Abstract
The synthesis and application of sodium trithiocarbonate (Na2CS3) for the treatment of real galvanic wastewater in order to remove heavy metals (Cu, Cd and Zn) was investigated. A Central Composite Design/Response Surface Methodology (CCD/RSM) was employed to optimize the removal of heavy metals from industrial wastewater. Adequacy of approximated data was verified using Analysis of Variance (ANOVA). The calculated coefficients of determination (R2 and R2adj) were 0.9119 and 0.8532, respectively. Application of Na2CS3 conjugated with CCD/RSM allowed Cu, Cd and Zn levels to be decreased and, as a consequence, ∑Cu,Cd,Zn decreased by 99.80%, 97.78%, 99.78%, and 99.69%, respectively, by using Na2CS3 at 533 mg/L and pH 9.7, within 23 min. Implementation of conventional metal precipitation reagents (NaOH, Ca(OH)2 and CaO) at pH 11 within 23 min only decreased ∑Cu,Cd,Zn by 90.84%, 93.97% and 93.71%, respectively. Rotifer Brachionus plicatilis was used to conduct the assessment of wastewater toxicity. Following the application of Na2CS3, after 60 min the mortality of B. plicatilis was reduced from 90% to 25%. Engagement of Na2CS3 under optimal conditions caused the precipitation of heavy metals from the polluted wastewater and significantly decreased wastewater toxicity. In summary, Na2CS3 can be used as an effective heavy metal precipitating agent, especially for Cu, Cd and Zn.
1. Introduction
Heavy metal salts are widely used throughout industry. Rapid transformation of traditional societies into industrial ones, the introduction of mechanization-based production, effective planning and management methods have resulted not only in improved effectiveness and quality of production processes, but have also exerted a negative impact on the natural environment. Due to such intensified industrialization trends, heavy metals from various branches of industry are released into ecosystems at a constantly increasing rate. As a consequence, metals such as Cd, Cr, Cu, Hg, Ni, Pb and Zn are detected in industrial wastewater from electroplating facilities, mining and metallurgy plants, tanning and petroleum industries, paint and pigment production sites, etc. [1,2].
Heavy metals are defined as metals characterized by a specific density of >5 g/cm3 and, in addition, which have an adverse impact on the natural environment including living organisms [3]. On the one hand, some metal cations at low physiological concentrations play important functions contributing to proper functioning of plants, animals and humans. On the other hand, at high concentrations that exceed some predefined threshold values, they are toxic and disrupt normal physiological processes. Due to their toxicity, persistence, as well as environmental mobility, heavy metals are one of the most problematic air, water and soil pollutants in the context of food production and ecological issues as well as evolutionary aspects [4,5].
The problems concerning toxicity of heavy metals should be considered individually for each metal, due to their unique physicochemical properties, which imply different mechanisms of interaction with cells and tissues of living organisms. Unfortunately, these mechanisms are not yet fully elucidated. It has been clearly demonstrated that heavy metal cations can variously affect cell organelles including cell membrane, lysosomes, endoplasmic reticulum as well as the cell nucleus. They can also modify the activity of certain enzymes that play important roles in metabolism, detoxification or damage repair due to toxic substances [6]. Moreover, heavy metal cations can interact with DNA and nuclear proteins and thus contribute to the formation of significant conformational changes, DNA damage and, as a result, to the processes of carcinogenesis and apoptosis [7,8,9]. Formation of Reactive Oxygen Species (ROS) in the cells of living organisms and the phenomenon of oxidative stress have a significant impact on the degree of toxicity and carcinogenicity of heavy metal cations, as was shown for As [10], Cd [11], Cr [12], Pb [13] and Hg [14], among others.
Chemical and electrochemical metal deposition processes are engaged in many industrial plants for technical and decorative purposes and are preceded by several preliminary operations such as cleaning, degreasing, etching and activation, respectively. The purpose of these preliminary processes is to properly prepare the workpiece surface prior to covering with a metal layer. These operations and processes generate wastewater that contains heavy metal cations. Such wastewater must be treated when it is reused in production processes or before discharging into sewage systems or water courses.
Untreated galvanic wastewater is very toxic due to the presence of cyanides (CN−), hexavalent chromium (Cr6+) and heavy metals such as Cu, Zn, As, Be, Cd, Pb, Ni, etc. [15,16,17]. Additionally, galvanic wastewater contains various concentrations of metals, which depends on the type of workpiece subjected to treatment (e.g., made of steel, brass, etc.), the type of process they come from (e.g., electrochemical copper plating, final surface finishing operations, etc.), type of metallization technology used (chemical, electrochemical processes, etc.), the type of rinsing methods implemented (cascade rinsing, intermittent scrubbers, etc.) and the employment of certain recovery and reuse methods in the rinsing water (ion exchange methods, membrane methods, etc.), among others.
Example data show that wastewater from the chrome plating process (pH = 4) contained 0.105 mg/L Cu, 24.53 mg/L Cr, 3.380 mg/L Ni, 7.528 mg/L Zn and 1.188 mg/L Pb. Galvanic wastewater (pH = 4) from electrochemical processes using cyanide baths contained (apart from CN− ions) 5.194 mg/L Cu, 2.113 mg/L Cr, 35.56 mg/L Ni, 75.86 mg/L Zn and 0.013 mg/L Pb, while the acid–alkaline wastewater from washing processes contained small amounts of heavy metals—i.e., 0.621 mg/L Cu, 0.240 mg/L Cr, 2.970 mg/L Ni, 4.810 mg/L Zn and 0.025 mg/L Pb [18]. On the other hand, the wastewater from chemical and electrochemical processing of printed circuit boards (PCBs) revealed different concentrations of copper, depending on the type of the derived process—i.e., 3–20 mg/L (after alkaline and acid etching processes), 0.1–0.5 mg/L (after chemical copper plating), 0.5–3.0 mg/L (after electrolytic copper plating) and 10–60 mg/L (after brushing) [19]. Wastewater from the manufacturing of PCBs was characterized by pH and COD (Chemical Oxygen Demand) in the range of 2.4–9.6 and 12–280 mg O2/L (after chemical and electrochemical copper plating), 1.8–2.5 and 74–577 mg O2/L (after acid etching), 10.2–13.0 and 10.860–25.680 mg O2/L (after photopolymer development and stripping) and 7.6–7.8 and 113–364 mg O2/L (after brushing). For the above processes, copper concentrations in the wastewater ranged from 9–91 mg/L, 405–919 mg/L, 11–147 mg/L and 72–230 mg/L, respectively [20]. Additionally, the analysis of the influence of wastewater containing copper compounds (1200 mg/L) from the alkaline etching processes on the respiratory properties of the activated sludge (the so-called respirometric analysis) showed 100% inhibition of the respiratory properties of the activated sludge [21]. On the other hand, the wastewater from the continuous electrochemical tinning of copper wires was strongly acidic (pH < 2) and contained: 3100 mg/L Sn, 27.6 mg/L Fe, 2.41 mg/L Ni and 1.46 mg/L Pb [22] among others.
