The Photostability of Novel Boron Hydride Blue Emitters in Solution and Polystyrene Matrix
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Substrates
2.2. Sample Preparation and Characterization
2.3. Quantum Yield Determination and Photostability Experiment
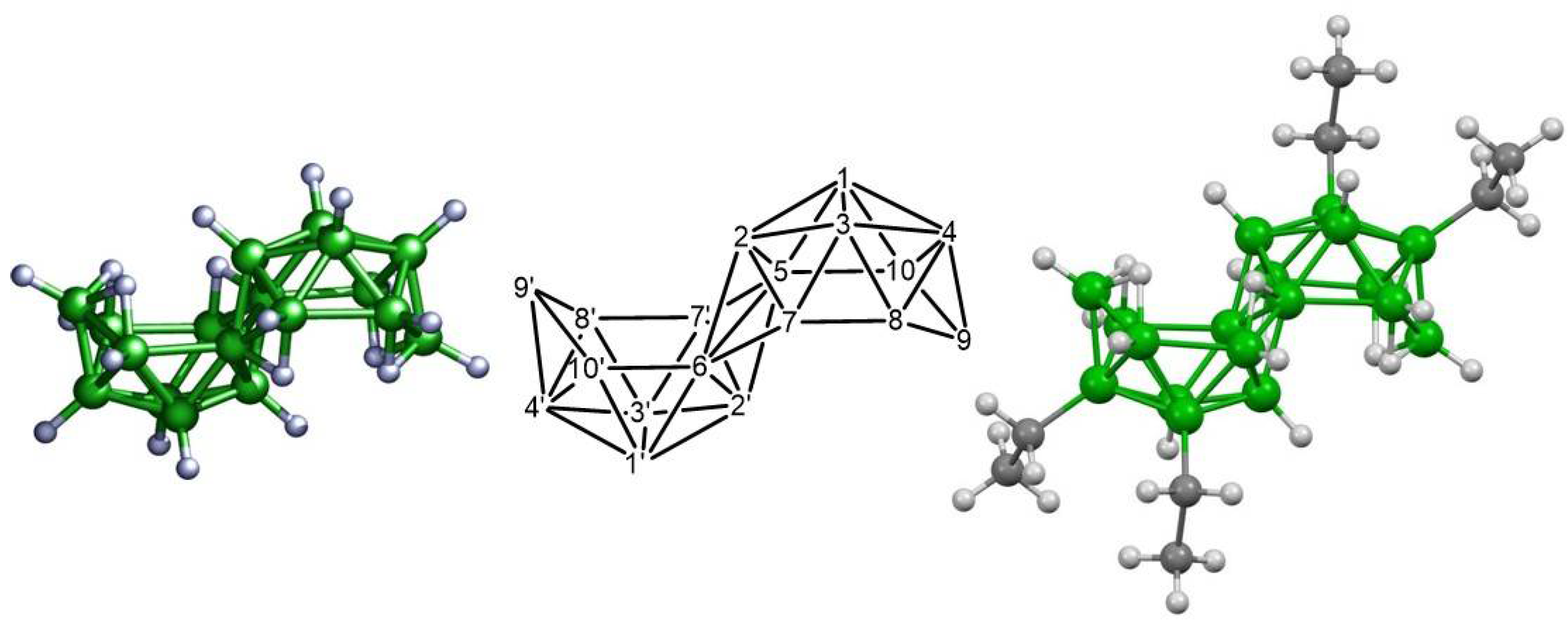
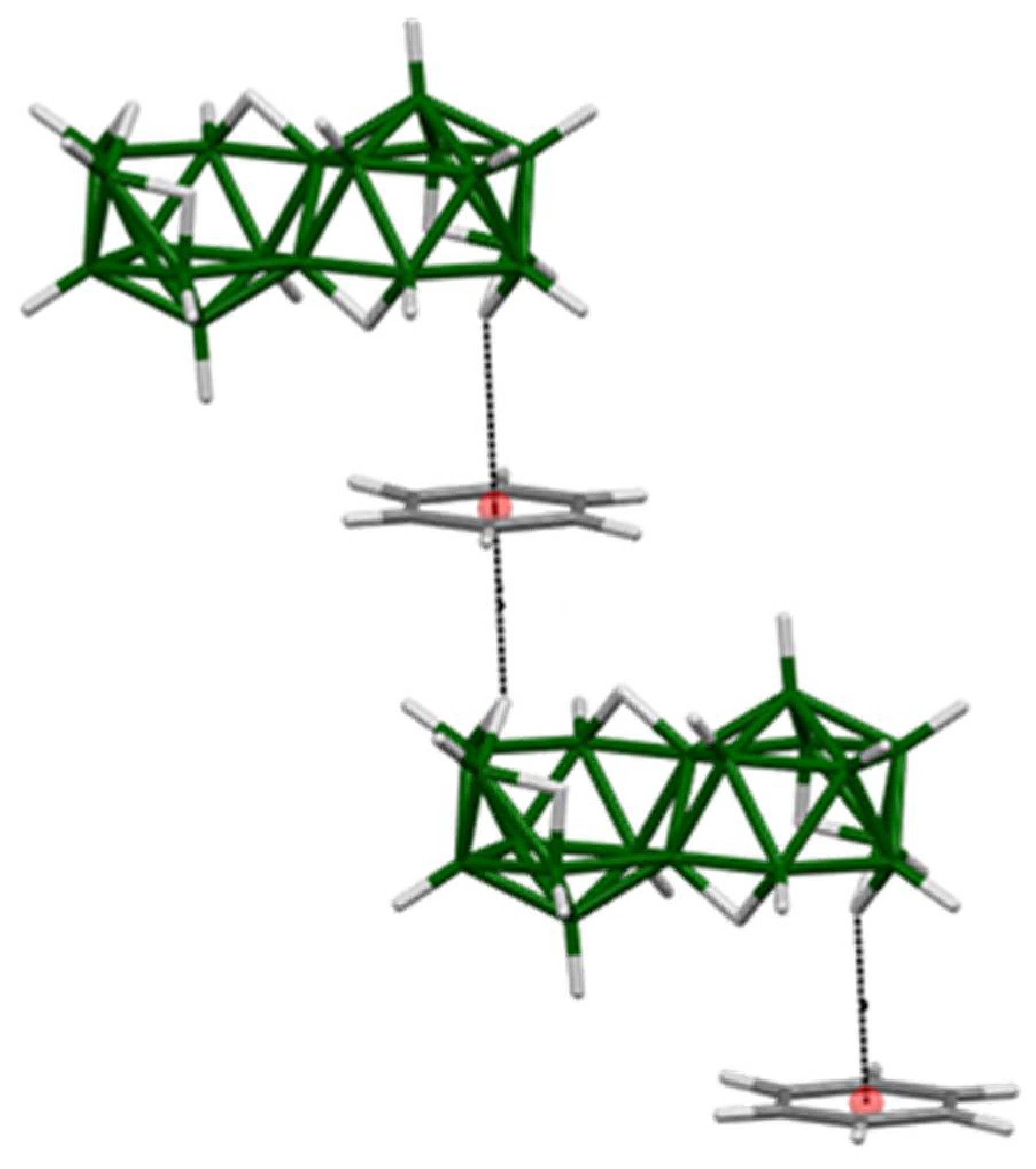
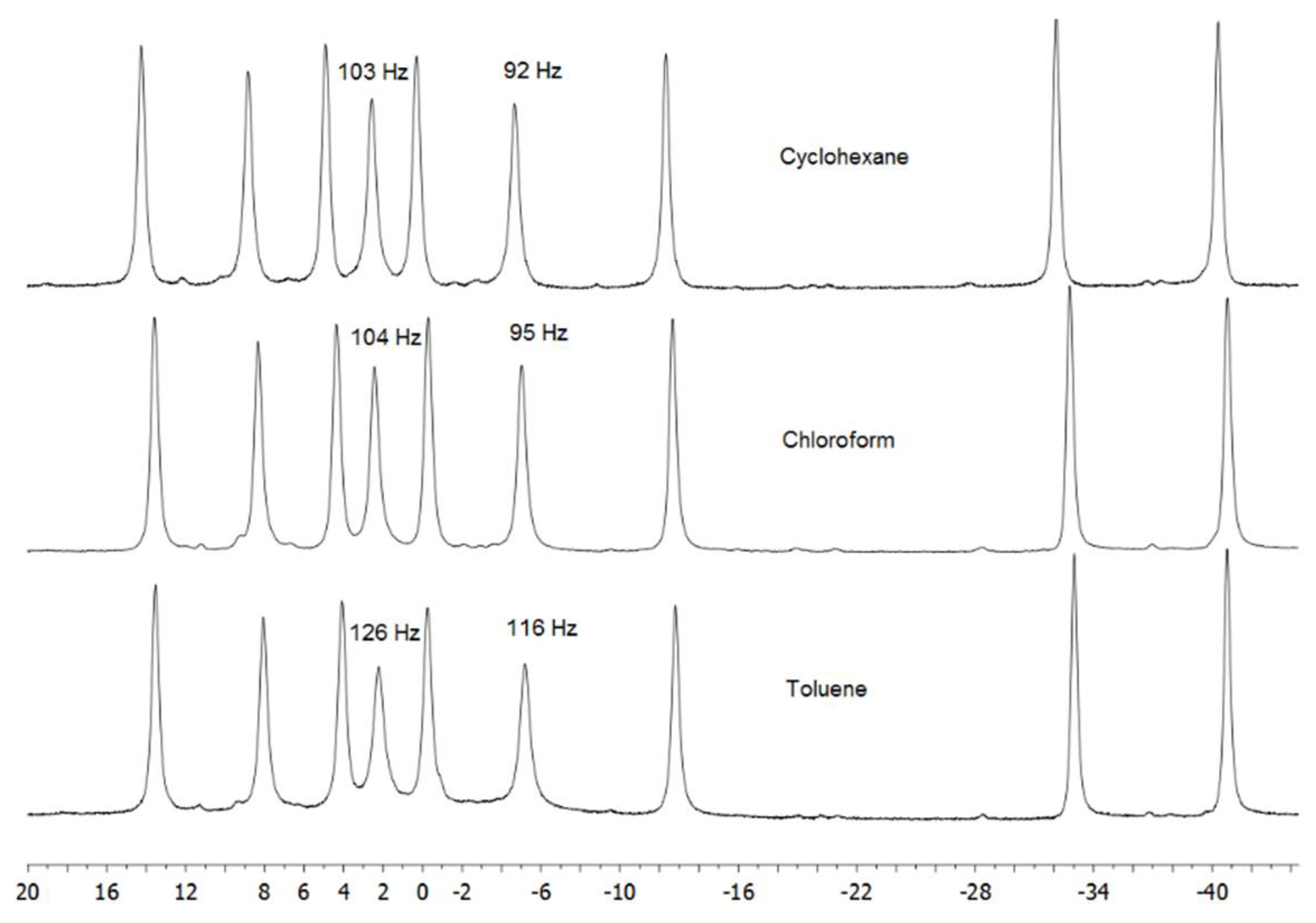
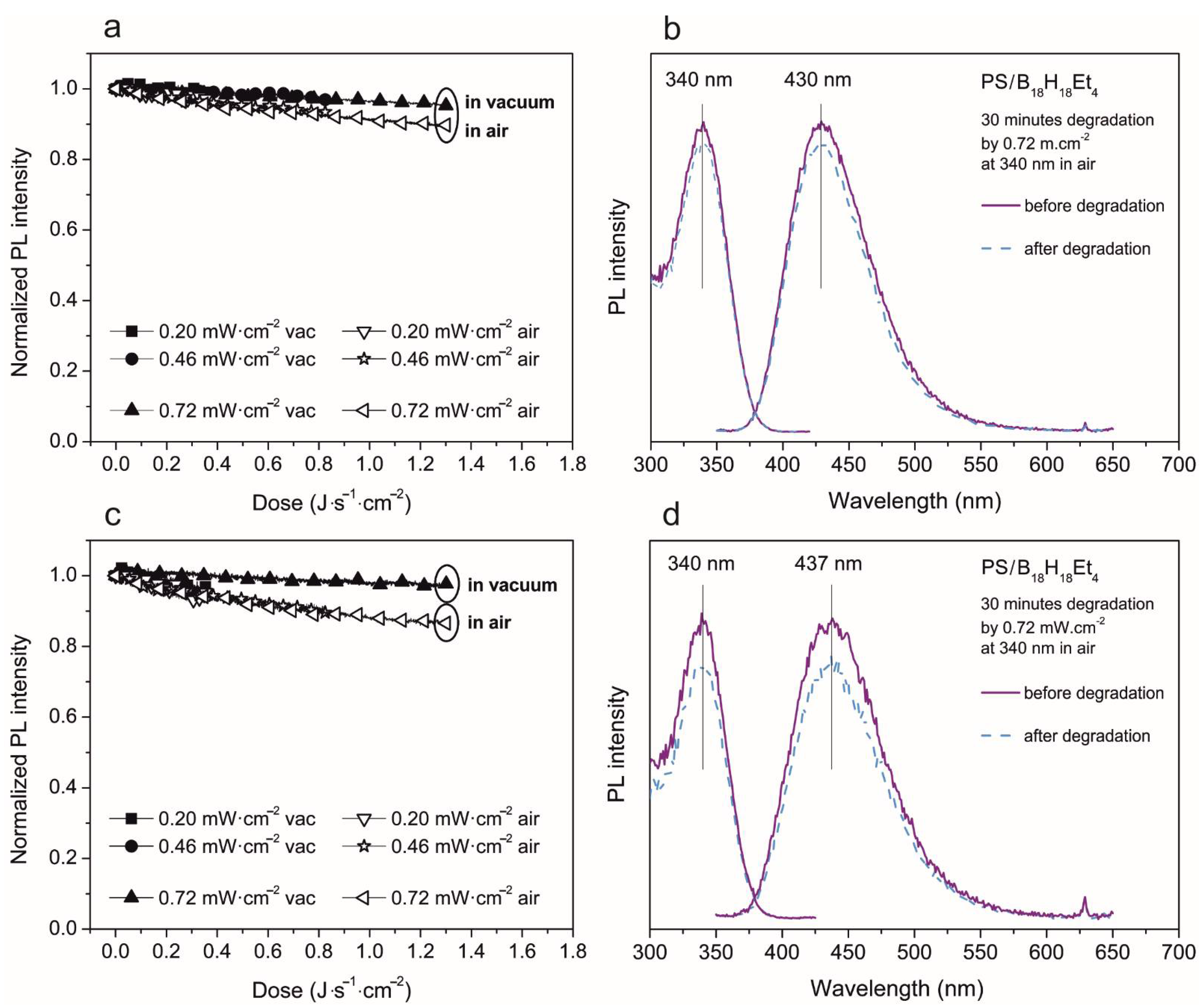
3. Results and Discussion
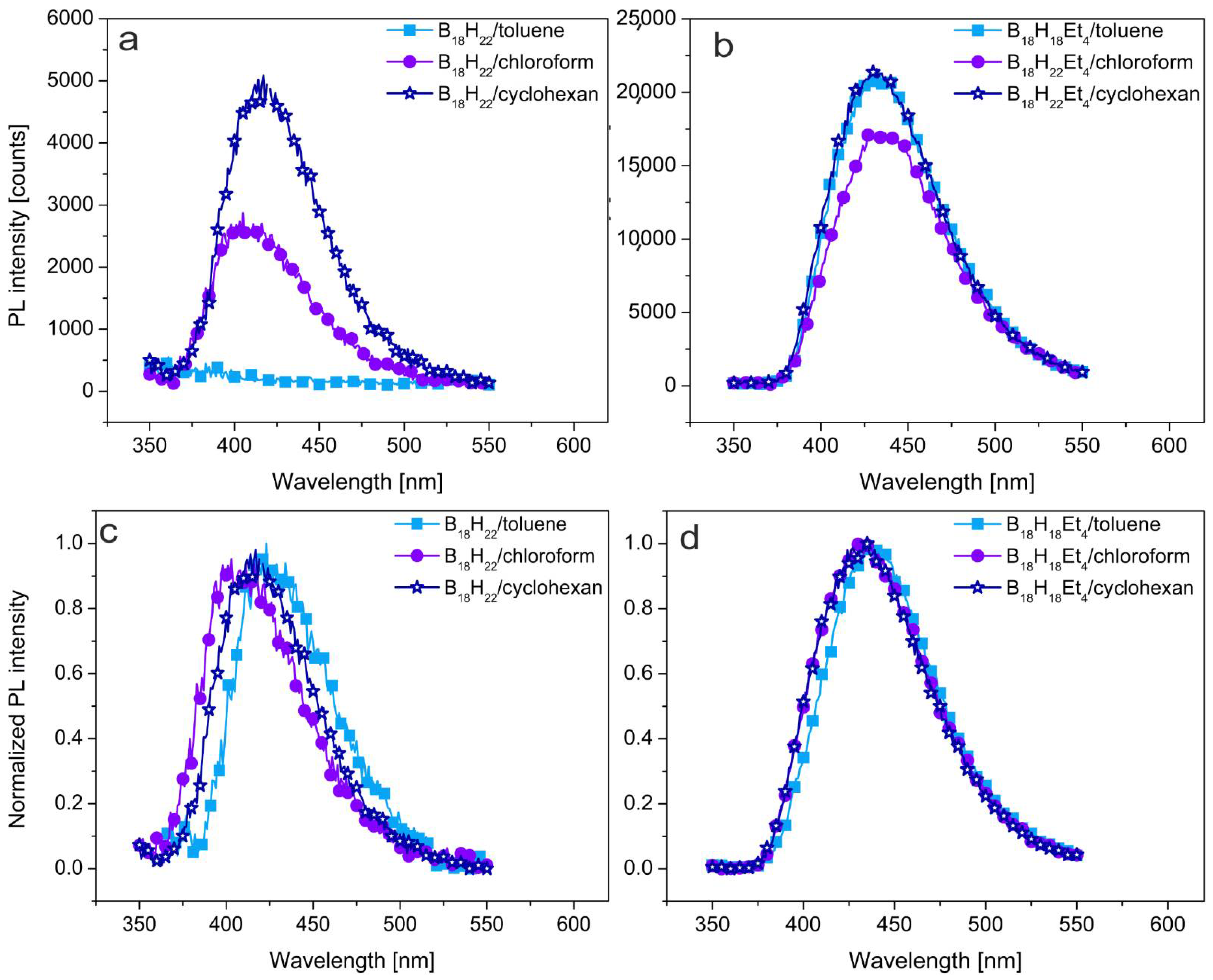
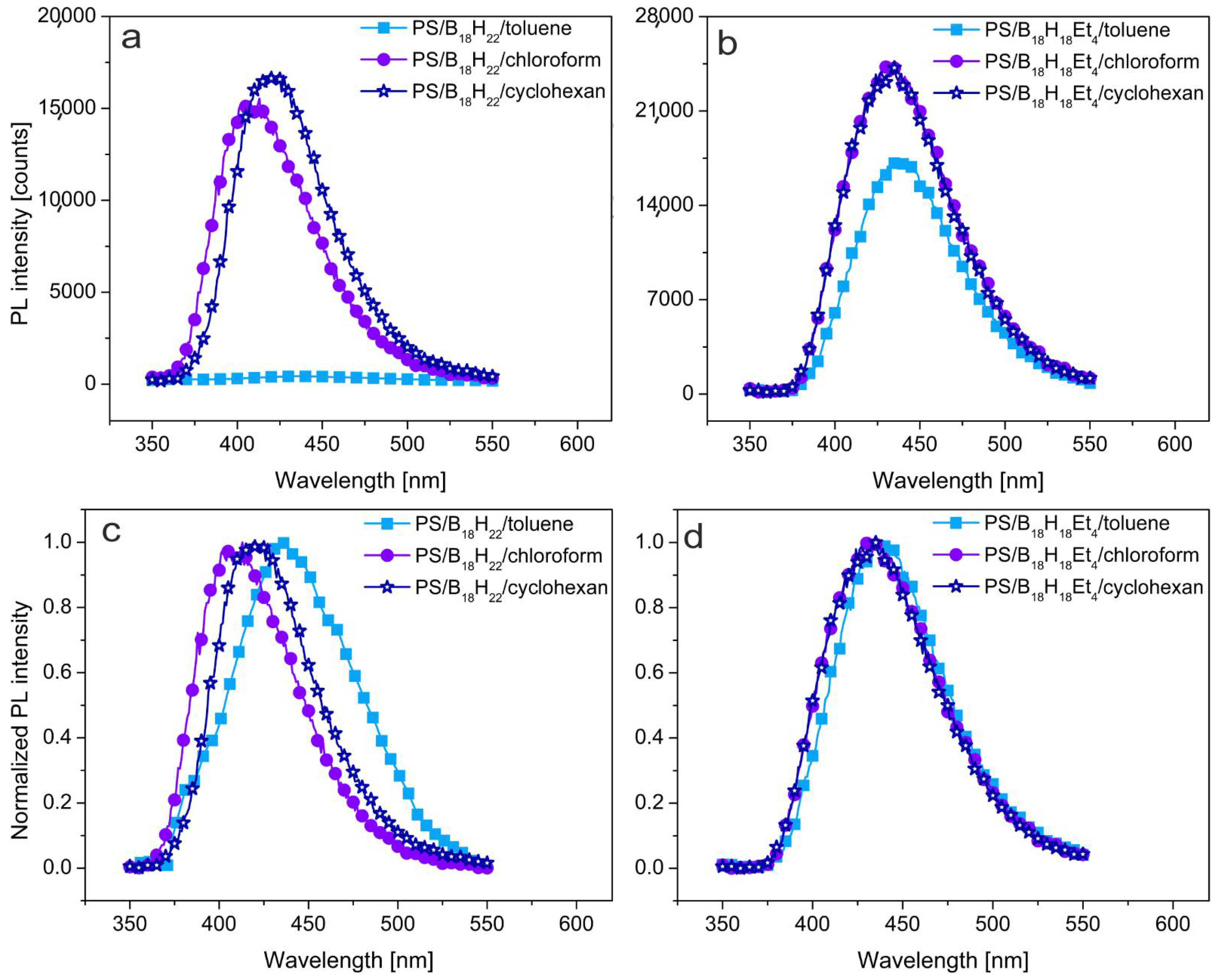
3.1. Photoluminescent Properties in Solution
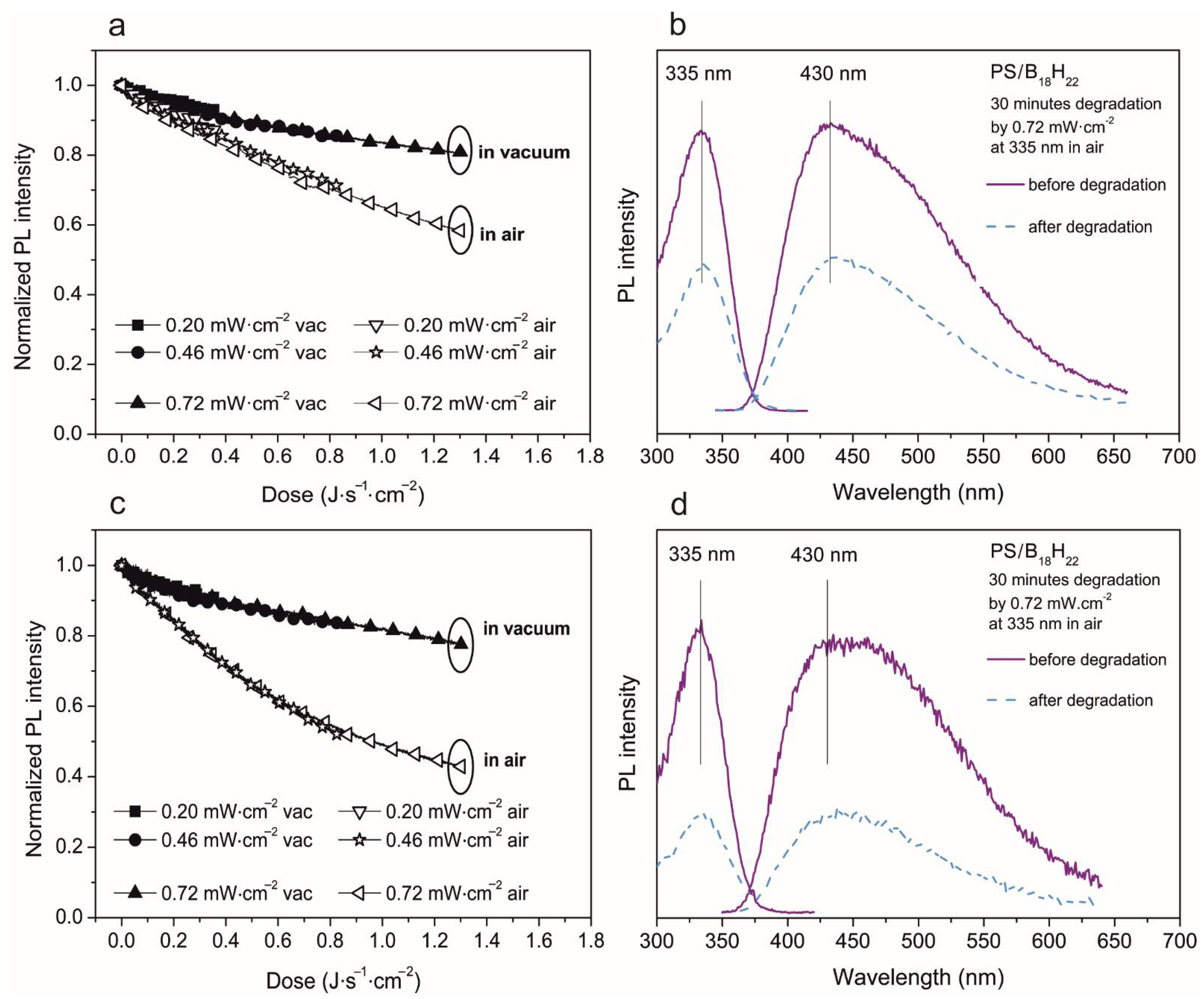
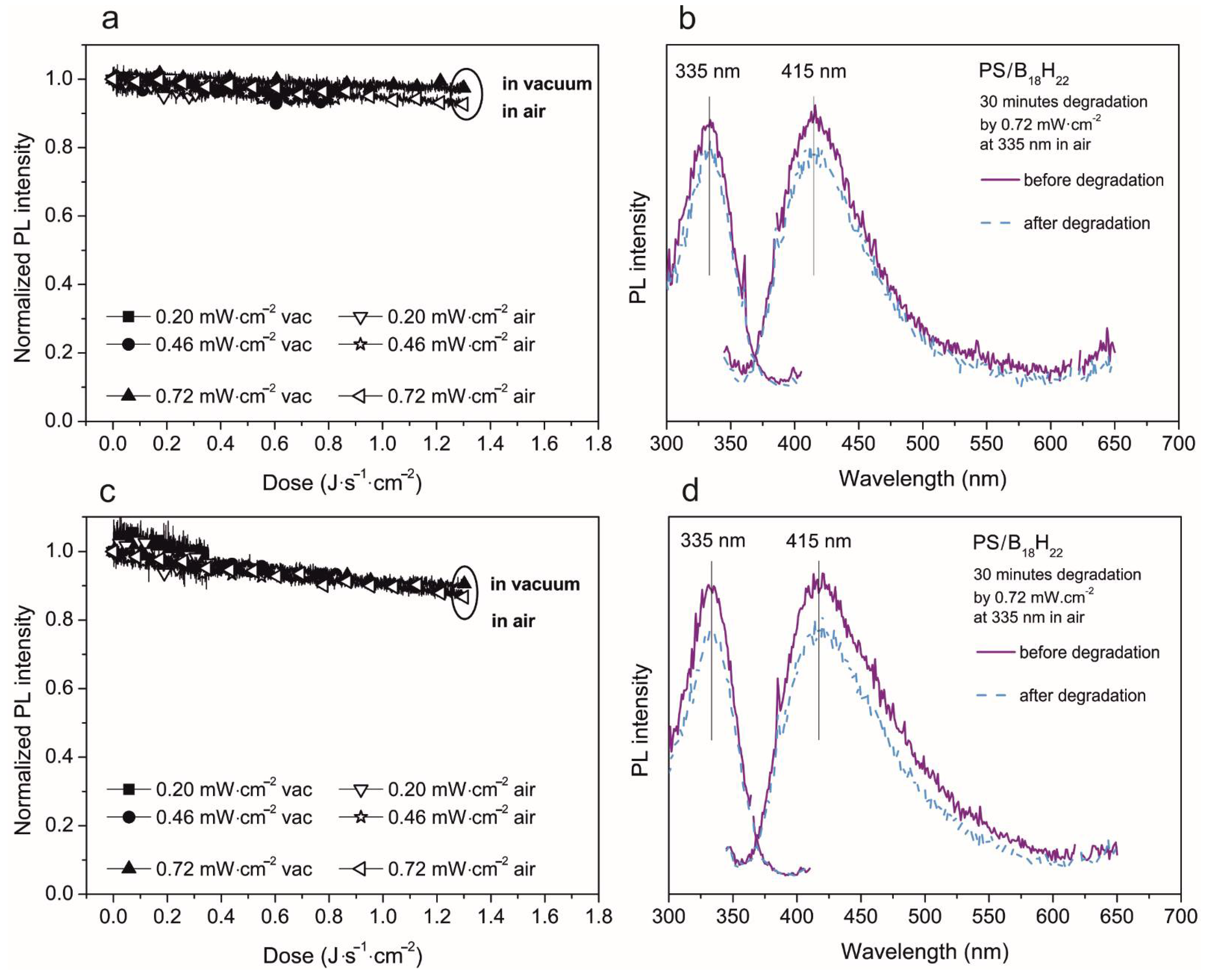
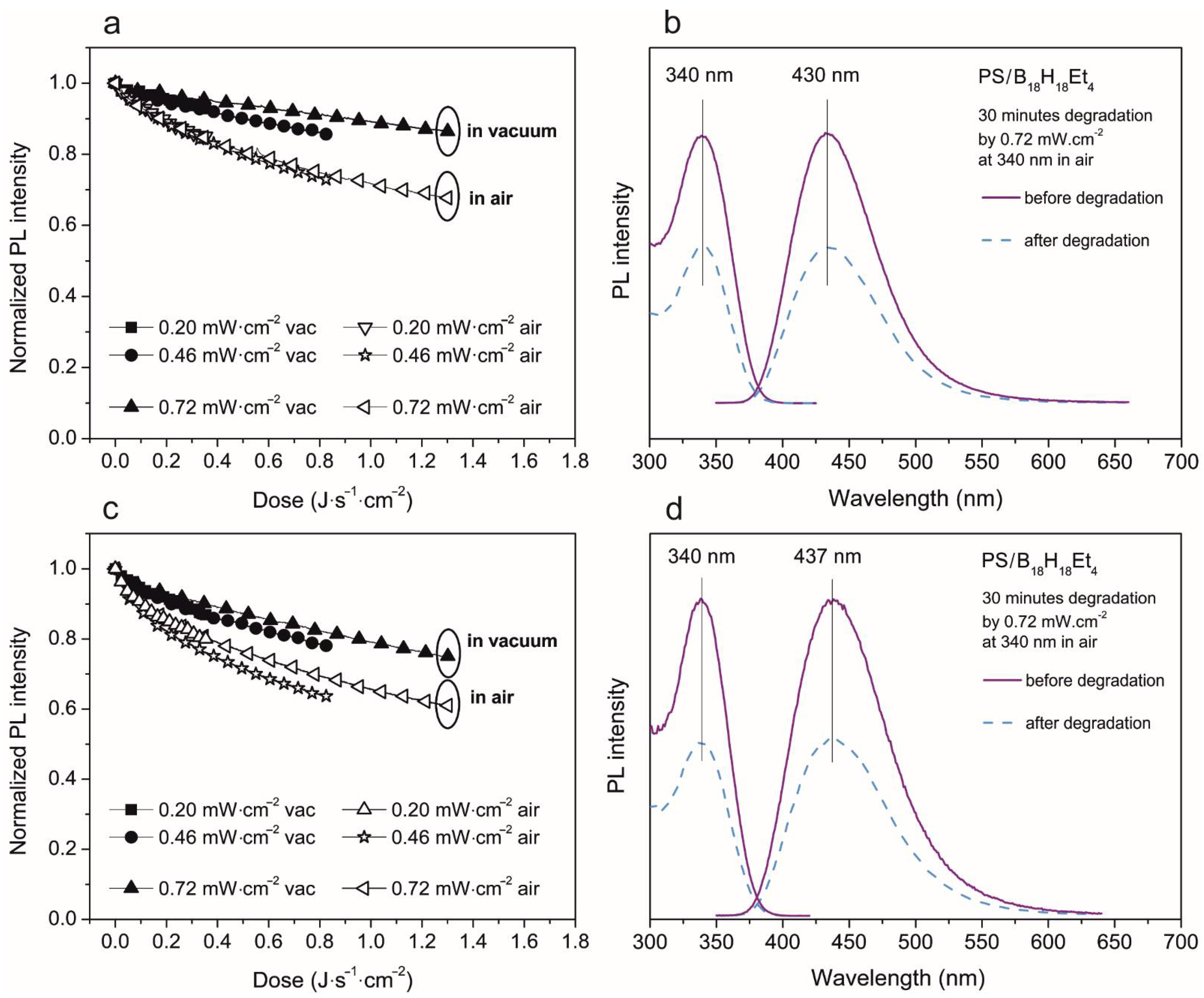
3.2. Photoluminescent Properties in Polystyrene Thin Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goushi, K.; Yoshida, K.; Sato, K.; Adachi, C. Organic light-emitting diodes employing efficient reverse intersystem crossing for triplet-to-singlet state conversion. Nat. Photonics 2012, 6, 253–258. [Google Scholar] [CrossRef]
- Lee, J.-H.; Chen, C.-H.; Lee, P.-H.; Lin, H.-Y.; Leung, M.-K.; Chiu, T.-L.; Lin, C.-F. Blue organic light-emitting diodes: Current status, challenges, and future outlook. J. Mater. Chem. C 2019, 7, 5874–5888. [Google Scholar] [CrossRef]
- Li, W.; Pan, Y.; Xiao, R.; Peng, Q.; Zhang, S.; Ma, D.; Li, F.; Shen, F.; Wang, Y.; Yang, B.; et al. Employing ∼100% Excitons in OLEDs by Utilizing a Fluorescent Molecule with Hybridized Local and Charge-Transfer Excited State. Adv. Funct. Mater. 2014, 24, 1609–1614. [Google Scholar] [CrossRef]
