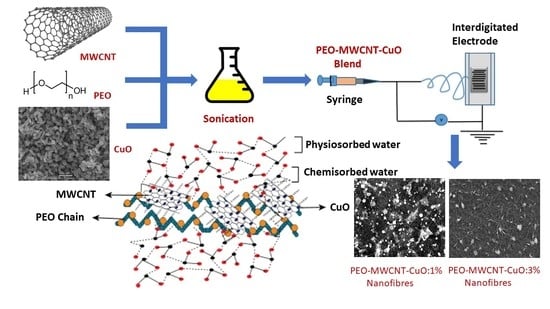
Highly Sensitive Humidity Sensors Based on Polyethylene Oxide/CuO/Multi Walled Carbon Nanotubes Composite Nanofibers
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Synthesis of CuO Nanoparticles
2.3. Oxidation of MWCNTs
2.4. Preparation of the Polymer Composite Blend
2.5. Nanofibers Development via Electrospinning
2.6. Characterization of PEO–CuO–MWCNT Composite Nanofibers
2.7. Humidity Sensing Experiments
3. Results and Discussion
3.1. Characterization of Composite Nanofibers
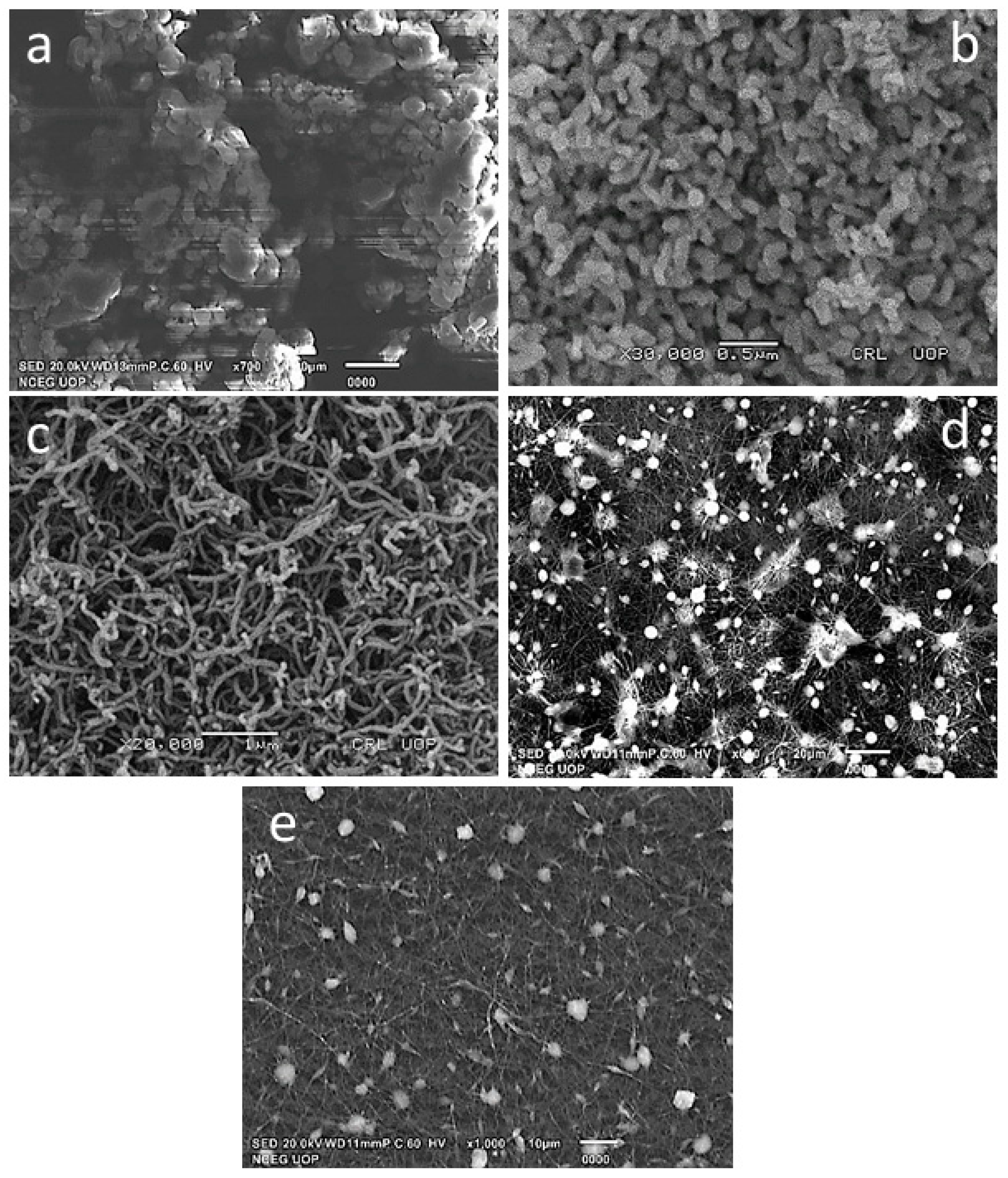
3.1.1. SEM Analysis
3.1.2. X-ray Diffraction Analysis
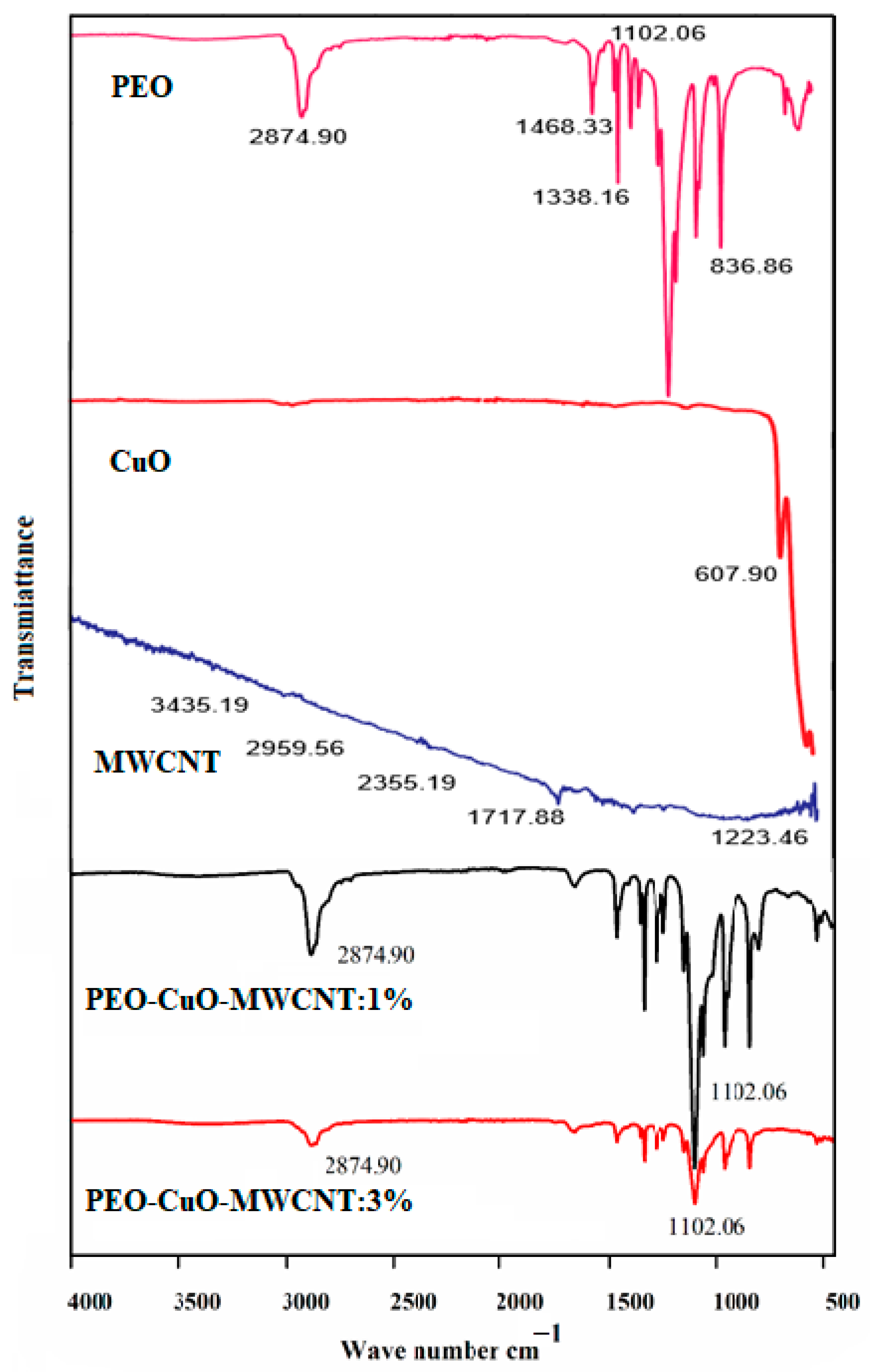
3.1.3. FTIR Spectroscopy
3.1.4. EDS Analysis
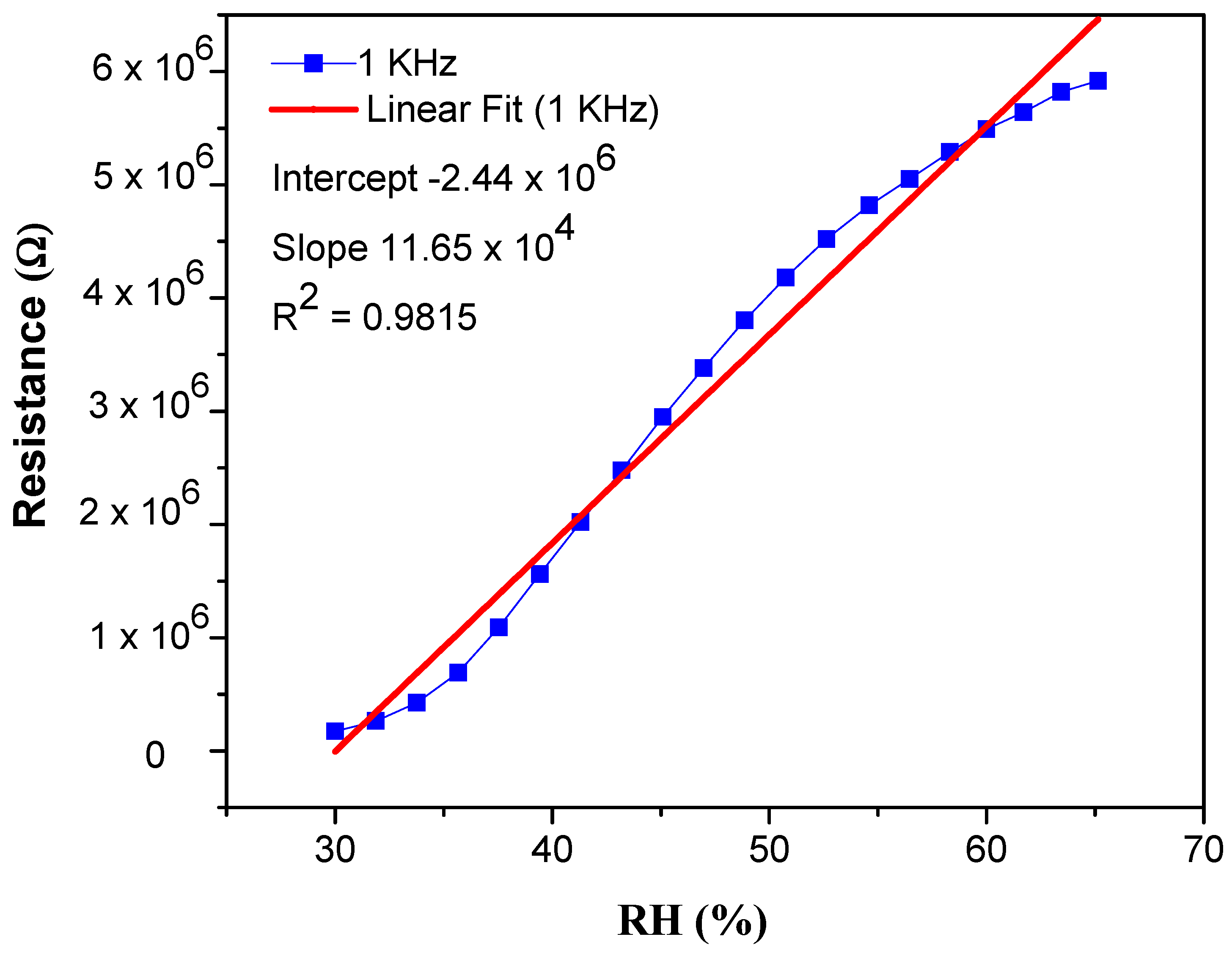
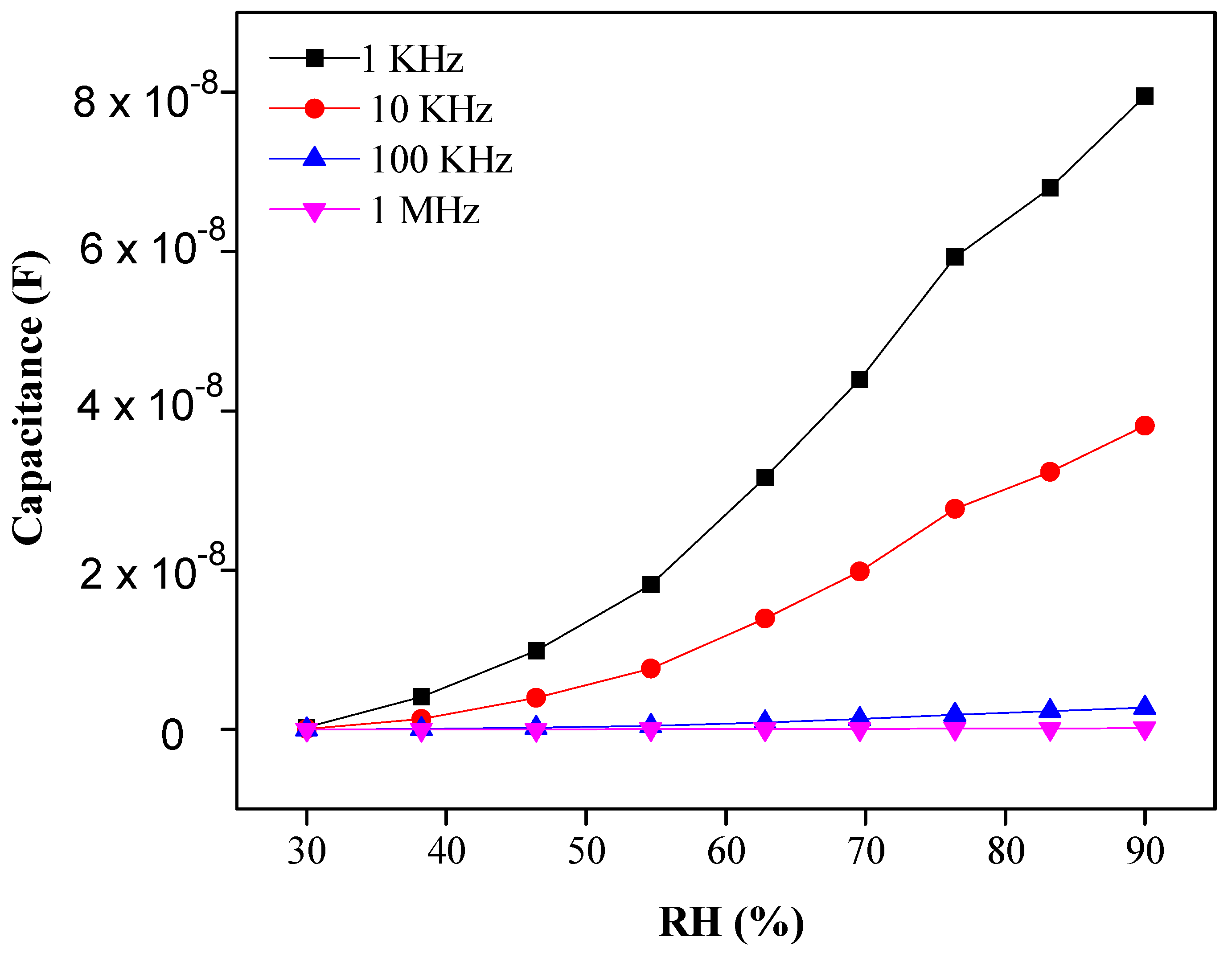
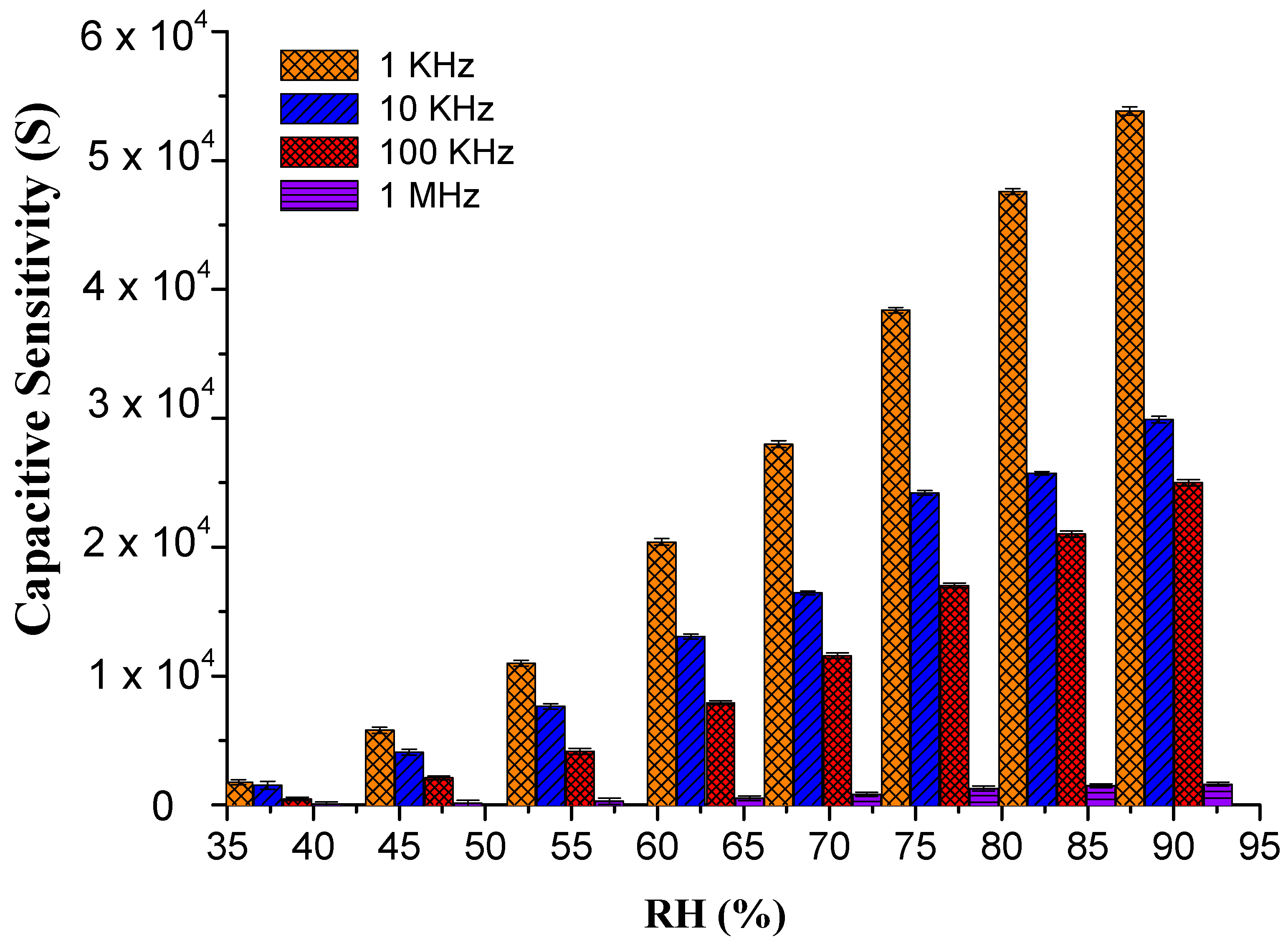
3.2. Humidity Sensing Efficiency of PEO–CuO–MWCNT Composite Nanofibers
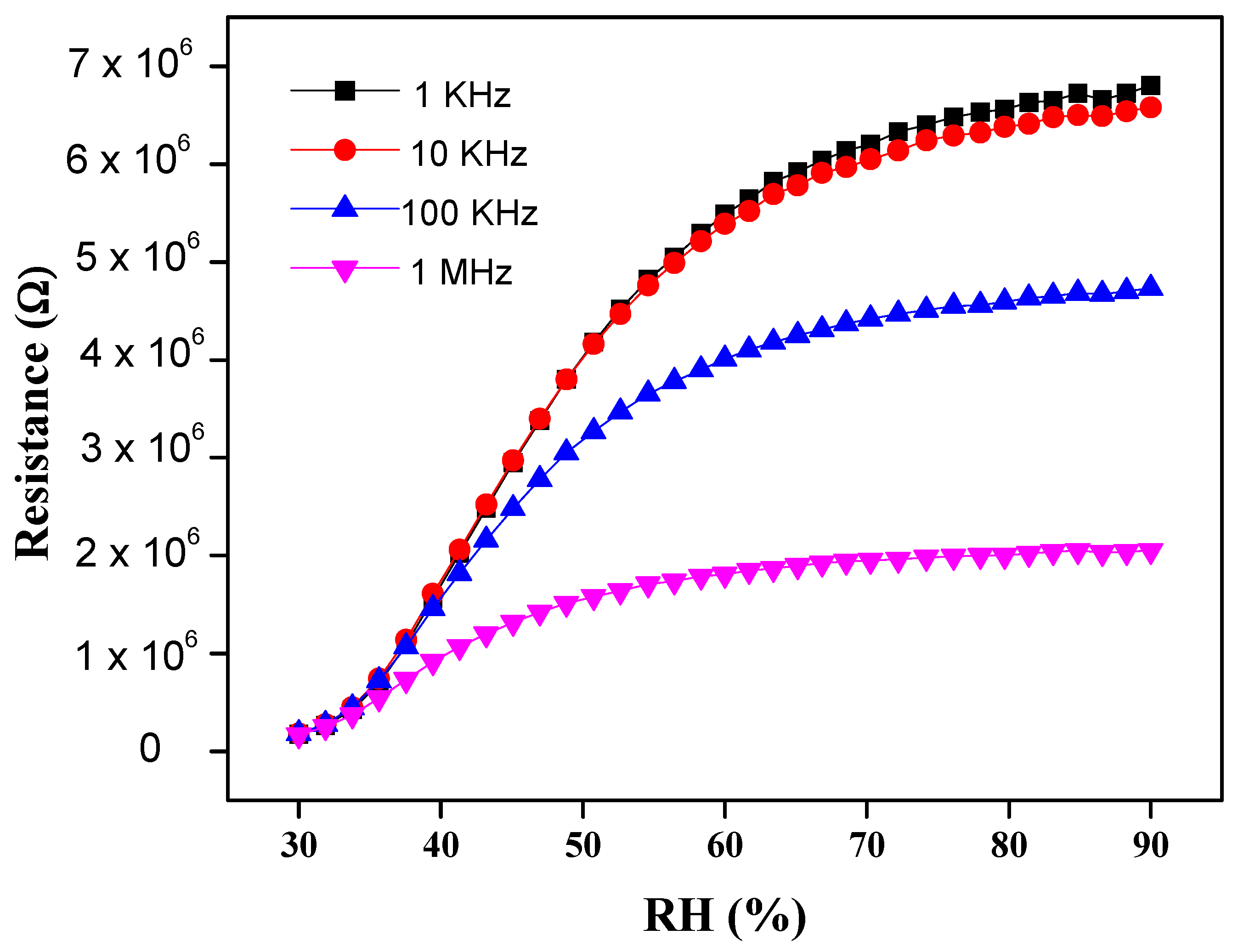
3.2.1. Sensitivity and Response
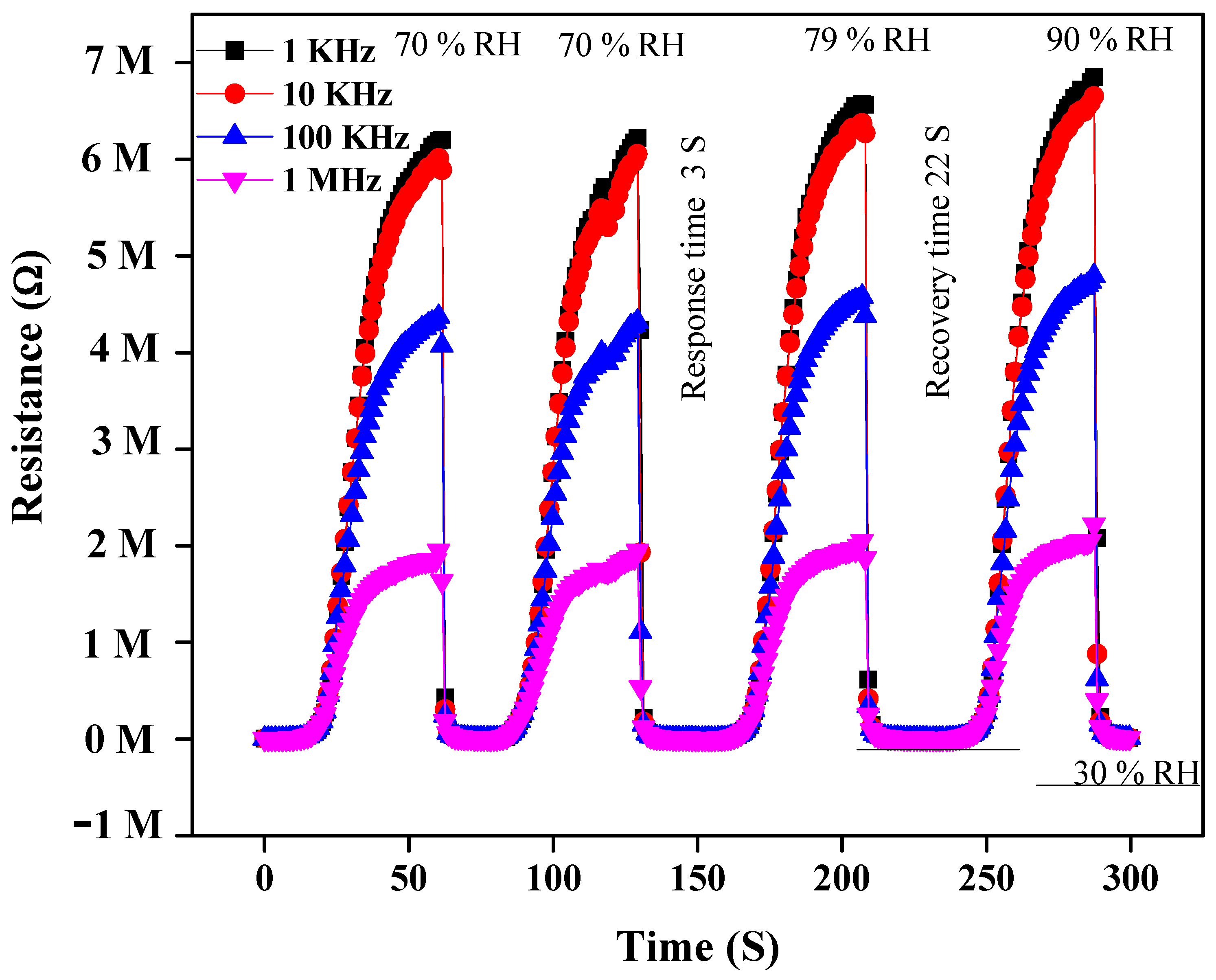
3.2.2. Response and Recovery Time
3.2.3. Humidity Sensing Mechanism
3.3. Comparative Efficiencies of the Sensors
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shah, G.; Ullah, A.; Arshad, M.; Khan, R.; Ullah, B.; Ahmad, I. Resistive-and capacitive-type humidity and temperature sensors based on a novel caged nickel sulfide for environmental monitoring. J. Mater. Sci. Mater. Electron. 2020, 31, 3557–3563. [Google Scholar]
- Cai, B.; Yin, H.; Huo, T.; Ma, J.; Di, Z.; Li, M.; Hu, N.; Yang, Z.; Zhang, Y.; Su, Y. Semiconducting single-walled carbon nanotube/graphene van der Waals junctions for highly sensitive all-carbon hybrid humidity sensors. J. Mater. Chem. C 2020, 8, 3386–3394. [Google Scholar] [CrossRef]
- Kundu, S.; Majumder, R.; Ghosh, R.; Pradhan, M.; Roy, S.; Singha, P.; Ghosh, D.; Banerjee, A.; Banerjee, D.; Chowdhury, M.P. Relative humidity sensing properties of doped polyaniline-encased multiwall carbon nanotubes: Wearable and flexible human respiration monitoring application. J. Mater. Sci. 2020, 55, 3884–3901. [Google Scholar] [CrossRef]
- Chen, Z.; Lu, C. Humidity sensors: A review of materials and mechanisms. Sens. Lett. 2005, 3, 274–295. [Google Scholar] [CrossRef]