Wastewater from electroplating processes requires the use of effective treatment methods in order to precipitate heavy metals (and other pollutants) and, consequently, minimize their potential negative impact on the natural environment. For the treatment of galvanic wastewater, a number of methods of varying effectiveness are employed, such as conventional methods of chemical precipitation of metals (including coagulation, sedimentation and flocculation of the formed sediments), in the form of hydroxides (using NaOH, Ca(OH)2 etc.) [23], sulfides (using Na2S, NaHS, FeS to generate H2S at pH < 3, etc.) [24], as well as combined methods involving the use of chemical methods in the first stage, followed by nanofiltration or ion exchange [25,26]. There are also processes that use the phenomenon of adsorption on activated carbon [27], low-cost adsorbents, e.g., lignin [28], diatomite [29], zeolites [30] and biosorbents, obtained from nonliving biomass (bark, lignin, shrimp, krill etc.), algal biomass and microbial biomass (e.g., bacteria, fungi and yeast) [31]. Membrane methods are also applied, including micellar enhanced ultrafiltration (MEUF), polymer enhanced ultrafiltration (PEUF) [32,33], reverse osmosis (RO) [34] and nanofiltration (NF) [35]. Electrochemical methods such as electrocoagulation (EC) are also used to remove heavy metals from wastewater [36].
Chemical precipitation methods are usually effective and are widely used due to the possibility of applying simple technological systems and low costs of wastewater treatment. However, the effectiveness of chemical methods decreases in the case of wastewater containing complex compounds of heavy metals. In these cases, highly effective organic or inorganic precipitants, such as sodium diethyl- and dimethylodithiocarbamate (NaS2CN(C2H5)2, NaS2CN(CH3)2, respectively), trimercapto-s-triazine, trisodium salt (C3N3S3Na3) and sodium trithiocarbonate (Na2CS3) are applied [37,38]. Sodium diethyl- and dimethylodithiocarbamate and trimercapto-s-triazine trisodium salts are widely used at an industrial scale, while Na2CS3 is less frequently employed, despite the fact that it is equally effective, and the produced metal trithiocarbonate deposits are characterized by very good sedimentation and filtration properties [39]. So far, its use (also at an industrial scale) for the treatment of industrial wastewater from the production of PCBs containing Cu, Ni and Sn, in the presence of complexing compounds such as Na2EDTA (ethylenediaminetetraacetic acid, disodium salt), NH3(aq), NH2-CS-NH2 (thiourea), Na3MGDA (methylglycinediacetic acid, trisodium salt), Na4GLDA (N,N-dicarboxymethyl glutamic acid, tetrasodium salt) [37,38,39] and for the precipitation of Rare Earth Elements (REEs) from Acid Mine Drainage (AMD) [40]. The wide application possibilities of Na2CS3 result from patents [41]; however, there are no scientific investigations presenting the engagement of Na2CS3 for the treatment of real galvanic wastewater, containing metals other than copper, nickel and tin, in various concentrations and the assessment of the toxicity of treated wastewater after the usage of the precipitant. This is necessary to investigate the potential and effectiveness of this precipitating agent when used to remove metals from a particularly complex matrix such as galvanic wastewater.
As a matter of fact, the paper presents a method for the synthesis of Na2CS3 from Na2S and CS2 and the optimization of its use for the removal of a mixture of Cu, Cd and Zn cations from real galvanic wastewater originating from a manufacturing plant where copper, cadmium and zinc plating are used. Additionally, Central Composite Design/Response Surface Methodology (CCD/RSM) was implemented to optimize the metal removal process and the toxicity of untreated and treated wastewaters was assessed using the rotifer B. plicatilis.
2. Materials and Methods
2.1. Chemicals and Synthesis of Sodium Trithiocarbonate (Na2CS3)
All reagents, unless otherwise stated, were of analytical grade (AvantorTM), Gliwice, Poland). For pH adjustment, 10% and 20% NaOH and H2SO4 solutions (AvantorTM) and 15% suspension of CaO (CaO + MgO ≥ 91%, highly reactive burned lime, technical grade, and Ca(OH)2 ≥ 93%, technical grade (Trzuskawica, Nowiny, Poland) were used. A 0.10% solution of Praestol 2640 anionic flocculant in distilled water (SolenisTM, Capelle aan den IJssel, Holland) was used for flocculation of the precipitates. The artificial sea water contained 58.490% NaCl, 26.460% MgCl2·6H2O, 9.750% Na2SO4, 2.765% CaCl2, 1.645% KCl, 0.477% NaHCO3, 0.238% KBr, 0.071% H3BO3, 0.095% SrCl2·6H2O, 0.007% NaF and distilled water, prepared as described previously [42]. Additionally, deionized water (<2 µS/cm) was used to prepare and dilute the solutions. The synthesis of the Na2CS3 solution was carried out by a modified method, based on the information on the synthesis of K2CS3 [43] and Na2CS3 [43,44,45,46,47]. Na2S was used for synthesis (approx. 60% Na2S, ≤2% Na2SO3, ≤0.004% of substances insoluble in water, ≤2% Na2S2O3, ≤2% Na2CO3, ≤0.001% Fe, technical grade, Brenntag, Kędzierzyn-Koźle, Poland), CS2 (99.9% CS2, technical grade, Siarkopol, Grzybów, Poland) and saturated NaOH solution (approx. 50%) (AvantorTM). The synthesis of Na2CS3 was carried out in such a way that 50 mL of water (2.77 mol) and 37 g of CS2 (0.49 mol) were introduced into a three-necked flask (500 mL) equipped with a reflux condenser, stirrer, dropping funnels and nitrogen inlet (technical grade, Air Liquide, Kraków, Poland) and was vigorously stirred, keeping the temperature of the mixture at a maximum of 30–35 °C. Then, 5.7 g of Na2S (0.07 mol) was added every hour—a total of 45.6 g (0.58 mol). Then, 1.62 g (0.04 mol) of NaOH as an approx. 50% saturated solution was carefully introduced. The prepared reaction mixture was stirred for eight hours and then left to separate the phases. The lower layer constituting the Na2CS3 solution was separated and filtered through a fritted funnel to remove solid impurities, and selected physicochemical parameters of the product obtained were determined using the methods described in Section 2.4.
2.2. Origin and Physicochemical Parameters of Galvanic Wastewater
In this study, galvanic wastewater originated from an industrial plant located in eastern Poland was used. In the electroplating plant, chemical and electrochemical copper plating, cadmium plating and zinc plating processes are applied. Unit samples were taken from the storage tank collecting all raw industrial wastewater generated in the plant, and averaged by mixing prior to pumping into reactors where treatment processes are carried out. One-liter unit samples were taken manually, every hour during a 24-h period. The average sample used in the tests was obtained by mixing unit samples. The average daily sample was not fixed and until the tests were performed, it was stored at 4 °C. Basic physicochemical parameters such as pH, specific conductivity, salinity, content of complex compounds (expressed as EDTA) and heavy metal concentrations (Cu, Cd, Zn) were determined in the wastewater sample using the methods described in Section 2.4.
2.3. Apparatus and Experiment Conditions
All experiments were conducted at a constant temperature (19 ± 1 °C) in beakers containing 1000 ± 5 mL of wastewater, which during the research was mixed with a magnetic stirrer at a constant speed of 250 rpm (at the metal precipitation stage) and 50 rpm for 1 min at the stage of flocculation of precipitated sediments. The research was carried out in such a way that 10% or 20% NaOH solutions were added to 1000 ± 5 mL of wastewater in order to adjust the pH to the value assumed in the experimental plan; then, the assumed volume of 40.8% Na2CS3 solution (corresponding to the amount of pure Na2CS3 presented in the experimental plan) was added using a micropipette and the pH was corrected again to the assumed value with 10% or 20% H2SO4 solution and precipitation was carried out for the assumed time. Next, the precipitates were flocculated with 1.0 mL of 0.1% Praestol 2640 (anionic flocculant) solution and, after mixing (1 min/50 rpm), the precipitates were sedimented for 30 min. After this time, samples of treated wastewater were collected from above the sludge, filtered through a 0.45 µm membrane filter and subjected to the tests described in Section 2.4. For the most favorable metal removal conditions determined, a verification experiment was performed and the wastewater was subjected to the physicochemical and toxicological tests described in Section 2.4. Tests using conventional precipitants (20% NaOH, 15% Ca(OH)2 suspension or CaO) were conducted as above except that 1000 ± 5 mL of wastewater was alkalized to pH = 11 with the selected reagent.
