Intermetallic Phases Identification and Diffusion Simulation in Twin-Roll Cast Al-Fe Clad Sheet
Abstract
:1. Introduction
1.1. Aluminium-Steel Interface
1.2. Diffusion
2. Materials and Methods
3. Results and Discussion
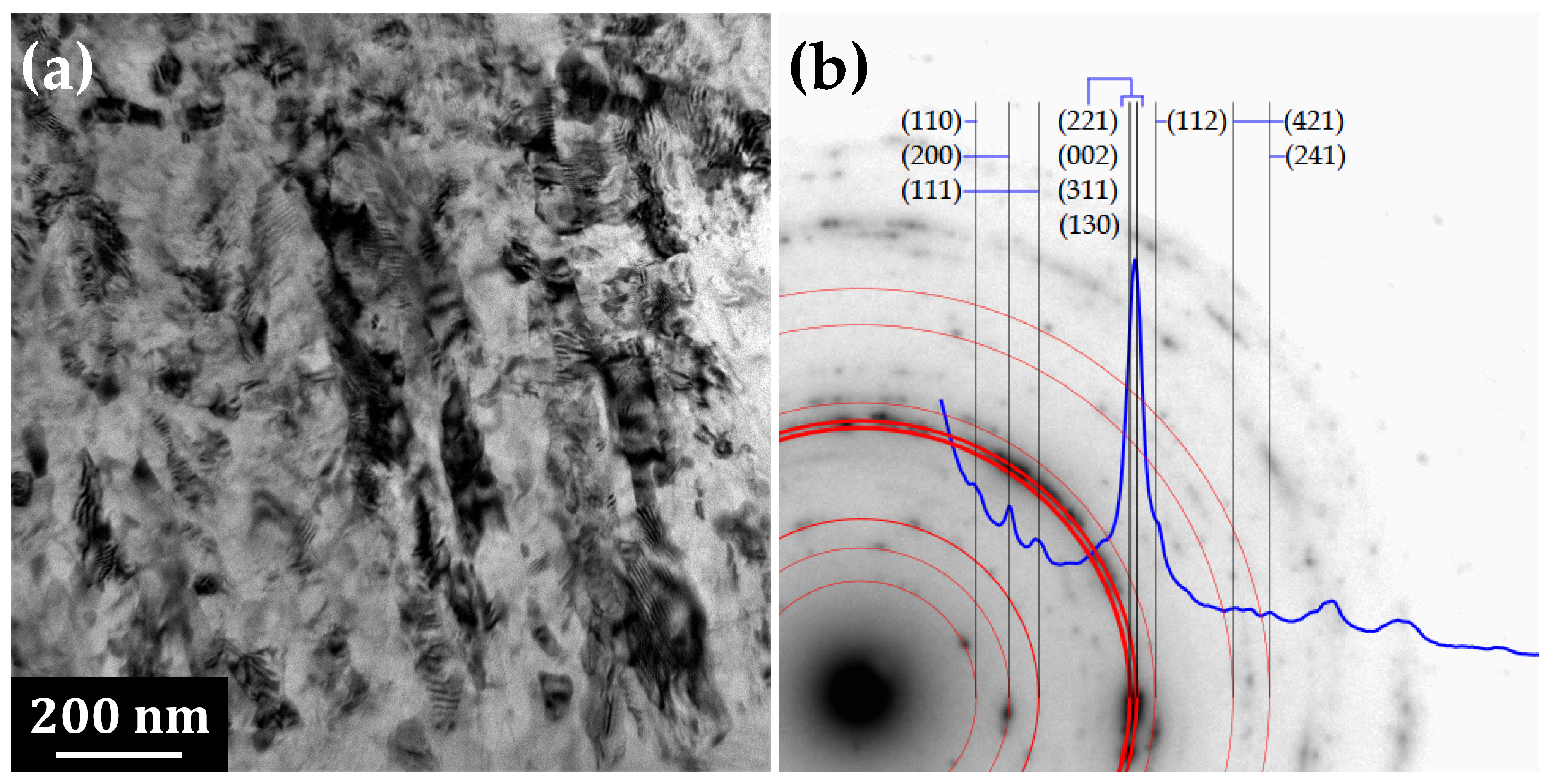
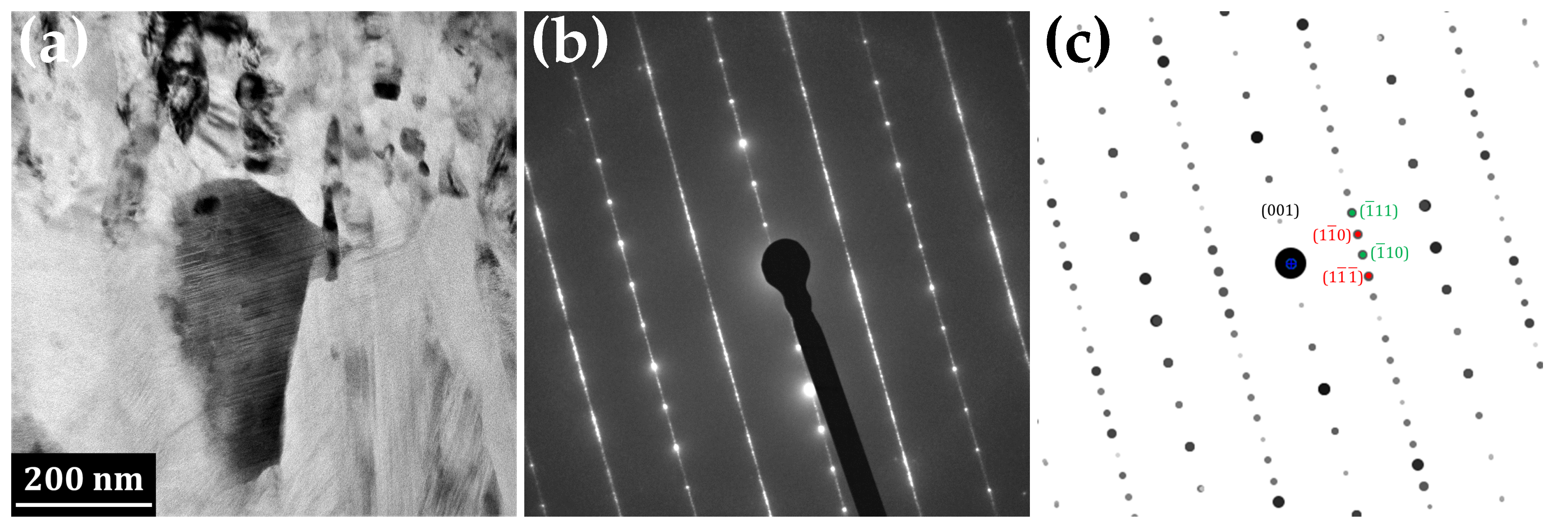
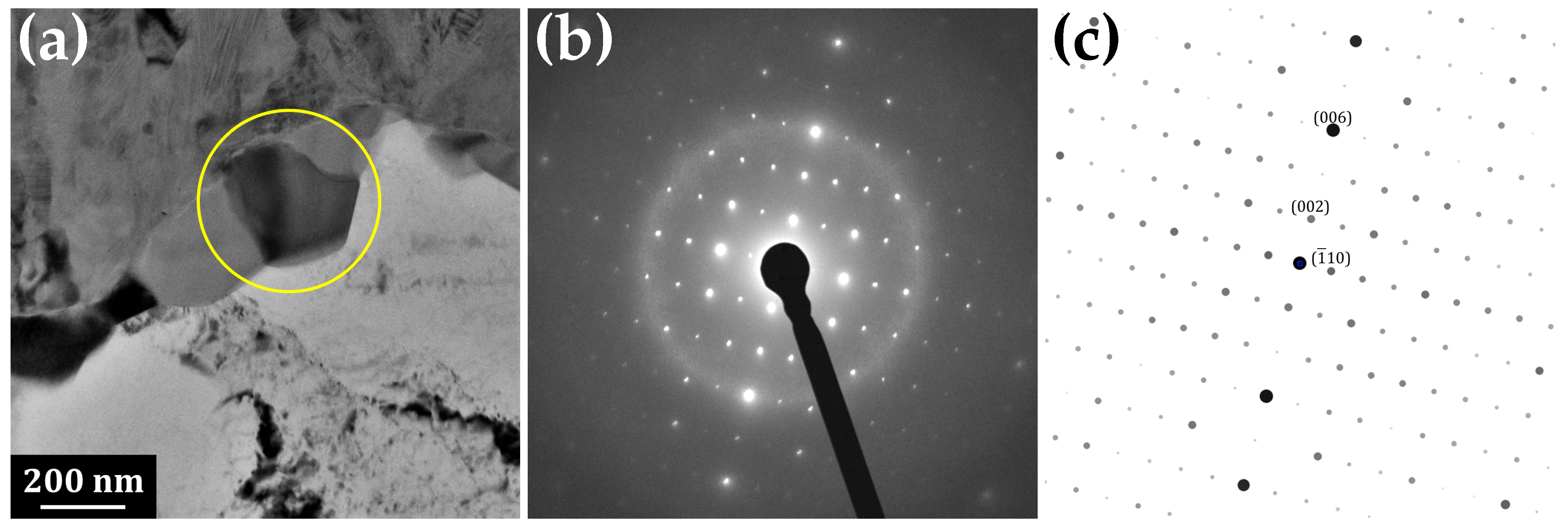
3.1. Transmission Electron Microscopy
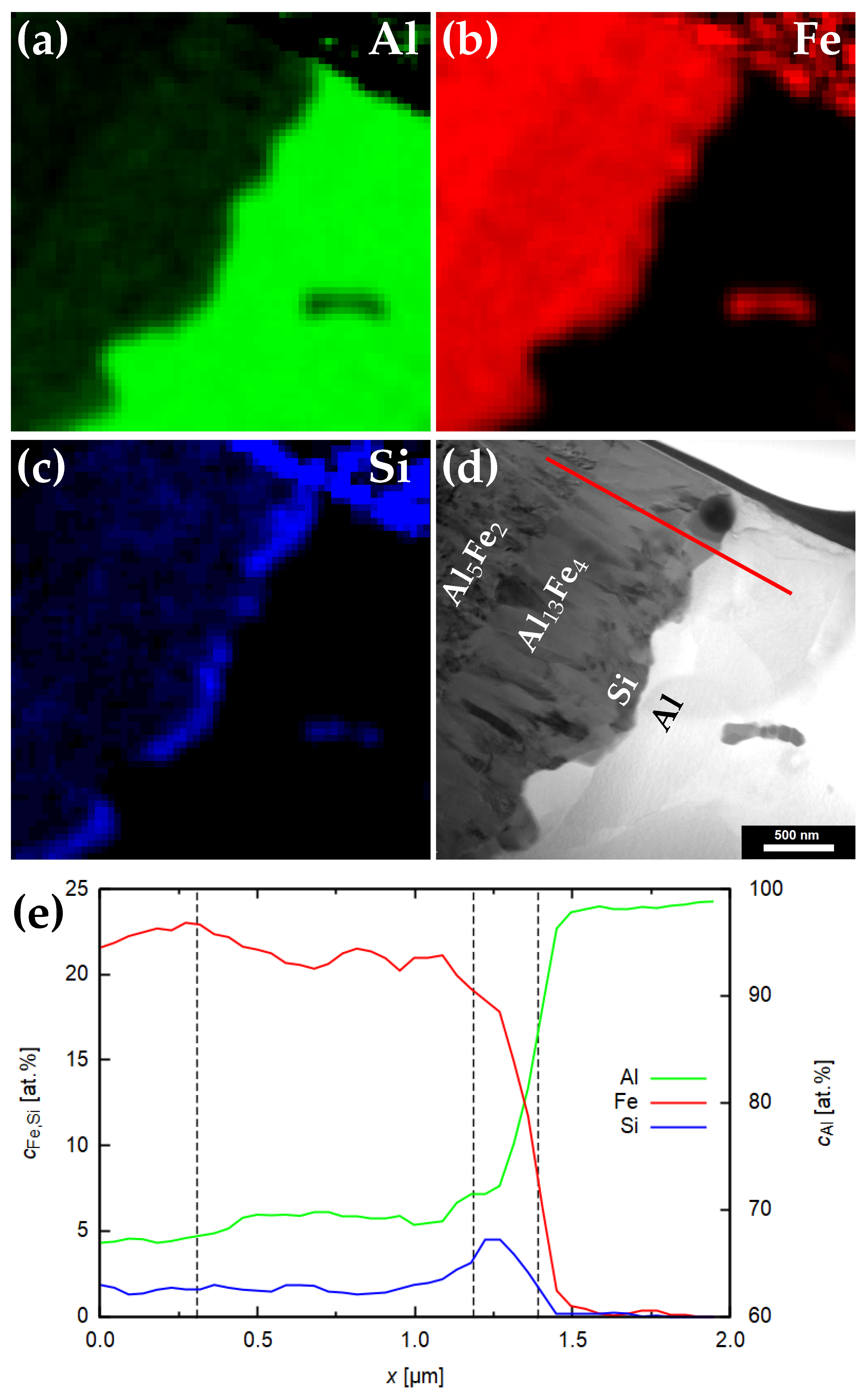
3.2. Chemical Analysis
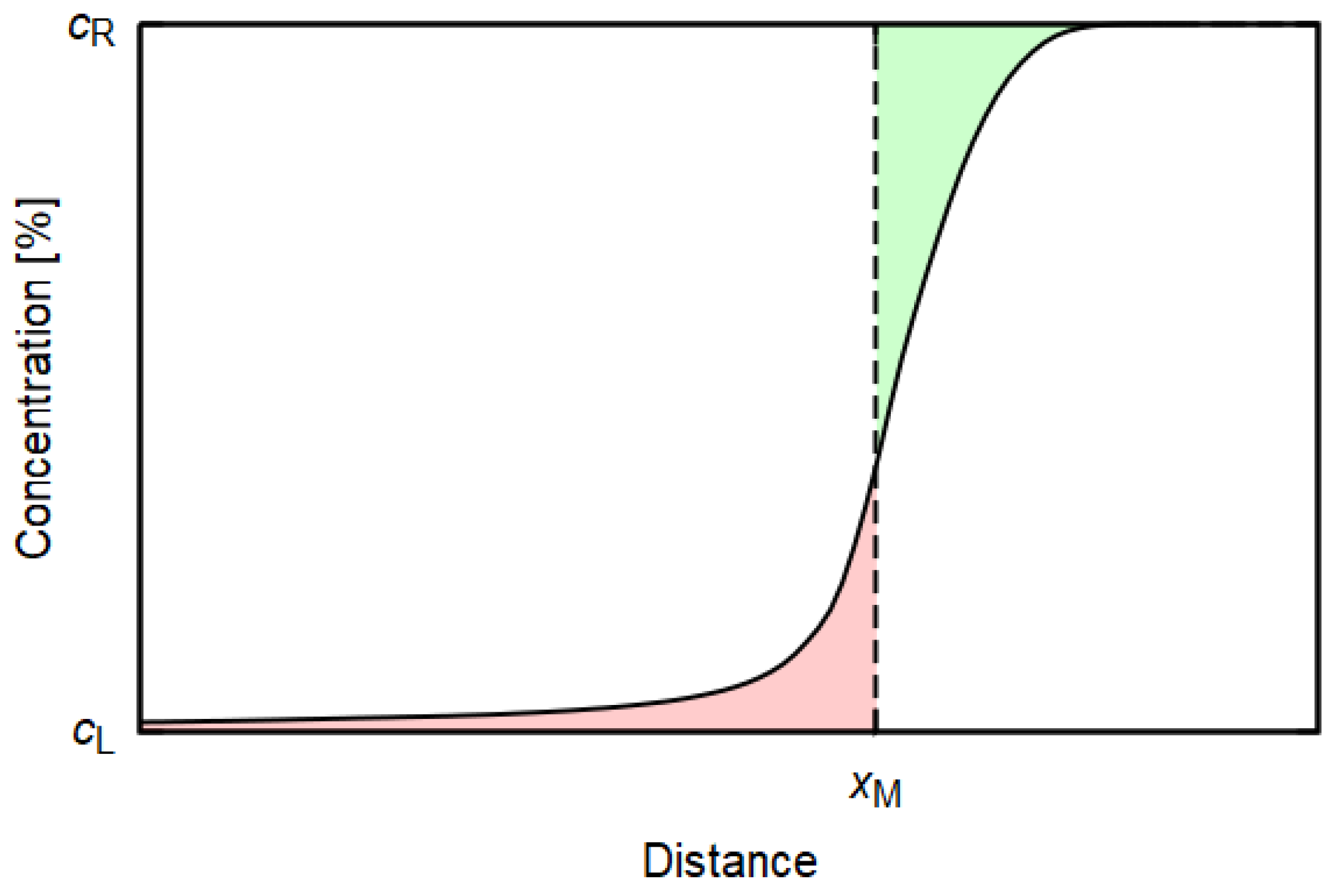
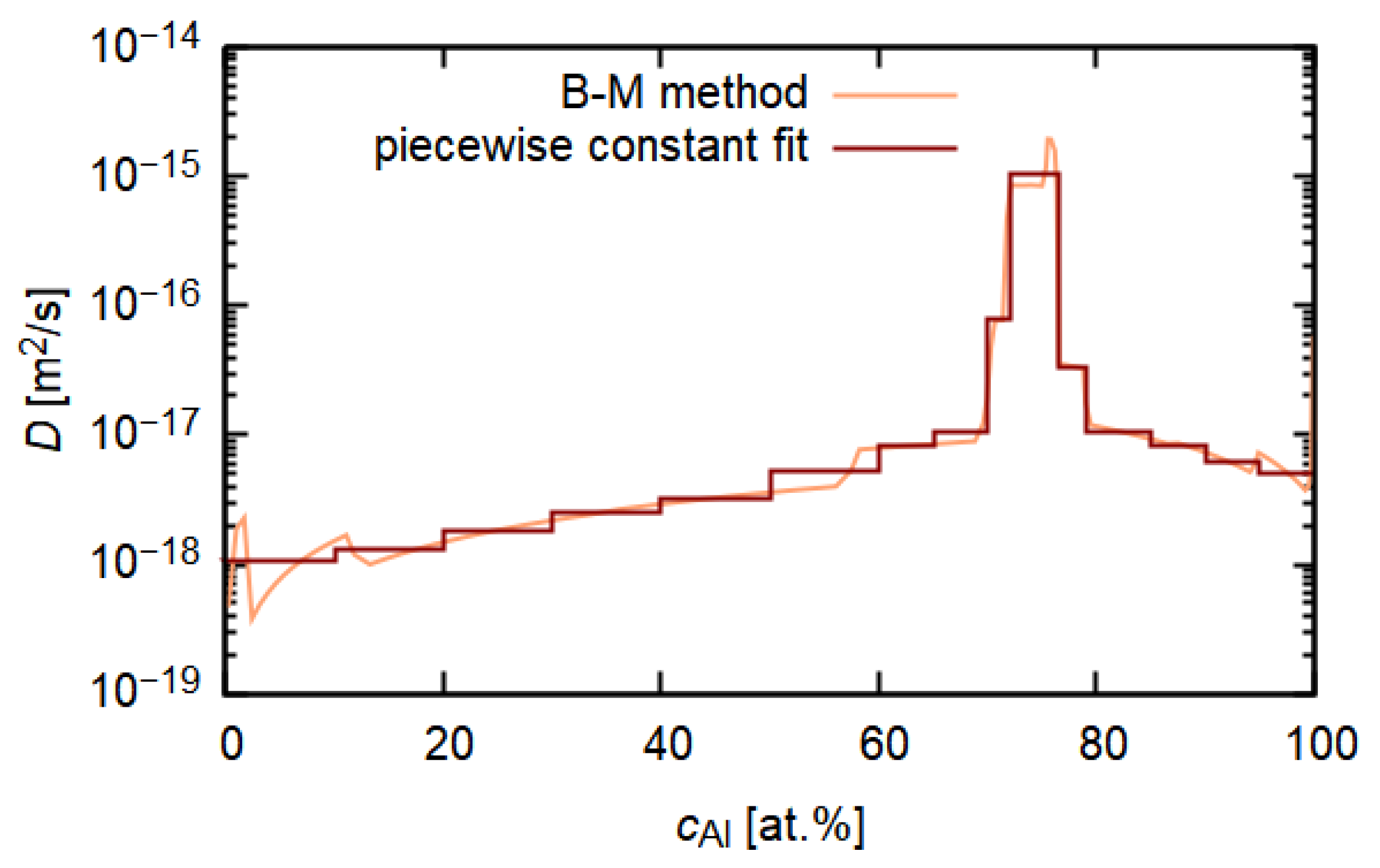
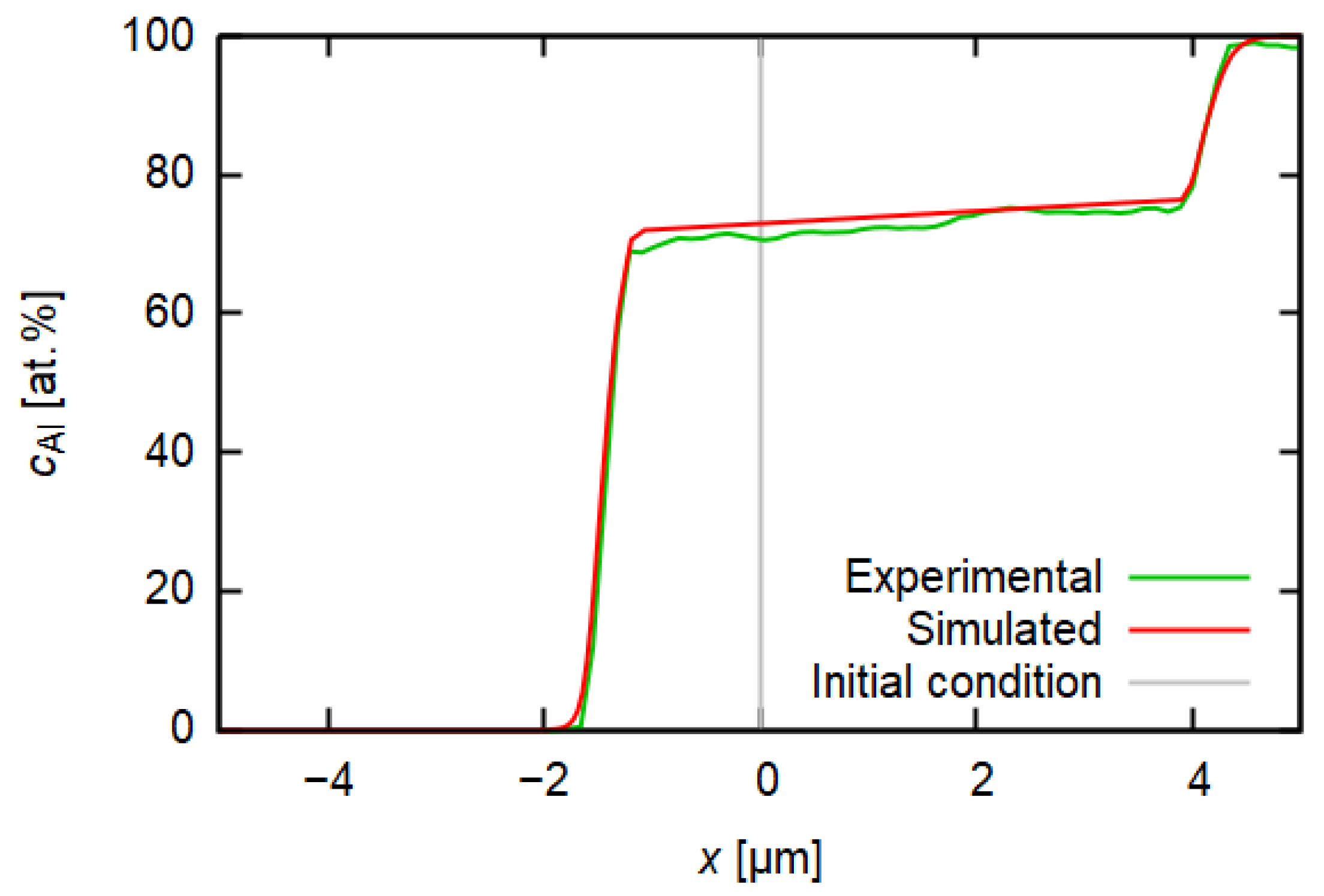
3.3. Diffusion Simulation
4. Conclusions
- Orthorhombic phase AlFe at the steel side of the interface;
- Monoclinic phase AlFe with columnar grains and high density of planar faults and twins;
- Cubic phase isostructural with AlFeMnSi decorating interface between AlFe and aluminium in a discontinuous layer.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yang, Y.; Zhang, F.; He, J.; Qin, Y.; Liu, B.; Yang, M.; Yin, F. Microstructure, growth kinetics and mechanical properties of interface layer for roll bonded aluminum-steel clad sheet annealed under argon gas protection. Vacuum 2018, 151, 189–196. [Google Scholar] [CrossRef]
