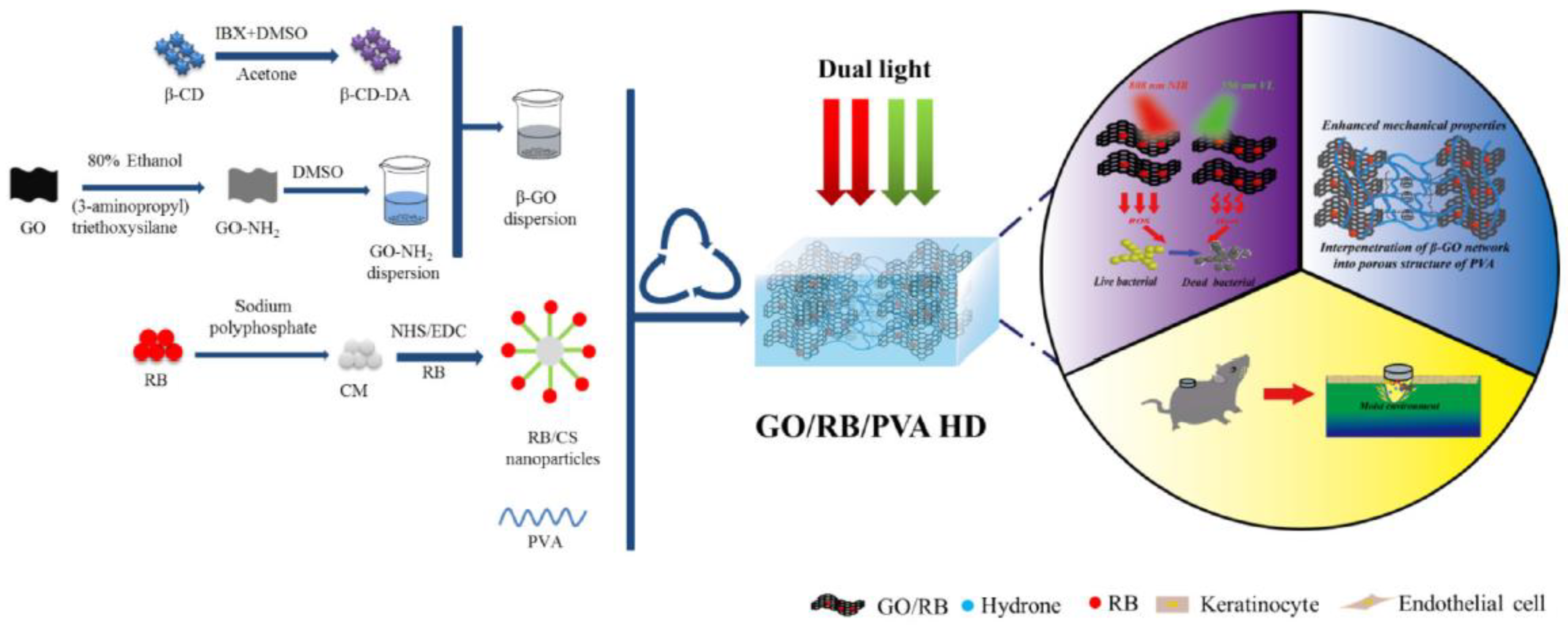
- Li, Y.; Wang, J.; Yang, Y.; Shi, J.; Zhang, H.; Yao, X.; Chen, W.; Zhang, X. A Rose Bengal/Graphene Oxide/PVA Hybrid Hydrogel with Enhanced Mechanical Properties and Light-Triggered Antibacterial Activity for Wound Treatment. Mater. Sci. Eng. C 2021, 118, 111447. [Google Scholar] [CrossRef]
- Zakia, M.; Koo, J.M.; Kim, D.; Ji, K.; Huh, P.; Yoon, J.; Yoo, S.I. Development of Silver Nanoparticle-Based Hydrogel Composites for Antimicrobial Activity. Green Chem. Lett. Rev. 2020, 13, 34–40. [Google Scholar] [CrossRef]
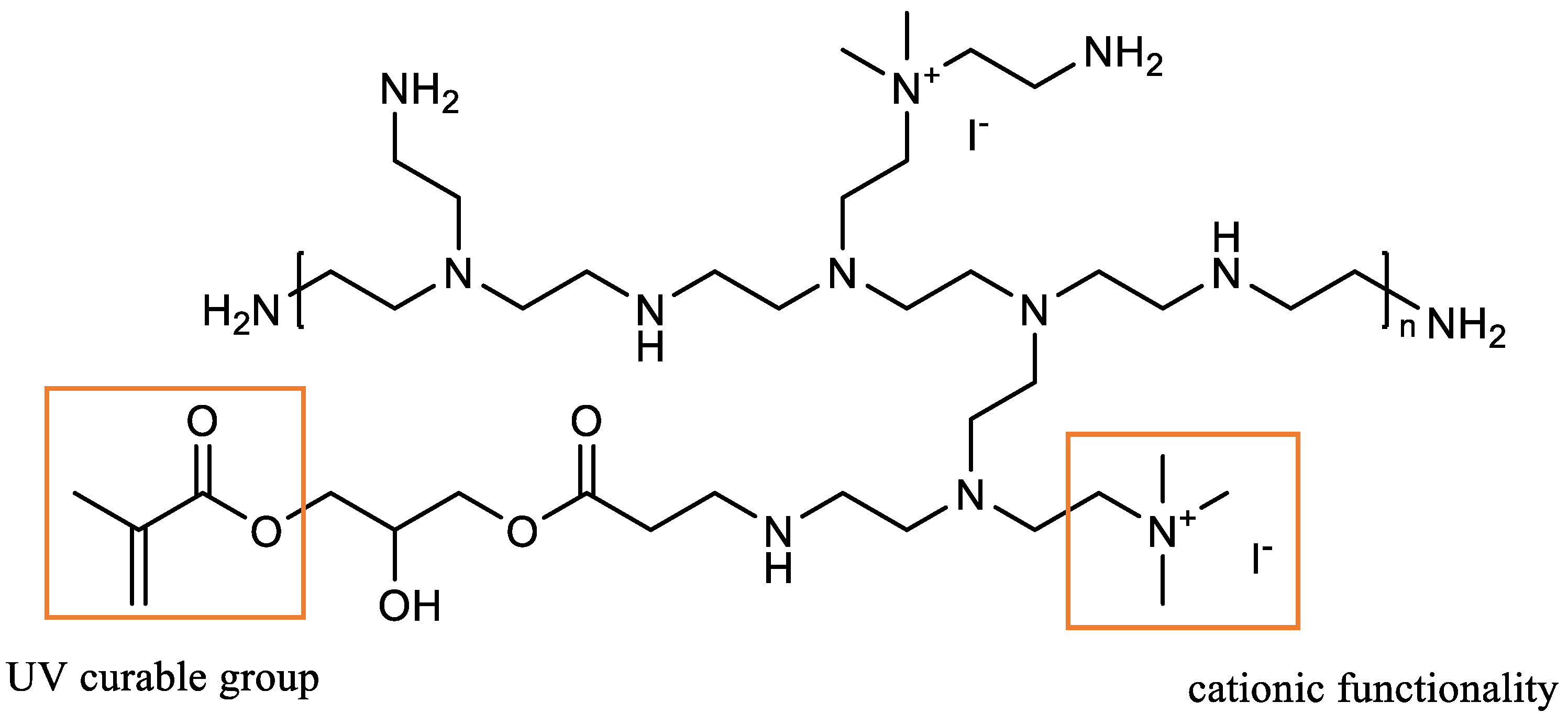
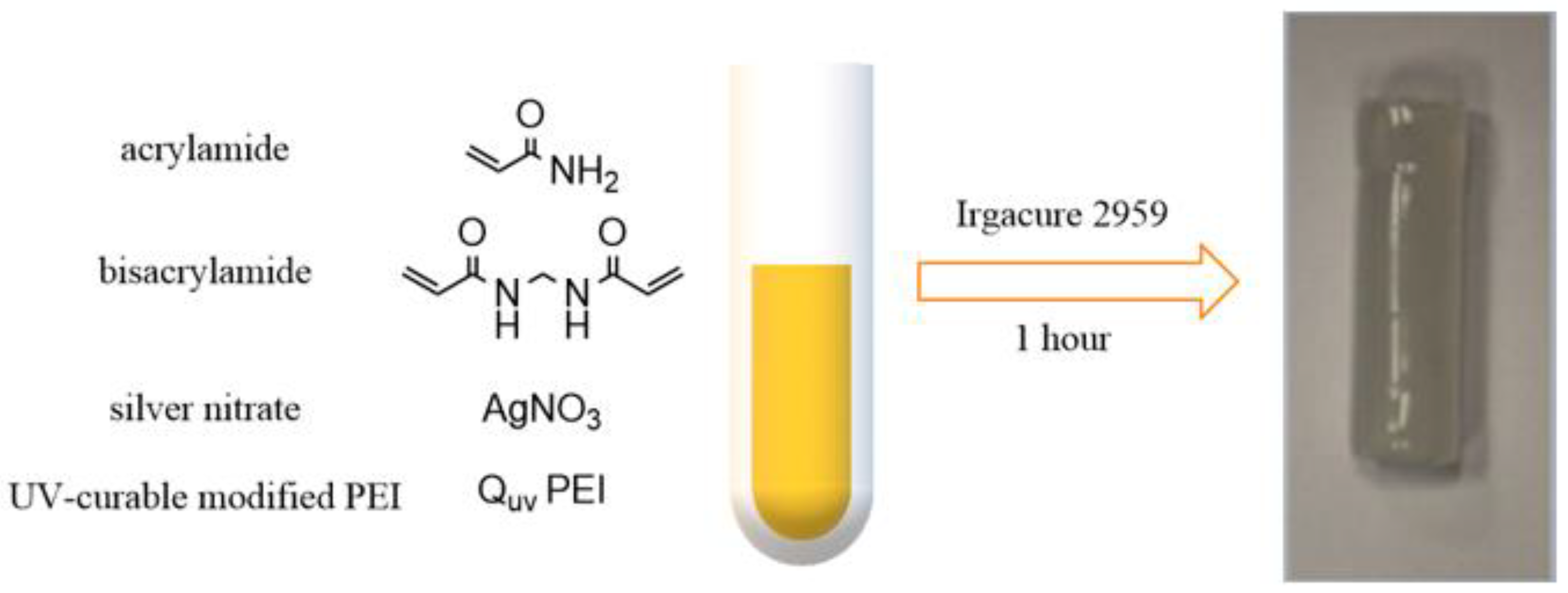
- Palantoken, A.; Sari Yilmaz, M.; Altikatoğlu Yapaöz, M.; Yenigül Tulunay, E.; Eren, T.; Piskin, S. Dual Antimicrobial Effects Induced by Hydrogel Incorporated with UV-Curable Quaternary Ammonium Polyethyleneimine and AgNO3. Mater. Sci. Eng. C 2016, 68, 494–504. [Google Scholar] [CrossRef]
- Zhao, C.; Zhou, L.; Chiao, M.; Yang, W. Antibacterial Hydrogel Coating: Strategies in Surface Chemistry. Adv. Colloid Interface Sci. 2020, 285, 102280. [Google Scholar] [CrossRef]
- Parsons, C.; McCoy, C.P.; Gorman, S.P.; Jones, D.S.; Bell, S.E.J.; Brady, C.; McGlinchey, S.M. Anti-Infective Photodynamic Biomaterials for the Prevention of Intraocular Lens-Associated Infectious Endophthalmitis. Biomaterials 2009, 30, 597–602. [Google Scholar] [CrossRef]
- Silva, D.; de Sousa, H.C.; Gil, M.H.; Santos, L.F.; Amaral, R.; Saraiva, J.A.; Oom, M.S.; Alvarez-Lorenzo, C.; Serro, A.P.; Saramago, B. Imprinted Hydrogels with LbL Coating for Dual Drug Release from Soft Contact Lenses Materials. SSRN J. 2021, 120, 111687. [Google Scholar] [CrossRef]
