(Ti,Sn) Solid Solution Based Gas Sensors for New Monitoring of Hydraulic Oil Degradation
Abstract
1. Introduction
2. Materials and Methods
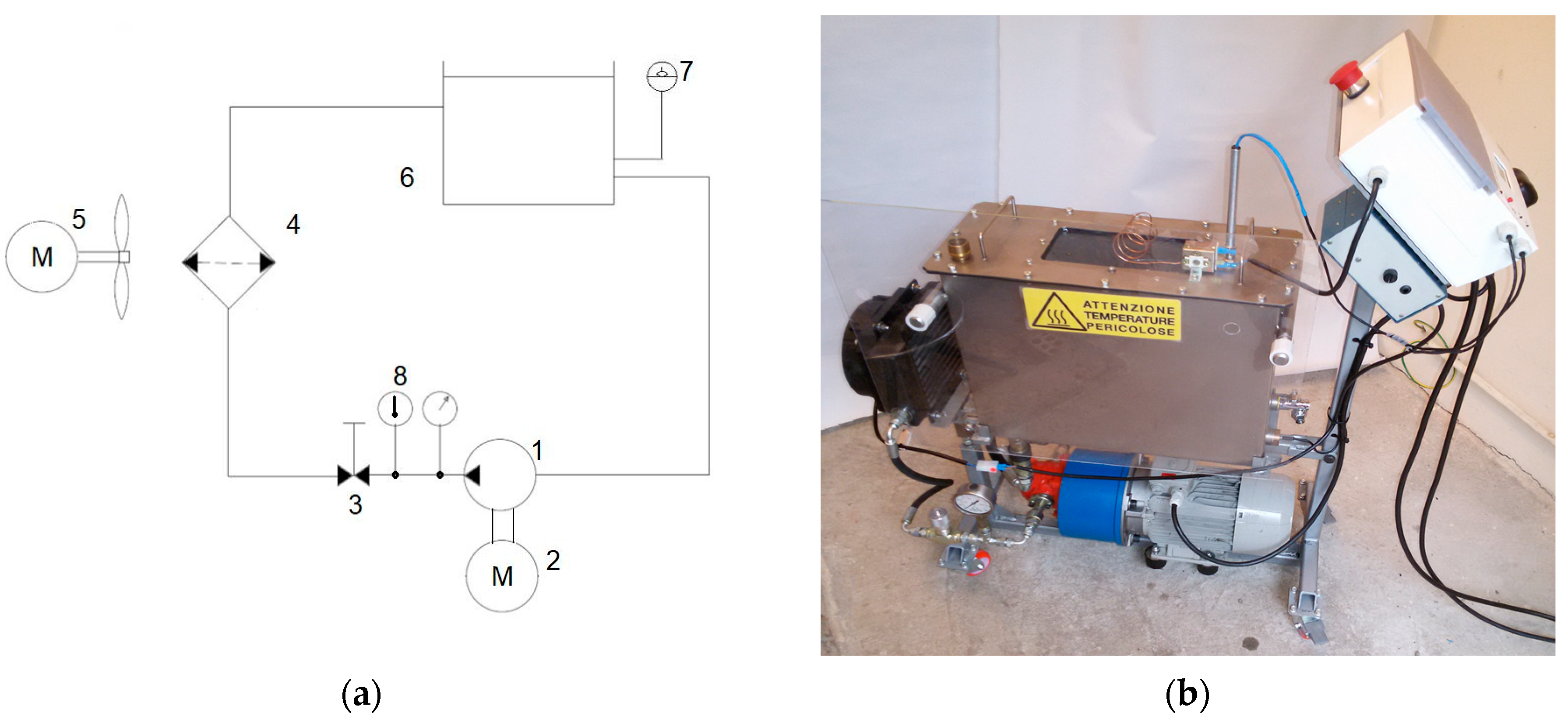
2.1. Hydraulic Oil and Its Ageing
2.2. Array of MOX Gas Sensors
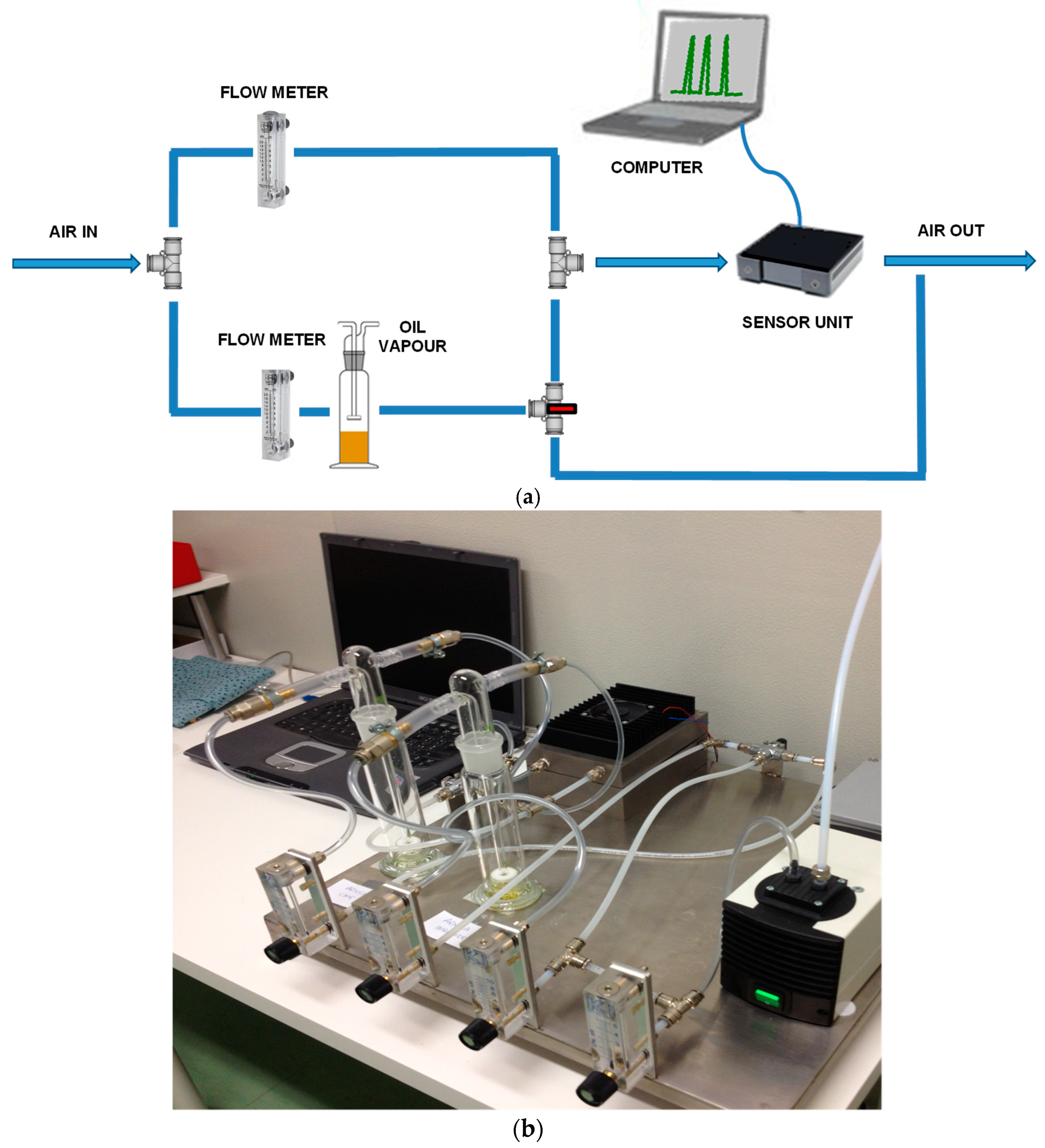
