Catalytic Activity of New Oxovanadium(IV) Microclusters with 2-Phenylpyridine in Olefin Oligomerization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Syntheses
2.3. Physicochemical Characteristics
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Higman, C.S.; Lummiss, J.A.M.; Fogg, D.E. Olefin Metathesis at the Dawn of Implementation in Pharmaceutical and Specialty-Chemicals Manufacturing. Angew. Chem. Int. Ed. 2016, 55, 3552–3565. [Google Scholar] [CrossRef]
- Grubbs, R.G.; Wenzel, A.G.; O’Leary, D.J.; Khosravi, E. Handbook of Metathesis, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2015. [Google Scholar]
- Mulhaupt, R.; Ovenall, D.W.; Ittel, S.D. Control of composition in ethylene copolymerizations using magnesium mhloride supported Ziegler–Natta catalysts. J. Polym. Sci. Part A Polym. Chem. 1988, 26, 2487–2500. [Google Scholar] [CrossRef]
- Tsujino, T.; Saegusa, T.; Furukawa, J. Polymerization of norbornene by modified Ziegler-catalysts. Die Makromol. Chem. 2003, 85, 71–79. [Google Scholar] [CrossRef]
- Adisson, E.; Deffieux, A.; Fontanille, M. Polymerization of ethylene at high temperature by vanadium-based heterogeneous Ziegler–Natta catalysts. I. study of the deactivation process. J. Polym. Sci. Part A Polym. Chem. 1993, 31, 831–839. [Google Scholar] [CrossRef]
- Calderon, N.; Ofstead, E.A.; Ward, J.P.; Judy, W.A.; Scott, K.W. Olefin metathesis. I. Acyclic vinylenic hydrocarbons. J. Am. Chem. Soc. 1968, 90, 4133–4140. [Google Scholar] [CrossRef]
- Natta, G.; Dall’Asta, G.; Mazzanti, G. Stereospecific homopolymerization of cyclopentene. Angew. Chem. Int. Ed. 1964, 3, 723–729. [Google Scholar] [CrossRef]
- Mol, J.C.; Ivin, K.J. Olefin Metathesis and Metathesis Polymerization; Academic Press: London, UK, 1997. [Google Scholar]