- Gwon, K.; Han, I.; Lee, S.; Kim, Y.; Lee, D.N. Novel Metal–Organic Framework-Based Photocrosslinked Hydrogel System for Efficient Antibacterial Applications. ACS Appl. Mater. Interfaces 2020, 12, 20234–20242. [Google Scholar] [CrossRef]
- Bellotti, E.; Schilling, A.L.; Little, S.R.; Decuzzi, P. Injectable Thermoresponsive Hydrogels as Drug Delivery System for the Treatment of Central Nervous System Disorders: A Review. J. Control. Release 2021, 329, 16–35. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Wu, L.; Li, T.; Xu, D.; Lin, X.; Wu, C. Facile Preparation of Biomass Lignin-Based Hydroxyethyl Cellulose Super-Absorbent Hydrogel for Dye Pollutant Removal. Int. J. Biol. Macromol. 2019, 137, 939–947. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Huang, S.; Zheng, J.; Qiu, Z.; Lin, X.; Qin, Y. Synthesis and Characterization of Biomass Lignin-Based PVA Super-Absorbent Hydrogel. Int. J. Biol. Macromol. 2019, 140, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Barthes, J.; Lagarrigue, P.; Riabov, V.; Lutzweiler, G.; Kirsch, J.; Muller, C.; Courtial, E.-J.; Marquette, C.; Projetti, F.; Kzhyskowska, J.; et al. Biofunctionalization of 3D-Printed Silicone Implants with Immunomodulatory Hydrogels for Controlling the Innate Immune Response: An in Vivo Model of Tracheal Defect Repair. Biomaterials 2021, 268, 120549. [Google Scholar] [CrossRef]
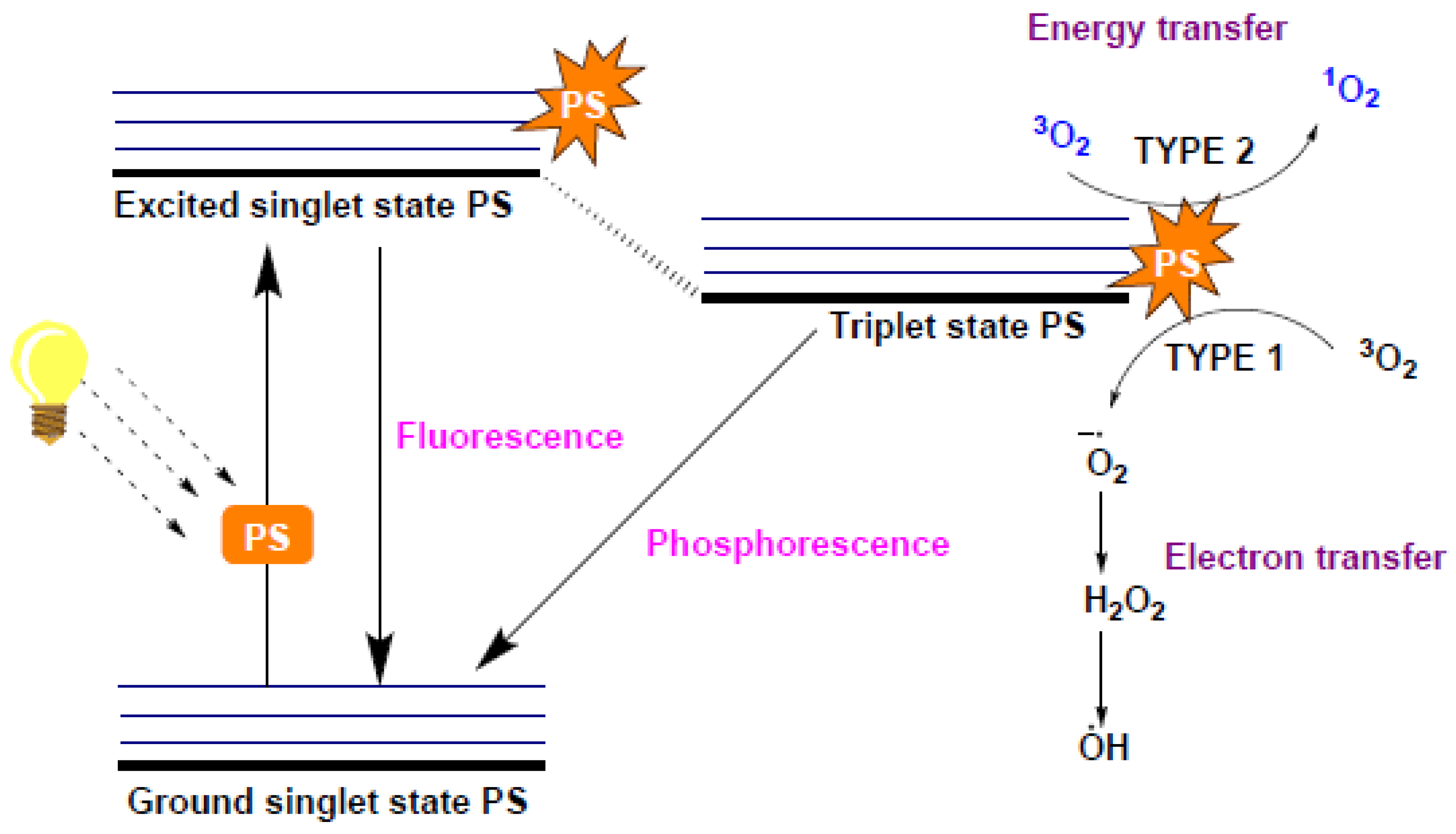
- Dharmaratne, P.; Sapugahawatte, D.N.; Wang, B.; Chan, C.L.; Lau, K.-M.; Lau, C.B.; Fung, K.P.; Ng, D.K.P.; Ip, M. Contemporary Approaches and Future Perspectives of Antibacterial Photodynamic Therapy (APDT) against Methicillin-Resistant Staphylococcus Aureus (MRSA): A Systematic Review. Eur. J. Med. Chem. 2020, 200, 112341. [Google Scholar] [CrossRef]
- Pinelli, F.; Magagnin, L.; Rossi, F. Progress in Hydrogels for Sensing Applications: A Review. Mater. Today Chem. 2020, 17, 100317. [Google Scholar] [CrossRef]