2.3. Laboratory MOX Sensors System
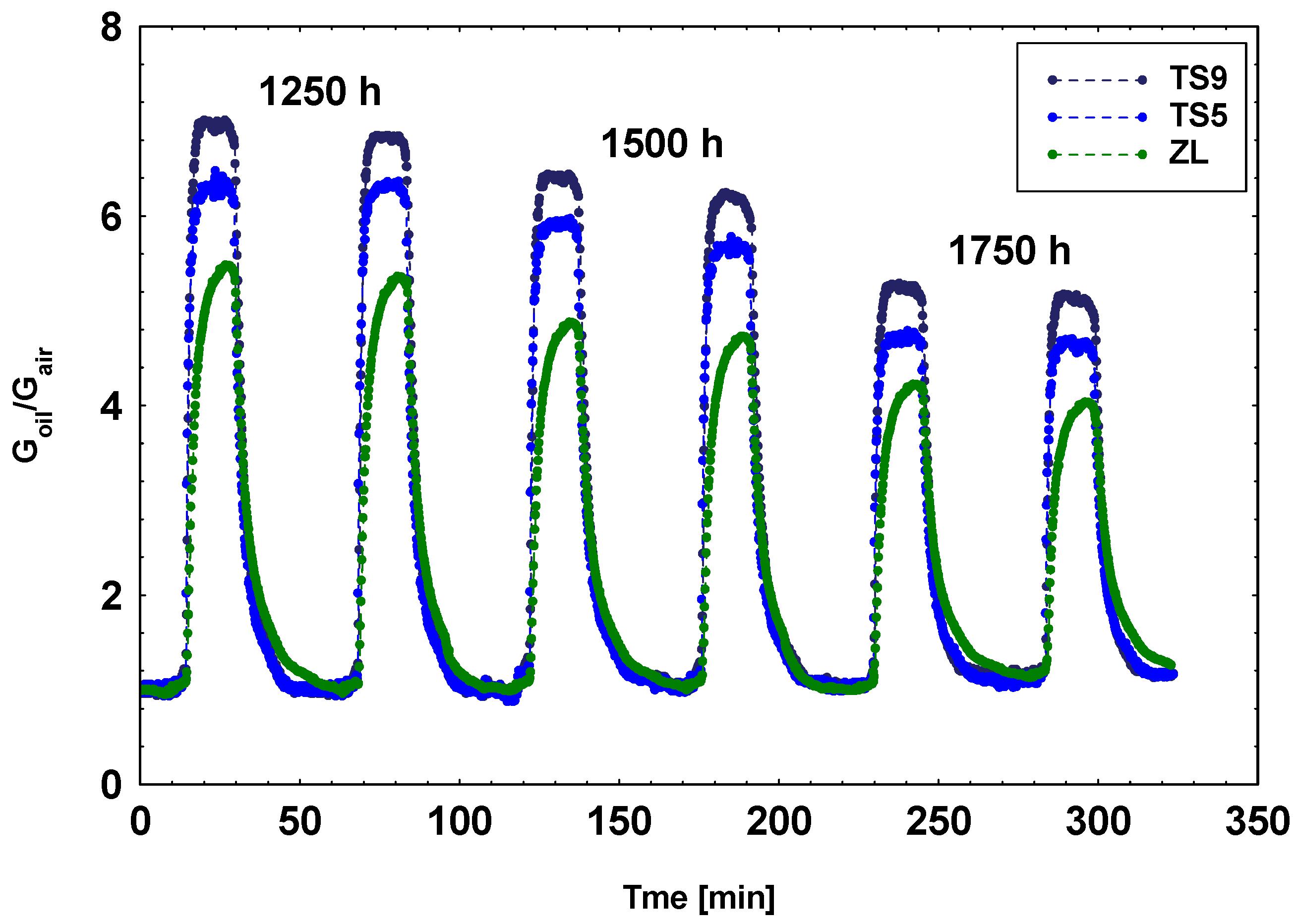
3. Results and Discussion
3.1. Hydraulic Oil Ageing Samples
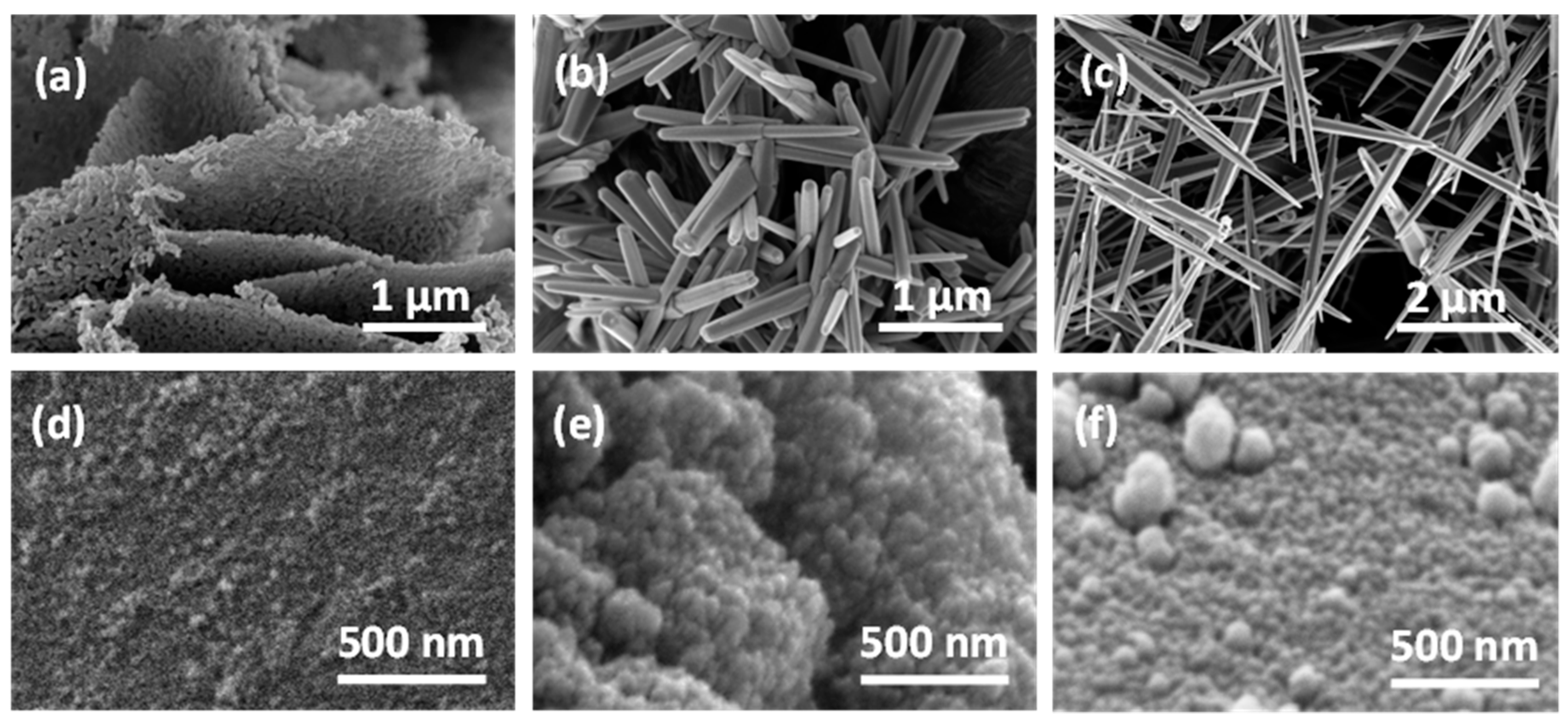
3.2. Characterizations of Sensors’ Materials
- -
- SnO2, SnO2_Au, and SnO2_Pd from 20 to 40 nm agglomerated in a roundish structure of about 100–200 nm,
- -
- STN from 10 to 30 nm,
- -
- TiO2 from 20 to 40 nm agglomerated in a roundish structure of about 50–100 nm,
- -
- TTV from 20 to 40 nm,
- -
- T-Ta from 30 to 60 nm,
- -
- WO3 from 60 to 90 nm, and
- -
- LaFeO3 from 50 to 80 nm agglomerated in a hexagonal structure of about 1 μm.
