Use of a Handheld X-ray Fluorescence Analyser to Quantify Chloride Ions In Situ: A Case Study of Structural Repair
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
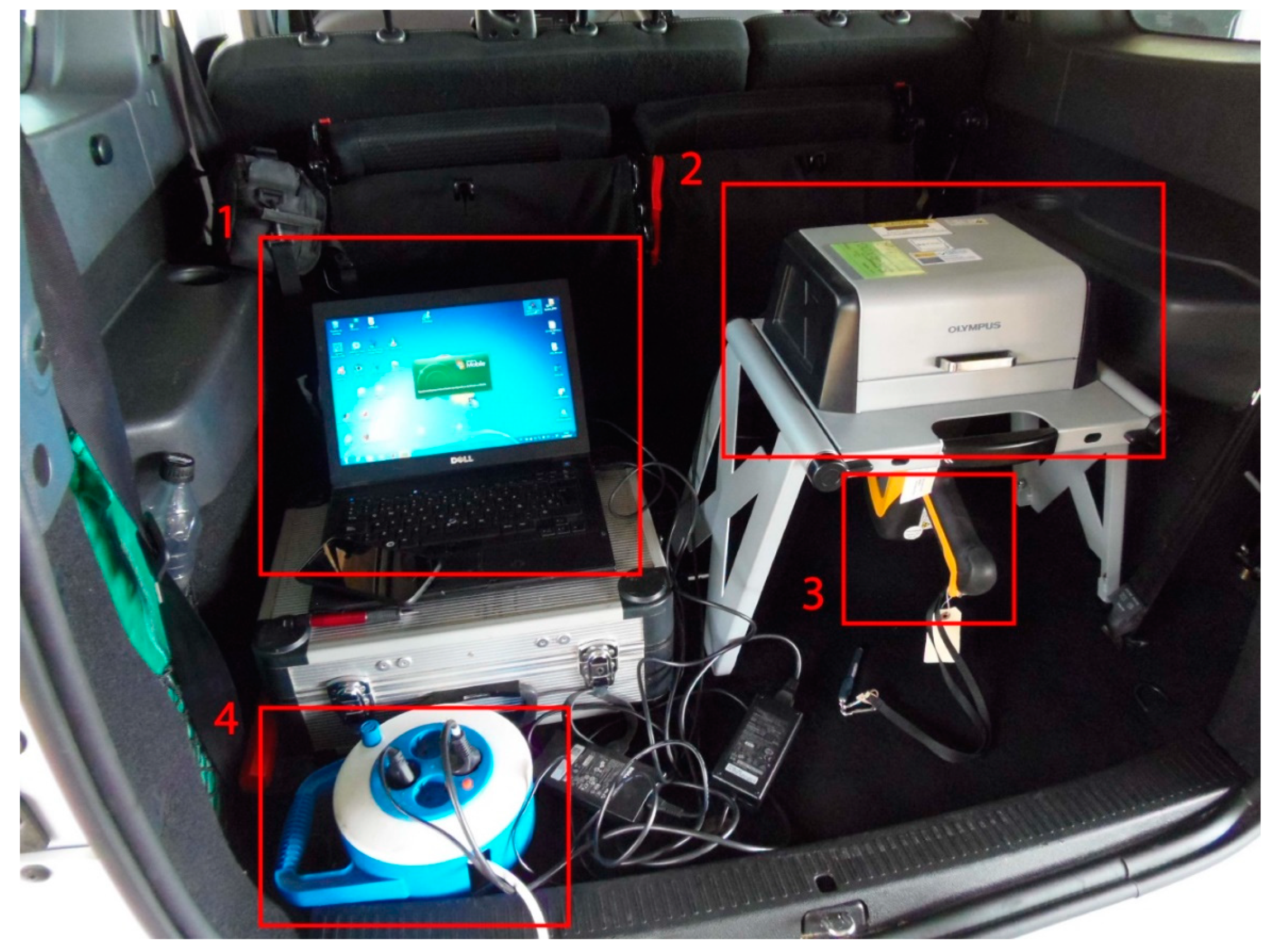
2.2. On-Site Use of the hXRF
3. Results and Discussion
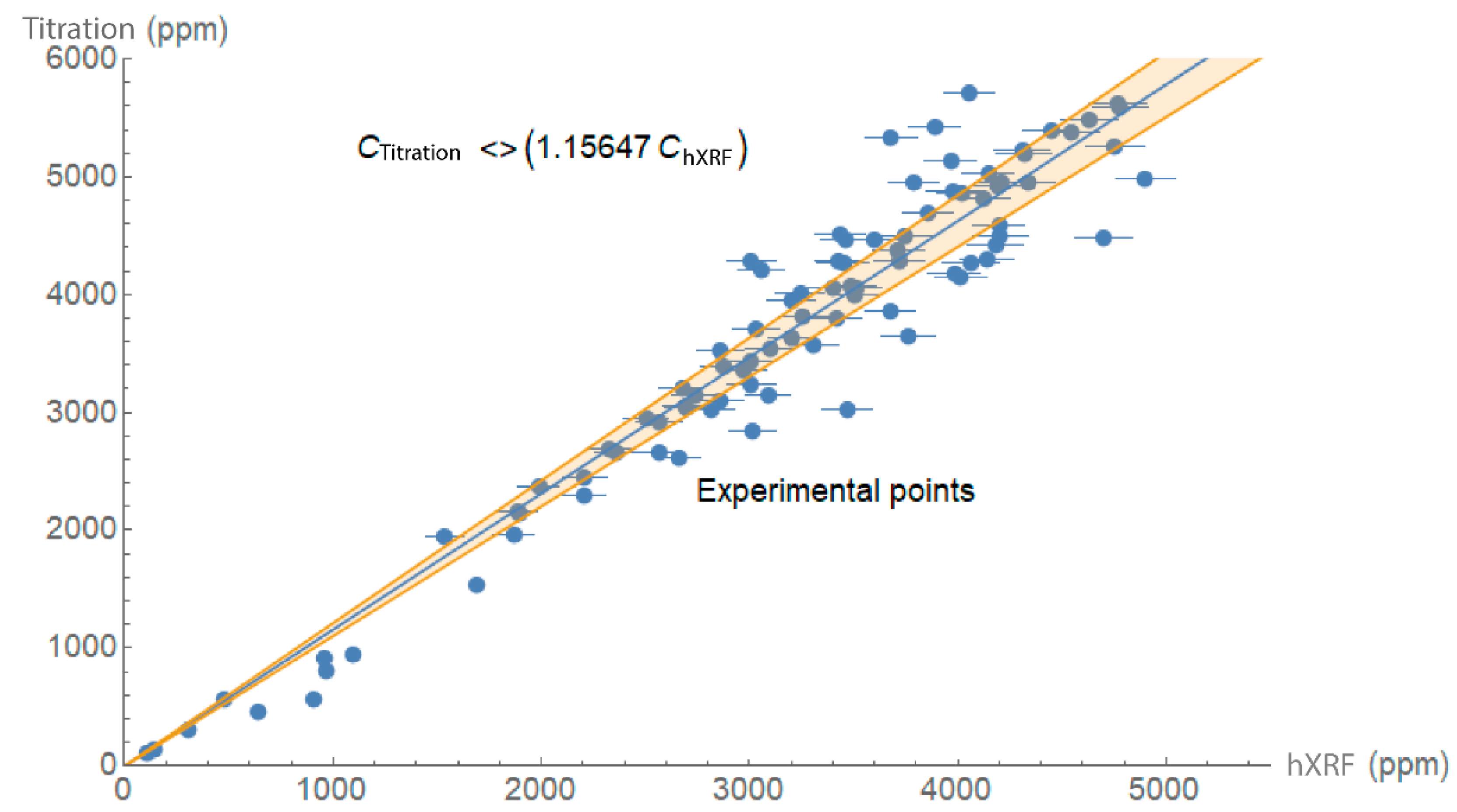
3.1. Laboratory Calibration
3.2. On-Site Use of the hXRF Analyser
4. Conclusions
- Chloride ions were quantified with a handheld X-ray fluorescence analyser in a large number of mortar and concrete prepared with OPC samples with differing Cl- content. A comparison of the findings to the data delivered by another standardised analytical technique showed the readings to be highly accurate and the precision of the two techniques to be similar. The correction factor calculated for the analyser was 1.16.
