Effect of pH-Regulation on the Capture of Lipopolysaccharides from E. coli EH100 by Four-Antennary Oligoglycines in Aqueous Medium
Abstract
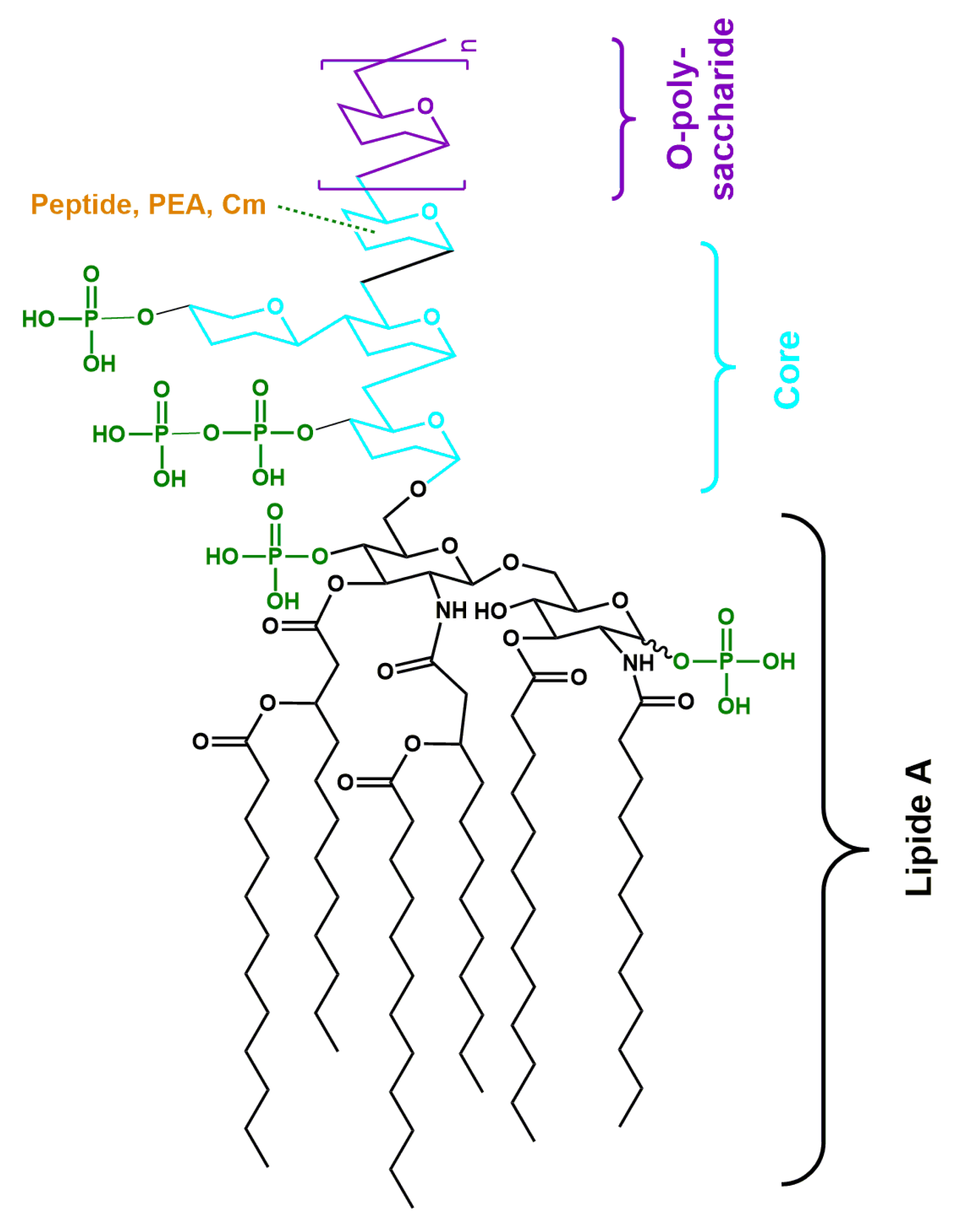
1. Introduction
2. Materials and Methods
3. Results
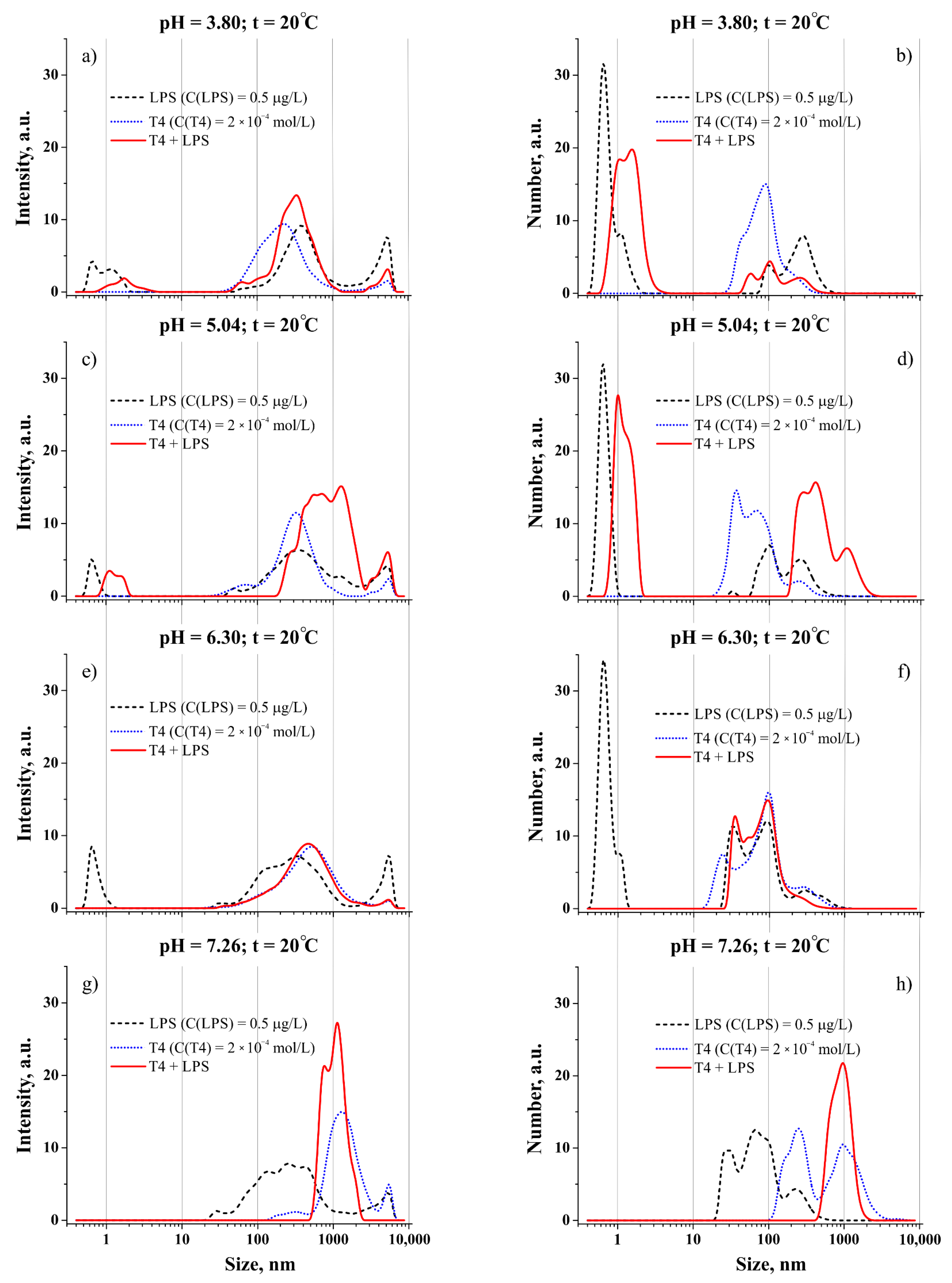
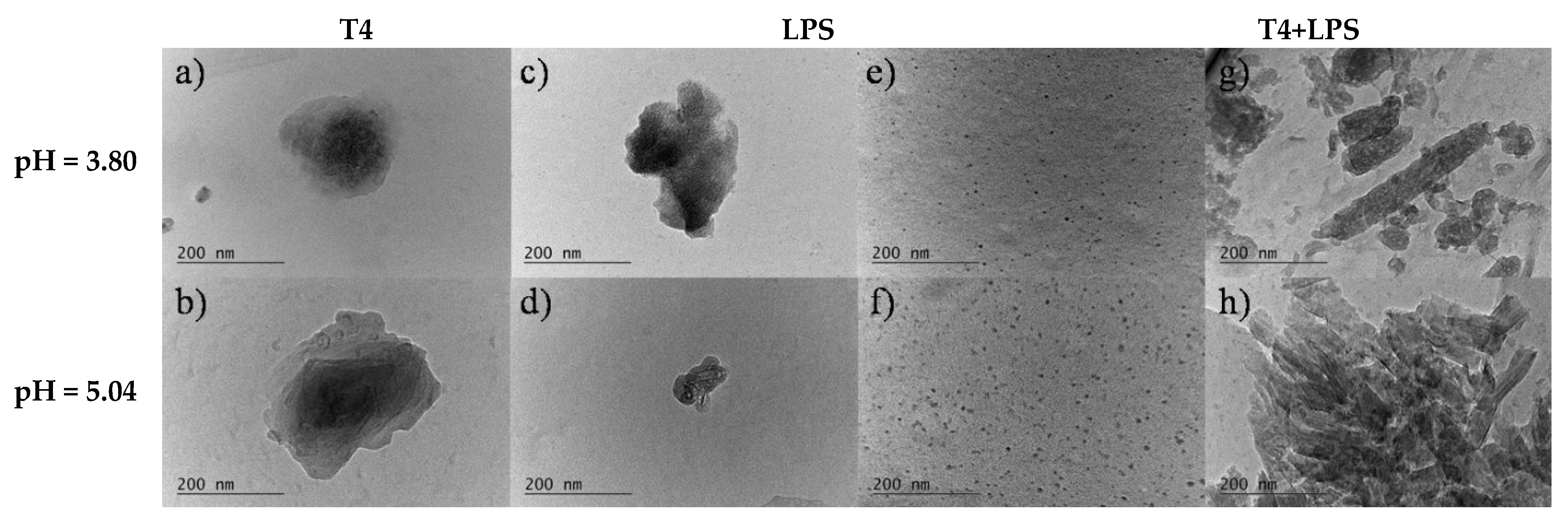
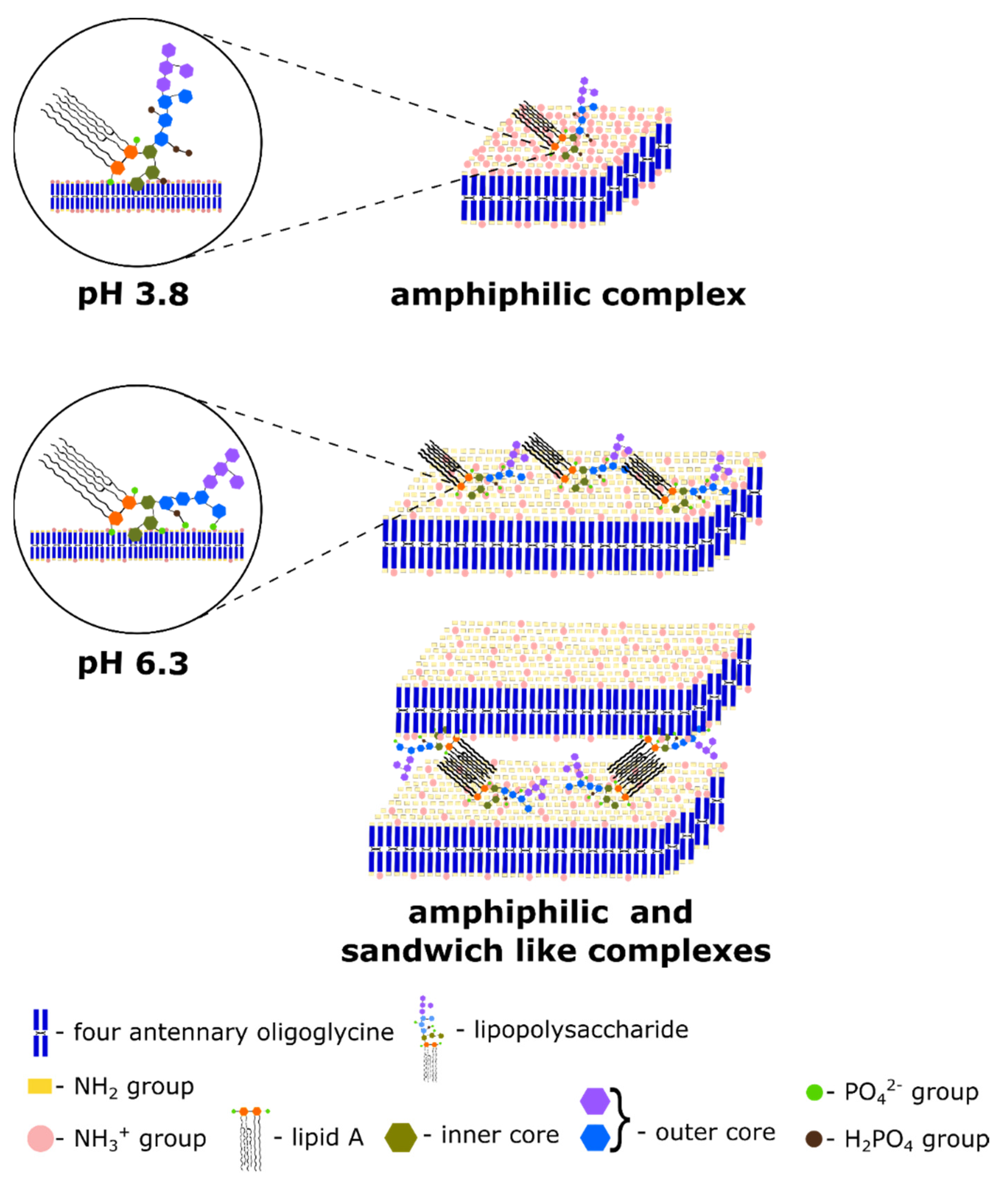
3.1. Effect of pH on the Bulk Self-Assembly Phenomena
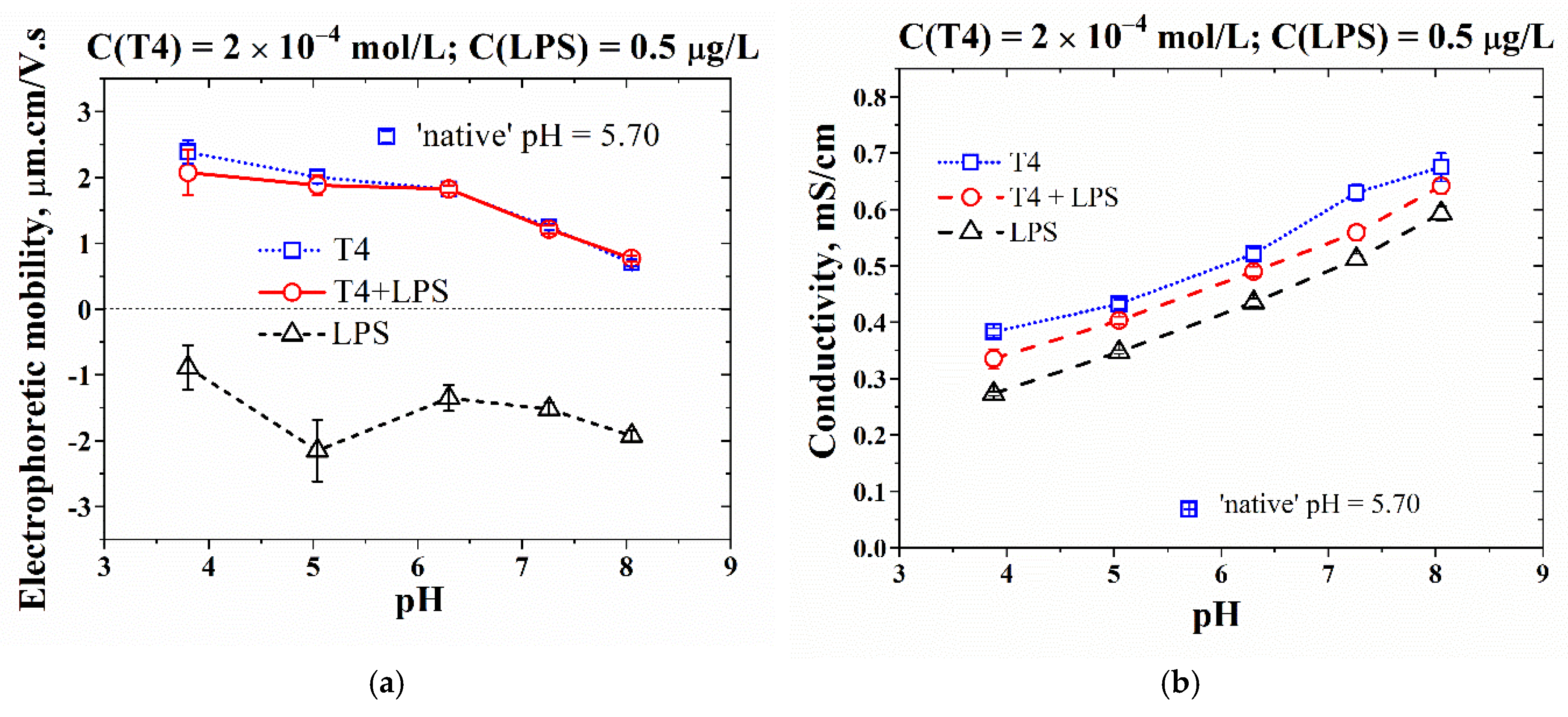
3.2. Effect of pH on the Adsorption-Layer Properties at Air/Solution Interface
3.3. Effect of pH on the Drainage Peculiarities of Microscopic Foam Films
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Erridge, C.; Bennett-Guerrero, E.; Poxton, I.R. Structure and function of lipopolysaccharides. Microbes Infect. 2002, 4, 837–851. [Google Scholar] [CrossRef]
- Clifton, L.A.; Skoda, M.W.A.; Daulton, E.L.; Huges, A.V.; Le Brun, A.P.; Lakey, J.H.; Holt, S.A. Asymmetric phospholipid: Lipopolysaccharide bilayers; a Gram-negative bacterial outer membrane mimic. J. R. Soc. Interface 2013, 10, 20130810. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, W.; Rosselet, A. Function of the outer membrane of Escherichia coli as a permeability barrier to beta-lactam antibiotics. Antimicrob. Agents Chemother. 1977, 12, 368–372. [Google Scholar] [CrossRef] [PubMed]
- Bergstrand, A.; Svanberg, C.; Langton, M. Nyd´en M. Aggregation behavior and size of lipopolysaccharide from Escherichia coli O55:B5. Colloids Surf. B Biointerfaces 2006, 53, 9–14. [Google Scholar] [CrossRef]
- Wang, X.; Quinn, P.J. Endotoxins: Lipopolysaccharides of Gram-Negative bacteria. In Endotoxins: Structure, Function and Recognition, Subcellular Biochemistry; Wang, X., Quinn, P.J., Eds.; Springer: Dordrecht, The Netherlands; Heidelberg, Germany; London, UK; New York, NY, USA, 2010; Volume 53, pp. 3–26. [Google Scholar]