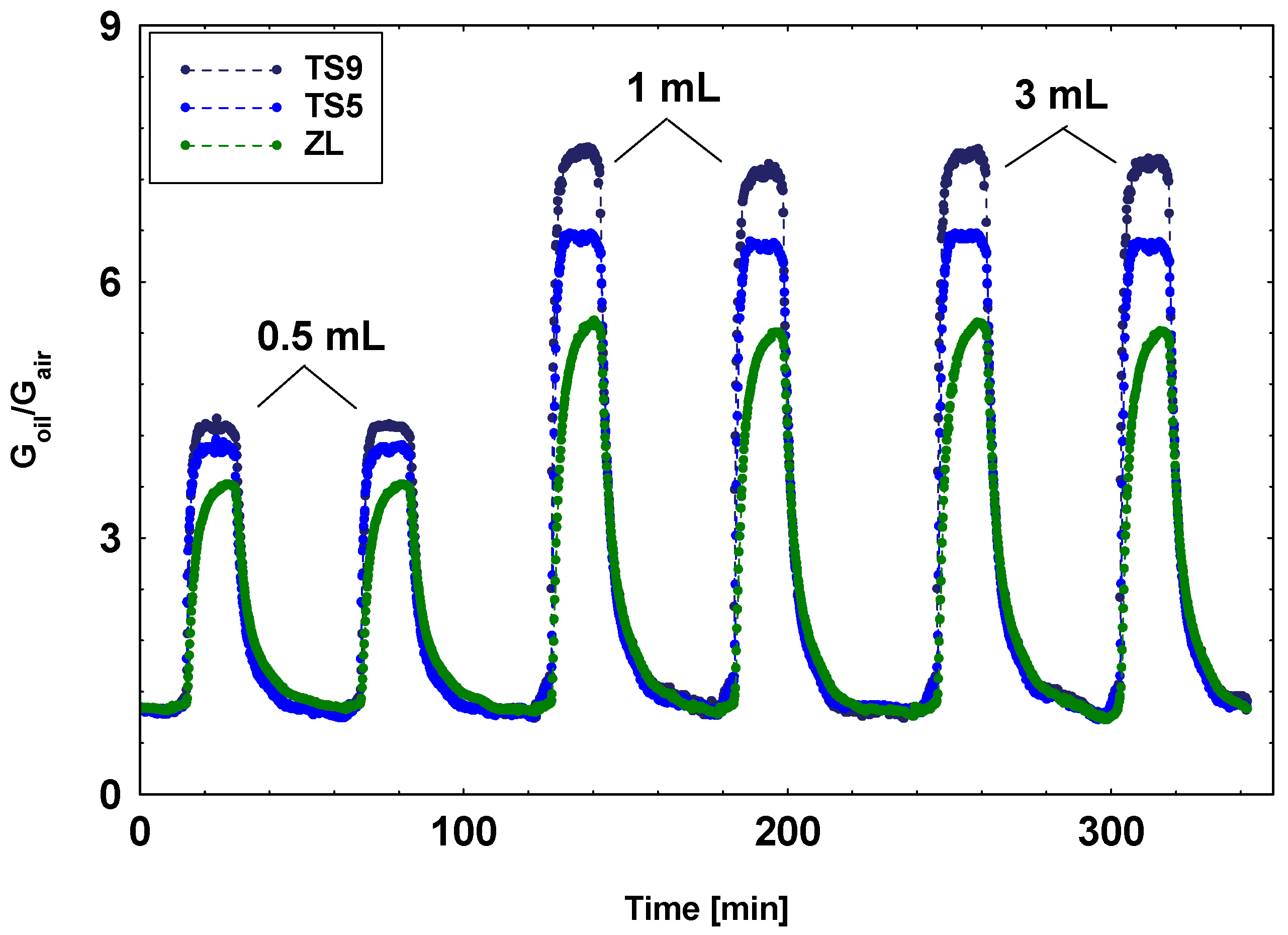
3.3. Laboratory MOX Sensors System: Oil Ageing Characterization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baczewski, K.; Szczawiński, P. Investigation of the process of ageing of hydraulic oil during its use. Arch. Automot. Eng. Arch. Motoryz. 2016, 73, 5–18. [Google Scholar] [CrossRef]
- Totten, G.E. Handbook of Hydraulic Fluid Technology; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Majumdar, S.R. Oil Hydraulic Systems: Principles and Maintenance; McGraw-Hill: New York, NY, USA, 2003. [Google Scholar]
- Lovrec, D.; Tič, V. On-line condition monitoring systems for hydraulic machines, UDC 621.22. Facta Univ. Ser. Mech. Eng. 2012, 10, 81–89. [Google Scholar]
- Höhn, B.R.; Michaellis, K. Influence of Lubricant Ageing on Gear Performance. Available online: http://www.oetg.at/fileadmin/Dokumente/oetg/Proceedings/WTC_2001_files/html/M-28-02-339-HOEHN.pdf (accessed on 9 December 2019).