- Sajid, M.; Kim, H.B.; Lim, J.H.; Choi, K.H. Liquid-assisted exfoliation of 2D hBN flakes and their dispersion in PEO to fabricate highly specific and stable linear humidity sensors. J. Mater. Chem. C 2018, 6, 1421–1432. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Zou, F.; Jiang, C.; Mou, C.; Wang, T. Humidity sensor based on a long-period fiber grating coated with polymer composite film. Sensors 2019, 19, 2263. [Google Scholar] [CrossRef]
- Ahmadi Tabar, F.; Nikfarjam, A.; Tavakoli, N.; Nasrollah Gavgani, J.; Mahyari, M.; Hosseini, S.G. Chemical-resistant ammonia sensor based on polyaniline/CuO nanoparticles supported on three-dimensional nitrogen-doped graphene-based framework nanocomposites. Microchim. Acta 2020, 187, 1–13. [Google Scholar] [CrossRef]
- Ze, L.; Yueqiu, G.; Xujun, L.; Yong, Z. MoS2-modified ZnO quantum dots nanocomposite: Synthesis and ultrafast humidity response. Appl. Surf. Sci. 2017, 399, 330–336. [Google Scholar] [CrossRef]
- Ali, S.S.; Pauly, A.; Brunet, J.; Varenne, C.; Ndiaye, A.L. MWCNTs/PMMA/PS composites functionalized PANI: Electrical characterization and sensing performance for ammonia detection in a humid environment. Sens. Actuators B Chem. 2020, 128364. [Google Scholar]
- Wang, X.; Ding, B.; Yu, J.; Wang, M. Highly sensitive humidity sensors based on electro-spinning/netting a polyamide 6 nano-fiber/net modified by polyethyleneimine. J. Mater. Chem. 2011, 21, 16231–16238. [Google Scholar] [CrossRef]
- Konstantaki, M.; Papaioannou, G.; Pissadakis, S.; Pispas, S.; Madamopoulos, N.; Vainos, N. Optical fiber long-period grating humidity sensor utilizing PEO/CoCl2 outcladding overlayers. In Optical Fibers: Application Proceedings of Congress of Optics and Optoelectronics, Warsaw, Poland, 28 August–2 September 2005; SPIE: Bellingham, WA, USA, 2015. [Google Scholar]
- Wang, Y.; Shen, C.; Lou, W.; Shentu, F. Fiber optic humidity sensor based on the graphene oxide/PVA composite film. Opt. Commun. 2016, 372, 229–234. [Google Scholar] [CrossRef]
- Vu, D.L.; Li, Y.-Y.; Lin, T.-H.; Wu, M.-C. Fabrication and humidity sensing property of UV/ozone treated PANI/PMMA electrospun fibers. J. Taiwan Inst. Chem. Eng. 2019, 99, 250–257. [Google Scholar] [CrossRef]