- Yang, J. Freezing-Tolerant and Robust Gelatin-Based Supramolecular Conductive Hydrogels with Double-Network Structure for Wearable Sensors. Polym. Test. 2021, 93, 106879. [Google Scholar] [CrossRef]
- Dai, L. A Multifunctional Self-Crosslinked Chitosan/Cationic Guar Gum Composite Hydrogel and Its Versatile Uses in Phosphate-Containing Water Treatment and Energy Storage. Carbohydr. Polym. 2020, 244, 116472. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhai, S.; Yuan, Z.; Chen, J.; Yu, Z.; Pei, Z.; Liu, F.; Li, X.; Wei, L.; Chen, Y. Drying Graphene Hydrogel Fibers for Capacitive Energy Storage. Carbon 2020, 164, 100–110. [Google Scholar] [CrossRef]
- Sinha, V.; Chakma, S. Advances in the Preparation of Hydrogel for Wastewater Treatment: A Concise Review. J. Environ. Chem. Eng. 2019, 7, 103295. [Google Scholar] [CrossRef]
- Sharshir, S.W.; Algazzar, A.M.; Elmaadawy, K.A.; Kandeal, A.W.; Elkadeem, M.R.; Arunkumar, T.; Zang, J.; Yang, N. New Hydrogel Materials for Improving Solar Water Evaporation, Desalination and Wastewater Treatment: A Review. Desalination 2020, 491, 114564. [Google Scholar] [CrossRef]
- Qu, B.; Luo, Y. Chitosan-Based Hydrogel Beads: Preparations, Modifications and Applications in Food and Agriculture Sectors – A Review. Int. J. Biol. Macromol. 2020, 152, 437–448. [Google Scholar] [CrossRef]
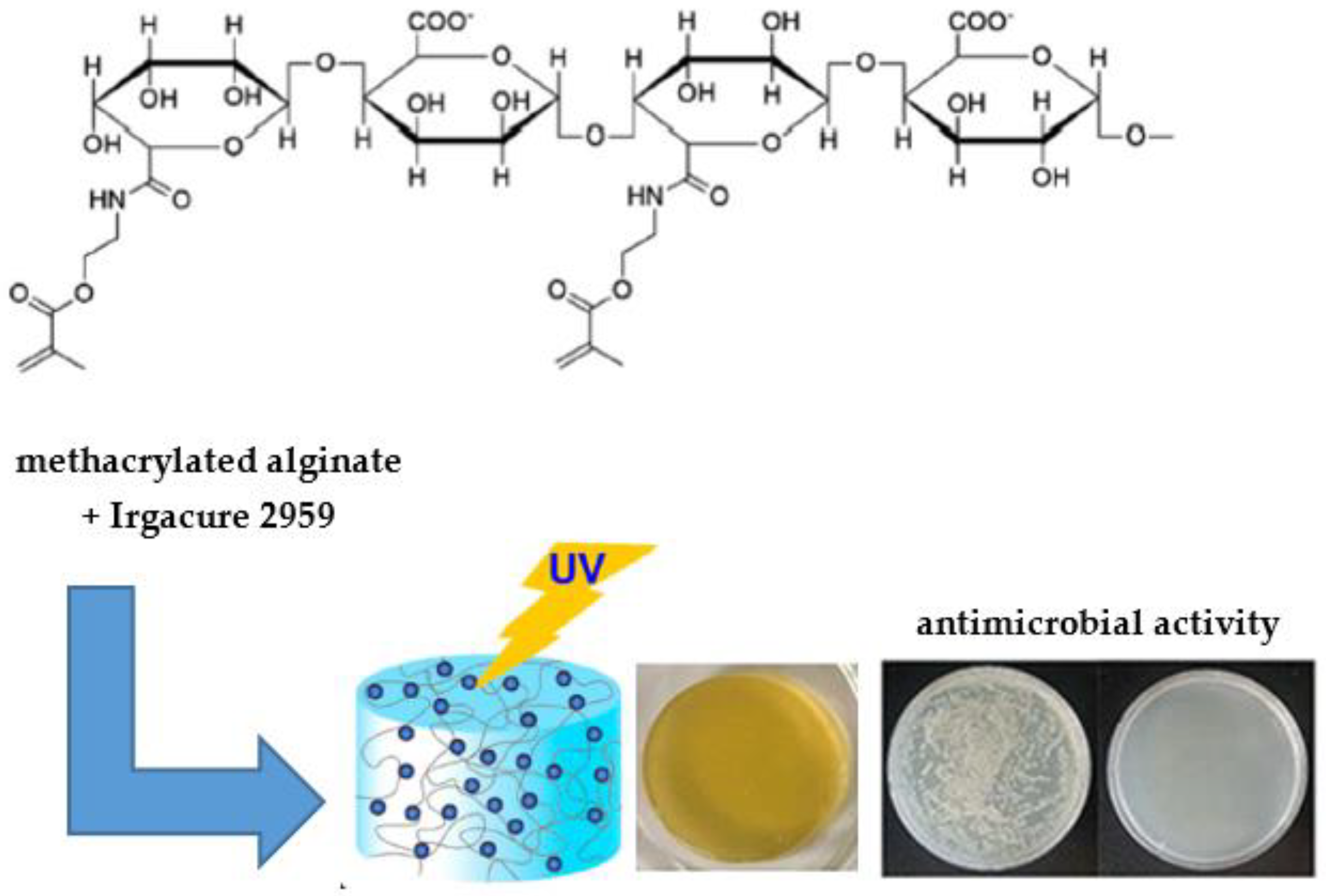
- Sautrot-Ba, P.; Jockusch, S.; Nguyen, T.-T.-T.; Grande, D.; Chiapionne, A.; Abbad-Andaloussi, S.; Pan, M.; Méallet-Renault, R.; Versace, D.-L. Photoinduced Synthesis of Antibacterial Hydrogel from Aqueous Photoinitiating System. Eur. Polym. J. 2020, 138, 109936. [Google Scholar] [CrossRef]