- Lopez, P.; Mabe, J.; Miró, G.; Etxeberria, L. Low cost photonic sensor for in-line oil quality monitoring: Methodological development process towards uncertainty mitigation. Sensors 2018, 18, 2015. [Google Scholar] [CrossRef]
- Pacchioni, G. Oxygen vacancy: The invisible agent on oxide surfaces. ChemPhysChem 2003, 4, 1041–1047. [Google Scholar] [CrossRef]
- Barsan, N.; Koziej, D.; Weimar, U. Metal oxide-based gas sensor research: How to? Sens. Actuators B Chem. 2007, 121, 18–35. [Google Scholar] [CrossRef]
- Trani, F.; Causà, M.; Ninno, D.; Cantele, G.; Barone, V. Density functional study of oxygen vacancies at the SnO2 surface and subsurface sites. Phys. Rev. B 2008, 77, 245410. [Google Scholar] [CrossRef]
- Trani, F.; Causà, M.; Lettieri, S.; Setaro, A.; Ninno, D.; Barone, V.; Maddalena, P. Role of Surface oxygen vacancies in photoluminescence of tin dioxide nanobelts. Microelectron. J. 2009, 40, 236–238. [Google Scholar] [CrossRef]
- Pallotti, D.K.; Orabona, E.; Amoruso, S.; Aruta, C.; Bruzzese, R.; Chiarella, F.; Tuzi, S.; Maddalena, P.; Lettieri, S. Multi-Band photoluminescence in TiO2 nanoparticles-assembled films produced by femtosecond pulsed laser deposition. J. Appl. Phys. 2013, 114, 043503. [Google Scholar] [CrossRef]
- Miklos, D.B.; Remy, C.; Jekel, M.; Linden, K.G.; Drewes, J.E.; Hübner, U. Evaluation of advanced oxidation processes for water and wastewater Treatment—A critical review. Water Res. 2018, 139, 118–131. [Google Scholar] [CrossRef]
- Abdel-Maksoud, Y.K.; Imam, E.; Ramadan, A.R. TiO2 water-bell photoreactor for wastewater treatment. Sol. Energy 2018, 170, 323–335. [Google Scholar] [CrossRef]
- Acar, C.; Dincer, I.; Naterer, G.F. Review of Photocatalytic water-splitting methods for sustainable hydrogen production: Review: Photocatalysis for sustainable hydrogen. Int. J. Energy Res. 2016, 40, 1449–1473. [Google Scholar] [CrossRef]
- Zhang, X.; Peng, T.; Song, S. Recent advances in dye-sensitized semiconductor systems for photocatalytic hydrogen production. J. Mater. Chem. A 2016, 4, 2365–2402. [Google Scholar] [CrossRef]
- He, H.; Lin, J.; Fu, W.; Wang, X.; Wang, H.; Zeng, Q.; Gu, Q.; Li, Y.; Yan, C.; Tay, B.K.; et al. MoS2/TiO2 edge-on heterostructure for efficient photocatalytic hydrogen evolution. Adv. Energy Mater. 2016, 6, 1600464. [Google Scholar] [CrossRef]
- Chen, B.; Meng, Y.; Sha, J.; Zhong, C.; Hu, W.; Zhao, N. Preparation of MoS2/TiO2 based nanocomposites for photocatalysis and rechargeable batteries: Progress, challenges, and perspective. Nanoscale 2018, 10, 34–68. [Google Scholar] [CrossRef] [PubMed]
- Lettieri, S.; Gargiulo, V.; Pallotti, D.K.; Vitiello, G.; Maddalena, P.; Alfè, M.; Marotta, R. Evidencing opposite charge-transfer processes at TiO2/graphene-related materials interface through a combined EPR, photoluminescence and photocatalysis assessment. Catal. Today 2018, 315, 19–30. [Google Scholar] [CrossRef]
- Tang, B.; Chen, H.; Peng, H.; Wang, Z.; Huang, W. Graphene modified TiO2 composite photocatalysts: Mechanism, progress and perspective. Nanomaterials 2018, 8, 105. [Google Scholar] [CrossRef] [PubMed]
- Mujahid, A.; Dickert, F.L. Monitoring automotive oil degradation: Analytical tools and onboard sensing technologies. Anal. Bioanal. Chem. 2012, 404, 1197–1209. [Google Scholar] [CrossRef]