- Singh, P.; Shukla, S. Structurally optimized cupric oxide/polyaniline nanocomposites for efficient humidity sensing. Surf. Interfaces 2020, 18, 100410. [Google Scholar] [CrossRef]
- Megha, R.; Ravikiran, Y.; Chethan, B.; Prakash, H.R.; Kumari, S.V.; Thomas, S. Effect of mechanical mixing method of preparation of polyaniline-transition metal oxide composites on DC conductivity and humidity sensing response. J. Mater. Sci. Mater. Electron. 2018, 29, 7253–7261. [Google Scholar] [CrossRef]
- Wang, S.-B.; Hsiao, C.-H.; Chang, S.-J.; Lam, K.-T.; Wen, K.-H.; Young, S.-J.; Hung, S.-C.; Huang, B.-R. CuO nanowire-based humidity sensor. IEEE Sens. J. 2011, 12, 1884–1888. [Google Scholar] [CrossRef]
- Blank, T.A.; Eksperiandova, L.P.; Belikov, K.N. Recent trends of ceramic humidity sensors development: A review. Sens. Actuators B Chem. 2016, 228, 416–442. [Google Scholar] [CrossRef]
- Silva, S.C.; Cardoso, R.M.; Richter, E.M.; Munoz, R.A.; Nossol, E. Reduced graphene oxide/multi-walled carbon nanotubes/prussian blue nanocomposites for amperometric detection of strong oxidants. Mater. Chem. Phys. 2020, 123011. [Google Scholar] [CrossRef]
- Lin, Q.; Li, Y.; Yang, M. Highly sensitive and ultrafast response surface acoustic wave humidity sensor based on electrospun polyaniline/poly (vinyl butyral) nanofibers. Anal. Chim. Acta 2012, 748, 73–80. [Google Scholar] [CrossRef]
- Mallick, S.; Ahmad, Z.; Touati, F.; Shakoor, R. Improvement of humidity sensing properties of PVDF-TiO2 nanocomposite films using acetone etching. Sens. Actuators B Chem. 2019, 288, 408–413. [Google Scholar] [CrossRef]
- Fratoddi, I.; Bearzotti, A.; Venditti, I.; Cametti, C.; Russo, M. Role of nanostructured polymers on the improvement of electrical response-based relative humidity sensors. Sens. Actuators B Chem. 2016, 225, 96–108. [Google Scholar] [CrossRef]
- Sakai, Y.; Sadaoka, Y.; Matsuguchi, M. Humidity sensors based on polymer thin films. Sens. Actuators B Chem. 1996, 35, 85–90. [Google Scholar] [CrossRef]
- Sakai, Y.; Sadaoka, Y.; Matsuguchi, M.; Sakai, H. Humidity sensor durable at high humidity using simultaneously crosslinked and quaternized poly (chloromethyl styrene). Sens. Actuators B Chem. 1995, 25, 689–691. [Google Scholar] [CrossRef]