- Yang, Z.; Gao, M.; Wu, W.; Yang, X.; Sun, X.W.; Zhang, J.; Wang, H.-C.; Liu, R.-S.; Han, C.-Y.; Yang, H.; et al. Recent advances in quantum dot-based light-emitting devices: Challenges and possible solutions. Mater. Today 2019, 24, 69–93. [Google Scholar] [CrossRef]
- Giovanella, U.; Pasini, M.; Botta, C. Organic Light-Emitting Diodes (OLEDs): Working Principles and Device Technology; Springer International Publishing: Cham, Switzerland, 2016; ISBN 9783319316710. [Google Scholar]
- Anikeeva, P.O.; Madigan, C.F.; Halpert, J.E.; Bawendi, M.; Bulović, V. Electronic and excitonic processes in light-emitting devices based on organic materials and colloidal quantum dots. Phys. Rev. B 2008, 78, 1–8. [Google Scholar] [CrossRef]
- Yang, X.; Zhou, G.; Wong, W.-Y. ChemInform Abstract: Functionalization of Phosphorescent Emitters and Their Host Materials by Main-Group Elements for Phosphorescent Organic Light-Emitting Devices. Chem. Soc. Rev. 2016, 47, 8484–8575. [Google Scholar] [CrossRef]
- Ho, C.-L.; Li, H.; Wong, W.-Y. Red to near-infrared organometallic phosphorescent dyes for OLED applications. J. Organomet. Chem. 2014, 751, 261–285. [Google Scholar] [CrossRef]
- Jamatia, T.; Škoda, D.; Urbanek, P.; Sevcik, J.; Maslik, J.; Munster, L.; Kalina, L.; Kuřitka, I. Microwave-assisted synthesis of FexZn1−xO nanoparticles for use in MEH-PPV nanocomposites and their application in polymer light-emitting diodes. J. Mater. Sci. Mater. Electron. 2019, 30, 11269–11281. [Google Scholar] [CrossRef]
- Škoda, D.; Urbanek, P.; Sevcik, J.; Munster, L.; Nádaždy, V.; Cullen, D.A.; Bazant, P.; Antos, J.; Kuritka, I. Colloidal cobalt-doped ZnO nanoparticles by microwave-assisted synthesis and their utilization in thin composite layers with MEH-PPV as an electroluminescent material for polymer light emitting diodes. Org. Electron. 2018, 59, 337–348. [Google Scholar] [CrossRef]