2.4. Analytical Procedures
The Na2CS3 content (%) in the synthesized solution was determined by the modified method presented previously [48], which consisted of calculating the percentage of Na2CS3 content as the difference between the total content of reducing substances and the content of reducing impurities (SO32− and S2O32−), The total content of reducing substances was determined by introducing the test sample into the standard I2 solution and back-titrating the excess I2 with the standard Na2S2O3 solution against starch as an indicator. The content of reducing admixtures was determined by direct titration of the sample with standard I2 solution after prior separation of S2− and CS32− in the form of ZnS and ZnCS3. Density of Na2CS3 at 19 °C was determined using the standard pycnometric method. The pH values, salinity and temperature were measured using an Inolab® pH/Ion/Cond/Temp 750 m and SenTix® 81 electrodes (WTW, Weilheim in Oberbayern, Germany) [49]. The content of complex compounds expressed as EDTA (mg/L) was determined with the Nanocolor® Organic Complexing Agents test kit (Macherey Nagel, Düren, Germany) by using photometric determination through decoloration of the bismuth xylenol orange complex [50]. The content of Cu, Cd and Zn was determined using ICP-OES in accordance with ISO 11885: 2007 [51].
To determine the toxicity of untreated and treated wastewaters (after neutralization of samples to pH 7–7.5) using Na2CS3 under the most favorable conditions and NaOH, 15% suspension of Ca(OH)2 and CaO, a test was performed using a method described previously [52,53,54] with modifications, using neonates of rotifer B. plicatilis. The organisms were incubated at 25 °C for 15, 30, 45 and 60 min in the dark. Synthetic sea water (9.0 mL), untreated or treated wastewater tested (1.0 mL) and 10 neonates of rotifer B. plicatilis were introduced into sterile glass vials. The control sample contained 10.0 mL of synthetic seawater. After an incubation period of 15, 30, 45 and 60 min, the number of living and dead organisms in each culture was calculated using the Eclipse E200–LED trinocular microscope (Nikon Instruments Europe B.V., Amsterdam, The Netherlands) and reported as the rotifer mortality percent.
2.5. Optimization of the Experiments
Central Composite Design (CCD) and Response Surface Methodology (RSM) were used to optimize the process of Cu, Cd and Zn precipitation from the tested wastewater. Literature data show that the lowest concentration of metal ions in the treated wastewater following precipitation of Cu(OH)2, Cd(OH)2 and Zn(OH)2 was obtained at pH = 9.1, 11.2 and 9.2, respectively. In the case of sulfide precipitation, the most favorable pH values for the precipitation of CuS, CdS and ZnS are approx. 6.5, 11 and 11, respectively [55]. Therefore, it was assumed that some metal ions would precipitate as metal hydroxides as a result of wastewater alkalization, which would probably be beneficial regarding the reduction in the stoichiometric amount of Na2CS3 (497 mg/L of wastewater, corresponding to 0.376 mL of 40.8% Na2CS3) required for complete precipitation of metals. At the same time, Polish requirements for the pH of wastewater discharged into sewage disposal systems are set at 6.5–9.5 [56]. The adopted precipitation pH value should therefore take into account these requirements and ensure that, as far as possible, there is no need to readjust the pH of the wastewater.
Based on the presented data and several preliminary experiments, it was assumed that three independent parameters would be optimized—i.e., pH, Na2CS3 dose (mg) and reaction time (min). The sum of metal concentrations, i.e., ∑Cu,Cd,Zn (mg/L), after the wastewater treatment process with Na2CS3 was adopted as a dependent parameter. The following ranges of independent parameters were finally adopted: pH 7–9, Na2CS3 dose 300–500 mg and reaction time 15–30 min. CCD was used to plan the experiments and the corresponding input parameters were obtained for 16 experiments, as shown in Table 1. Sixteen experiments were performed (in triplicate). The wastewater tests were carried out as described in Section 2.3 and the values of the ∑Cu,Cd,Zn (mg/L) parameter were determined for each of the experiments. The obtained experimental results were statistically analyzed using the Statistica 13 package (StatSoft, Kraków, Poland) in order to determine the influence of the independent parameters (pH, Na2CS3 dose (mg) and the reaction time (min)) on the value of the dependent parameter—i.e., ∑Cu,Cd,Zn (mg/L). The relationships are presented using the response surface plots. For the most favorable conditions, the model was verified experimentally and toxicity tests were also performed.

Table 1.
Empirical conditions for the Central Composite Design/Response Surface Methodology (CCD/RSM) and results (∑Cu,Cd,Zn (mg/L)) for real galvanic wastewater.
3. Results and Discussion
3.1. Physicochemical Parameters of Synthesized Na2CS3 Solution
Table 2 shows the selected physicochemical properties of sodium trithiocarbonate solution obtained by direct synthesis from Na2S and CS2.

Table 2.
Determined physicochemical parameters of Na2CS3 solution.
We used the direct reaction of Na2S with CS2 under synthesis conditions, with the formation of Na2CS3 in accordance with Equation (1):
Due to the fact that technical grade Na2S containing NaHS was used for the synthesis, in the second stage approximately 50% saturated NaOH solution was added in order to convert the NaHS present in the reaction medium into an additional amount of Na2S according to Equation (2):
Since the density of the resulting Na2CS3 solution is greater than that of CS2, accumulation of excess CS2 on the surface of the Na2CS3 solution was observed. The obtained Na2CS3 solution was characterized by an intense, dark red color and a strongly alkaline reaction (pH > 13). Previously conducted studies confirmed that trithiocarbonates have the form of yellow or red-brown crystals, that are well soluble in water, and their solutions are intensely red [43,45,47].
Various methods of determining trithiocarbonates are known from the literature, e.g., with application one-step titration wit potassium ferricyanide, using Fe(II)–dimethylglyoxyme or sodium nitroprusside as indicator [57], direct titrimetric in presence of sulfite, thiosulfate and thiocyanate by titration with o-hydroxymercuribenzoate with sodium nitroprusside as indicator [58] (in both methods, sulfides are determined together with trithiocarbonates) or a method with the separation of sulfur compounds by means of a hexane solution of tributhyl tin (TBT) followed with o-hydroxymercuribenzoic acid titration in the presence of dithizone as indicator [59]; however, this is a multistage, time-consuming method and requires application of KCN in addition to TBT. Due to the fact that the reaction efficiency is practically 100% with respect to the sum of sulfides (Na2S and NaHS) [44] and the use of an excess of CS2, a simplified method was employed to determine the concentration of Na2CS3 that is based on determining the concentration of Na2CS3 as the total content of reducing substances (Na2CS3 + SO32− + S2O32−) decreased by the concentration of reducing admixtures (SO32− and S2O32−) [48]; the method is quite sufficient to determine the concentration of Na2CS3 in solution in the context of its further use in wastewater treatment. According to this method, a 40.8% Na2CS3 solution was obtained, which was utilized in the presented research.