- Choi, S.; Lee, H.M.; Kim, H.S. Effect of molecular weight on humidity-sensitive characteristics of electrospun polyethylene oxide. Sens. Actuators A Phys. 2019, 294, 194–202. [Google Scholar] [CrossRef]
- Zhang, D.; Tong, J.; Xia, B.; Xue, Q. Ultrahigh performance humidity sensor based on layer-by-layer self-assembly of graphene oxide/polyelectrolyte nanocomposite film. Sens. Actuators B Chem. 2014, 203, 263–270. [Google Scholar] [CrossRef]
- Mazhar, S.; Qarni, A.A.; Haq, Y.U.; ul Haq, Z.; Murtaza, I.; Ahmad, N.; Jabeen, N.; Amin, S. Electrospun PVA/TiC Nanofibers for High Performance Capacitive Humidity Sensing. Microchem. J. 2020, 104974. [Google Scholar] [CrossRef]
- Waber, T.; Sax, M.; Pahl, W.; Stufler, S.; Leidl, A.; Günther, M.; Feiertag, G. Fabrication and characterization of a piezoresistive humidity sensor with a stress-free package. J. Sens. Sens. Syst. 2014, 3, 167. [Google Scholar] [CrossRef]
- Likhite, R.; Banerjee, A.; Majumder, A.; Karkhanis, M.; Kim, H.; Mastrangelo, C.H. Parametrically amplified low-power MEMS capacitive humidity sensor. Sensors 2019, 19, 3954. [Google Scholar] [CrossRef]
- Reddy, S.; Swamy, B.K.; Jayadevappa, H. CuO nanoparticle sensor for the electrochemical determination of dopamine. Electrochim. Acta 2012, 61, 78–86. [Google Scholar] [CrossRef]
- Lima, A.P.; Catto, A.C.; Longo, E.; Nossol, E.; Richter, E.M.; Munoz, R.A. Investigation on acid functionalization of double-walled carbon nanotubes of different lengths on the development of amperometric sensors. Electrochim. Acta 2019, 299, 762–771. [Google Scholar] [CrossRef]
- Ardeshirzadeh, B.; Anaraki, N.A.; Irani, M.; Rad, L.R.; Shamshiri, S. Controlled release of doxorubicin from electrospun PEO/chitosan/graphene oxide nanocomposite nanofibrous scaffolds. Mater. Sci. Eng. 2015, 48, 384–390. [Google Scholar] [CrossRef]
- Rajeh, A.; Morsi, M.; Elashmawi, I. Enhancement of spectroscopic, thermal, electrical and morphological properties of polyethylene oxide/carboxymethyl cellulose blends: Combined FT-IR/DFT. Vacuum 2019, 159, 430–440. [Google Scholar] [CrossRef]
- Radhakrishnan, A.A.; Beena, B.B. Structural and optical absorption analysis of CuO nanoparticles. Indian J. Adv. Chem. Sci. 2014, 2, 158–161. [Google Scholar]