- Škoda, D.; Urbánek, P.; Sevcik, J.; Munster, L.; Antos, J.; Kuřitka, I. Microwave-assisted synthesis of colloidal ZnO nanocrystals and their utilization in improving polymer light emitting diodes efficiency. Mater. Sci. Eng. B 2018, 2018, 22–32. [Google Scholar] [CrossRef]
- Lee, H.; Maeng, M.-J.; Hong, J.-A.; Najnin, R.; Moon, J.; Cho, H.; Lee, J.; Yu, B.-G.; Park, Y.; Cho, N.S. Highly efficient green, blue, and white phosphorescent inverted organic light-emitting diodes by improving charge injection and balance. J. Mater. Chem. C 2017, 5, 9911–9919. [Google Scholar] [CrossRef]
- Londesborough, M.G.S.; Hnyk, D.; Bould, J.; Serrano-Andrés, L.; Sauri, V.; Oliva-Enrich, J.M.; Kubát, P.; Polívka, T.; Lang, K. Distinct Photophysics of the Isomers of B18H22 Explained. Inorg. Chem. 2012, 51, 1471–1479. [Google Scholar] [CrossRef] [PubMed]
- Cooke, P.A.; O’Dowd, C.; Londesborough, M.G.S.; Holub, J.; Štíbr, B.; Thornton-Pett, M.; Clegg, W.; Teat, S.J.; Kennedy, J.D. B-frame supported bimetallics. “Composite cluster” compounds and the structures of [2,7-(η5-C5Me5)2-nido-2,7,8,6-Ir2CSB6H8] and its 9-chloro derivative. Synchrotron and conventional X-ray studies. J. Organomet. Chem. 2000, 614–615, 57–60. [Google Scholar] [CrossRef]
- Saurí, V.; Oliva, J.M.; Hnyk, D.; Bould, J.; Braborec, J.; Merchán, M.; Kubát, P.; Císařová, I.; Lang, K.; Londesborough, M.G.S. Tuning the Photophysical Properties of anti-B18H22: Efficient Intersystem Crossing between Excited Singlet and Triplet States in New 4,4′-(HS)2-anti-B18H20. Inorg. Chem. 2013, 52, 9266–9274. [Google Scholar] [CrossRef]