- Grydin, O.; Stolbchenko, M.; Schaper, M. Impact of steel substrate preheating on microstructure and properties of twin-roll cast aluminium-steel clad strips. Procedia Eng. 2017, 207, 1695–1700. [Google Scholar] [CrossRef]
- Qian, W.; Leng, X.S.; Yang, T.H.; Yan, J.C. Effects of Fe—Al intermetallic compounds on interfacial bonding of clad materials. Trans. Nonferrous Met. Soc. China 2014, 24, 279–284. [Google Scholar]
- Springer, H.; Kostka, A.; dos Santos, J.; Raabe, D. Influence of intermetallic phases and Kirkendall-porosity on the mechanical properties of joints between steel and aluminium alloys. Mater. Sci. Eng. A 2011, 528, 4630–4642. [Google Scholar] [CrossRef]
- Wang, H.; Yang, F.; Zhang, Z.; Liu, L. Bonding mechanism of Al/steel interface formed by laser-TIG welding assisted riveting technology. Mater. Today Commun. 2020, 25, 101487. [Google Scholar] [CrossRef]
- Cui, L.; Wei, Z.; Ma, B.; He, D.; Huang, H.; Cao, Q. Microstructure inhomogeneity of dissimilar steel/Al butt joints produced by laser offset welding. J. Manuf. Process. 2020, 50, 561–572. [Google Scholar] [CrossRef]
- Kumamoto, K.; Kosaka, T.; Kobayashi, T.; Shohji, I.; Kamakoshi, Y. Microstructure and Fatigue Behaviors of Dissimilar A6061/Galvannealed Steel Joints Fabricated by Friction Stir Spot Welding. Materials 2021, 14, 3877. [Google Scholar] [CrossRef] [PubMed]