- Takassi, M.; Zadehnazari, A. Nanocomposites of triazole functionalized multi-walled carbon nanotube with chemically grafted polyimide: Preparation, characterization, and properties. Fuller 2016, 24, 128–138. [Google Scholar] [CrossRef]
- Xianhua, C.; Khanmirzaei, M.H.; Omar, F.S.; Kasi, R.; Subramaniam, R.T. The effect of incorporation of multi-walled carbon nanotube into poly (ethylene oxide) gel electrolyte on the photovoltaic performance of dye-sensitized solar cell. Polym. -Plast. Technol. Mater. 2019, 58, 97–104. [Google Scholar] [CrossRef]
- Phiwdang, K.; Suphankij, S.; Mekprasart, W.; Pecharapa, W. Synthesis of CuO nanoparticles by precipitation method using different precursors. Energy Procedia 2013, 34, 740–745. [Google Scholar] [CrossRef]
- Li, F.; Gao, J.; Li, X.; Li, Y.; He, X.; Chen, L.; Zhang, Y. Preparation of magnetic molecularly imprinted polymers functionalized carbon nanotubes for highly selective removal of aristolochic acid. J. Chromatogr. A 2019, 1602, 168–177. [Google Scholar] [CrossRef]
- Lin, Y.W.; Wu, T.M. Fabrication of water-soluble polyaniline/poly (ethylene oxide)/carbon nanotube electrospun fibers. J. Appl. Polym. Sci. 2012, 126, E123–E129. [Google Scholar] [CrossRef]
- Banitaba, S.N.; Semnani, D.; Heydari-Soureshjani, E.; Rezaei, B.; Ensafi, A.A. Nanofibrous poly (ethylene oxide)-based structures incorporated with multi-walled carbon nanotube and graphene oxide as all-solid-state electrolytes for lithium ion batteries. Polym. Int. 2019, 68, 1787–1794. [Google Scholar] [CrossRef]
- Prakash, L.; Tirupathi, C. Synthesis and Characterization of Pure and Rare-Earth Metal Gd Doped SnO 2-CuO Nanoparticles by Co-Precipitation Method. J. Nanosci. Technol. 2018, 478–482. [Google Scholar] [CrossRef]
- Morsi, M.A.; Rajeh, A.; Al-Muntaser, A.A. Reinforcement of the optical, thermal and electrical properties of PEO based on MWCNTs/Au hybrid fillers: Nanodielectric materials for organoelectronic devices. Compos. Part B Eng. 2019, 173, 106957. [Google Scholar] [CrossRef]
- Naghdi Sedeh, N.; Entezam, M.; Hassan Jafari, S.; Khonakdar, H.A.; Abdouss, M. Morphology, drug release behavior, thermal, and mechanical properties of poly (ethylene oxide)(PEO)/poly (vinyl pyrrolidone)(PVP) blends. J. Appl. Polym. Sci. 2018, 135, 46403. [Google Scholar] [CrossRef]