- Magalhães, P.O.; Lopes, A.M.; Mazzola, P.G.; Rangel-Yagui, C.; Penna, T.C.V.; Pessoa, A., Jr. Methods of endotoxin removal from biological preparations. J. Pharm. Pharmaceut. Sci. 2007, 10, 388–404. [Google Scholar]
- Caroff, M.; Novikov, A. LPS Structure, Function, and Heterogeneity. In Endotoxin Detection and Control in Pharma, Limulus, and Mammalian Systems; Williams, K.L., Ed.; Springer Nature: Cham, Switzerland, 2019; pp. 53–93. [Google Scholar]
- Zhang, G.E.; Meredith, T.C.; Kahne, D. On the essentiality of lipopolysaccharide to Gram-negative bacteria. Curr. Opin. Microbiol. 2013, 16, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Comstock, L.E.; Kasper, D.L. Bacterial glycans: Key mediators of diverse host immune responses. Cell 2006, 126, 847–850. [Google Scholar] [CrossRef] [PubMed]
- Opal, S.M. Endotoxins and Other Sepsis Triggers. In Contrib Nephrol. Endotoxemia and Endotoxin Shock: Disease, Diagnosis and Therapy; Ronco, C., Piccinni, P., Rosner, M.H., Eds.; Karger: Basel, Switzerland, 2010; Volume 167, pp. 14–24. [Google Scholar]
- Van der Poll, T.; Opal, S.M. Host–pathogen interactions in sepsis. Lancet Infect. Dis. 2008, 8, 32–43. [Google Scholar] [CrossRef]
- Martin, G.S.; Mannino, D.M.; Eaton, S.; Moss, M. The epidemiology of sepsis in the United States from 1979 through 2000. N. Engl. J. Med. 2003, 348, 1546–1554. [Google Scholar] [CrossRef] [PubMed]