- Sautrot-Ba, P.; Razza, N.; Breloy, L.; Andaloussi, S.A.; Chiappone, A.; Sangermano, M.; Hélary, C.; Belbekhouche, S.; Coradin, T.; Versace, D.-L. Photoinduced Chitosan–PEG Hydrogels with Long-Term Antibacterial Properties. J. Mater. Chem. B 2019, 7, 6526–6538. [Google Scholar] [CrossRef] [PubMed]
- Monnereau, C.; Andraud, C. Thérapie photodynamique: Évolution chimique des photosensibilisateurs. 2013, p. 16. Available online: https://www.techniques-ingenieur.fr/base-documentaire/innovation-th10/innovations-en-analyses-et-mesures-42112210/therapie-photodynamique-evolution-chimique-des-photosensibilisateurs-in161/ (accessed on 20 January 2021).
- Yang, K.; Han, Q.; Chen, B.; Zheng, Y.; Zhang, K.; Li, Q.; Wang, J. Antimicrobial Hydrogels: Promising Materials for Medical Application. Int. J. Nanomed. 2018, 13, 2217–2263. [Google Scholar] [CrossRef]
- Uygun, M.; Kahveci, M.U.; Odaci, D.; Timur, S.; Yagci, Y. Antibacterial Acrylamide Hydrogels Containing Silver Nanoparticles by Simultaneous Photoinduced Free Radical Polymerization and Electron Transfer Processes. Macromol. Chem. Phys. 2009, 210, 1867–1875. [Google Scholar] [CrossRef]
- La, Y.-H.; McCloskey, B.D.; Sooriyakumaran, R.; Vora, A.; Freeman, B.; Nassar, M.; Hedrick, J.; Nelson, A.; Allen, R. Bifunctional Hydrogel Coatings for Water Purification Membranes: Improved Fouling Resistance and Antimicrobial Activity. J. Membr. Sci. 2011, 372, 285–291. [Google Scholar] [CrossRef]
- Kahveci, M.U.; Ciftci, M.; Evran, S.; Timur, S.; Yagci, Y. Photoinduced in Situ Formation of Clickable PEG Hydrogels and Their Antibody Conjugation. Des. Monomers Polym. 2015, 18, 129–136. [Google Scholar] [CrossRef]
- Fouassier, J.-P.; Lalevée, J. Photoinitiators for Polymer Synthesis: Scope, Reactivity and Efficiency; Wiley-VCH: Weinheim, Germany, 2012. [Google Scholar]