- Ng, F.; Harding, J.A.; Glass, J. Improving hydraulic excavator performance through in line hydraulic oil contamination monitoring. Mech. Syst. Signal Process. 2017, 83, 176–193. [Google Scholar] [CrossRef]
- Zebing, M.; Zhao, J.; Xuan, W.; Wang, W.; Luo, J.; Xie, J. Distilling determination of water content in hydraulic oil with a ZnO/glass surface acoustic wave device. Microsyst. Technol. 2017, 23, 1841–1845. [Google Scholar] [CrossRef]
- Sepcic, K.; Josowicz, M.; Janata, J.; Selby, T. Diagnosis of used engine oil based on gas phase analysis. Analyst 2004, 129, 1070–1075. [Google Scholar] [CrossRef]
- Capone, S.; Zuppa, M.; Presicce, D.S.; Francioso, L.; Casino, F.; Siciliano, P. Metal oxide gas sensor array for the detection of diesel fuel in engine oil. Sens. Actuators B Chem. 2008, 131, 125–133. [Google Scholar] [CrossRef]
- Fioravanti, A.; Bonanno, A.; Gherardi, S.; Carotta, M.C.; Skouloudis, A.N. A portable air-quality station based on thick film gas sensors for real time detection of traces of atmospheric pollutants. IOP Conf. Ser. Mater. Sci. Eng. 2016, 2016 108, 012005. [Google Scholar] [CrossRef]
- Galstyan, V. Porous TiO2-Based gas sensors for cyber chemical systems to provide security and medical diagnosis. Sensors 2017, 17, 2947. [Google Scholar] [CrossRef]
- Lee, S.-H.; Galstyan, V.; Ponzoni, A.; Gonzalo-Juan, I.; Riedel, R.; Dourges, M.-A.; Nicolas, Y.; Toupance, T. Finely tuned SnO2 nanoparticles for efficient detection of reducing and oxidizing gases: The influence of alkali metal cation on gas-sensing properties. ACS Appl. Mater. Interfaces 2018, 10, 10173–10184. [Google Scholar] [CrossRef]
- Shimizu, Y.; Matsunaga, N.; Hyodo, T.; Egashira, M. Improvement of SO2 sensing properties of WO3 by noble metal loading. Sens. Actuators B Chem. 2001, 77, 35–40. [Google Scholar] [CrossRef]
- Abdullah, N.A.; Khusaimi, Z.; Mohammad Rusop, M. A review on zinc oxide nanostructures: Doping and gas sensing. Adv. Mater. Res. 2013, 667, 329–332. [Google Scholar] [CrossRef]
- Ambrosone, G.; Coscia, U.; Lettieri, S.; Maddalena, P.; Minarini, C. Optical, structural and electrical properties of Μc-Si:H films deposited by SiH4+H2. Mater. Sci. Eng. B 2003, 101, 236–241. [Google Scholar] [CrossRef]
- Pallotti, D.K.; Passoni, L.; Gesuele, F.; Maddalena, P.; Di Fonzo, F.; Lettieri, S. Giant O2-induced photoluminescence modulation in hierarchical titanium dioxide nanostructures. ACS Sens. 2017, 2, 61–68. [Google Scholar] [CrossRef]
- Orabona, E.; Pallotti, D.; Fioravanti, A.; Gherardi, S.; Sacerdoti, M.; Carotta, M.C.; Maddalena, P.; Lettieri, S. On mechanism of NO2 detection by ZnO excitonic luminescence. Sens. Actuators B Chem. 2015, 210, 706–711. [Google Scholar] [CrossRef]
- Fioravanti, A.; Bonanno, A.; Mazzocchi, M.; Carotta, M.C.; Sacerdoti, M. Enhanced gas sensing properties of different ZnO 3D hierarchical structures. Adv. Sci. Technol. 2017, 99, 48–53. [Google Scholar] [CrossRef]
- Carotta, M.C.; Fioravanti, A.; Gherardi, S.; Malagù, C.; Sacerdoti, M.; Ghiotti, G.; Morandi, S. (Ti, Sn) solid solutions as functional materials for gas sensing. Sens. Actuators B Chem. 2014, 194, 195–205. [Google Scholar] [CrossRef]
- John Deere-HY-GARD JDM J20C. Available online: https://www.deere.it/it/ricambi-e-assistenza/ricambi/ricambi-manutenzione/lubrificanti/fluidi-idraulici-per-trasmissione/ (accessed on 14 December 2020).