- Mohawk, K.L.; O’Brien, A.D. Mouse models of Escherichia coli O157:H7 infection and shiga toxin injection. J Biomed. Biotechnol. 2011, 2011, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Hamesch, K.; Borkham-Kamphorst, E.; Strnad, P.; Weiskirchen, R. Lipopolysaccharide-induced inflammatory liver injury in mice. Lab. Anim. 2015, 49, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Opal, S.M. The host response to endotoxin, antilipopolysaccharide strategies, and the management of severe sepsis. Int. J. Med. Microbiol. 2007, 297, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Gorbet, M.B.; Sefton, M.V. Endotoxin: The uninvited guest. Biomaterials 2005, 26, 6811–6817. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, S.; Peterbauer, A.; Schindler, S.; Fennrich, S.; Poole, S.; Mistry, Y.; Montag-Lessing, T.; Spreitzer, I.; Loschner, B.; van Aalderen, M.; et al. International validation of novel pyrogen tests based on human monocytoid cells. J. Immunol. Methods 2005, 298, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Zubova, S.V.; Ivanov, A.Y.; Prokhorenko, I.R. The effect of composition of the core region of Escherichia coli K-12 lipopolysaccharides on the surface properties of the cells. Microbiology 2008, 77, 293–297. [Google Scholar] [CrossRef]
- Richter, W.; Vogel, V.; Howe, J.; Steiniger, F.; Brauser, A.; Koch, M.H.J.; Roessle, M.; Gutsmann, T.; Garide, P.; Maentele, W.; et al. Morphology, size distribution and aggregate structure of lipopolysaccharides and lipid A dispersions from enterobacterial origin. Innate Immun. 2011, 17, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Le Brun, A.P.; Clifton, L.A.; Halbert, C.E.; Lin, B.; Meron, M.; Holden, P.J.; Lakey, J.H.; Holt, S.A. Structural characterization of a model Gram-negative bacterial surface using Lipopolysaccharides from rough strains of Escherichia coli. Biomacromolecules 2013, 14, 2014–2022. [Google Scholar] [CrossRef]