- Herbert, M.B.; Grubbs, R.H. Z-Selective Cross Metathesis with Ruthenium Catalysts: Synthetic Applications and Mechanistic Implications. Angew. Chem. Int. Ed. 2015, 54, 5018–5024. [Google Scholar] [CrossRef] [PubMed]
- Awang, N.W.; Tsutsumi, K.; Hustakova, B.; Yusoff, S.F.M.; Nomura, K.; Yamin, B.M. Cross metathesis of methyl oleate (MO) with terminal, internal olefins by ruthenium catalysts: Factors affecting the efficient MO conversion and the selectivity. RSC Adv. 2016, 6, 100925–100930. [Google Scholar] [CrossRef]
- Liniger, M.; Neuhaus, C.M.; Altmann, K.H. Ring-Closing Metathesis Approaches towards the Total Synthesis of Rhizoxins. Molecules 2020, 25, 4527. [Google Scholar] [CrossRef] [PubMed]
- Choinopoulos, I. Grubbs’ and Schrock’s Catalysts, Ring Opening Metathesis Polymerization and Molecular Brushes—Synthesis, Characterization, Properties and Applications. Polymers 2019, 11, 298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chołuj, A.; Nogaś, W.; Patrzałek, M.; Krzesiński, P.; Chmielewski, M.J.; Kajetanowicz, A.; Grela, K. Preparation of Ruthenium Olefin Metathesis Catalysts Immobilized on MOF, SBA-15, and 13X for Probing Heterogeneous Boomerang Effect. Catalysts 2020, 10, 438. [Google Scholar] [CrossRef]
- Jasiński, R.; Dresler, E. On the Question of Zwitterionic Intermediates in the [3+2] Cycloaddition Reactions: A Critical Review. Organics 2020, 1, 49–69. [Google Scholar] [CrossRef]
- Schmitt, C.C.; Belén, M.; Reolon, G.; Zimmermann, M.; Raffelt, K.; Grunwaldt, J.D.; Dahmen, N. Synthesis and Regeneration of Nickel-Based Catalysts for Hydrodeoxygenation of Beech Wood Fast Pyrolysis Bio-Oil. Catalysts 2018, 8, 449. [Google Scholar] [CrossRef] [Green Version]
- Gawdzik, B.; Kamizela, A.; Szyszkowska, A. Lactones with a fragrance properties. Chemist 2015, 69, 346–349. [Google Scholar]