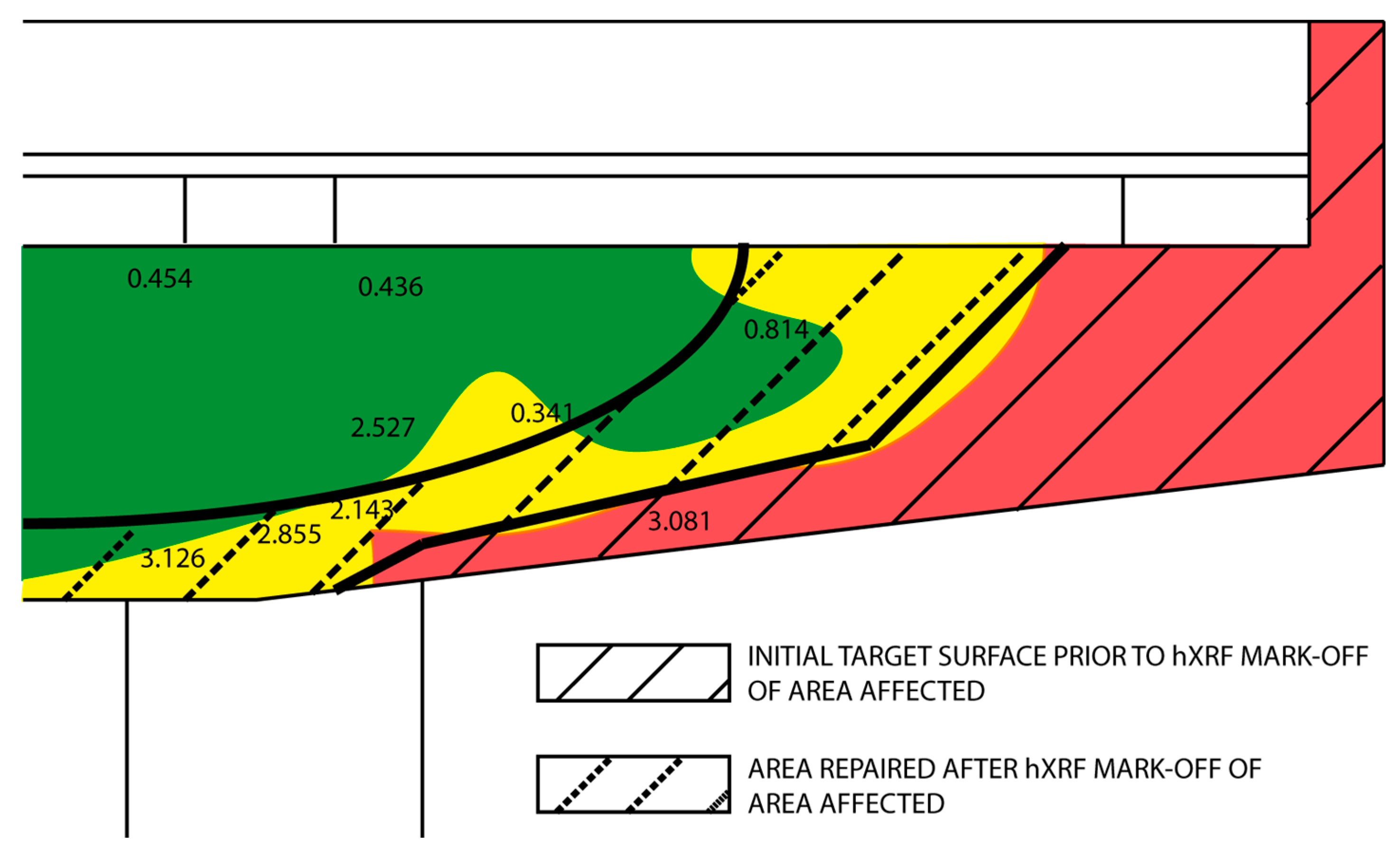
- On the grounds of the 0.6% chloride threshold (by cement weight) laid down in the legislation applied, a value of 900 ppm was established as a conservative threshold above which chloride ion-mediated corrosion may be deemed to be underway or potentially underway. The methodology described is equally applicable for use with other standards or codes with different thresholds for calculating the respective chloride concentration threshold.