- Santos, N.C.; Silva, A.C.; Castanho, M.A.R.B.; Martins-Silva, J.; Saldanha, C. Evaluation of lipopolysaccharide aggregation by light scattering spectroscopy. ChemBioChem. 2003, 4, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Aurell, C.A.; Wistrom, A.O. Critical aggregation concentrations of Gram-negative bacterial lipopolysaccharides (LPS). Biochem. Biophys. Res. Comm. 1998, 253, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Stromberg, L.R.; Mendez, H.M.; Mukundan, H. Detection Methods for Lipopolysaccharides: Past and Present. In Escherichia coli-Recent Advances on Physiology, Pathogenesis and Biotechnological Applications; Samie, A., Ed.; InTechOpen: Rijeka, Croatia, 2017; pp. 141–168. [Google Scholar]
- Sondhi, P.; Maruf, M.H.U.; Stine, K.J. Nanomaterials for biosensing lipopolysaccharide. Biosensors 2020, 10, 2–28. [Google Scholar] [CrossRef]
- Su, W.; Ding, X. Methods of Endotoxin Detection. J. Lab. Autom. 2015, 20, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Tian, F.; Zhang, M.; Zhang, Z.; Bai, M.; Guo, G.; Zheng, W.; Wanq, Q.; Shi, Y.; Wang, L. Endotoxin contamination, a potentially important inflammation factor in water and wastewater: A review. Sci. Total Environ. 2019, 681, 365–378. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Boraschi, D. Endotoxin contamination: A key element in the interpretation of nanosafety studies. Nanomedicine 2016, 11, 269–287. [Google Scholar] [CrossRef]
- Schneier, M.; Razdan, S.; Miller, A.M.; Briceno, M.E.; Barua, S. Current technologies to endotoxin detection and removal for biopharmaceutical purification. Biotechnol. Bioeng. 2020, 117, 1–22. [Google Scholar] [CrossRef]