- King, R.B. Three-dimensional aromaticity in polyhedral boranes and related molecules. Chem. Rev. 2001, 101, 1119–1152. [Google Scholar] [CrossRef] [PubMed]
- Londesborough, M.G.S.; Dolanský, J.; Cerdán, L.; Lang, K.; Jelínek, T.; Oliva, J.M.; Hnyk, D.; Roca-Sanjuán, D.; Francés-Monerris, A.; Martinčík, J.; et al. Thermochromic Fluorescence from B18H20(NC5H5)2: An Inorganic–Organic Composite Luminescent Compound with an Unusual Molecular Geometry. Adv. Opt. Mater. 2017, 5, 1–16. [Google Scholar] [CrossRef]
- Cerdán, L.; Braborec, J.; Garcia-Moreno, I.; Costela, A.; Londesborough, M.G.S. A borane laser. Nat. Commun. 2015, 6, 1–7. [Google Scholar] [CrossRef]
- Bould, J.; Lang, K.; Kirakci, K.; Cerdán, L.; Roca-Sanjuán, D.; Francés-Monerris, A.; Clegg, W.; Waddell, P.G.; Fuciman, M.; Polivka, T.; et al. A Series of Ultra-Efficient Blue Borane Fluorophores. Inorg. Chem. 2020, 59, 17058–17070. [Google Scholar] [CrossRef]
- Miller, R.D.; Michl, J. Polysilane High Polymers. Chem. Rev. 1989, 89, 1359–1410. [Google Scholar] [CrossRef]
- Tan, C.H.; Zhang, B.K.; Chen, J.; Zhang, L.N.; Huang, X.G. Study of Hydrolysis Kinetic of New Laser Material [anti-B18H22]. Russ. J. Inorg. Chem. 2019, 64, 1359–1364. [Google Scholar]
- Lin, N.; Qiao, J.; Duan, L.; Wang, L.; Qiu, Y. Molecular Understanding of the Chemical Stability of Organic Materials for OLEDs: A Comparative Study on Sulfonyl, Phosphine-Oxide, and Carbonyl-Containing Host Materials. J. Phys. Chem. C 2014, 118, 7569–7578. [Google Scholar] [CrossRef]