3.2. Physicochemical Parameters of Galvanic Wastewater
Table 3 presents selected physicochemical parameters of the actual galvanic wastewater used in the investigation.

Table 3.
Determined physicochemical parameters of real galvanic wastewater.
The tested wastewater was acidic (pH = 3.4) and was characterized by a high electrical conductivity (EC) and salinity value (25,800 µS/cm and 12,850 mg NaCl/L, respectively). Additionally, it contained complexing compounds, determined as EDTA (180 mg/L) and heavy metal ions such as Cu2+, Cd2+ and Zn2+, with the highest concentration of copper (59 mg/L). Research reports indicate that the values of pollution parameters for galvanic wastewater vary in a wide range [18,19,20,22], both in terms of qualitative and quantitative compositions, which depend on technical and technological factors. The reported studies indicate that the concentration of copper in galvanic wastewater can be as high as 30,000 mg/L [60], while that of zinc reaching 1.392 mg/L at low concentrations of copper (0.74 mg/L), nickel (1.37 mg/L), chromium (0.28 mg/L) and iron (1.91 mg/L) [61]. In addition, the study of wastewater from PCB production showed that it contained 20.9 and 128.5 mg/L chelating agents determined as Na2EDTA [37,38]. In the case of the tested wastewater (180 mg/L as EDTA), the type of complexing compound was not known, but considering the chelating compounds most commonly used in electroplating, it can be assumed that EDTA, NTA and some organic acids were present—e.g., succinic acid [62].
3.3. CCD/RSM Results
Sodium trithiocarbonate (Na2CS3) is a compound that is highly soluble in water and therefore undergoes electrolytic dissociation, and as a salt of a strong base and weak trithiocarbonic acid (H2CS3). In addition, it also undergoes hydrolysis and, additionally, in the presence of oxygen from the air, it decomposes very slowly, according to Equations (3)–(5):
Na2CS3(aq) → 2Na + + CS32−
Na2CS3 + 3H2O → Na2CO3 + 3H2S↑
2Na2CS3 + 2H2O + 2O2 → Na2CO3 + Na2S2O3 + CS2↑ + 2H2S↑
Moreover, it should be noted that as a result of wastewater alkalization (by adding NaOH and Na2CS3), precipitation of sparingly soluble metal hydroxides, i.e., Cu(OH)2, Cd(OH)2 and Zn(OH)2 will occur. Therefore, taking into account the specific properties of Na2CS3 (Equations (3), (4), and, under certain conditions, Equation (5)), it should be concluded that the precipitated sludge will be a mixture of mainly sparingly soluble metal hydroxides, trithiocarbonates and sulfides, in accordance with Equations (6)–(8):
Me2+ + 2OH− → Me(OH)2↓
Me2+ + S2− → MeS↓
Me2+ + CS32− → MeCS3↓
Table 1 shows the results of 16 experiments performed for various pH values, Na2CS3 doses and reaction times. The analysis of the presented experimental data shows that the lowest values of ∑Cu,Cd,Zn were obtained for experiments with a more alkaline reaction environment and a higher dose of Na2CS3 than in the remaining experiments (Equations (7), (8) and (12)). For Equations (7) and (8) (pH 10, Na2CS3 500 mg/L), which differed only in the reaction time (10 and 30 min, respectively), a slightly lower value of ∑Cu,Cd,Zn was obtained with extended reaction times (0.68 and 0.57 mg/L, respectively). This may suggest that for the effective precipitation of metals it is necessary to carry out the precipitation reaction in a certain sufficiently long time. Table 4 presents statistical evaluation of the independent parameters (pH, Na2CS3, time) and their influence on the value of the dependent parameter (∑Cu,Cd,Zn).

Table 4.
Statistical parameters of the experiments using CCD/RSM—evaluation of the effects.
Moreover, a high value of the corrected coefficient of determination (85.32%) indicates a high degree of model fit to another sample of data from the same population. The obtained values (R2) are comparable to the other available data regarding heavy metal removal from real power plant wastewater using electrocoagulation (R2 = 99.5% and R2 = 99.6% for iron and nickel, respectively) [63], biosorption of Hg (II) ions from aqueous media by Polyporus squamosus (R2 = 92.4%) [64] and optimization of coagulation–flocculation process for wastewater originating from automotive industry and containing Fe, Cr, Cu ions (R2 = 92.08%, R2 = 93.62%, R2 = 71.87% for Fe, Cr, Cu removal, respectively) [65] and also for organic substances (R2 = 0.8477, R2adj = 0.7462) [66,67]. Table 5 presents the results of the model adequacy verification using an analysis of variance.

Table 5.
Analysis of the CCD/RSM—verification of the adequacy of the model using analysis of variance (ANOVA).
The obtained results indicate that three out of the six analyzed independent parameters are statistically significant, which means that they have a significant impact on the value of the investigated dependent parameter (∑Cu,Cd,Zn). These data correspond with the bar chart of standardized effects presented in Figure 1.
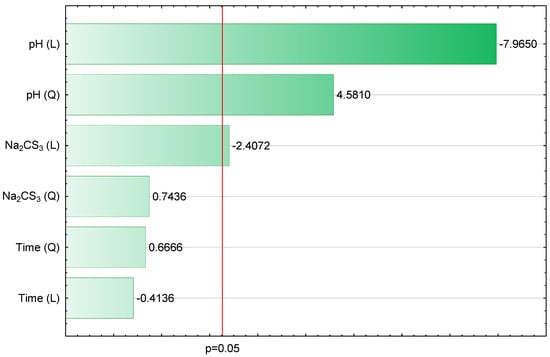
Figure 1.
Bar chart of standardized effects (∑Cu,Cd,Zn, mg/L, R2 = 0.9119, R2adj = 0.8532, 3 Parameters, 1 Block, 16 Experiments, MS = 0.1219, L—linear effect, Q—quadratic effect and p—the absolute value of the standardized effect evaluation).
Figure 1 is a visual representation of the model validation and shows estimates of standardized effects grouped by their absolute values. The lengths of the horizontal bars are proportional to the absolute value of the standardized effects, while the vertical line indicates the absolute value (p = 0.05) of the standardized effect evaluation. The statistically significant parameters show absolute values above the adopted significance level. The positive and negative signs describe the cases where the value of ∑ Cu,Cd,Zn is enhanced or weakened, respectively, when going from the lowest to the highest level set for a specific variable. Therefore, it should be assumed that the values of ∑Cu,Cd,Zn are most influenced (in descending order) by pH (L), pH (Q) and Na2CS3 (L). Accordingly, Na2CS3 (Q), Time (Q) and Time (L) have an inconsiderable impact on the ∑Cu,Cd,Zn value. Figure 2 shows the relationship between the observed values and those approximated from the model.
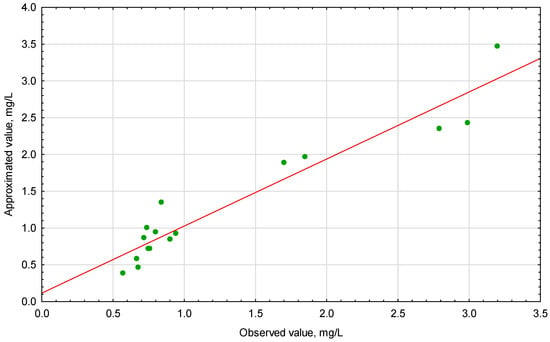
Figure 2.