- Gyurova, A.Y.; Stoyanov, S.V.; Mileva, E. Interaction of four-antennary oligoglycines and lipopolysaccharides in aqueous media. Colloids Surf. A 2014, 460, 130–136. [Google Scholar]
- Gyurova, A.Y.; Stoyanov, S.V.; Mileva, E. Capture of LPS traces in aqueous solutions by tectomers of four-antennary oligoglycines. Colloids Surf. A 2017, 520, 914–921. [Google Scholar]
- Bovin, N.V.; Tuzikov, A.B.; Chinarev, A.A. Oligoglycines: Materials with unlimited potential for nanotechnologies. Nanotechnologies Russ. 2008, 3, 291–302. [Google Scholar] [CrossRef]
- Tuzikov, A.B.; Chinarev, A.A.; Gambaryan, A.S.; Oleinikov, V.A.; Klinov, D.V.; Matsko, N.V.; Kadykov, V.A.; Ermishov, M.A.; Demin, I.V.; Demin, V.V.; et al. Polyglycine II nanosheets: Supramolecular antivirals. ChemBioChem 2003, 4, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Tsygankova, S.V.; Chinarev, A.A.; Tuzikov, A.B.; Zaitsev, I.S.; Severin, N.; Kalachev, A.A.; Rabe, J.P.; Bovin, N.V. Assembly of oligoglycine layers on mica surface. J. Biomater. Nanotechnol. 2011, 2, 90–96. [Google Scholar] [CrossRef]
- Gyurova, A.Y.; Michna, A.; Nikolov, L.; Mileva, E. Self-assembly of four- and two-antennary oligoglycines in aqueous medium. Colloids and Surfaces A 2017, 519, 106–116. [Google Scholar] [CrossRef]
- Bamford, C.H.; Brown, L.; Cant, E.M.; Ellliott, A.; Hanby, W.E.; Malcolm, B.R. Structure of polyglycine. Nature 1955, 176, 396–397. [Google Scholar] [CrossRef]
- Crick, F.H.C.; Rich, A. Structure of Polydlycine II. Nature 1955, 176, 780–781. [Google Scholar] [CrossRef]
- Ramachandran, G.N.; Sasisekharan, V.; Ramakrishnan, C. Molecular structure of polyglycine II. Biochim. Biophys. Acta 1966, 112, 168–170. [Google Scholar] [CrossRef][Green Version]