- Springer, H.; Kostka, A.; Payton, E.; Raabe, D.; Kaysser-Pyzalla, A.; Eggeler, G. On the formation and growth of intermetallic phases during interdiffusion between low-carbon steel and aluminum alloys. Acta Mater. 2011, 59, 1586–1600. [Google Scholar] [CrossRef]
- Li, X.; Scherf, A.; Heilmaier, M.; Stein, F. The Al-Rich Part of the Fe-Al Phase Diagram. J. Phase Equilibria Diffus. 2016, 37, 162–173. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Yang, Q.; Wang, Y. Intereaction and intermetallic phase formation between aluminum and stainless steel. Results Phys. 2019, 12, 514–524. [Google Scholar] [CrossRef]
- Sapanathan, T.; Sabirov, I.; Xia, P.; Monclús, M.; Molina-Aldareguía, J.; Jacques, P.; Simar, A. High temperature in situ SEM assessment followed by ex situ AFM and EBSD investigation of the nucleation and early growth stages of Fe-Al intermetallics. Scr. Mater. 2021, 200, 113910. [Google Scholar] [CrossRef]
- Matysik, P.; Jóźwiak, S.; Czujko, T. Characterization of Low-Symmetry Structures from Phase Equilibrium of Fe-Al System—Microstructures and Mechanical Properties. Materials 2015, 8, 914–931. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Chen, M.; Shi, Y.; Huang, J.; Yang, F. Analysis and modeling of the growth of intermetallic compounds in aluminum–steel joints. RSC Adv. 2017, 7, 37797–37805. [Google Scholar] [CrossRef] [Green Version]