- Kamizela, A.; Gawdzik, B.; Urbaniak, M.; Lechowicz, Ł.; Białońska, A.; Gonciarz, W.; Chmiela, M. Synthesis, Characterization, Cytotoxicity, and Antibacterial Properties of trans-γ-Halo-δ-lactones. ChemistryOpen 2018, 7, 543–550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drzeżdżon, J.; Piotrowska-Kirschling, A.; Malinowski, J.; Kloska, A.; Gawdzik, B.; Chmurzyński, L.; Jacewicz, D. Antimicrobial, cytotoxic, and antioxidant activities and physicochemical characteristics of chromium (III) complexes with picolinate, dipico-linate, oxalate, 2, 2′-bipyridine, and 4,4′-dimethoxy-2,2′-bipyridine as ligands in aqueous solutions. J. Mol. Liq. 2019, 282, 441–447. [Google Scholar] [CrossRef]
- Gawdzik, B.; Iwanek, W. Synthesis, structure, and stereochemistry of the bora derivatives of 1-[(2-hydroxy-1-naphthyl) methyl] proline. Tetrahedron Asymmetry 2005, 16, 2019–2202. [Google Scholar] [CrossRef]
- Prezhdo, O.; Gawdzik, B.; Zubkova, V.V.; Prezhdo, V.V. Molecular structure and electrical properties of some phosphonates, phosphine-oxides and phosphates. J. Mol. Struct. 2009, 919, 146–153. [Google Scholar] [CrossRef]
- Hearne, N.; Turnbull, M.M.; Landee, C.P.; van der Merwe, E.M.; Rademeyer, M. Halide-bi-bridged polymers of amide substituted pyridines and -pyrazines: Polymorphism, structures, thermal stability and magnetism. CrystEngComm 2019, 21, 1910–1927. [Google Scholar] [CrossRef]
- Kwiatek, D.; Kubicki, M.; Skokowski, P.; Gruszczyńska, J.; Lis, S.; Hnatejko, Z. Five subsequent new pyridine carboxamides and their complexes with d-electron ions. Synthesis, spectroscopic characterization and magnetic properties. J. Mol. Struct. 2019, 1178, 669–681. [Google Scholar] [CrossRef]