- Chatani, S.; Kloxin, C.J.; Bowman, C.N. The Power of Light in Polymer Science: Photochemical Processes to Manipulate Polymer Formation, Structure, and Properties. Polym. Chem. 2014, 5, 2187–2201. [Google Scholar] [CrossRef]
- Fouassier, J.P.; Lalevée, J. Photoinitiators: Structures, Reactivity and Applications in Polymerization; Wiley-VCH: Weinheim, Germany, 2021. [Google Scholar]
- Yagci, Y.; Jockusch, S.; Turro, N.J. Photoinitiated Polymerization: Advances, Challenges, and Opportunities. Macromolecules 2010, 43, 6245–6260. [Google Scholar] [CrossRef]
- Sangermano, M.; Razza, N.; Crivello, J.V. Cationic UV-Curing: Technology and Applications: Cationic UV-Curing: Technology and Applications. Macromol. Mater. Eng. 2014, 299, 775–793. [Google Scholar] [CrossRef]
- Williams, C.G.; Malik, A.N.; Kim, T.K.; Manson, P.N.; Elisseeff, J.H. Variable Cytocompatibility of Six Cell Lines with Photoinitiators Used for Polymerizing Hydrogels and Cell Encapsulation. Biomaterials 2005, 26, 1211–1218. [Google Scholar] [CrossRef]
- Hoyle, C.E.; Bowman, C.N. Thiol–Ene Click Chemistry. Angew. Chem. Int. Ed. 2010, 49, 1540–1573. [Google Scholar] [CrossRef]
- Lowe, A.B. Thiol-Ene “Click” Reactions and Recent Applications in Polymer and Materials Synthesis. Polym. Chem. 2010, 1, 17–36. [Google Scholar] [CrossRef]
- Sinha, A.K.; Equbal, D. Thiol−Ene Reaction: Synthetic Aspects and Mechanistic Studies of an Anti-Markovnikov-Selective Hydrothiolation of Olefins. Asian J. Org. Chem. 2019, 8, 32–47. [Google Scholar] [CrossRef]
- Fairbanks, B.D.; Love, D.M.; Bowman, C.N. Efficient Polymer-Polymer Conjugation via Thiol-Ene Click Reaction. Macromol. Chem. Phys. 2017, 218, 1700073. [Google Scholar] [CrossRef]
- Sawicki, L.A.; Kloxin, A.M. Design of Thiol–Ene Photoclick Hydrogels Using Facile Techniques for Cell Culture Applications. Biomater. Sci. 2014, 2, 1612–1626. [Google Scholar] [CrossRef]