- Lehn, J.-M. Toward complex matter: Supramolecular chemistry and self-organization. PNAS 2002, 99, 4763–4768. [Google Scholar] [CrossRef] [PubMed]
- Britton, H.T.K.; Robinson, R.A. Universal buger solutions and the dissociation constant of veronal. J. Chem. Soc. 1931, 75, 1456–1462. [Google Scholar] [CrossRef]
- Mie, G. Beiträge zur Optik trüber Medien, speziell kolloidaler Metallösungen. Annalen der Physik 1908, 330, 377–445. [Google Scholar] [CrossRef]
- Øgendal, L. Light Scattering Demystified: Theory and Practice; University of Copenhagen: Copenhagen, Denmark, 2013. [Google Scholar]
- Loglio, G.; Pandolfini, P.; Miller, R.; Makievski, A.; Ravera, F.; Ferrari, M.; Liggieri, L. Drop and Bubble Shape Analysis as a Tool for Dilational Rheological Studies of Interfacial Layers. In Novel Methods to Study of Interfacial Layers; Moebius, D., Miller, R., Eds.; Elsevier Science, B.V.: Amsterdam, The Netherlands, 2001; Chapter 25; pp. 439–483. [Google Scholar]
- Liggieri, L.; Miller, R. Relaxation of surfactants adsorption layers at liquid interfaces. Curr. Opin. Coll. Interf. Sci. 2010, 15, 256–263. [Google Scholar] [CrossRef]
- Liggieri, L.; Mileva, E.; Miller, R. The Surface Layer as the Basis for Foam Formation and Stability. In Foam Films and Foams; Exerowa, D., Gochev, G., Platikanov, D., Liggieri, L., Miller, R., Eds.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2019; Chapter 1; pp. 3–55. [Google Scholar]
- Exerowa, D.; Kruglyakov, P.M. Experimental methods involved in the study of foam films. In Foams and Foam Films; Elsevier: Amsterdam, The Netherlands, 1998; Chapter 2; pp. 42–87. [Google Scholar]