- Oxford Diffraction Ltd. CrysAlis CCD and CrysAlis RED; Version 1.171.36.24; Oxford Diffraction Ltd.: Yarnton, UK, 2012. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Spek, A.L. Structure validation in chemical crystallography. Acta Crystallogr. D 2009, 65, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.K. ORTEP II, Report ORNL-5138; Oak Ridge National Laboratory: Oak Ridge, TN, USA, 1976. [Google Scholar]
- Motherwell, S.; Clegg, S. PLUTO-78, Program for Drawing and Molecular Structure; University of Cambridge: Cambridge, UK, 1978. [Google Scholar]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; van de Streek, J.; Wood, P.A. Mercury CSD 2.0—New features for the visualization and investigation of crystal structures. J. Appl. Crystallogr. 2008, 41, 466–470. [Google Scholar] [CrossRef]
- Britovsek, G.J.; Gibson, V.C.; Wass, D.F. The search for new-generation olefin polymerization catalysts: Life beyond metallocenes. Angew. Chem. Int. Ed. 1999, 38, 428–447. [Google Scholar] [CrossRef]
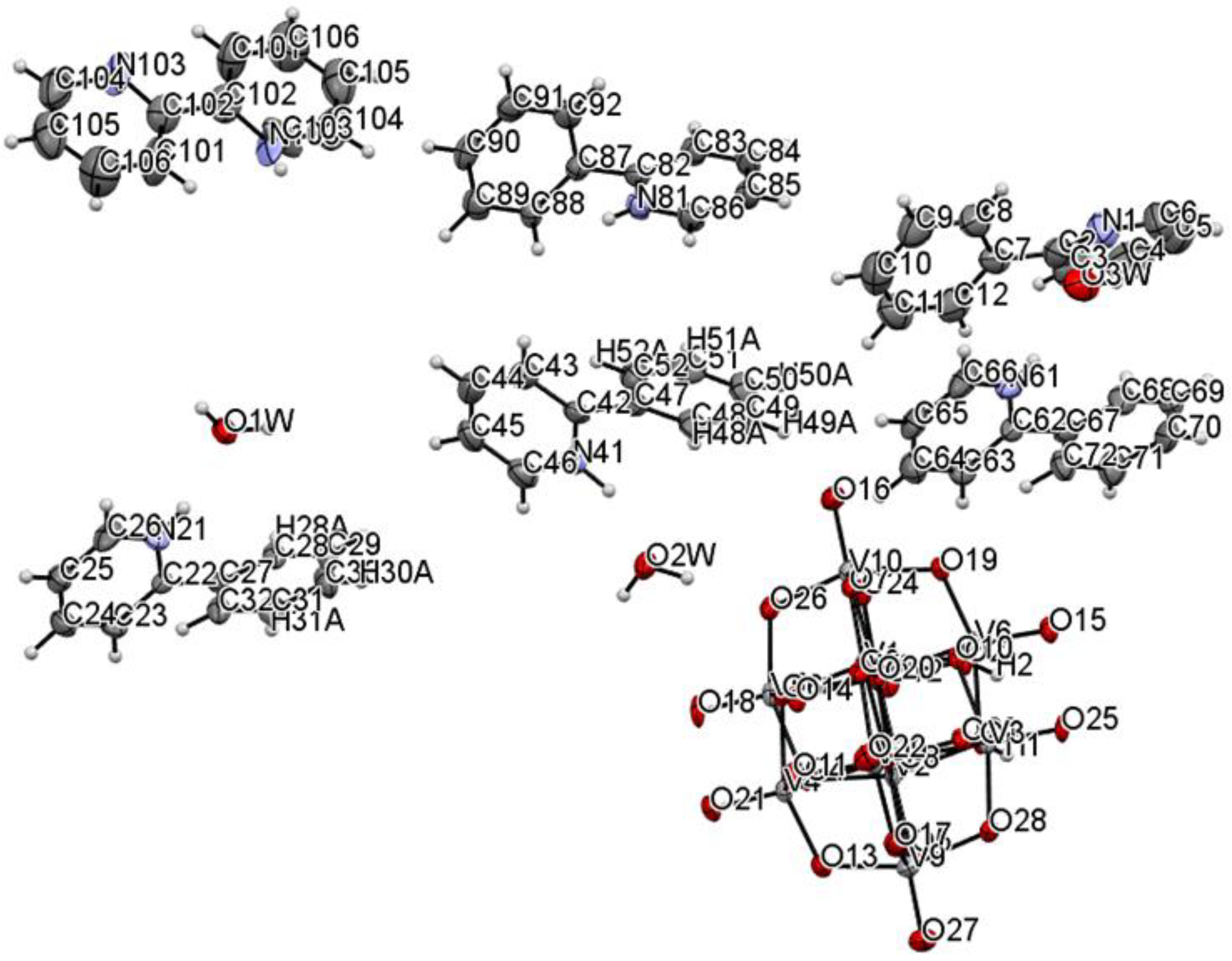
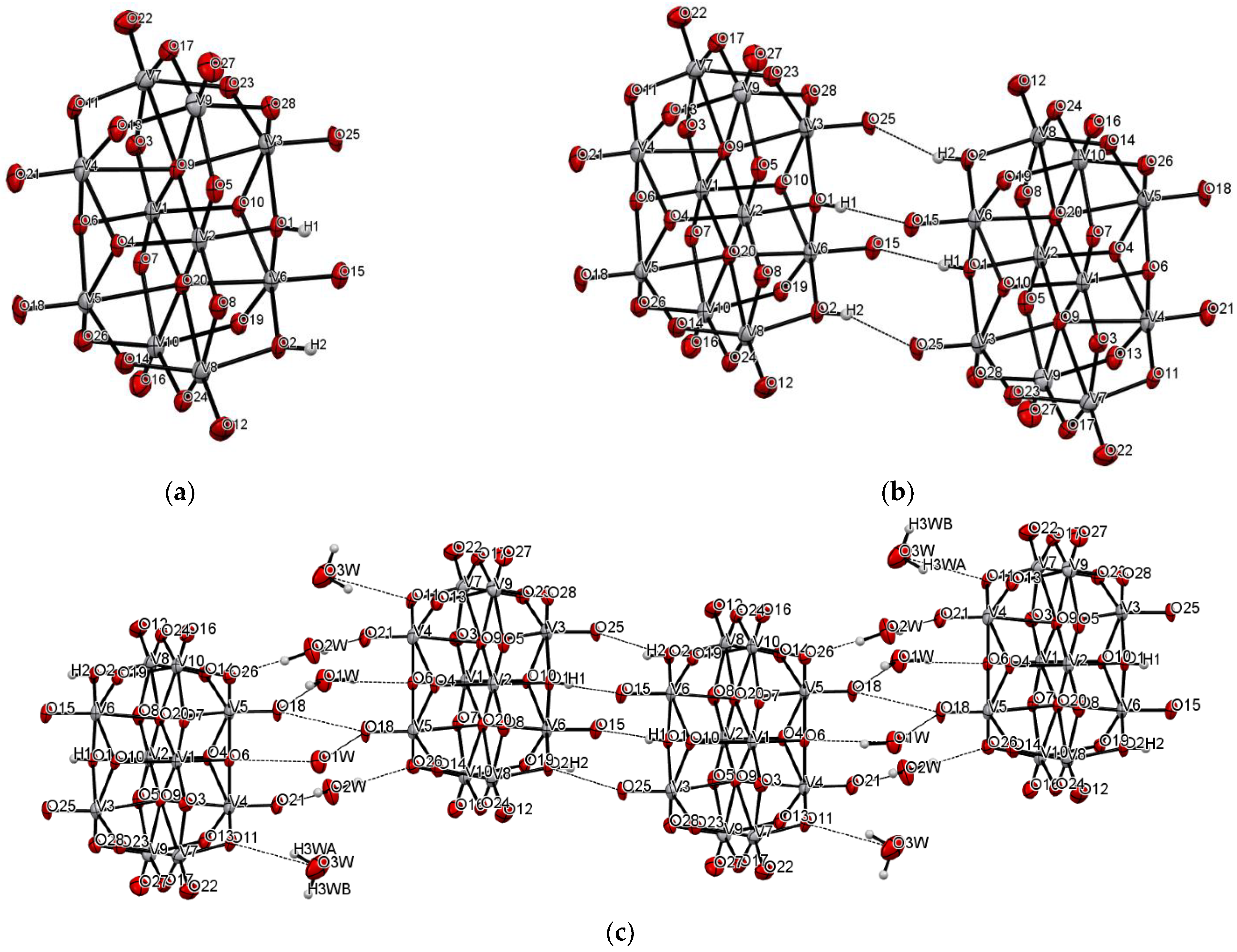



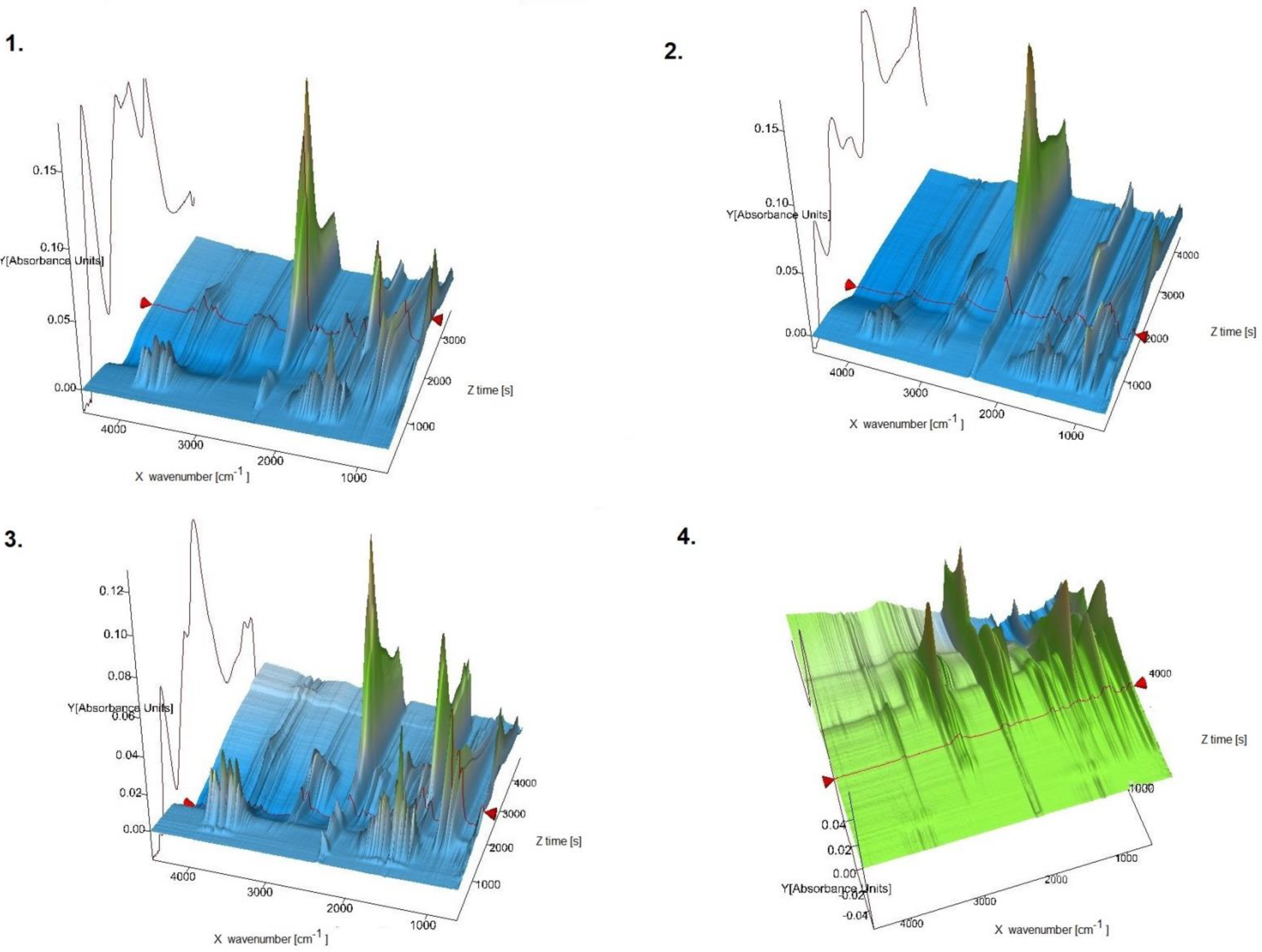
| Chemical Formula | V20C121H123N11O62 |
|---|---|
| Formula weight/g·mol−1 | 3742.10 |
| Crystal system | monoclinic |
| Space group | P21/c |
| a/Å | 15.2074(5) |
| b/Å | 18.4168(6) |
| c/Å | 24.2763(7) |
| α/° | 90 |
| β/° | 91.304(3) |
| γ/° | 90 |
| V/Å3 | 6797.4(4) |
| Z | 2 |
| T/K | 293(2) |
| λMo/Å | 0.71073 |
| ρcalc/g·cm–3 | 1.828 |
| F(000) | 3762 |
| µ/mm−1 | 1.403 |
| θ range/° | 3.32–25.00 |
| Completeness θ/% | 99.8 |
| Reflections collected | 53,867 |
| Reflections unique | 11,941 [Rint = 0.1078] |
| Data/restraints/parameters | 11,941/6/1011 |
| Goodness of fit on F2 | 1.037 |
| Final R1 value (I > 2σ(I)) | 0.0639 |