- Traversa, E.; Di Vona, M.L.; Licoccia, S.; Sacerdoti, M.; Carotta, M.C.; Gallana, M.; Martinelli, G. Sol-Gel nanosized semiconducting titania-based powders for thick-film gas sensors. J. Sol Gel Sci. Technol. 2000, 19, 193–196. [Google Scholar] [CrossRef]
- Carotta, M.C.; Vincenzo, G.; Malagù, C.; Vendemiati, B.; Zanni, A.; Martinelli, G.; Sacerdoti, M.; Licoccia, S.; Di Vona, M.L.; Traversa, E. Vanadium and tantalum-doped titanium oxide (TiTaV): A novel material for gas sensing. Sens. Actuators B Chem. 2005, 108, 89–96. [Google Scholar] [CrossRef]
- Cavicchi, B.; Carotta, M.C.; Ferroni, M.; Martinelli, G.; Piga, M.; Gherardi, S. Preparation and characterization of thick films of Au-, Pd-, Pt-doped SnO2 for gas sensing. Sens. Microsyst. 2002, 154–157. [Google Scholar] [CrossRef]
- Carotta, M.C.; Benetti, M.; Guidi, V.; Gherardi, S.; Malagu’, C.; Vendemiati, B.; Martinelli, G. Nanostructured (Sn,Ti,Nb)O2 solid solution for hydrogen sensing. MRS Proc. 2006, 915, 1–6. [Google Scholar] [CrossRef]
- Morandi, S.; Amodio, A.; Fioravanti, A.; Giacomino, A.; Mazzocchi, M.; Sacerdoti, M.; Carotta, M.C.; Skouloudis, A.N. Operational functionalities of air-quality W-Sn metal-oxide sensors correlating semiconductor defect levels and surface potential barriers. Sci. Total Environ. 2020, 706, 135731. [Google Scholar] [CrossRef] [PubMed]
- Morandi, S.; Fioravanti, A.; Cerrato, G.; Lettieri, S.; Sacerdoti, M.; Carotta, M.C. Facile synthesis of ZnO nano-structures: Morphology influence on electronic properties. Sens. Actuators B Chem. 2017, 249, 581–589. [Google Scholar] [CrossRef]
- Martinelli, G.; Carotta, M.C.; Ferroni, M.; Sadaoka, Y.; Traversa, E. Screen-Printed perovskite-type thick films as gas sensors for environmental monitoring. Sens. Actuators B Chem. 1999, 55, 99–110. [Google Scholar] [CrossRef]
- Wiles, D.B.; Young, R.A. A new computer program for Rietveld analysis of X-ray powder diffraction patterns. J. Appl. Crystallogr. 1981. [Google Scholar] [CrossRef]
- Prudenziati, M.; Hormadaly, J. Printed Films: Materials Science and Applications in Sensors, Electronics and Photonics; Elsevier: Amsterdam, The Netherlands, 2012; ISBN 9781845699888. [Google Scholar]
- Yang, W.; Feng, L.; He, S.H.; Liu, L.Y.; Liu, S. Density gradient strategy for preparation of broken In2O3 microtubes with remarkably selective detection of triethylamine vapor. ACS Appl. Mater. Interfaces 2018, 10, 27131–27140. [Google Scholar] [CrossRef]
- Byrne, C.; Fagan, R.; Hinder, S.; McCormack, D.E.; Pillai, S.C. New approach of modifying the anatase to rutile transition temperature in TiO2 photocatalysts. RSC Adv. 2016, 6, 95232–95238. [Google Scholar] [CrossRef]
- Fioravanti, A.; Carotta, M.C. Year 2020: A snapshot of the last progress in flexible printed gas sensors. Appl. Sci. 2020, 10, 1741. [Google Scholar] [CrossRef]
- Fioravanti, A.; Morandi, S.; Carotta, M.C. Chemoresistive gas sensors for sub-ppm acetone detection. Proc. Eng. 2016, 168, 485–488. [Google Scholar] [CrossRef]
- Fioravanti, A.; Bonanno, A.; Carotta, M.C.; Gherardi, S.; Lettieri, S.; Maddalena, P.; Orabona, E.; Pallotti, D.K.; Paoluzzi, R.; Sacerdoti, M. ZnO as functional material for sub-ppm acetone detection. In Proceedings of the International Conference on IEEE Sensor, Valencia, Spain, 2–5 November 2014; pp. 803–806. [Google Scholar] [CrossRef]


| Sample Name | Oxide | Reagents, Medium, and Catalyst | Calcination Process | Ref. |
|---|---|---|---|---|
| T | TiO2 | Ti(IV) n-butoxide, hydroalcoholic media | 2 h at 450 °C | [34] |
| T-Ta | TiO2_10%atTa | Titanium(IV) isopropoxide, Tantalum(V) ethoxide in hydroalcoholic media | 2 h at 400 °C | [36] |