Approximated versus observed values plots for ∑Cu,Cd,Zn, mg/L.
The analyzed data showed a linear relationship between the observed values and the values approximated from the model. Moreover, the points reflecting the experimental data are generally randomly distributed around the estimated relationship, which proves the adequacy of the model. Figure 3A–C illustrates the response surface plots for ∑Cu,Cd,Zn with respect to pH, Na2CS3 concentration and Time.
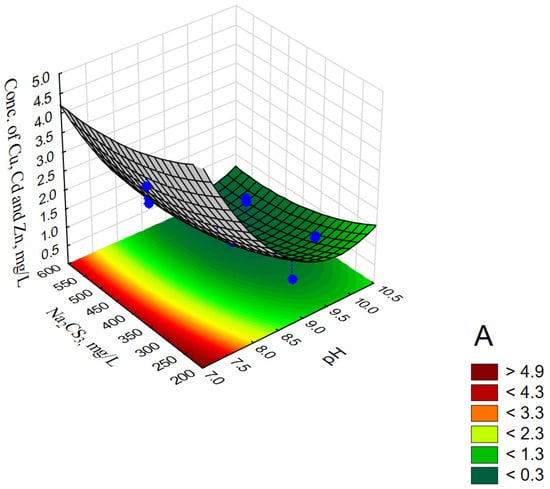
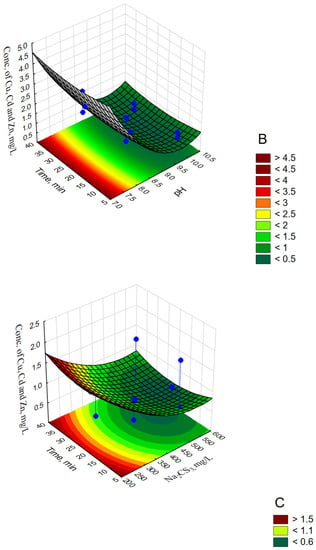
Figure 3.
Response surface plots for ∑Cu,Cd,Zn, mg/L with respect to pH and Na2CS3, mg/L (A); pH and Time, min (B); Na2CS3, mg/L and time, min (C).
The performed model tests (CCD/RSM) showed that at a constant reaction time (Time 20 min), the smallest values (<0.3 mg/L) of ∑Cu,Cd,Zn were obtained at pH 9.3–10.3 and Na2CS3 concentration > 400 mg/L (see Figure 3A). For a constant precipitant concentration (Na2CS3 concentration 400 mg/L), the lowest values (<0.5 mg/L) of ∑Cu,Cd,Zn were obtained at pH 9.25–10.1 during 17–33 min (see Figure 3B). Similar values for the reaction time at constant pH 9 and Na2CS3 concentration in the range of 490–600 mg/L (see Figure 3C) made it possible to obtain values of ∑Cu,Cd,Zn < 0.6 mg/L.
The analysis of the effect of wastewater pH on the value of ∑Cu,Cd,Zn and, therefore, on the concentration of individual metals, suggests that excessive increase in the pH value in the case of the tested wastewater may be caused by an increase in the concentration of metals. This phenomenon may result both from the presence of amphoteric hydroxides (Zn(OH)2, Cu(OH)2) in the tested wastewater [55,66], as well as from the increased solubility of complexes (or salts formed in the presence of other substances in the wastewater, e.g., NH4+) of the CS32− ion with metals at pH > 10—e.g., [Cu(CS3)n]n−, [Zn(CS3)n]n−, [Cd(CS3)n]n− KCuCS3, NH4CuCS3, Zn(NH3)2CS and others [45,47]. The studies conducted so far have shown that high efficiency of the process of removing heavy metals from wastewater from PCBs production is achieved with the use of Na2CS3 in the pH range of 9–9.5 [37,38,39]. Table 6 presents the calculated coefficients of the approximating polynomial for the experimental data presented in Table 1.

Table 6.
Coefficients of the fitted model *.
Consequently, the changes in the ∑Cu,Cd,Zn value can be calculated according to the following formula:
∑Cu,Cd,Zn (mg/L) = 52.71329 − 10.21092 (pH) + 0.52547 (pH)2 − 0.00910 (Na2CS3) + 0.00001 (Na2CS3)2 − 0.03449 (Time) + 0.00076 (Time)2
For optimal values of the three independent parameters (pH 9.72, Na2CS3 concentration 533.32 mg/L, Time 22.56 min), the sum of heavy metals in the treated wastewater (∑Cu,Cd,Zn = 0.29 mg/L) was calculated and a verification experiment was performed. Under these conditions, the concentrations of Cu, Cd and Zn were 0.12 ± 0.01, 0.10 ± 0.01, and 0.05 ± 0.01 mg/L, respectively, and ∑Cu,Cd,Zn = 0.27 ± 0.03 mg/L, as shown in Table 7. Interpretation of the obtained experimental and calculated values from the model for ∑Cu,Cd,Zn (0.27 ± 0.03 versus 0.29 mg/L, respectively) requires the measurement uncertainty for the applied method of heavy metals determination in wastewater to be taken into account, which was ±10%. Therefore, the experimental value of ∑Cu,Cd,Zn can vary in the range of 0.24–0.30 mg/L and means that the estimated value from the model (0.29 mg/L) is within the given concentration range of ∑Cu,Cd,Zn, which indicates the adequacy of the model.

Table 7.
Selected physicochemical parameters of galvanic wastewater before and after treatment.
Additionally, studies of Cu, Cd and Zn precipitation were carried out with the employment of conventional reagents used in sewage treatment plants. Due to the presence of Cd and the need for its quantitative precipitation, the wastewater was alkalinized to pH = 11 because the lowest concentration of Cd in treated wastewater is obtained only at pH approximately 11.2, and in the case of Cu and Zn at approximately 9.1 and 9.2 [55,66]. Under these conditions, using NaOH, Ca(OH)2 and CaO, a lower metal removal efficiency was achieved compared to the method with Na2CS3 (∑Cu,Cd,Zn = 90.84, 93.97, 93.71 mg/L versus ∑Cu,Cd,Zn = 99.69 mg/L, respectively). Effective precipitation of metals from wastewater required the use of Na2CS3 dose higher (by 7.2%) than the stoichiometric dose (533 versus 497 mg/L), which was probably related to the presence of chelating agents in the wastewater (as EDTA 180 mg/L) and the formation of complexes of heavy metals. Chelating agents reduce the efficiency of metal precipitation processes in the form of hydroxides [55,66] by the formation of stable complex compounds such as CuNa-NTA, ZnNa2EDTA, and others (where H3NTA is nitrilotriacetic acid, H4EDTA is ethylenediaminetetraacetic acid) [68]. The previous studies indicate that chelating agents enhance the difficulty of removing heavy metals, such as Cu, Zn, Co, Ni, Cd and Pb [69] and result in incomplete precipitation. Moreover, the increase in Zn concentration in treated wastewater, especially after NaOH application, may also be associated with the amphotericity of Zn(OH)2 and the formation of complex ions [Zn(OH)4]2− [70,71]. Other investigators obtained a final concentration of Zn < 1 mg/L using Na2S together with chemical flocculant. They concluded that zinc removal by sodium sulfide is not affected by pH, that is an economic advantage as pH adjustment is not necessary [72]. Removal of chelated copper from wastewater by replacement precipitation by using ferrous salt was closely related to the molar ratio of Fe2+/Cu2+. When the Fe2+/Cu2+ ratio increased to 12, the Cu concentration in wastewater decreased from 25 to 0.38 mg/L, while the Cu2+/EDTA ratio in wastewater was 1:1 [73]. For Cd, application of coprecipitation with 100 mg/L FeCl3 at pH 9.0 removed 97% of Cd. The application of Al2(SO)4 instead of FeCl3 at pH 9.0 removed only 91.5% of Cd [74].