| Final wR2 value (I > 2σ(I)) | 0.0824 |
| Final R1 value (all data) | 0.1275 |
| Final wR2 value (all data) | 0.0968 |
| CCDC number | 2,117,153 |
| D–H···A | d(D–H) [Å] | d(H···A) [Å] | d(D⋯A) (Å) | ∠D–H⋯A (°) |
|---|---|---|---|---|
| O1–H1···O15 i | 0.75(2) | 2.03(5) | 2.745(5) | 175(7) |
| O1W–H1WA···O6 | 0.94(2) | 2.01(3) | 2.908(5) | 158(4) |
| O2–H2···O25 i | 0.80(7) | 2.05(7) | 2.810(5) | 159(6) |
| O1W–H1WB···O18 ii | 0.92(4) | 2.01(4) | 2.910(5) | 164(5) |
| O2W–H2WA···O21 iii | 0.93(4) | 1.99(5) | 2.881(5) | 161(5) |
| O2W–H2WB···O26 iv | 0.93(3) | 1.88(3) | 2.807(5) | 172(5) |
| O3W–H3WA···O11 v | 0.95(5) | 2.08(6) | 3.899(6) | 144(4) |
| O3W–H3WA···O21 v | 0.95(5) | 2.37(5) | 2.160(6) | 141(4) |
| O3W–H3WB···N1 | 0.93(4) | 2.03(5) | 2.910(7) | 158(6) |
| N21–H21A···O1W | 0.87(5) | 1.86(5) | 2.718(6) | 166(5) |
| N41–H41A···O2W | 0.98(5) | 1.77(5) | 2.697(6) | 156(5) |
| N61–H61A···O3W | 0.76(5) | 1.99(5) | 2.699(7) | 158(5) |
| N81–H81A···O28 | 0.80(5) | 2.05(5) | 2.831(6) | 167(5) |
| C23–H23A··· O23 vi | 0.93 | 2.51 | 3.334(6) | 148 |
| C26–H26A··· O4 ii | 0.93 | 2.20 | 3.023(6) | 147 |
| C28–H28A···O1W | 0.93 | 2.59 | 3.202(6) | 124 |
| C31–H31A···O15 vi | 0.93 | 2.57 | 3.300(6) | 136 |
| C31–H31A···O8 iii | 0.93 | 2.57 | 3.188(6) | 124 |
| C32–H32A··· O10 vi | 0.93 | 2.56 | 3.480(6) | 171 |
| C45–H45A··· O27 iii | 0.93 | 2.57 | 3.453(8) | 158 |
| C46–H46A··· O13 iii | 0.93 | 2.30 | 2.994(8) | 131 |
| C49–H49A··· O16 iv | 0.93 | 2.50 | 3.300(7) | 144 |
| C63–H63A··· O19 iv | 0.93 | 2. 48 | 3.389(6) | 166 |
| C65–H65··· O12 i | 0.93 | 2.55 | 3.270(7) | 135 |
| C66–H66A··· O14 i | 0.93 | 2.56 | 3.230(6) | 129 |
| C68–H68B··· O3W | 0.93 | 2.58 | 3.384(7) | 144 |
| C69–H69A·····O22 v | 0.93 | 2.48 | 3.353(8) | 157 |