- Aziz, H.; Popovic, Z.D. Degradation Phenomena in Small-Molecule Organic Light-Emitting Devices. Chem. Mater. 2004, 16, 4522–4532. [Google Scholar] [CrossRef]
- So, F.; Kondakov, D. Degradation mechanisms in small-molecule and polymer organic light-emitting diodes. Adv. Mater. 2010, 22, 3762–3777. [Google Scholar] [CrossRef] [PubMed]
- Cerdán, L.; Francés-Monerris, A.; Roca-Sanjuán, D.; Bould, J.; Dolanský, J.; Fuciman, M.; Londesborough, M.G.S. Unveiling the role of upper excited electronic states in the photochemistry and laser performance of: Anti-B18H22. J. Mater. Chem. C 2020, 8, 12806–12818. [Google Scholar] [CrossRef]
- Gibb, T.C.; Kennedy, J.D. Proton and boron-11 nuclear spin relaxation and the molecular tumbling of nido-decaborane in perdeuterotoluene solution. An interesting transition in solute–solvent interaction behaviour. J. Chem. Soc. 1982, 78, 525–536. [Google Scholar] [CrossRef]
- Fontaine, X.L.R.; Greenwood, N.N.; Kennedy, J.D.; MacKinnon, P. Boron-11 and proton nuclear magnetic resonance study of anti-B18H22 and its anions, anti-[B18H21]− and anti-[B18H20]2−. The crystal and molecular structure of [NMe4]2[anti-B18H20]. J. Chem. Soc. Dalton Trans. 1988, 1785–1793. [Google Scholar] [CrossRef]
- Hamilton, E.J.M.; Kultyshev, R.G.; Du, B.; Meyers, E.A.; Liu, S.; Hadad, C.M.; Shore, S.G. A stacking interaction between a bridging hydrogen atom and aromatic π density in the n-B18H22-benzene system. Chem.-A Eur. J. 2006, 12, 2571–2578. [Google Scholar] [CrossRef]
- Londesborough, M.G.S.; Lang, K.; Clegg, W.; Waddell, P.G.; Bould, J. Swollen Polyhedral Volume of the anti-B18H22 Cluster via Extensive Methylation: Anti-B18H8Cl2Me12. Inorg. Chem. 2020, 59, 2651–2654. [Google Scholar] [CrossRef]