| TTV | Ti:Ta:V 100:15:5 | Ti(IV) n-butoxidein hydroalcoholic media | 2 h at 400 °C | [37] |
| S | SnO2 | Tin(II)2-ethylexanoate, hydroalcoholic media, and diluted HNO3 | 2 h at 550 °C | [34] |
| S-Au | SnO2_0.4 wt.%-Au | SnO2 nanopowders, Gold(III) bromide, in water solution | 3 h at 120 °C 1 | [38] |
| S-Pd | SnO2_0.4 wt.%-Pd | SnO2 nanopowders, Palladium(II) nitrate hydrate, in water solution | 3 h at 120 °C 1 | [38] |
| TS1, TS3, TS5, TS7, TS9 | TixSn1-xO2 x = 0.1, 0.3, 0.5, 0.7, 0.9 | Ti(IV) n-butoxide, Tin(II)2-ethylexanoate, hydroalcoholic media, and diluted HNO3 | 2 h at 550 °C | [34] |
| STN | Sn:Ti:Nb 100:42:5 | Tin(II)2-ethylexanoate, Ti(IV) n-butoxide, Niobium(V) bromide, hydroalcoholic media, and diluted HNO3 | 2 h at 550 °C | [39] |
| W | WO3 | Tungsten hexachloride, alcoholic media, and 2,4-pentanedione | 2 h at 650 °C | [40] |
| ZL | ZnO leaves | Zinc nitrate hexahydrate, water, and ammonia solution | 2 h at 450 °C | [41] |
| ZN | ZnO needles | Zinc nitrate hexahydrate, water, and ammonia solution | not necessary | [41] |
| ZP | ZnO prisms | Zinc nitrate hexahydrate, water, and HMTA | not necessary | [41] |
| LF | LaFeO3 | Potassium hexacyanoferrate(III), Lanthanum nitrate, and water solution | 30 min at 700 °C | [42] |
| Oxide | Crystalline Phase | Space Group | Crystallite Size (nm) |
|---|---|---|---|
| TiO2 | Anatase 100% | I41/amd | 12.0 |
| T-Ta | Anatase (Rutile traces) | I41/amd | 15.0 |
| TTV | Anatase ≈ 70% Rutile ≈ 30% | I41/amd P42/mnm | 20.0 |
| SnO2, SnO2_Au, SnO2_Pd | Rutile | P42/mnm | 10.6 |
| TixSn1–xO2 x = 0.1, 0.3, 0.5, 0.7, and 0.9 | Rutile | P42/mnm | 7.8, 4.4, 4.4, 6.3, 11.7 |
| STN | Rutile | P42/mnm | 21.0 |
| WO3 | Monoclinic pseudo-cubic | P121/n1 | 87.0 |
| ZnO leaves | Hexagonal wurtzite | P63mc | 26.0 |
| ZnO needles | Hexagonal wurtzite | P63mc | 36.0 |
| ZnO prisms | Hexagonal wurtzite | P63mc | 46.0 |
| LaFeO3 | Orthorhombic Perovskite | Pbnm | 48.0 |
| Sensor Type | Best Working Temperature (°C) | R0h/R3000h | Selected Sensor (R0h/R3000h ≥ 2) |
|---|---|---|---|
| T | 450 | 1.2 | - |
| T-Ta | 450 | 1.2 | - |
| TTV | 400 | 1.4 | - |
| S | 450 | 1.0 | - |
| S-Au | 450 | 1.0 | - |
| S-Pd | 450 | 1.0 | - |
| TS1 | 500 | 1.0 | - |
| TS3 | 500 | 1.1 | - |
| TS5 | 500 | 2.4 | X |
| TS7 | 500 | 1.6 | - |
| TS9 | 500 | 2.4 | X |
| STN | 500 | 1.0 | - |
| W | 450 | 1.2 | - |
| ZL | 400 | 2.0 | X |
| ZN | 450 | 1.6 | - |
| ZP | 450 | 1.8 | - |
| LF | 350 | 1.1 | - |
| Sensor Type | Response Time (min) | Recovery Time (min) |
|---|---|---|
| TS9 | 1.0 | 9.3 |
| TS5 | 1.4 | 10.3 |
| ZL | 3.0 | 13.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fioravanti, A.; Marani, P.; Massarotti, G.P.; Lettieri, S.; Morandi, S.; Carotta, M.C. (Ti,Sn) Solid Solution Based Gas Sensors for New Monitoring of Hydraulic Oil Degradation. Materials 2021, 14, 605. https://doi.org/10.3390/ma14030605
Fioravanti A, Marani P, Massarotti GP, Lettieri S, Morandi S, Carotta MC. (Ti,Sn) Solid Solution Based Gas Sensors for New Monitoring of Hydraulic Oil Degradation. Materials. 2021; 14(3):605. https://doi.org/10.3390/ma14030605
Chicago/Turabian StyleFioravanti, Ambra, Pietro Marani, Giorgio Paolo Massarotti, Stefano Lettieri, Sara Morandi, and Maria Cristina Carotta. 2021. "(Ti,Sn) Solid Solution Based Gas Sensors for New Monitoring of Hydraulic Oil Degradation" Materials 14, no. 3: 605. https://doi.org/10.3390/ma14030605
APA StyleFioravanti, A., Marani, P., Massarotti, G. P., Lettieri, S., Morandi, S., & Carotta, M. C. (2021). (Ti,Sn) Solid Solution Based Gas Sensors for New Monitoring of Hydraulic Oil Degradation. Materials, 14(3), 605. https://doi.org/10.3390/ma14030605