- Fick, A. On liquid diffusion. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1855, 10, 30–39. [Google Scholar] [CrossRef]
- Dekker, A. Solid State Physics; Macmillan & Co., Ltd.: New York, NY, USA, 1957; p. 70. [Google Scholar]
- Atkins, P.; de Paula, J. Physical Chemistry; Oxford University Press: Oxford, UK, 2006; p. 776. [Google Scholar]
- Mehrer, H. Diffusion in Solids; Springer: Berlin/Heidelberg, Germany, 2007; Volume 155. [Google Scholar] [CrossRef]
- Glicksman, M.E. Diffusion in Solids: Field Theory, Solid-State Principles and Applications; Wiley-Interscience: Hoboken, NJ, USA, 1999. [Google Scholar]
- Crank, J. The Mathematics of Diffusion; Oxford University Press: Oxford, UK, 1979. [Google Scholar]
- Alnæs, M.; Blechta, J.; Hake, J.; Johansson, A.; Kehlet, B.; Logg, A.; Richardson, C.; Ring, J.; Rognes, M.E.; Wells, G.N. The FEniCS Project Version 1.5. Arch. Numer. Softw. 2015, 3. [Google Scholar] [CrossRef]
- Logg, A.; Mardal, K.A.; Wells, G. (Eds.) Automated Solution of Differential Equations by the Finite Element Method; Lecture Notes in Computational Science and Engineering; Springer: Berlin/Heidelberg, Germany, 2012; Volume 84. [Google Scholar] [CrossRef]