- Hunter, E.P.L.; Lias, S.G. Evaluated Gas Phase Basicities and Proton Affinities of Molecules: An Update. J. Phys. Chem. Ref. Data 1998, 27, 413–656. [Google Scholar] [CrossRef]
- Henderson, D.J.; Rettner, C.T. Physical Chemistry. In Encyclopedia of Physical Science and Technology; Elsevier: Amsterdam, The Netherlands, 2003; pp. 159–176. [Google Scholar]
- Barthel, J.; Neueder, R. Chemical Thermodynamics. In Encyclopedia of Physical Science and Technology; Elsevier BV: Tarzana, LA, USA, 2003; pp. 767–786. [Google Scholar]
- Karelson, M.; Shibuya, T.I.; Wielgus, M.; Bartkowiak, W. Electronic and electrical effects of solvents. In Handbook of Solvents, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2014; Volume 1, pp. 649–723. ISBN 9781895198782. [Google Scholar]
- Safi, Z.S.; Omar, S. Proton affinity and molecular basicity of m-and p-substituted benzamides in gas phase and in solution: A theoretical study. Chem. Phys. Lett. 2014, 610, 321–330. [Google Scholar] [CrossRef]
- Hansen, C.M. Hansen Solubility Parameters Second edition: A User’s Handbook; Taylor & Francis Group: Boca Raton, FL, USA, 2007; ISBN-10: 0-8493-7248-8. [Google Scholar]
- Kuřitka, I.; Schauer, F.; Sáha, P.; Zemek, J.; Jiricek, P.; Nešpůrek, S. UV degradability of polysilanes for nanoresists examined by electron spectroscopies and photoluminescence. Czechoslov. J. Phys. 2006, 56, 41–50. [Google Scholar] [CrossRef]
- Urbánek, P.; Kuřitka, I. Thickness dependent structural ordering, degradation and metastability in polysilane thin films: A photoluminescence study on representative σ-conjugated polymers. J. Lumin. 2015, 168, 261–268. [Google Scholar] [CrossRef]