3.4. Toxicity Assessment
Figure 4 shows the changes in the toxicity of wastewater to rotifer B. plicatilis, which is used to assess the toxicity of the marine environment [52]. Taking into account the significant salinity of galvanic wastewater, it is also useful for assessing the toxicity of the tested wastewater. The conducted tests showed that the use of Na2CS3 allowed the reduction in the toxicity of wastewater (expressed as percentage mortality of B. plicatilis) from 90% (untreated wastewater) to 25% (treated wastewater) after 60 min exposure time. In the case of conventional methods, the toxicity of treated wastewater was in the range of 30%–35%. Another study indicated the LC50 values (24 h) for B. plicatilis exposed to CuSO4 and CdSO4 to be 0.40 and 35.0 mg/L, respectively. These data show that B. plicatilis is more sensitive to Cu than to Cd by two orders of magnitude [75]. In the case of tested wastewater, reduction in toxicity is related to the removal of heavy metals, while final toxicity results from the presence of other compounds in the wastewater (including organic ones), that negatively affect the vital functions of B. plicatilis.
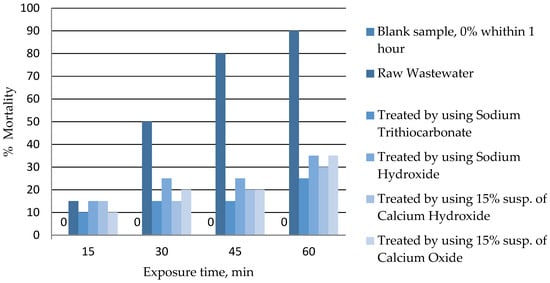
Figure 4.
The results of the wastewater toxicity assessment against rotifer B. plicatilis.
4. Conclusions
- The use of Na2CS3 under optimal conditions determined with the use of CCD/RSM enables effective precipitation of heavy metals such as Cu, Cd and Zn from actual galvanic wastewater, even in the presence of complexing compounds.
- The conventional methods of metal precipitation used so far, which consist of the alkalization of wastewater to the appropriate pH value in order to precipitate metal hydroxides, are less effective compared to the proposed methodology.
- The use of rotifer B. plicatilis to assess the toxicity of treated wastewater indicated that it has decreased significantly, which is beneficial from an environmental point of view.
- The application of Na2CS3 is not complicated and can be easily used in metal surface treatment plants.
Thus, Na2CS3 can be recommended as an effective compound to precipitate Cu, Cd and Zn from galvanic wastewater.
Author Contributions
Conceptualization, M.T.; methodology, M.T., A.B., K.B., and V.K.; statistical analysis, M.T.; formal analysis, M.T., V.K., J.J.-R. and A.B.; data curation, M.T., V.K., J.J.-R. and A.B.; writing—original draft preparation, M.T.; writing—review and editing, M.T., V.K., J.J.-R., K.B., and A.B.; visualization, M.T.; supervision, K.B. and J.J.; project administration, V.K., J.J., J.J.-R. and A.B.; funding acquisition, V.K., A.B. and J.J. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Ministry of Science and Higher Education Republic of Poland within statutory funds. This work was also supported by the Slovak Research and Development Agency (APVV-17–0373, APVV-17–0318) and the Slovak Grant Agency for Science (VEGA 1/0787/18).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable.
Acknowledgments
The authors would like to thank the anonymous reviewers for their helpful comments.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kadirvelu, K.; Thamaraiselvi, K.; Namasivayam, C. Removal of heavy metals from industrial wastewaters by adsorption onto activated carbon prepared from an agricultural solid waste. Bioresour. Technol. 2001, 76, 63–65. [Google Scholar] [CrossRef]
- Williams, C.J.; Aderhold, D.; Edyvean, G.J. Comparison between biosorbents for the removal of metal ions from aqueous solutions. Water Res. 1998, 32, 216–224. [Google Scholar] [CrossRef]
- Järup, L. Hazards of heavy metal contamination. Br. Med. Bull. 2003, 68, 167–182. [Google Scholar] [CrossRef] [PubMed]
- Nagajyoti, P.C.; Lee, K.D.; Sreekanth, T.V.M. Heavy metals, occurrence and toxicity for plants: A review. Environ. Chem. Lett. 2010, 8, 199–216. [Google Scholar] [CrossRef]
- Jaishankar, M.; Mathew, B.B.; Shah, M.S.; Gowda, K.R.S. Biosorption of Few Heavy Metal Ions Using Agricultural Wastes. J. Environ. Pollut. Hum. 2014, 2, 1–6. [Google Scholar]
- Wang, S.; Shi, X. Molecular mechanisms of metal toxicity and carcinogenesis. Mol. Cell. Biochem. 2001, 222, 3–9. [Google Scholar] [CrossRef]
- Chang, L.W.; Magos, L.; Suzuki, T. Toxicology of Metals; CRC Press: London, UK, 1996. [Google Scholar]
- Davidson, T.; Ke, Q.; Costa, M. Selected Molecular Mechanisms of Metal Toxicity and Carcinogenicity. In Handbook on the Toxicology of Metals, 4th ed.; Nordberg, G., Fowler, B., Nordberg, M., Eds.; Academic Press: Cambridge, MA, USA, 2015; Chapter 9; pp. 173–196. [Google Scholar]
- Beyersmann, D.; Hartwig, A. Carcinogenic metal compounds: Recent insight into molecular and cellular mechanisms. Arch. Toxicol. 2008, 82, 493–512. [Google Scholar] [CrossRef]
- Yedjou, G.C.; Tchounwou, P.B. In vitro cytotoxic and genotoxic effects of arsenic trioxide on human leukemia cells using the MTT and alkaline single cell gel electrophoresis (comet) assays. Mol. Cell. Biochem. 2007, 301, 123–130. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Ishaque, A.; Schneider, J. Cytotoxicity and transcriptional activation of stress genes in human liver carcinoma cells (HepG2) exposed to cadmium chloride. Mol. Cell. Biochem. 2001, 222, 21–28. [Google Scholar] [CrossRef]
- Patlolla, A.; Barnes, C.; Field, J.; Hackett, D.; Tchounwou, P.B. Potassium dichromate-induced cytotoxicity, genotoxicity and oxidative stress in human liver carcinoma (HepG2) cells. Int. J. Environ. Res. Public Health 2009, 6, 643–653. [Google Scholar] [CrossRef]
- Yedjou, G.C.; Tchounwou, P.B. N-acetyl-cysteine affords protection against lead-induced cytotoxicity and oxidative stress in human liver carcinoma (HepG2) cells. Int. J. Environ. Res. Public Health 2008, 4, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Sutton, D.J.; Tchounwou, P.B. Mercury induces the externalization of phosphatidylserine in human proximal tubule (HK-2) cells. Int. J. Environ. Res. Public Health 2007, 4, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Vasudevan, S.; Oturan, M.A. Electrochemistry: As cause and cure in water pollution–An overview. Environ. Chem. Lett. 2014, 12, 97–108. [Google Scholar] [CrossRef]
- Salles, F.J.; Sato, A.P.S.; Luz, M.S.; Fávaro, D.I.T.; Ferreira, F.J.; da Silva Paganini, W.; Olympio, K.P.K. The environmental impact of informal and home productive arrangement in the jewelry and fashion jewelry chain on sanitary sewer system. Environ. Sci. Pollut. Res. 2018, 25, 10701–10713. [Google Scholar] [CrossRef] [PubMed]
- Giurlani, W.; Zangari, G.; Gambinossi, F.; Passaponti, M.; Salvietti, E.; Di Benedetto, F.; Caporali, S.; Innocenti, M. Electroplating for Decorative Applications: Recent Trends in Research and development. Coatings 2018, 8, 260. [Google Scholar] [CrossRef]