- Crank, J.; Nicolson, P. A practical method for numerical evaluation of solutions of partial differential equations of the heat-conduction type. Math. Proc. Camb. Philos. Soc. 1947, 43, 50–67. [Google Scholar] [CrossRef]
- Courant, R.; Lewy, H.; Friedrichs, K. On the Partial Difference Equations of Mathematical Physics; Courant Institute of Mathematical Sciences, New York University: New York, NY, USA, 1956. [Google Scholar]
- Akramifard, H.; Mirzadeh, H.; Parsa, M.H. Microstructural Evolution of Roll Bonded Al-Clad Stainless Steel Sheets at Elevated Temperatures. Int. J. Iron Steel Soc. Iran 2016, 13, 38–44. [Google Scholar]
- Křivská, B.; Šlapáková, M.; Minárik, P.; Fekete, K.; Králík, R.; Stolbchenko, M.; Schaper, M.; Grydin, O. In-situ TEM Observation of Intermetallic Phase Growth in Al-Steel Clad Sheet. AIP Conf. Proc. 2021, 2411, 030002. [Google Scholar] [CrossRef]
- Šlapáková, M.; Křivská, B.; Fekete, K.; Králík, R.; Grydin, O.; Stolbchenko, M.; Schaper, M. The Influence of Surface on Direction of Diffusion in Al-Fe Clad Material. Mater. Charact. 2022, 185. submitted. [Google Scholar]
- Recko, K.; Dobrzynski, L.; Senyshyn, A.; Fuess, H.; Szymanski, K.; Kotur, B.Y.; Suski, W. Structural and magnetic properties of Sc1.1Fe3.9Al8 alloy. J. Magn. Magn. Mater. 2011, 323, 1860–1867. [Google Scholar] [CrossRef]