- Tan, C.; Chen, J.; Zhang, L.; Zhang, B.; Huang, X.; Meng, H. The preparation and characterization of n-B18H22-beta cyclodextrin inclusion complex. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Taoyuan City, Taiwan, 2019; Volume 233, p. 022005. [Google Scholar]
- Londesborough, M.G.S.; Dolanský, J.; Jelínek, T.; Kennedy, J.D.; Císařová, I.; Kennedy, R.D.; Roca-Sanjuán, D.; Francés-Monerris, A.; Lang, K.; Clegg, W. Substitution of the laser borane: Anti-B18H22 with pyridine: A structural and photophysical study of some unusually structured macropolyhedral boron hydrides. Dalton Trans. 2018, 47, 1709–1725. [Google Scholar] [CrossRef] [PubMed]
- Alder, R.W.; Bryce, M.R.; Goode, N.C.; Miller, N.; Owen, J. Preparation of a range of NNN′N′-tetrasubstituted-1,8-diaminonaphthalenes. J. Chem. Soc. Perkin Trans. 1 1981, 2840–2847. [Google Scholar] [CrossRef]
| Slit Size [μm] | UV Irradiance [mW·cm−2] |
|---|---|
| 1 | 0.20 |
| 2 | 0.46 |
| 3 | 0.72 |
| Solvent | QY | Emission Maxima (nm) | ||
|---|---|---|---|---|
| anti-B18H22 | 3,3′,4,4′-Et4-anti-B18H18 | anti-B18H22 | 3,3′,4,4′-Et4-anti-B18H18 | |
| Cyclohexane | 0.96 ± 0.03 | 0.99 ± 0.01 | 417 | 434 |
| Chloroform | 0.88 ± 0.03 | 0.85 ± 0.02 | 407 | 434 |
| Toluene | 0.05 ± 0.01 | 0.95 ± 0.02 | 425 | 439 |
| Solvent | QY | Emission Maxima (nm) | ||
|---|---|---|---|---|
| anti-B18H22 | 3,3′,4,4′-Et4-anti-B18H18 | anti-B18H22 | 3,3′,4,4′-Et4-anti-B18H18 | |
| Cyclohexane | 0.82 ± 0.05 | 0.99 ± 0.01 | 420 | 434 |
| Chloroform | 0.73 ± 0.02 | 0.99 ± 0.01 | 409 | 434 |
| Toluene | 0.03 ± 0.02 | 0.95 ± 0.02 | 437 | 439 |