- This study discusses the use of a handheld X-ray fluorescence analyser to support analysis in repairs, where the primary cause of deterioration is reinforcement steel oxidation due to the presence of a high chloride concentration. Such instruments furnish speedy and accurate on-site information on ion concentration in concrete that can, then, be applied to more accurately define the areas in need repair.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Angst, U.; Elsener, B.; Larsen, C.K.; Vennesland, Ø. Critical chloride content in reinforced concrete—A review. Cem. Concr. Res. 2009, 39, 1122–1138. [Google Scholar] [CrossRef]
- Markeset, G. Critical chloride content and its influence on service life predictions. Mater. Corros. 2009, 60, 593–596. [Google Scholar] [CrossRef]
- Pachón-Montaño, A.; Sánchez-Montero, J.; Andrade, C.; Fullea, J.; Moreno, E.; Matres, V. Threshold concentration of chlorides in concrete for stainless steel reinforcement: Classic austenitic and new duplex stainless steel. Constr. Build. Mater. 2018, 186, 495–502. [Google Scholar] [CrossRef]
- Iordachescu, M.; Valiente, A.; Pérez-Guerrero, M.; Elices, M. Environment-assisted failure of structural tendons for construction and building applications. Constr. Build. Mater. 2018, 159, 499–507. [Google Scholar] [CrossRef]
- Keßler, S.; Fischer, J.; Straub, D.; Gehlen, C. Updating of service-life prediction of reinforced concrete structures with potential mapping. Cem. Concr. Compos. 2014, 47, 47–52. [Google Scholar] [CrossRef]
- Morales, J.A.; Torres, J.; Rebolledo, N.; Montero, J.S. Experimental and statistical analysis of the corrosion in tendons in contact with water. Front. Mater. 2019, 6. [Google Scholar] [CrossRef]
- Sanchez, J.; Fullea, J.; Andrade, C. Corrosion-induced brittle failure in reinforcing steel. Theor. Appl. Fract. Mech. 2017, 92, 229–232. [Google Scholar] [CrossRef]
- Sanchez, J.; Fullea, J.; Andrade, C. Fracto-surface mobility mechanism in high-strength steel wires. Eng. Fract. Mech. 2017, 186, 410–422. [Google Scholar] [CrossRef]
- Bastidas-Arteaga, E.; Chateauneuf, A.; Sánchez-Silva, M.; Bressolette, P.; Schoefs, F. A comprehensive probabilistic model of chloride ingress in unsaturated concrete. Eng. Struct. 2011, 33, 720–730. [Google Scholar] [CrossRef]
- Pang, L.; Li, Q. Service life prediction of RC structures in marine environment using long term chloride ingress data: Comparison between exposure trials and real structure surveys. Constr. Build. Mater. 2016, 113, 979–987. [Google Scholar] [CrossRef]
- AENOR. UNE_112011 Corrosión en Armaduras: Determinación de la Profundidad de Carbonatación en Hormigones Endurecidos y Puestos en Servicio; AENOR: Madrid, Spain, 2011. [Google Scholar]
- Atkins, C.; Scantlebury, J.; Nedwell, P.; Blatch, S. Monitoring chloride concentrations in hardened cement pastes using ion selective electrodes. Cem. Concr. Res. 1996, 26, 319–324. [Google Scholar] [CrossRef]
- Montemor, M.F.; Alves, J.; Simões, A.; Fernandes, J.; Lourenço, Z.; Costa, A.; Appleton, A.; Ferreira, M. Multiprobe chloride sensor for in situ monitoring of reinforced concrete structures. Cem. Concr. Compos. 2006, 28, 233–236. [Google Scholar] [CrossRef]