- Khaidar, M.; Allibert, C.H.; Driole, J. Phase Eaquilibria of the Fe-Ni-Al System for Al Content above 50 at.% and Crystal Structures of some Ternary Phases. Int. J. Mater. Res. 1982, 73, 433–438. [Google Scholar] [CrossRef]
- Black, P.J. The structure of FeAl3. I. Acta Crystallogr. 1955, 8, 43–48. [Google Scholar] [CrossRef]
- Tsuchimori, M.; Ishimasa, T.; Fukano, Y. Crystal structures of small Al-rich Fe alloy particles formed by a gas-evaporation technique. Philos. Mag. B 1992, 66, 89–108. [Google Scholar] [CrossRef]
- Xu, P.; Hua, X.; Shen, C.; Mou, G.; Li, F. Formation of Fe5Si3 precipitate in the Fe2Al5 intermetallic layer of the Al/steel dissimilar arc welding joint: A transmission electron microscopy (TEM) study. Mater. Charact. 2021, 178, 111236. [Google Scholar] [CrossRef]
- Cooper, M. The crystal structure of the ternary alloy α (AlFeSi). Acta Crystallogr. 1967, 23, 1106–1107. [Google Scholar] [CrossRef]
- Hwang, J.Y.; Doty, H.W.; Kaufman, M.J. Crystallographic studies on the iron-containing intermetallic phases in the 319-type aluminium casting alloys. Philos. Mag. 2008, 88, 607–619. [Google Scholar] [CrossRef]
- Kang, K.; Liu, Y.; Li, J.; Liu, C.; Zhen, Z.; Wang, Y.; Sun, Q. Microstructure and Mechanical Properties of Al/Steel Butt Joint by Hybrid CMT Welding with External Axial Magnetic Field. Materials 2020, 13, 3601. [Google Scholar] [CrossRef]