| C72–H72A··· O19 iv | 0.93 | 2.57 | 3.476(7) | 166 |
| C83–H83A··· O27 vii | 0.93 | 2.56 | 3.116(7) | 118 |
| C85–H85A··· O24 i | 0.93 | 2.27 | 3.098(7) | 148 |
| C86–H86A··· O2 i | 0.93 | 2.47 | 3.304(7) | 150 |
| C89–H89A···O22 | 0.93 | 2.49 | 2.295(7) | 145 |
| Entry | Olefin | Amt of V (µmol) | t (min) | Molar Ratio Compound: MMAO | Activity (g∙mmol−1∙h−1) |
|---|---|---|---|---|---|
| 1 | 3-buten-1-ol | 3 | 35 | 1:1000 | 2571 |
| 2 | 2-chloro-2-propen-1-ol | 3 | 28 | 1:1000 | 643 |
| 3 | allyl alcohol | 3 | 29 | 1:1000 | 3724 |
| 4 | 2,3-dibromo-2-propen-1-ol | 3 | 25 | 1:1000 | 3096 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gawdzik, B.; Drzeżdżon, J.; Siarhei, T.; Sikorski, A.; Malankowska, A.; Kowalczyk, P.; Jacewicz, D. Catalytic Activity of New Oxovanadium(IV) Microclusters with 2-Phenylpyridine in Olefin Oligomerization. Materials 2021, 14, 7670. https://doi.org/10.3390/ma14247670
Gawdzik B, Drzeżdżon J, Siarhei T, Sikorski A, Malankowska A, Kowalczyk P, Jacewicz D. Catalytic Activity of New Oxovanadium(IV) Microclusters with 2-Phenylpyridine in Olefin Oligomerization. Materials. 2021; 14(24):7670. https://doi.org/10.3390/ma14247670
Chicago/Turabian StyleGawdzik, Barbara, Joanna Drzeżdżon, Tatsiana Siarhei, Artur Sikorski, Anna Malankowska, Paweł Kowalczyk, and Dagmara Jacewicz. 2021. "Catalytic Activity of New Oxovanadium(IV) Microclusters with 2-Phenylpyridine in Olefin Oligomerization" Materials 14, no. 24: 7670. https://doi.org/10.3390/ma14247670
APA StyleGawdzik, B., Drzeżdżon, J., Siarhei, T., Sikorski, A., Malankowska, A., Kowalczyk, P., & Jacewicz, D. (2021). Catalytic Activity of New Oxovanadium(IV) Microclusters with 2-Phenylpyridine in Olefin Oligomerization. Materials, 14(24), 7670. https://doi.org/10.3390/ma14247670