- Mallakpour, S.; Mansourzadeh, S. Sonochemical synthesis of PVA/PVP blend nanocomposite containing modified CuO nanoparticles with vitamin B1 and their antibacterial activity against Staphylococcus aureus and Escherichia coli. Ultrason. Sonochemistry 2018, 43, 91–100. [Google Scholar] [CrossRef]
- Phan, D.-T.; Park, I.; Park, A.-R.; Park, C.-M.; Jeon, K.-J. Black P/graphene hybrid: A fast response humidity sensor with good reversibility and stability. Sci. Rep. 2017, 7, 1–7. [Google Scholar]
- Jiang, W.F.; Xiao, S.H.; Feng, C.Y.; Li, H.Y.; Li, X.J. Resistive humidity sensitivity of arrayed multi-wall carbon nanotube nests grown on arrayed nanoporous silicon pillars. Sens. Actuators B Chem. 2007, 125, 651–655. [Google Scholar] [CrossRef]
- Wang, J.; Wan, H.; Lin, Q. Properties of a nanocrystalline barium titanate on silicon humidity sensor. Meas. Sci. Technol. 2003, 14, 172. [Google Scholar] [CrossRef]
- Wang, Q.; Pan, Y.; Huang, S.; Ren, S.; Li, P.; Li, J. Resistive and capacitive response of nitrogen-doped TiO2 nanotubes film humidity sensor. Nanotechnology 2010, 22, 025501. [Google Scholar] [CrossRef]
- Parangusan, H.; Bhadra, J.; Ahmad, Z.; Mallick, S.; Touati, F.; Al-Thani, N. Capacitive type humidity sensor based on PANI decorated Cu–ZnS porous microspheres. Talanta 2020, 219, 121361. [Google Scholar] [CrossRef]
- Wang, Z.; Shi, L.; Wu, F.; Yuan, S.; Zhao, Y.; Zhang, M. The sol–gel template synthesis of porous TiO2 for a high performance humidity sensor. Nanotechnology 2011, 22, 275502. [Google Scholar] [CrossRef]
- Lee, J.; Cho, D.; Jeong, Y. A resistive-type sensor based on flexible multi-walled carbon nanotubes and polyacrylic acid composite films. Solid-State Electron. 2013, 87, 80–84. [Google Scholar] [CrossRef]
- Kingston, C.; Zepp, R.; Andrady, A.; Boverhof, D.; Fehir, R.; Hawkins, D.; Roberts, J.; Sayre, P.; Shelton, B.; Sultan, Y.; et al. Release characteristics of selected carbon nanotube polymer composites. Carbon 2014, 68, 33–57. [Google Scholar] [CrossRef]