- Platikanov, D.; Exerowa, D. Fundamentals of Foam Films. In Foam Films and Foams; Exerowa, D., Gochev, G., Platikanov, D., Liggieri, L., Miller, R., Eds.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2019; Chapter 3; pp. 77–98. [Google Scholar]
- Langevin, D.; Marques-Beltran, C.; Delacotte, J. Surface force measurements on freely suspended liquid films. Adv. Colloid Interface Sci. 2011, 168, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, G.; Langevin, D. Interfacial shear rheology of mixed polyelectrolyte-surfactant layers. Langmuir 2009, 25, 12201–12207. [Google Scholar] [CrossRef]
- Mileva, E.; Tchoukov, P. Surfactant Nanostructures in Foam Films. In Colloid Stability. The Role of Surface Forces, Part I; Tadros, T., Ed.; Wiley-VCH Verlag, GmbH&Co. KGaA: Weinheim, Germany, 2007; Chapter 8; pp. 187–206. [Google Scholar]
- Mileva, E.; Exerowa, D. Amphiphilic nanostructures in foam films. Curr. Opin. Coll. Interf. Sci. 2008, 13, 120–127. [Google Scholar] [CrossRef]
- Vargaftik, N.B.; Volkov, B.N.; Voljak, L.D. International tables of the surface tension of water. J. Phys. Chem. Ref. Data 1983, 12, 817–820. [Google Scholar] [CrossRef]
- Ongkudon, C.M.; Chew, J.H.; Liu, B.; Danquah, M.K. Chromatographic removal of endotoxins: A bioprocess engineer’s perspective. ISRN Chromatogr. 2012, 2012, 1–9. [Google Scholar] [CrossRef][Green Version]
- Serdakowski London, A.; Kerins, B.; Tschantz, W.R.; Mackay, K. Endotoxin removal and prevention for preclinical biologics production. Biotechnol. J. 2012, 7, 1509–1516. [Google Scholar] [CrossRef] [PubMed]
- Razdan, S.; Wang, J.C.; Barua, S. PolyBall: A new adsorbent for the efficient removal of endotoxin from biopharmaceuticals. Sci. Rep. 2019, 9, 8867. [Google Scholar] [CrossRef] [PubMed]

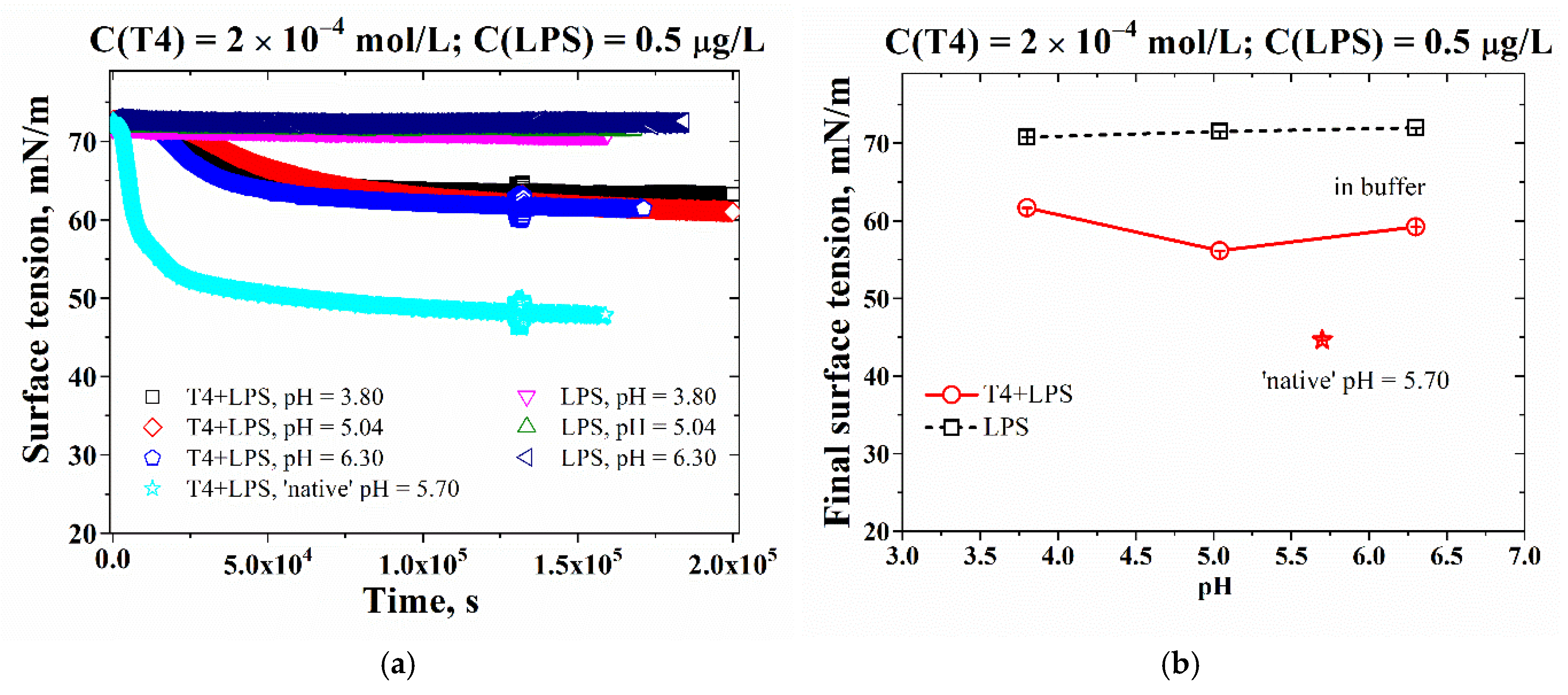