- Pargar, F.; Koleva, D.A.; Van Breugel, K. Determination of chloride content in cementitious materials: Fom fundamental aspects to application of Ag/AgCl chloride sensors. Sensors 2017, 17, 2482. [Google Scholar] [CrossRef] [PubMed]
- Climent, M.Á.; Viqueira-Pérez, E.; López-Atalaya, M.M. Embeddable Ag/AgCl sensors for In-Situ monitoring chloride contents in concrete. Cem. Concr. Res. 1996, 26, 1157–1161. [Google Scholar] [CrossRef]
- Zhang, Z.; Hu, J.; Ma, Y.; Wang, Y.; Huang, H.; Zhang, Z.; Wei, J.; Yin, S.; Yu, Q. A state-of-the-art review on Ag/AgCl ion-selective electrode used for non-destructive chloride detection in concrete. Compos. Part B Eng. 2020, 200, 108289. [Google Scholar] [CrossRef]
- European Standard. UNE-EN 196-2:2014. Métodos de Ensayo de Cementos. Parte 2: Análisis Químico de Cementos; European Standard: Madrid, Spain, 2014. [Google Scholar]
- Lemière, B. A review of pXRF (field portable X-ray fluorescence) applications for applied geochemistry. J. Geochem. Explor. 2018, 188, 350–363. [Google Scholar] [CrossRef]
- Glass, G.; Buenfeld, N. The presentation of the chloride threshold level for corrosion of steel in concrete. Corros. Sci. 1997, 39, 1001–1013. [Google Scholar] [CrossRef]
- Glass, G.; Buenfeld, N. The influence of chloride binding on the chloride induced corrosion risk in reinforced concrete. Corros. Sci. 2000, 42, 329–344. [Google Scholar] [CrossRef]
- Alonso, C.; Andrade, C.; Castellote, M.; Castro, P. Chloride threshold values to depassivate reinforcing bars embedded in a standardized OPC mortar. Cem. Concr. Res. 2000, 30, 1047–1055. [Google Scholar] [CrossRef]
- Ann, K.Y.; Song, H.-W. Chloride threshold level for corrosion of steel in concrete. Corros. Sci. 2007, 49, 4113–4133. [Google Scholar] [CrossRef]
- Angst, U.M.; Geiker, M.R.; Alonso, M.C.; Polder, R.; Isgor, O.B.; Elsener, B.; Wong, H.; Michel, A.; Hornbostel, K.; Gehlen, C.; et al. The effect of the steel–concrete interface on chloride-induced corrosion initiation in concrete: A critical review by RILEM TC 262-SCI. Mater. Struct. 2019, 52. [Google Scholar] [CrossRef]
- Figueira, R.B.; Sadovski, A.; Melo, A.P.; Pereira, E.V. Chloride threshold value to initiate reinforcement corrosion in simulated concrete pore solutions: The influence of surface finishing and pH. Constr. Build. Mater. 2017, 141, 183–200. [Google Scholar] [CrossRef]
- Mammoliti, L.T.; Brown, L.C.; Hansson, C.M.; Hope, B.B. The influence of surface finish of reinforcing steel and pH of the test solution on the chloride threshold concentration for corrosion initiation in synthetic pore solutions. Cem. Concr. Res. 1996, 26, 545–550. [Google Scholar] [CrossRef]
- Hussain, S.; Rasheeduzzafar; Al-Musallam, A.; Al-Gahtani, A. Factors affecting threshold chloride for reinforcement corrosion in concrete. Cem. Concr. Res. 1995, 25, 1543–1555. [Google Scholar] [CrossRef]
- Government of Spain, Ministry of Development. EHE-08 “Instrución de Hormigón Estructural”; Government of Spain, Ministry of Development: Madrid, Spain, 2008.