- Xia, H.; Zhao, X.; Tan, C.; Chen, B.; Song, X.; Li, L. Effect of Si content on the interfacial reactions in laser welded-brazed Al/steel dissimilar butted joint. J. Mater. Process. Technol. 2018, 258, 9–21. [Google Scholar] [CrossRef]
- Křivská, B.; Šlapáková, M.; Kihoulou, M.; Králík, R.; Bajtošová, L.; Cieslar, M.; Grydin, O.; Stolbchenko, M.; Schaper, M. Interdiffusion in Aluminum-Steel Clad Strip. In Proceedings of the 20th Conference of Czech and Slovak Physicists Proceedings, Praha, Czech Republic, 7–10 September 2020; Slovak Physical Society, Czech Physical Society: Košice, Slovakia, 2020; pp. 153–154. [Google Scholar]
- Stolbchenko, M.; Grydin, O.; Schaper, M. Manufacturing and Characterization of Twin-Roll Cast Aluminum-Steel Clad Strips. Adv. Eng. Mater. 2018, 21, 1800454. [Google Scholar] [CrossRef]
- Li, R.; Yuan, T.; Liu, X.; Zhou, K. Enhanced atomic diffusion of Fe–Al diffusion couple during spark plasma sintering. Scr. Mater. 2016, 110, 105–108. [Google Scholar] [CrossRef]