- Rahman, L.; Sarkar, S.M.; Yousef, M.M. Efficient removal of heavy metals from electroplating wastewater using polymer ligands. Front. Environ. Sci. Eng. 2016, 10, 352–361. [Google Scholar] [CrossRef]
- Thomas, M.; Białecka, B.; Zdebik, D. Sources of copper ions and selected methods of their removal from wastewater from the printed circuits board production. Inż. Ekol. 2014, 37, 31–49. [Google Scholar]
- Thomas, M.; Białecka, B.; Zdebik, D. Evaluation and application of coagulants containing divalent and trivalent iron to enhance removal of organic compounds and complexed copper and tin ions from industrial effluents. Inż. Ekol. 2014, 38, 167–180. [Google Scholar]
- Głodniok, M.; Zdebik, D.; Thomas, M.; Zawartka, P. A toxic effect of wastewater from the production of printed circuit boards on activated sludge from municipal wastewater treatment plant. Przem. Chem. 2016, 95, 1304–1309. [Google Scholar]
- Thomas, M.; Zdebik, D.; Świnder, H. Recovery of tin from electroplating sludges generated in the treatment of the concentrated wastewater from electrochemical tin plating. Przem. Chem. 2017, 96, 1296–1302. [Google Scholar]
- Huisman, J.L.; Schouten, G.; Schultz, C. Biologically produced sulphide for purification of process streams, effluent treatment and recovery of metals in the metal and mining industry. Hydrometallurgy 2006, 83, 106–113. [Google Scholar] [CrossRef]
- Özverdi, A.; Erdem, M. Cu2+, Cd2+ and Pb2+ adsorption from aqueous solutions by pyrite and synthetic iron sulphide. J. Hazard. Mater. 2006, 137, 626–632. [Google Scholar] [CrossRef] [PubMed]
- González-Muñoz, M.J.; Rodríguez, M.A.; Luquea, S.; Álvareza, J.R. Recovery of heavy metals from metal industry waste waters by chemical precipitation and nanofiltration. Desalination 2006, 200, 742–744. [Google Scholar] [CrossRef]
- Papadopoulos, A.; Fatta, D.; Parperis, K.; Mentzis, A.; Haralambous, K.J.; Loizidou, M. Nickel uptake from a wastewater stream produced in a metal finishing industry by combination of ion-exchange and precipitation methods. Sep. Purif. Technol. 2004, 39, 181–188. [Google Scholar] [CrossRef]
- Jusoh, A.; Shiung, L.S.; Ali, N.; Noor, M.J.M.M. A simulation study of the removal efficiency of granular activated carbon on cadmium and lead. Desalination 2007, 206, 9–16. [Google Scholar] [CrossRef]
- Reyes, I.; Villarroel, M.; Diez, M.C.; Navia, R. Using lignimerin (a recovered organic material from Kraft cellulose mill wastewater) as sorbent for Cu and Zn retention from aqueous solutions. Bioresour. Technol. 2009, 100, 4676–4682. [Google Scholar] [CrossRef] [PubMed]
- Sheng, G.D.; Wang, S.W.; Hua, J.; Lu, Y.; Li, J.X.; Dong, Y.H.; Wang, X.K. Adsorption of Pb(II) on diatomite as affected via aqueous solution chemistry and temperature. Colloid Surf. 2009, 339, 159–166. [Google Scholar] [CrossRef]
- Apiratikul, R.; Pavasant, P. Sorption of Cu2+, Cd2+ and Pb2+ using modified zeolite from coal fly ash. Chem. Eng. J. 2008, 144, 245–258. [Google Scholar] [CrossRef]
- Apiratikul, R.; Pavasant, P. Batch and column studies of biosorption of heavy metals by Caulerpa lentillifera. Bioresour. Technol. 2008, 99, 2766–2777. [Google Scholar] [CrossRef]
- Landaburu-Aguirre, J.; García, V.; Pongrácz, E.; Keiski, R.L. The removal of zinc from synthetic wastewaters by micellar-enhanced ultrafiltration: Statistical design of experiments. Desalination 2009, 240, 262–269. [Google Scholar] [CrossRef]
- Molinari, R.; Poerio, T.; Argurio, P. Selective separation of copper (II) and nickel (II) from aqueous media using the complexation-ultrafiltration process. Chemosphere 2008, 70, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Mohsen-Nia, M.; Montazeri, P.; Modarress, H. Removal of Cu2+ and Ni2+ from wastewater with a chelating agent and reverse osmosis processes. Desalination 2007, 217, 276–281. [Google Scholar] [CrossRef]
- Figoli, A.; Cassano, A.; Criscuoli, A.; Mozumder, M.S.I.; Uddin, M.T.; Islam, M.A.; Drioli, E. Influence of operating parameters on the arsenic removal by nanofiltration. Water Res. 2010, 44, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Kabdasli, I.; Arslan, T.; Olmez-Hanci, T.; Arslan-Alaton, I.; Tünay, O. Complexing agent and heavy metal removals from metal plating effluent by electrocoagulation with stainless steel electrodes. J. Hazard. Mater. 2009, 165, 838–845. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.; Białecka, B.; Zdebik, D. Removal of copper, nickel and tin from model and real industrial wastewater using sodium trithiocarbonate. The negative impact of complexing compounds. Arch. Environ. Prot. 2018, 44, 33–47. [Google Scholar]
- Thomas, M.; Zdebik, D.; Białecka, B. Use of sodium trithiocarbonate for remove of chelated copper ions from industrial wastewater originating from the electroless copper plating process. Arch. Environ. Prot. 2018, 44, 32–42. [Google Scholar]
- Thomas, M.; Zdebik, D.; Białecka, B. Using sodium trithiocarbonate to precipitate heavy metals from industrial wastewater—From the laboratory to industrial scale. Pol. J. Environ. Stud. 2018, 27, 1–11. [Google Scholar] [CrossRef]
- Thomas, M.; Białecka, B.; Cykowska, M.; Bebek, M.; Bauerek, A. Precipitation of rare earth elements from model and real solutions by using alkaline and sulfur compounds. Przem. Chem. 2017, 96, 2471–2475. [Google Scholar]
- Elfline, G.S. Method of Removing Heavy Metal from Wastewater Streams. U.S. Patent 4,678,584, 7 July 1987. [Google Scholar]
- American Society for Testing Materials. Standard Practice for the Preparation of Substitute Ocean Water; ASTM D1141–98; ASTM International: West Conshohocken, PA, USA, 2013. [Google Scholar]
- Supniewski, J. Inorganic Preparation; PWN Warsaw: Paris, France, 1985. [Google Scholar]
- Stechman, M.; Różycka, D.; Mateńko, H.; Marszałek, J.; Wojtycha, Z.; Jamroży, J.; Zaczkowski, L.; Sufczyński, T. Sodium Trithiocarbonate Manufacturing Method. PL 198,453, 1 November 2001. [Google Scholar]
- Gattow, G.; Behrendt, W. Carbon Sulfides and Their Inorganic and Complex Chemistry; Georg Thieme Publishers: Stuttgart, Germany, 1977. [Google Scholar]
- Bobrowska-Krajewska, K.; Dąbek, M.; Kmieć, B.; Krajewski, J. Possibility of removing a trace amounts of heavy metals from wastewater. Arch. Environ. Prot. 1994, 3–4, 73–87. [Google Scholar]
- Bobrowska-Krajewska, K.; Dąbek, M.; Kmieć, B.; Krajewski, J. Selected issues of synthesis and application of sodium triocarbonate. Chemik 1994, 6, 155–158. [Google Scholar]
- Polish Committee for Standardization. Sodium Sulphide for Industrial Use; PN-C-84042; Polish Committee for Standardization: Warsaw, Poland, 1997. [Google Scholar]
- International Organization for Standardization. Water Quality-Determination of pH; ISO 10523; IOS: Geneva, Switzerland, 2008. [Google Scholar]
- NANOCOLOR® Organische Komplexbildner 10. Available online: https://www.mn-net.com/media/pdf/6a/60/8c/Instruction-985052-Tube-test-NANOCOLOR-org-Complexing-agents-10.pdf (accessed on 20 January 2020).