- Kharkar, P.M.; Rehmann, M.S.; Skeens, K.M.; Maverakis, E.; Kloxin, A.M. Thiol–Ene Click Hydrogels for Therapeutic Delivery. ACS Biomater. Sci. Eng. 2016, 2, 165–179. [Google Scholar] [CrossRef]
- Lin, C.-C.; Raza, A.; Shih, H. PEG Hydrogels Formed by Thiol-Ene Photo-Click Chemistry and Their Effect on the Formation and Recovery of Insulin-Secreting Cell Spheroids. Biomaterials 2011, 32, 9685–9695. [Google Scholar] [CrossRef] [PubMed]
- Sajomsang, W.; Gonil, P.; Tantayanon, S. Antibacterial Activity of Quaternary Ammonium Chitosan Containing Mono or Disaccharide Moieties: Preparation and Characterization. Int. J. Biol. Macromol. 2009, 44, 419–427. [Google Scholar] [CrossRef]
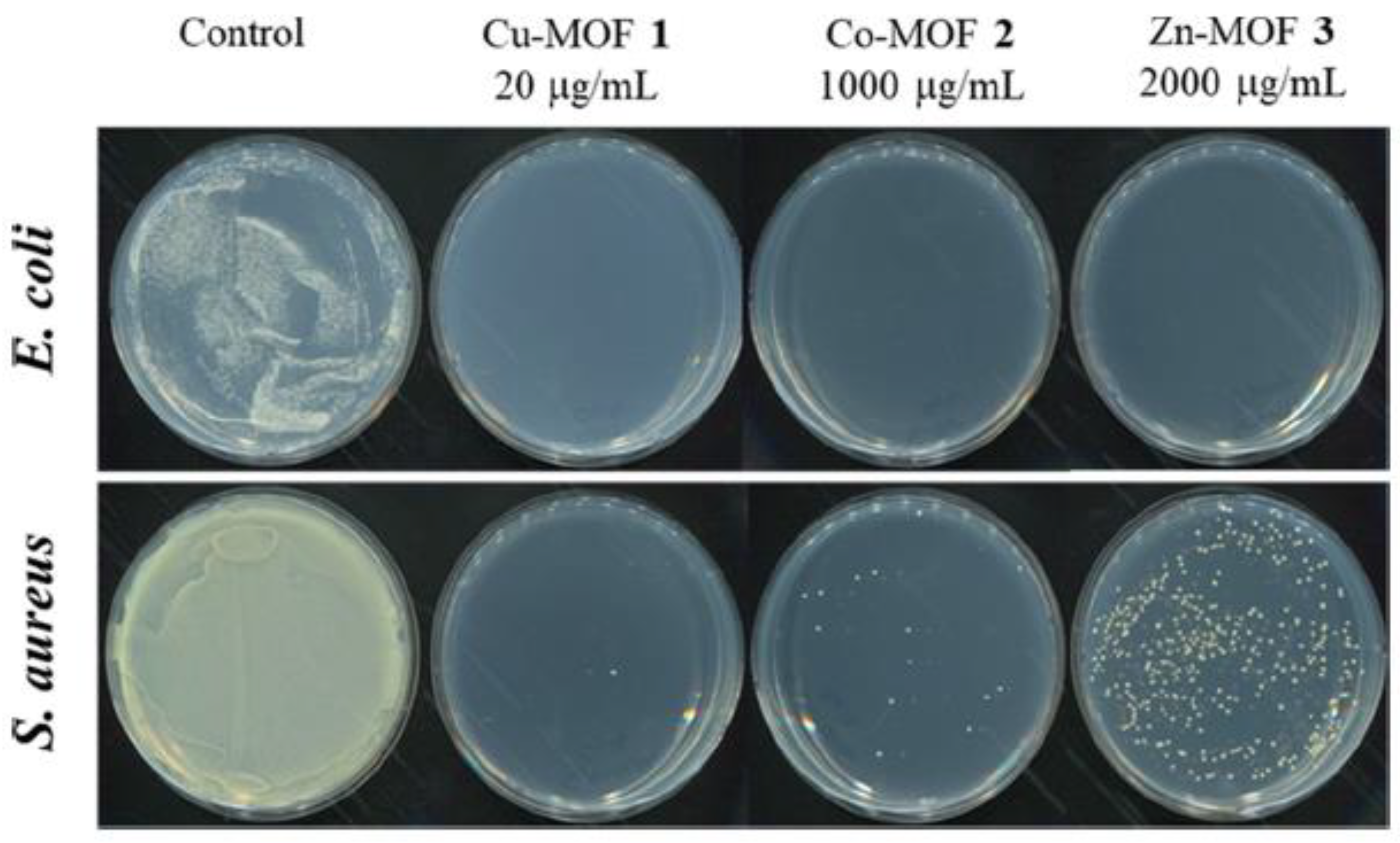
- Jo, J.H.; Kim, H.-C.; Huh, S.; Kim, Y.; Lee, D.N. Antibacterial Activities of Cu-MOFs Containing Glutarates and Bipyridyl Ligands. Dalton Trans. 2019, 48, 8084–8093. [Google Scholar] [CrossRef] [PubMed]
- Alarcon, E.I.; Udekwu, K.I.; Noel, C.W.; Gagnon, L.B.-P.; Taylor, P.K.; Vulesevic, B.; Simpson, M.J.; Gkotzis, S.; Islam, M.M.; Lee, C.-J.; et al. Safety and Efficacy of Composite Collagen–Silver Nanoparticle Hydrogels as Tissue Engineering Scaffolds. Nanoscale 2015, 7, 18789–18798. [Google Scholar] [CrossRef]
- Chen, H.; Peng, C.; Wang, L.; Li, X.; Yang, M.; Liu, H.; Qin, H.; Chen, W. Mechanically Tough, Healable Hydrogels Synergistically Reinforced by UV-Responsive Crosslinker and Metal Coordination Interaction for Wound Healing Application. Chem. Eng. J. 2021, 403, 126341. [Google Scholar] [CrossRef]
- Ochmann, M.; Hussain, A.; von Ahnen, I.; Cordones, A.A.; Hong, K.; Lee, J.H.; Ma, R.; Adamczyk, K.; Kim, T.K.; Schoenlein, R.W.; et al. UV-Photochemistry of the Disulfide Bond: Evolution of Early Photoproducts from Picosecond X-Ray Absorption Spectroscopy at the Sulfur K-Edge. J. Am. Chem. Soc. 2018, 140, 6554–6561. [Google Scholar] [CrossRef]