- Singh, P.; Kushwaha, C.S.; Shukla, S.K.; Dubey, G.C. Synthesis and Humidity Sensing Properties of NiO Intercalated Polyaniline Nanocomposite. Polym. -Plast. Technol. Mater. 2019, 58, 139–147. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, X.; Neri, G.; Pinna, N. Nanostructured materials for room-temperature gas sensors. Adv. Mater. 2016, 28, 795–831. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Koh, H.-J.; Ren, C.E.; Kwon, O.; Maleski, K.; Cho, S.-Y.; Anasori, B.; Kim, C.-K.; Choi, Y.-K.; Kim, J. Metallic Ti3C2T x MXene gas sensors with ultrahigh signal-to-noise ratio. Acs Nano 2018, 12, 986–993. [Google Scholar] [CrossRef] [PubMed]
- Aman Qazi, R.; Saleem Khan, M.; Siddiq, M.; Ullah, R.; Ali Shah, L.; Ali, M. Synthesis and characterization of functionalized MWCNTs/PMMA composites: Device fabrication for RH sensing. Polym. Plast. Technol. Mater. 2020, 1–13. [Google Scholar] [CrossRef]
- Rahim, I.; Shah, M.; Wahab, F.; Iqbal, M. Comparative study of microstructural and humidity-sensing properties of graphene-based nanocomposite thin film. Meas. Sci. Technol. 2019, 31, 035104. [Google Scholar] [CrossRef]
- Jeseentharani, V.; Jeyaraj, B.; Pragasam, J.; Dayalan, A.; Nagaraja, K.S. Humidity sensing properties of CuO, ZnO and NiO composites. Sens. Transducers 2010, 113, 48. [Google Scholar]
- Khanna, V.; Nahar, R. Surface conduction mechanisms and the electrical properties of Al2O3 humidity sensor. Appl. Surf. Sci. 1987, 28, 247–264. [Google Scholar] [CrossRef]
- Li, X.; Chen, X.; Chen, X.; Ding, X.; Zhao, X. High-sensitive humidity sensor based on graphene oxide with evenly dispersed multiwalled carbon nanotubes. Mater. Chem. Phys. 2018, 207, 135–140. [Google Scholar] [CrossRef]
- Sheng, L.; Dajing, C.; Yuquan, C. A surface acoustic wave humidity sensor with high sensitivity based on electrospun MWCNT/Nafion nanofiber films. Nanotechnology 2011, 22, 265504. [Google Scholar] [CrossRef]
- Yousaf, H.Z.; Kim, S.W.; Hassan, G.; Karimov, K.; Choi, K.H.; Sajid, M. Highly sensitive wide range linear integrated temperature compensated humidity sensors fabricated using Electrohydrodynamic printing and electrospray deposition. Sens. Actuators B Chem. 2020, 308, 127680. [Google Scholar] [CrossRef]
- Su, P.-G.; Chen, I.C.; Wu, R.-J. Use of poly (2-acrylamido-2-methylpropane sulfonate) modified with tetraethyl orthosilicate as sensing material for measurement of humidity. Anal. Chim. Acta 2001, 449, 103–109. [Google Scholar] [CrossRef]