- European Standard. Eurocode 2: Design of Concrete Structures -Part 1-1: General Rules and Rules for Buildings EN 1992-1-1; European Standard: Brussels, Belgium, 2014. [Google Scholar]
- Andrade, C.; Montero, J.S.; Fullea, J.; Rebolledo, N.; Tavares, F. On-site corrosion rate measurements: 3D simulation and representative values. Mater. Corros. 2012, 63, 1154–1164. [Google Scholar] [CrossRef]
- Andrade, C. Propagation of reinforcement corrosion: Principles, testing and modelling. Mater. Struct. 2019, 52, 2. [Google Scholar] [CrossRef]
- DELTA FamilyHandheld XRF Analyzer User’s Manual. Available online: https://www.olympus-ims.com/en/.downloads/download/?file=285217588&fl=en_US&inline (accessed on 26 January 2021).
- Vennesland, Ø.; Climent, M.Á.; Andrade, C. Recommendation of RILEM TC 178-TMC: Testing and modelling chloride penetration in concrete. Mater. Struct. 2012, 46, 337–344. [Google Scholar] [CrossRef]
- Andrade, C.; Alonso, C. Test methods for on-site corrosion rate measurement of steel reinforcement in concrete by means of the polarization resistance method. Mater. Struct. 2004, 37, 623–643. [Google Scholar] [CrossRef]
- Elsener, B.; Andrade, C.; Gulikers, J.; Polder, R.; Raupach, M. Half-cell potential measurements—Potential mapping on reinforced concrete structures. Mater. Struct. 2003, 36, 461–471. [Google Scholar] [CrossRef]
- Polder, R.B. Test methods for on site measurement of resistivity of concrete—A RILEM TC-154 technical recommendation. Constr. Build. Mater. 2001, 15, 125–131. [Google Scholar] [CrossRef]
- Wenner, F. A method of measuring earth resistivity. Bull. Bur. Stand. 1916, 12, 469. [Google Scholar] [CrossRef]
- Van Belleghem, B.; Kessler, S.; Heede, P.V.D.; van Tittelboom, K.; de Belie, N. Chloride induced reinforcement corrosion behavior in self-healing concrete with encapsulated polyurethane. Cem. Concr. Res. 2018, 113, 130–139. [Google Scholar] [CrossRef]