- Tian, J.; Huang, B.; Nawaz, M.H.; Zhang, W. Recent Advances of Multi-Dimensional Porphyrin-Based Functional Materials in Photodynamic Therapy. Coord. Chem. Rev. 2020, 420, 213410. [Google Scholar] [CrossRef]
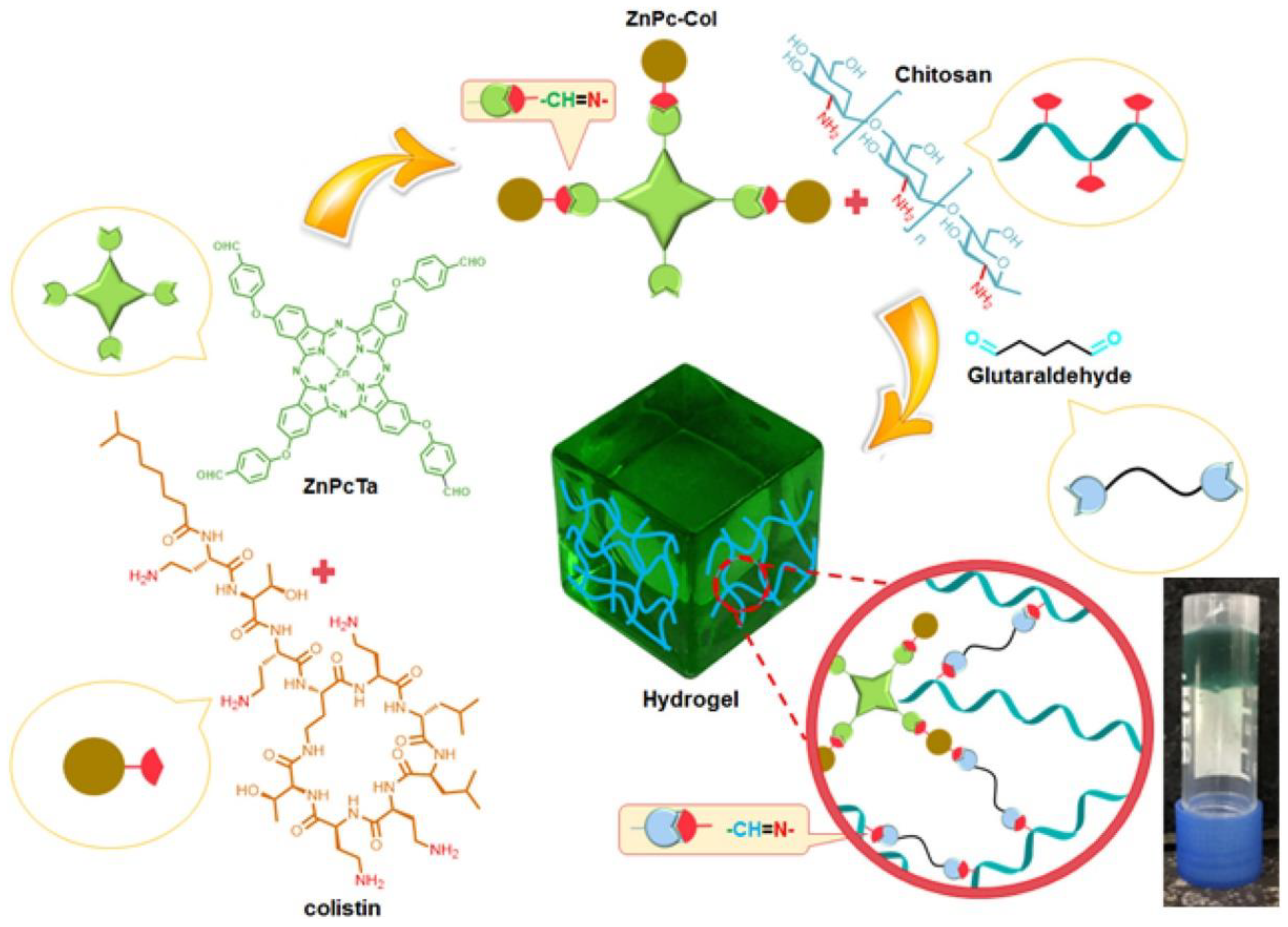
- Bayat, F.; Karimi, A.R. Design of Photodynamic Chitosan Hydrogels Bearing Phthalocyanine-Colistin Conjugate as an Antibacterial Agent. Int. J. Biol. Macromol. 2019, 129, 927–935. [Google Scholar] [CrossRef] [PubMed]
- Mao, C.; Xiang, Y.; Liu, X.; Cui, Z.; Yang, X.; Yeung, K.W.K.; Pan, H.; Wang, X.; Chu, P.K.; Wu, S. Photo-Inspired Antibacterial Activity and Wound Healing Acceleration by Hydrogel Embedded with Ag/Ag@AgCl/ZnO Nanostructures. ACS Nano 2017, 11, 9010–9021. [Google Scholar] [CrossRef]
- Zhu, P.; Weng, Z.; Li, X.; Liu, X.; Wu, S.; Yeung, K.W.K.; Wang, X.; Cui, Z.; Yang, X.; Chu, P.K. Biomedical Applications of Functionalized ZnO Nanomaterials: From Biosensors to Bioimaging. Adv. Mater. Interfaces 2016, 3, 1500494. [Google Scholar] [CrossRef]
- Yang, Q.; Pogue, J.M.; Li, Z.; Nation, R.L.; Kaye, K.S.; Li, J. Agents of Last Resort. Infect. Dis. Clin. N. Am. 2020, 34, 723–750. [Google Scholar] [CrossRef] [PubMed]
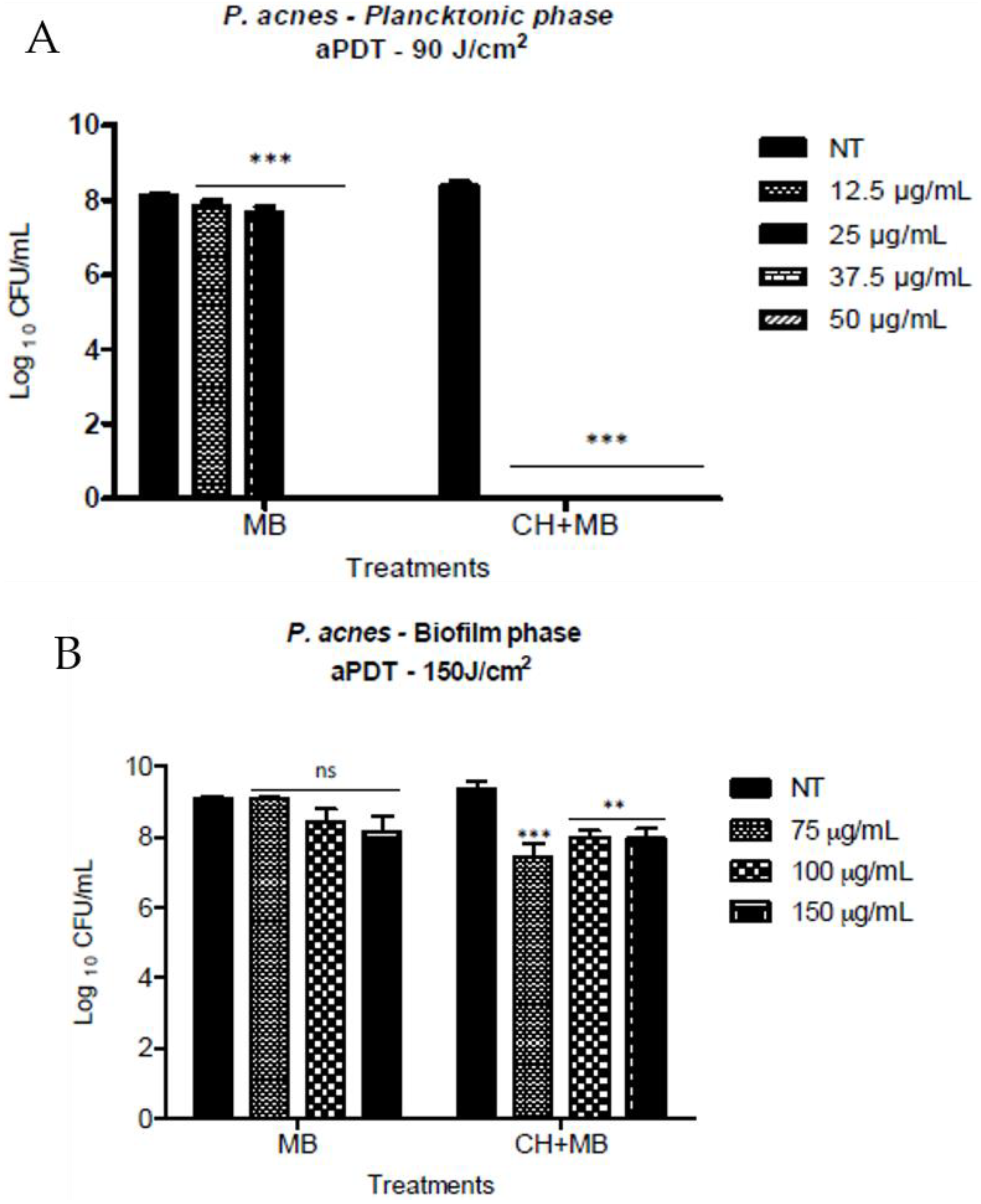
- Frade, M.L.; De Annuncio, S.R.; Fioramonti Calixto, G.M.; Damiani Victorelli, F.; Chorilli, M.; Fontana, C.R. Assessment of Chitosan-Based Hydrogel and Photodynamic Inactivation against Propionibacterium Acnes. Molecules 2018, 28, 473. [Google Scholar] [CrossRef] [PubMed]
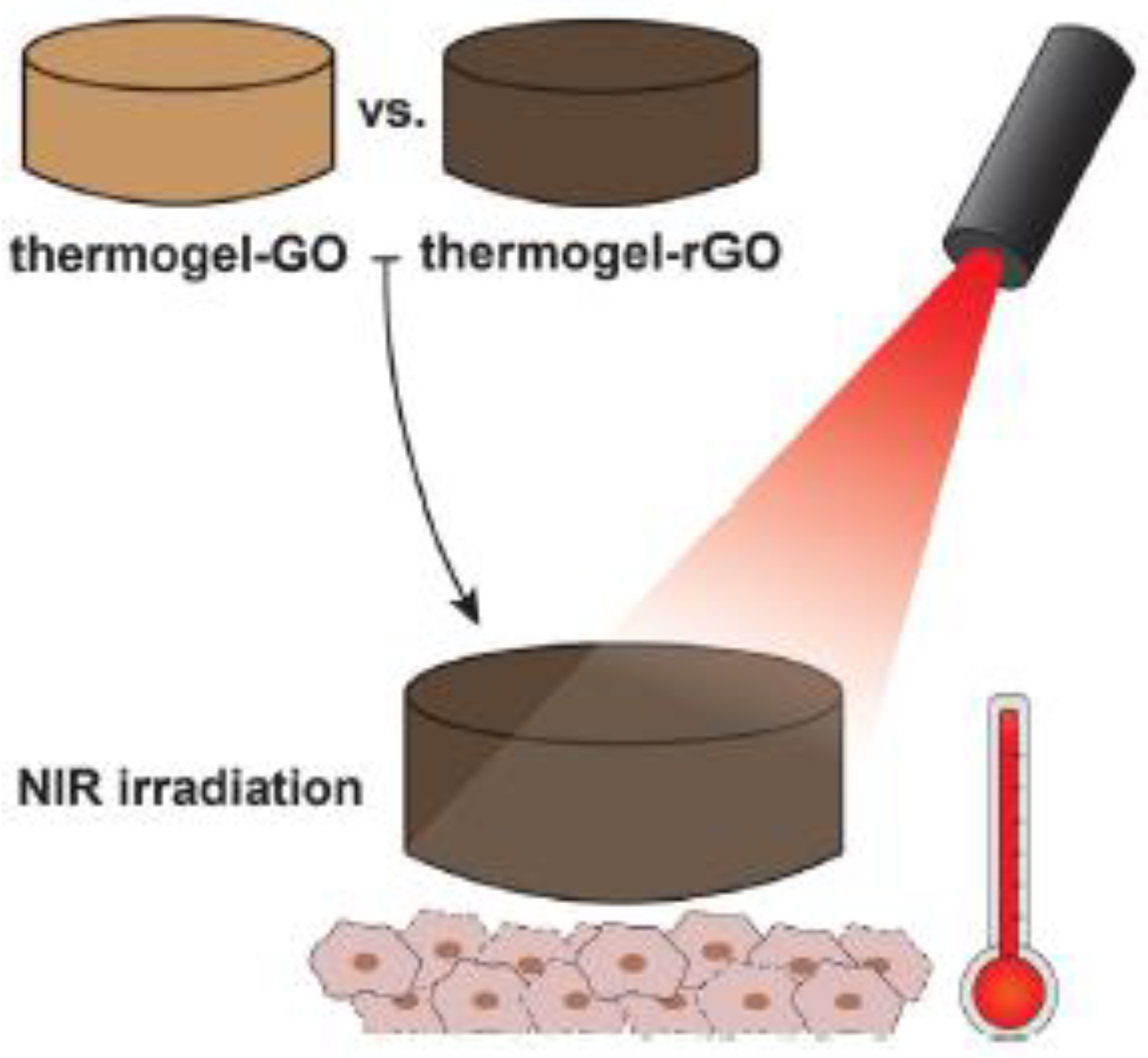