- Su, P.-G.; Uen, C.-L. A resistive-type humidity sensor using composite films prepared from poly (2-acrylamido-2-methylpropane sulfonate) and dispersed organic silicon sol. Talanta 2005, 66, 1247–1253. [Google Scholar] [CrossRef]


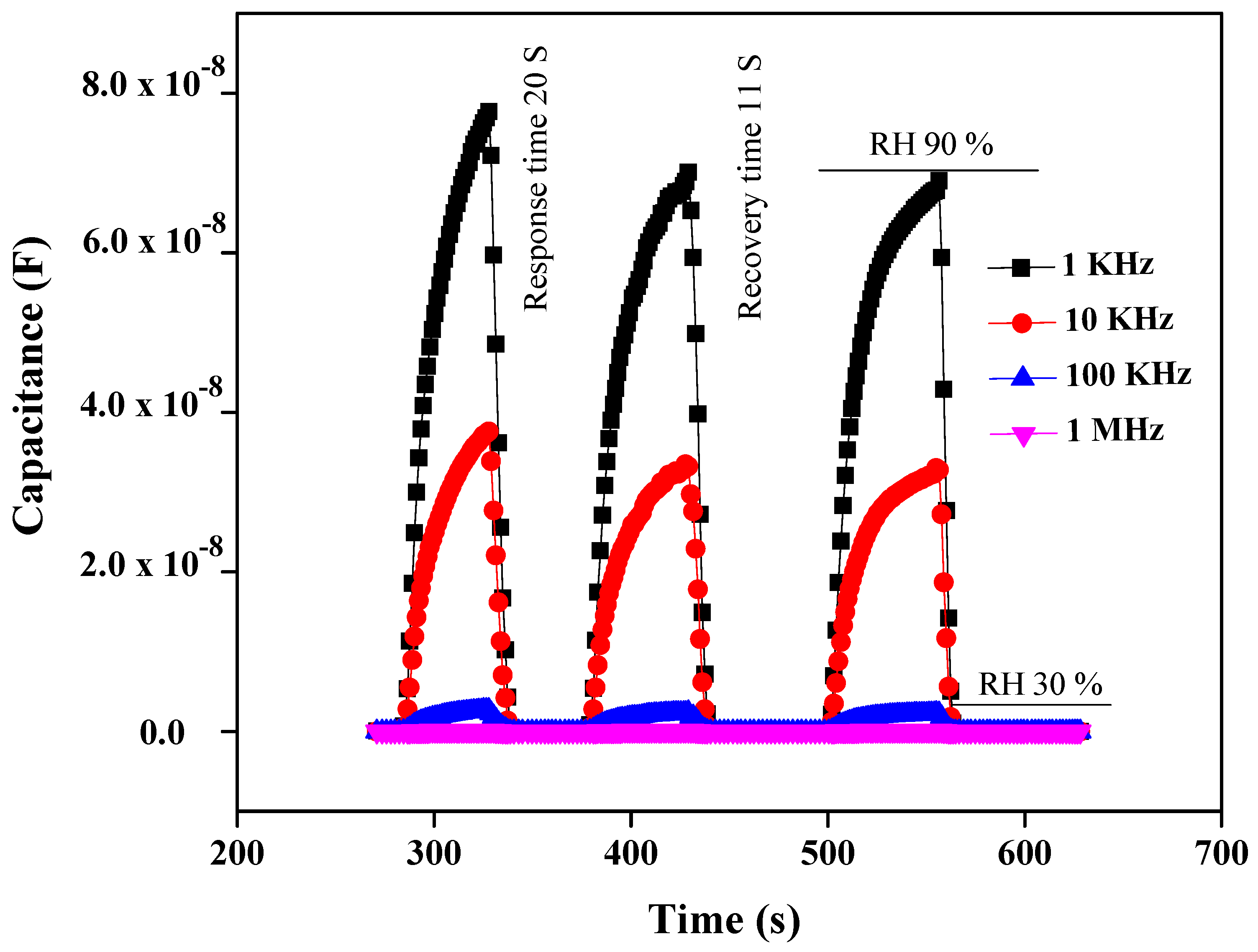
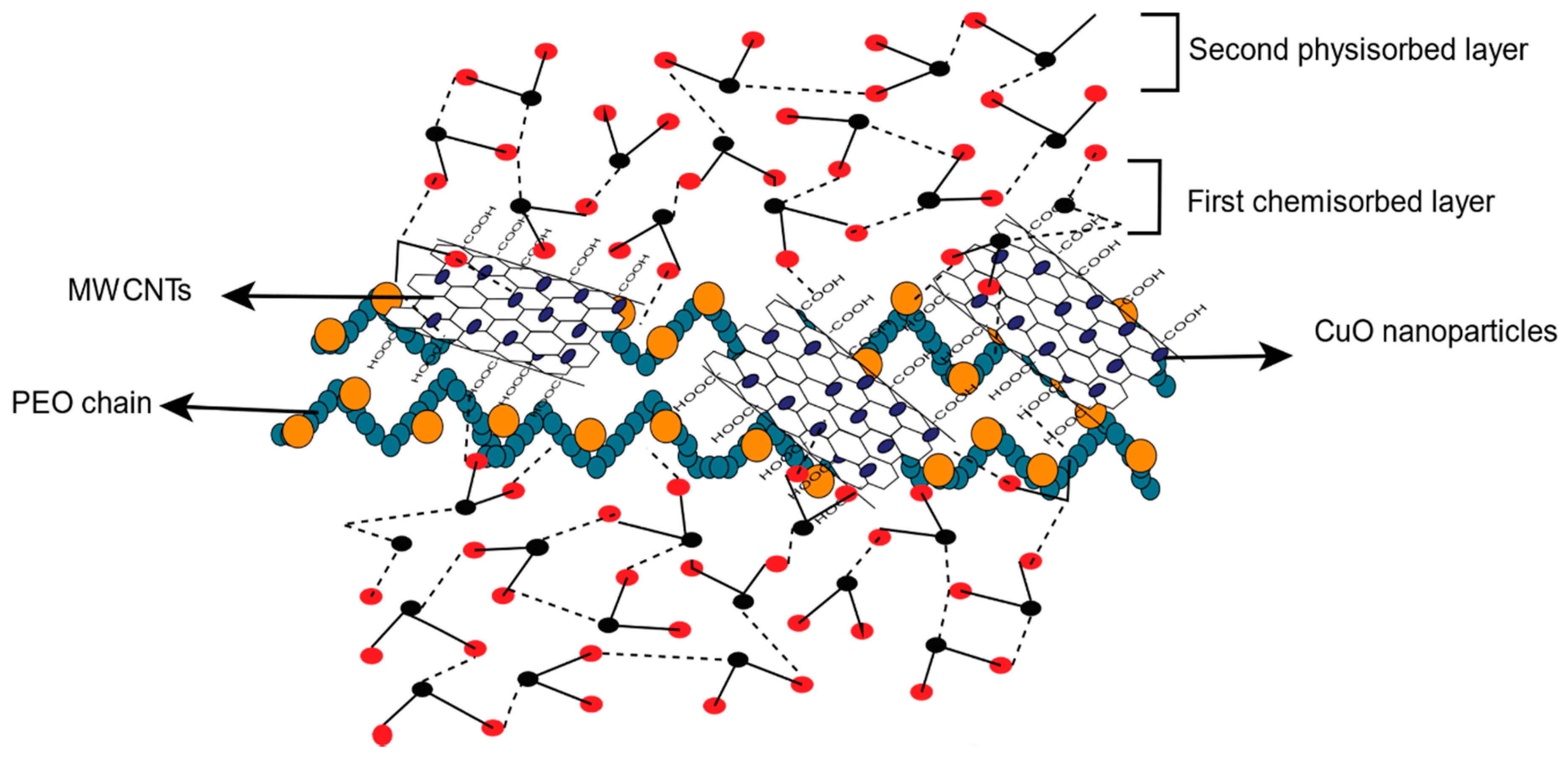
| Sensing Material | Type | RH (%) | Sensitivity | R2 | Response/Recovery Time | Ref. |
|---|---|---|---|---|---|---|
| GO/MWCNT | Capacitive | 11–97 | 7980% | - | 5 s /2.5 s | [59] |
| MWCNT/Nafion nanofibers film | SAWR | 10–80 | 427.6% | 0.987 | 3 s at 63% | [60] |
| PANI/PVB nanofibers | SAWR | 20–90 | ∼75 kHz/%RH | 0.927 | 1/2 | [19] |
| s2DMoS2 | Resistive | 0–80 | 85 KΩ%/RH | 0.999 | 0.6/0.3 | [61] |
| Poly-AMPS/ TEOS | Resistive | 30–90 | - | 0.998 | <2 min | [62] |
| Organic silicon sol/poly-AMPS | Resistive | 30–90 | - | 0.9491 | 30/60 s | [63] |
| PEO−CuO−MWCNT: 1% | Resistive | 30–90 | 3798.2% | 0.884 | 3/22 s | Current Study |
| PEO−CuO−MWCNT: 3% | Capacitive | 30–90 | 53837.6% | 0.961 | 20/11 s | Current Study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, W.; Jabbar, B.; Ahmad, I.; Mohamed Jan, B.; Stylianakis, M.M.; Kenanakis, G.; Ikram, R. Highly Sensitive Humidity Sensors Based on Polyethylene Oxide/CuO/Multi Walled Carbon Nanotubes Composite Nanofibers. Materials 2021, 14, 1037. https://doi.org/10.3390/ma14041037
Ahmad W, Jabbar B, Ahmad I, Mohamed Jan B, Stylianakis MM, Kenanakis G, Ikram R. Highly Sensitive Humidity Sensors Based on Polyethylene Oxide/CuO/Multi Walled Carbon Nanotubes Composite Nanofibers. Materials. 2021; 14(4):1037. https://doi.org/10.3390/ma14041037
Chicago/Turabian StyleAhmad, Waqas, Bushra Jabbar, Imtiaz Ahmad, Badrul Mohamed Jan, Minas M. Stylianakis, George Kenanakis, and Rabia Ikram. 2021. "Highly Sensitive Humidity Sensors Based on Polyethylene Oxide/CuO/Multi Walled Carbon Nanotubes Composite Nanofibers" Materials 14, no. 4: 1037. https://doi.org/10.3390/ma14041037
APA StyleAhmad, W., Jabbar, B., Ahmad, I., Mohamed Jan, B., Stylianakis, M. M., Kenanakis, G., & Ikram, R. (2021). Highly Sensitive Humidity Sensors Based on Polyethylene Oxide/CuO/Multi Walled Carbon Nanotubes Composite Nanofibers. Materials, 14(4), 1037. https://doi.org/10.3390/ma14041037