| Solvent Used for Thin Film Preparation | QY | Emission Maxima (nm) | ||
|---|---|---|---|---|
| anti-B18H22 | 3,3′,4,4′-Et4-anti-B18H18 | anti-B18H22 | 3,3′,4,4′-Et4-anti-B18H18 | |
| Cyclohexane | 0.13 ± 0.03 | 0.22 ± 0.04 | 415 | 432 |
| Chloroform | 0.67 ± 0.02 | 0.69 ± 0.06 | 405 | 436 |
| Toluene | 0.07 ± 0.04 | 0.29 ± 0.05 | 432 | 433 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ševčík, J.; Urbánek, P.; Hanulíková, B.; Čapková, T.; Urbánek, M.; Antoš, J.; Londesborough, M.G.S.; Bould, J.; Ghasemi, B.; Petřkovský, L.; et al. The Photostability of Novel Boron Hydride Blue Emitters in Solution and Polystyrene Matrix. Materials 2021, 14, 589. https://doi.org/10.3390/ma14030589
Ševčík J, Urbánek P, Hanulíková B, Čapková T, Urbánek M, Antoš J, Londesborough MGS, Bould J, Ghasemi B, Petřkovský L, et al. The Photostability of Novel Boron Hydride Blue Emitters in Solution and Polystyrene Matrix. Materials. 2021; 14(3):589. https://doi.org/10.3390/ma14030589
Chicago/Turabian StyleŠevčík, Jakub, Pavel Urbánek, Barbora Hanulíková, Tereza Čapková, Michal Urbánek, Jan Antoš, Michael G. S. Londesborough, Jonathan Bould, Bita Ghasemi, Lukáš Petřkovský, and et al. 2021. "The Photostability of Novel Boron Hydride Blue Emitters in Solution and Polystyrene Matrix" Materials 14, no. 3: 589. https://doi.org/10.3390/ma14030589
APA StyleŠevčík, J., Urbánek, P., Hanulíková, B., Čapková, T., Urbánek, M., Antoš, J., Londesborough, M. G. S., Bould, J., Ghasemi, B., Petřkovský, L., & Kuřitka, I. (2021). The Photostability of Novel Boron Hydride Blue Emitters in Solution and Polystyrene Matrix. Materials, 14(3), 589. https://doi.org/10.3390/ma14030589