- Keßler, S. Probabilistic corrosion condition assessment of a tunnel structure. Struct. Concr. 2020, 21, 1345–1355. [Google Scholar] [CrossRef]
- Gulikers, J. Theoretical considerations on the supposed linear relationship between concrete resistivity and corrosion rate of steel reinforcement. Mater. Corros. 2005, 56, 393–403. [Google Scholar] [CrossRef]
- Gulikers, J.J.W. A Simplified and Practical Approach Regarding Design for Durability of Reinforced Concrete Structures Based on Probabilistic Modeling of Chloride Ingress. In Concrete Repair, Rehabilitation and Retrofitting II, 1st ed.; Alexander, M.G., Beushausen, H.-D., Dehn, F., Moyo, P., Eds.; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Warkus, J.; Raupach, M.; Gulikers, J. Numerical modelling of corrosion–Theoretical backgrounds. Mater. Corros. 2006, 57, 614–617. [Google Scholar] [CrossRef]




| Resistivity (ρ) | Corrosion Risk |
|---|---|
| >1000–2000 Ω·m | Steel activity/passivity indistinguishable, low corrosion risk, irrespective of chloride content or degree of carbonation |
| >500–1000 Ω·m | Low |
| >100–500 Ω·m | Moderate to high in concretes exposed to carbonation or chloride attack |
| <100 Ω·m | Process not governed by resistivity, highest possible risk of concrete corrosion |
| Corrosion Current Density (icorr) (μA/cm2) | Corrosion Risk |
|---|---|
| <0.1 | Negligible |
| 0.1 a 0.5 | Low |
| 0.5 a 1 | Moderate |
| >1 | High |
| Point | Corrosion Potential and Resistivity Mapping | Corrosion Rate | Chloride Concentration | ||
|---|---|---|---|---|---|
| Ecorr (mVCu/CuSO4) (±10) | Resistivity (Ω·m) (±10) | Icorr (µA/cm2) (±0.002) | [Cl] (ppm) (±10) | [Cl] (wt % cem.) (±0.001) | |
| 1 | −129 | 8090 | 0.013 | 3738 | 3.126 |
| 2 | −25.8 | >9990 | |||
| 3 | 39.6 | >9990 | 543 | 0.454 | |
| 4 | −182 | 3320 | 3414 | 2.855 | |
| 4 | −182 | 3320 | 0.044 | 2562 | 2.143 |
| 5 | −15.6 | >9990 | 3021 | 2.527 | |
| 6 | 36.1 | >9990 | 521 | 0.436 | |
| 7 | −415 | 8480 | |||