- Lima-Sousa, R.; de Melo-Diogo, D.; Alves, C.G.; Cabral, C.S.D.; Miguel, S.P.; Mendonça, A.G.; Correia, I.J. Injectable in Situ Forming Thermo-Responsive Graphene Based Hydrogels for Cancer Chemo-Photothermal Therapy and NIR Light-Enhanced Antibacterial Applications. Mater. Sci. Eng. C 2020, 117, 111294. [Google Scholar] [CrossRef]
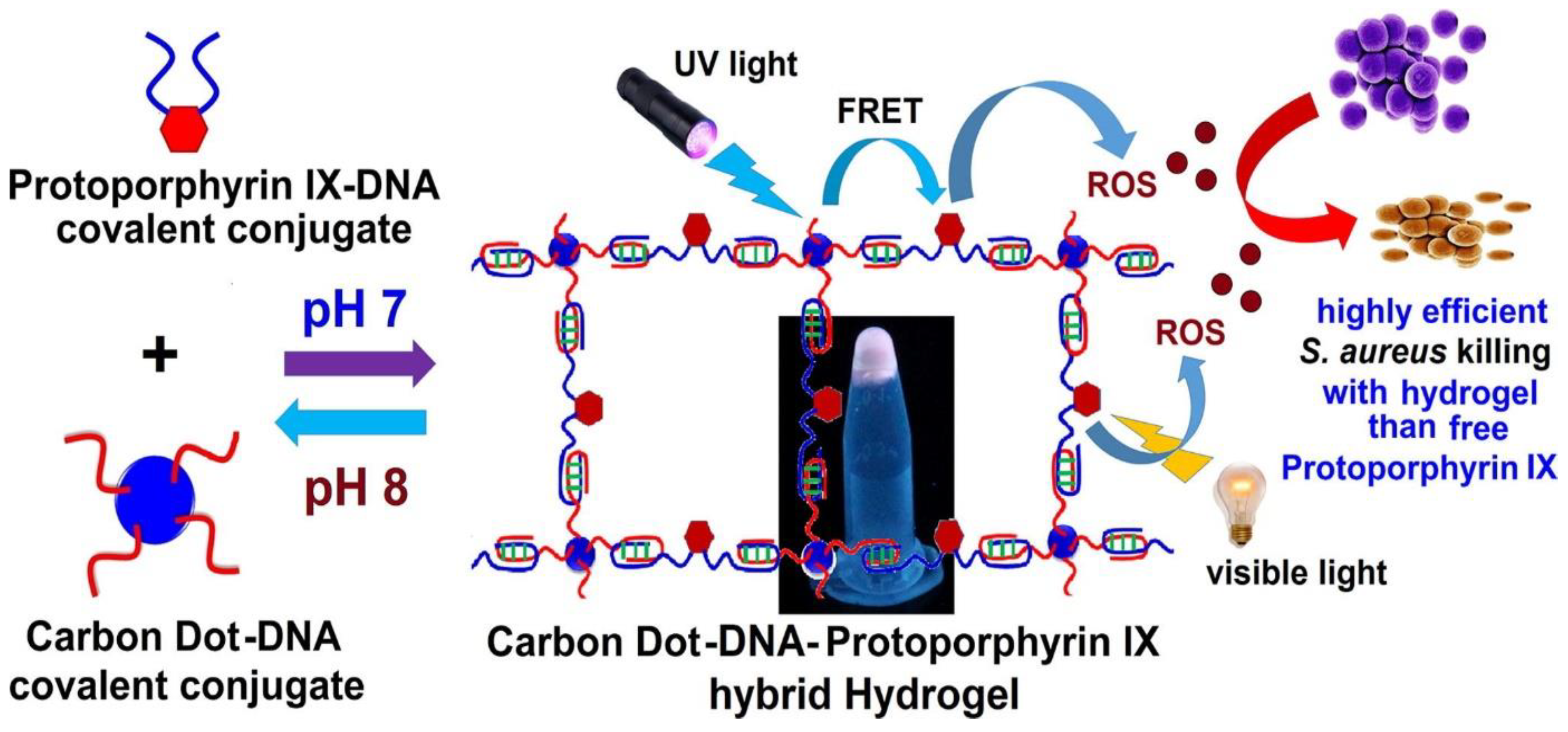
- Kumari, S.; Rajit Prasad, S.; Mandal, D.; Das, P. Carbon Dot-DNA-Protoporphyrin Hybrid Hydrogel for Sustained Photoinduced Antimicrobial Activity. J. Colloid Interface Sci. 2019, 553, 228–238. [Google Scholar] [CrossRef]
- Paoli, M.; Marles-Wright, J.; Smith, A. Structure–Function Relationships in Heme-Proteins. DNACell Biol. 2002, 21, 271–280. [Google Scholar] [CrossRef]
- Chen, J.; Xiao, G.; Duan, G.; Wu, Y.; Zhao, X.; Gong, X. Structural Design of Carbon Dots/Porous Materials Composites and Their Applications. Chem. Eng. J. 2020, 127743. [Google Scholar] [CrossRef]
- Xu, X.; Ray, R.; Gu, Y.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic Analysis and Purification of Fluorescent Single-Walled Carbon Nanotube Fragments. J. Am. Chem. Soc. 2004, 126, 12736–12737. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pierau, L.; Versace, D.-L. Light and Hydrogels: A New Generation of Antimicrobial Materials. Materials 2021, 14, 787. https://doi.org/10.3390/ma14040787
Pierau L, Versace D-L. Light and Hydrogels: A New Generation of Antimicrobial Materials. Materials. 2021; 14(4):787. https://doi.org/10.3390/ma14040787
Chicago/Turabian StylePierau, Lucie, and Davy-Louis Versace. 2021. "Light and Hydrogels: A New Generation of Antimicrobial Materials" Materials 14, no. 4: 787. https://doi.org/10.3390/ma14040787
APA StylePierau, L., & Versace, D.-L. (2021). Light and Hydrogels: A New Generation of Antimicrobial Materials. Materials, 14(4), 787. https://doi.org/10.3390/ma14040787