- International Organization for Standardization. Water Quality-Determination of Selected Elements by Inductively Coupled Plasma Optical Emission Spectrometry; ISO 11885:2007; IOS: Geneva, Switzerland, 2007. [Google Scholar]
- Hassaan, M.A.; El Nemr, A.; Madkour, F.F. Testing the advanced oxidation processes on the degradation of Direct Blue 86 dye in wastewater. Egypt. J. Aquat. Res. 2016, 43, 11–19. [Google Scholar] [CrossRef]
- Snell, T.W.; Persoone, G. Acute toxicity bioassays using rotifers. I. A test for brackish and marine environment with Brachionus Plicatilis. Aquat. Toxicol. 1989, 14, 65–80. [Google Scholar] [CrossRef]
- Thomas, M.; Zdebik, D. Treatment of Real Textile Wastewater by Using Potassium Ferrate(VI) and Fe(III)/H2O2. Application of Aliivibrio fischeri and Brachionus plicatilis Tests for Toxicity Assessment. Fibres Text. East. Eur. 2019, 3, 78–84. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. Treatability Manual Volume III Technologies for Comntrol/Removal of Pollutants, Office of Research and Development; USEPA: Washington, DC, USA, 1981.
- Regulation of the Minister of Construction on the manner of fulfilling the obligations of industrial wastewater suppliers and the conditions of introduction of wastewater to sewage systems. 2016; 1757, 1–9. (In Polish)
- Singh, K.; Bhatia, P.G.; Gupta, R.D. Direct Oxidimetric determination of thiocarbonate sulfur with ferricyanide using iron(II)-dimethylglyoxime or sodium nitroprusside as indicator. Talanta 1982, 29, 47–48. [Google Scholar] [CrossRef]
- Singh, K.; Fodeke, B.A. Determination of thiocarbonate in the presence of sulphite, thiosulphate and thiocyanate by titration with o-hydroxymercuribenzoate. Talanta 1983, 30, 693–694. [Google Scholar] [CrossRef]
- Wroński, M. Determination of sulphide, polysulphide, thiocarbonates and sulphur by extraction with tributyltin hydroxide and of thiosulphate by hydrogenation. Talanta 1981, 28, 173–176. [Google Scholar] [CrossRef]
- Chen, T.-C.; Priambodo, R.; Huang, R.-L.; Huang, Y.-H. The effective electrolytic recovery of dilute copper from industrial wastewater. J. Waste Manag. 2013, 2013, 164780. [Google Scholar] [CrossRef]
- Jon, M.; Heuss-Assbichler, S.; Ullrich, A. Recovery of Zn from wastewater of zinc plating industry by precipitation of doped ZnO nanoparticles. Int. J. Environ. Sci. Technol. 2016, 13, 2127–2134. [Google Scholar] [CrossRef]
- Tunay, O.; Kabdasli, N.I. Hydroxide precipitation of complexed metals. Water Res. 1994, 28, 2117–2124. [Google Scholar] [CrossRef]
- Beiramzadeh, Z.; Baqersad, M.; Aghababaei, M. Application of the Response Surface Methodology (RSM) in Heavy Metal Removal from Real Power Plant Wastewater Using Electrocoagulation. Available online: https://doi.org/10.1080/19648189.2019.1640139 (accessed on 19 December 2020).
- Uzun, Y.; Sahan, T. Optimization with Response Surface Methodology of biosorption conditions of Hg(II) ions from aqueous media by Polyporus squamosus fungi as a new biosorbent. Arch. Environ. Prot. 2017, 43, 37–43. [Google Scholar] [CrossRef][Green Version]
- Bakar, A.F.A.; Halim, A.A.; Hanafiah, M.M. Optimization of Coagulation-Flocculation Process for Automotive Wastewater Treatment using Response Surface Methodology. Nat. Environ. Pollut. 2015, 14, 567–572. [Google Scholar]
- Summary Report Control and Treatment Technology for the Metal Finishing Industry. Sulfide Precipitation; Industrial Environmental Research Laboratory: Cincinnati, OH, USA, 1980.
- Thomas, M.; Kozik, V.; Barbusiński, K.; Sochanik, A.; Jampilek, J.; Bąk, A. Potassium Ferrate (VI) as the Multifunctional Agent in the Treatment of Landfill Leachate. Molecules 2020, 13, 5017. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.; Brauch, H.-J. Impact of Aminocarboxylates on Aquatic Organisms and Eutrophication: Overview of Available Data. Environ. Toxicol. 2004, 19, 620–637. [Google Scholar] [CrossRef] [PubMed]
- Kołodyńska, D.; Siek, M.; Gęca, M.; Hubicki, Z. Role of Chelating Agents of a New Generation in Sorption of Metal Ions. Wastewater Treatment. In Proceedings of the 15th ICHMET, Gdańsk, Poland, 19–23 September 2010. [Google Scholar]
- Bielański, A. Fundamentals of Inorganic Chemistry; PWN: Warsaw, Poland, 2020. [Google Scholar]
- Lipiec, T.; Szmal, Z. Analytical Chemistry with the Basics of Instrumental Analysis; PZWL: Warsaw, Poland, 1996. [Google Scholar]
- Subbiah, R.M.; Sastry, C.A.; Agamuthu, P. Removal of Zink from Rubber Thread Manufacturing Industry Wastewater using Chemical Precipitation/Flocculant. Environ. Prog. 2000, 19, 299–304. [Google Scholar] [CrossRef]
- Jiang, S.; Qu, J.; Xiong, Y. Removal of chelated copper from wastewaters by Fe2+-based replacement-precipitation. Environ. Chem. Lett. 2010, 8, 339–342. [Google Scholar] [CrossRef]
- El-Gohary, F.; Lasheen, M.R.; Abdel-Shafy, H.I. Trace metal removal from wastewater via chemical treatment. In Proceedings of the 1st International Conference Management and Control of Heavy Metals in the Environment, London, UK, September 1979. [Google Scholar]
- Gvozdov, A.O. Phototaxis as a test function in bioassay. Hydrobiol. J. 1986, 22, 65–68. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).