| 8 | −115 | >9990 | 408 | 0.341 | |
| 9 | 8.9 | >9990 | |||
| 10 | −375 | 2280 | 0.493 | 3684 | 3.081 |
| 11 | −94 | 3810 | |||
| 11 | −94 | 3810 | 0.801 | 973 | 0.814 |
| 12 | −248 | 2970 | |||
| 13 | −277 | 3840 | |||
| 13 | −277 | 3840 | 0.393 | ||
| 14 | −248 | 280 | |||
| 15 | −248 | 7990 | |||
| 16 | −319 | 301 | |||
| 17 | −393 | 350 | 0.865 | ||
| 18 | −266 | 380 | |||
| 19 | −265 | 290 | |||
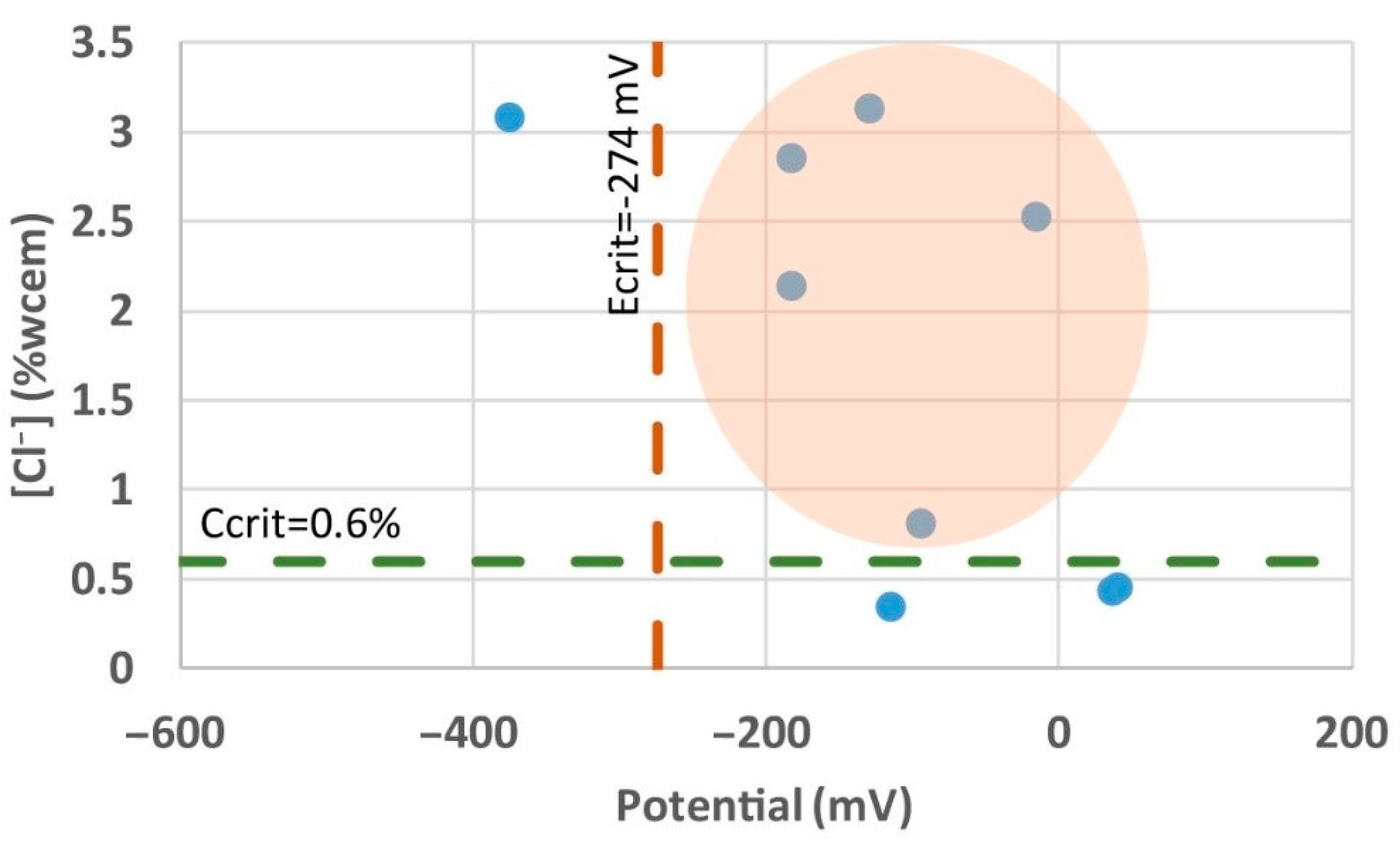
| Potential (mV) | Ccorrosion Risk | Saturated Calomel Elec−Trode (SCE) | Ag/AgCl Electrode (KCl3M−Saturated) | Cu/CuSO4 Electrode (Cu2+−Saturated) |
|---|---|---|---|---|
| Passivity | 10% | >−200 | >−157 | >−274 |
| Intermediate status | 50% | −200 to −350 | −157 to −307 | −274 to −424 |
| High risk | 90% | <−350 | <−307 | <−424 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chinchón-Payá, S.; Torres Martín, J.E.; Rebolledo Ramos, N.; Sánchez Montero, J. Use of a Handheld X-ray Fluorescence Analyser to Quantify Chloride Ions In Situ: A Case Study of Structural Repair. Materials 2021, 14, 571. https://doi.org/10.3390/ma14030571
Chinchón-Payá S, Torres Martín JE, Rebolledo Ramos N, Sánchez Montero J. Use of a Handheld X-ray Fluorescence Analyser to Quantify Chloride Ions In Situ: A Case Study of Structural Repair. Materials. 2021; 14(3):571. https://doi.org/10.3390/ma14030571
Chicago/Turabian StyleChinchón-Payá, Servando, Julio E. Torres Martín, Nuria Rebolledo Ramos, and Javier Sánchez Montero. 2021. "Use of a Handheld X-ray Fluorescence Analyser to Quantify Chloride Ions In Situ: A Case Study of Structural Repair" Materials 14, no. 3: 571. https://doi.org/10.3390/ma14030571
APA StyleChinchón-Payá, S., Torres Martín, J. E., Rebolledo Ramos, N., & Sánchez Montero, J. (2021). Use of a Handheld X-ray Fluorescence Analyser to Quantify Chloride Ions In Situ: A Case Study of Structural Repair. Materials, 14(3), 571. https://doi.org/10.3390/ma14030571