- Matano, C. On the Relation between the Diffusion-Coefficients and Concentrations of Solid Metals (The Nickel-Copper System). Jpn. J. Phys. 1933, 8, 109–113. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Křivská, B.; Šlapáková, M.; Veselý, J.; Kihoulou, M.; Fekete, K.; Minárik, P.; Králík, R.; Grydin, O.; Stolbchenko, M.; Schaper, M. Intermetallic Phases Identification and Diffusion Simulation in Twin-Roll Cast Al-Fe Clad Sheet. Materials 2021, 14, 7771. https://doi.org/10.3390/ma14247771
Křivská B, Šlapáková M, Veselý J, Kihoulou M, Fekete K, Minárik P, Králík R, Grydin O, Stolbchenko M, Schaper M. Intermetallic Phases Identification and Diffusion Simulation in Twin-Roll Cast Al-Fe Clad Sheet. Materials. 2021; 14(24):7771. https://doi.org/10.3390/ma14247771
Chicago/Turabian StyleKřivská, Barbora, Michaela Šlapáková, Jozef Veselý, Martin Kihoulou, Klaudia Fekete, Peter Minárik, Rostislav Králík, Olexandr Grydin, Mykhailo Stolbchenko, and Mirko Schaper. 2021. "Intermetallic Phases Identification and Diffusion Simulation in Twin-Roll Cast Al-Fe Clad Sheet" Materials 14, no. 24: 7771. https://doi.org/10.3390/ma14247771
APA StyleKřivská, B., Šlapáková, M., Veselý, J., Kihoulou, M., Fekete, K., Minárik, P., Králík, R., Grydin, O., Stolbchenko, M., & Schaper, M. (2021). Intermetallic Phases Identification and Diffusion Simulation in Twin-Roll Cast Al-Fe Clad Sheet. Materials, 14(24), 7771. https://doi.org/10.3390/ma14247771