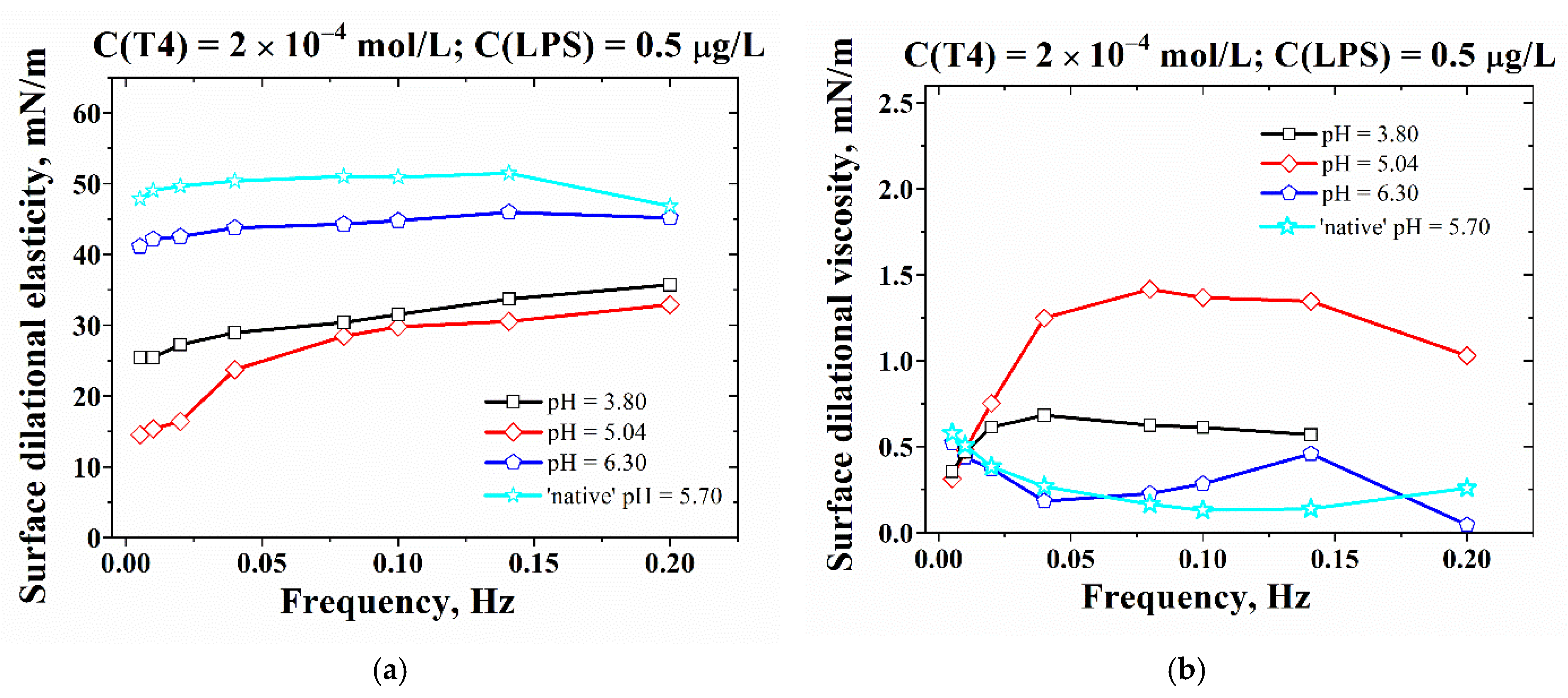
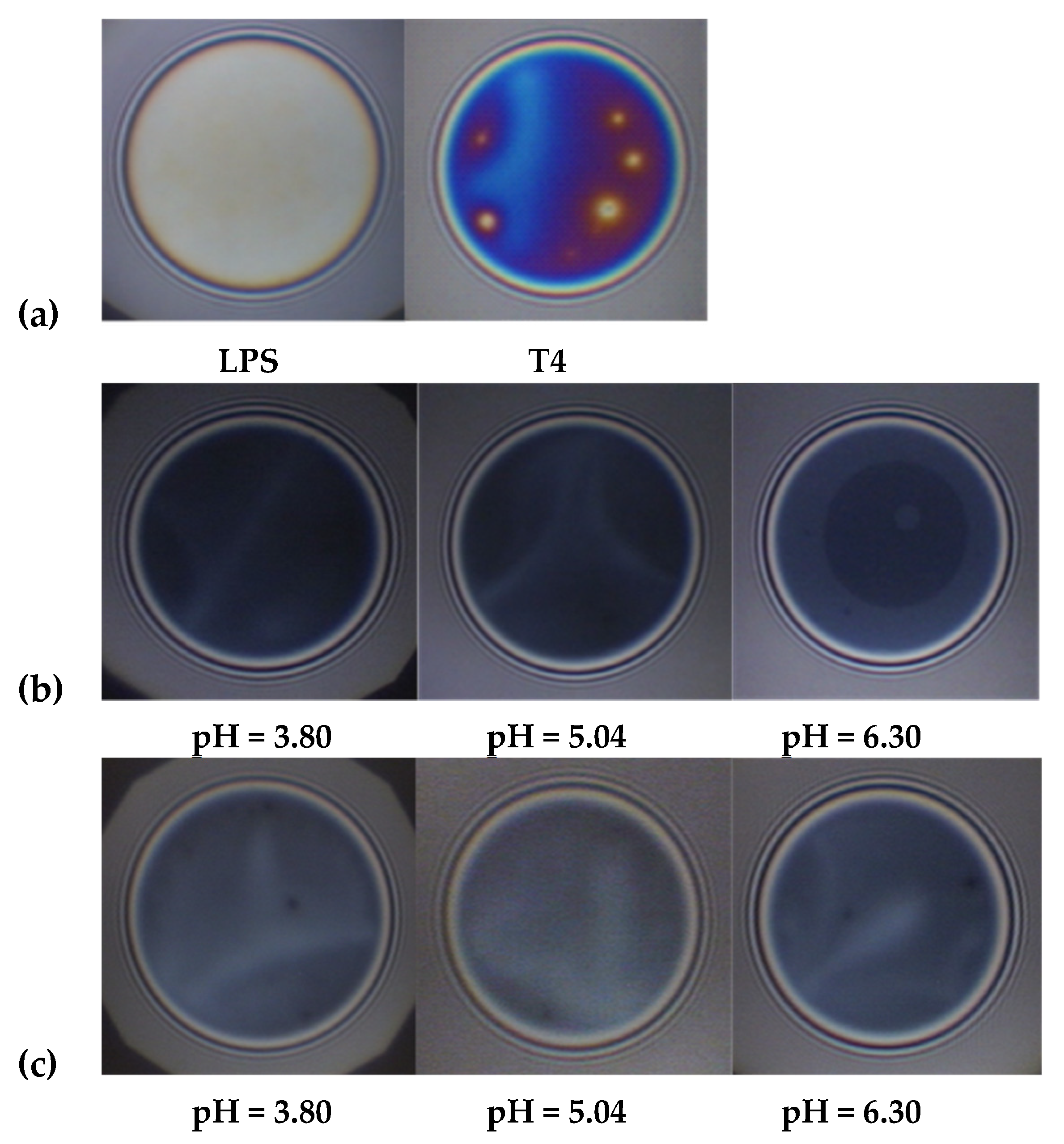
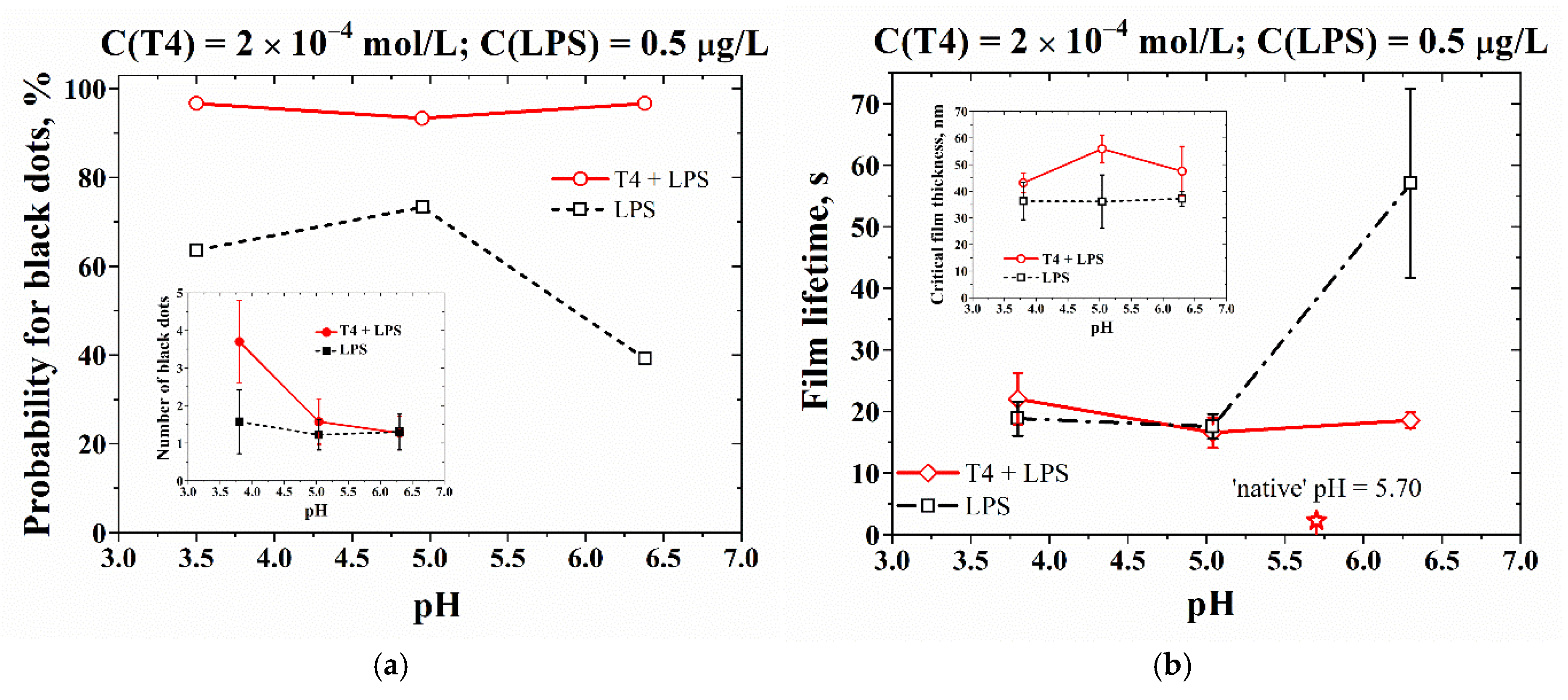
| pH | PdI (T4) | PdI (LPS) | PdI (T4+LPS) |
|---|---|---|---|
| 3.80 | 0.447 ± 0.062 | 0.577 ± 0.157 | 0.446 ± 0.091 |
| 5.04 | 0.516 ± 0.075 | 0.472 ± 0.123 | 0.539 ± 0.093 |
| 6.30 | 0.427 ± 0.020 | 0.379 ± 0.084 | 0.429 ± 0.033 |
| 7.26 | 0.394 ± 0.063 | 0.475 ± 0.151 | 0.208 ± 0.122 |
| 8.05 | 0.373 ± 0.118 | 0.431 ± 0.098 | 0.371 ± 0.128 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gyurova, A.Y.; Berberov, K.; Chinarev, A.; Nikolov, L.; Karashanova, D.; Mileva, E. Effect of pH-Regulation on the Capture of Lipopolysaccharides from E. coli EH100 by Four-Antennary Oligoglycines in Aqueous Medium. Materials 2021, 14, 7659. https://doi.org/10.3390/ma14247659
Gyurova AY, Berberov K, Chinarev A, Nikolov L, Karashanova D, Mileva E. Effect of pH-Regulation on the Capture of Lipopolysaccharides from E. coli EH100 by Four-Antennary Oligoglycines in Aqueous Medium. Materials. 2021; 14(24):7659. https://doi.org/10.3390/ma14247659
Chicago/Turabian StyleGyurova, Anna Y., Kaloyan Berberov, Alexander Chinarev, Ljubomir Nikolov, Daniela Karashanova, and Elena Mileva. 2021. "Effect of pH-Regulation on the Capture of Lipopolysaccharides from E. coli EH100 by Four-Antennary Oligoglycines in Aqueous Medium" Materials 14, no. 24: 7659. https://doi.org/10.3390/ma14247659
APA StyleGyurova, A. Y., Berberov, K., Chinarev, A., Nikolov, L., Karashanova, D., & Mileva, E. (2021). Effect of pH-Regulation on the Capture of Lipopolysaccharides from E. coli EH100 by Four-Antennary Oligoglycines in Aqueous Medium. Materials, 14(24), 7659. https://doi.org/10.3390/ma14247659







