Unconventional Stoichiometries of Na–O Compounds at High Pressures
Abstract
:1. Introduction
2. Computational Methods and Details
3. Results and Discussion
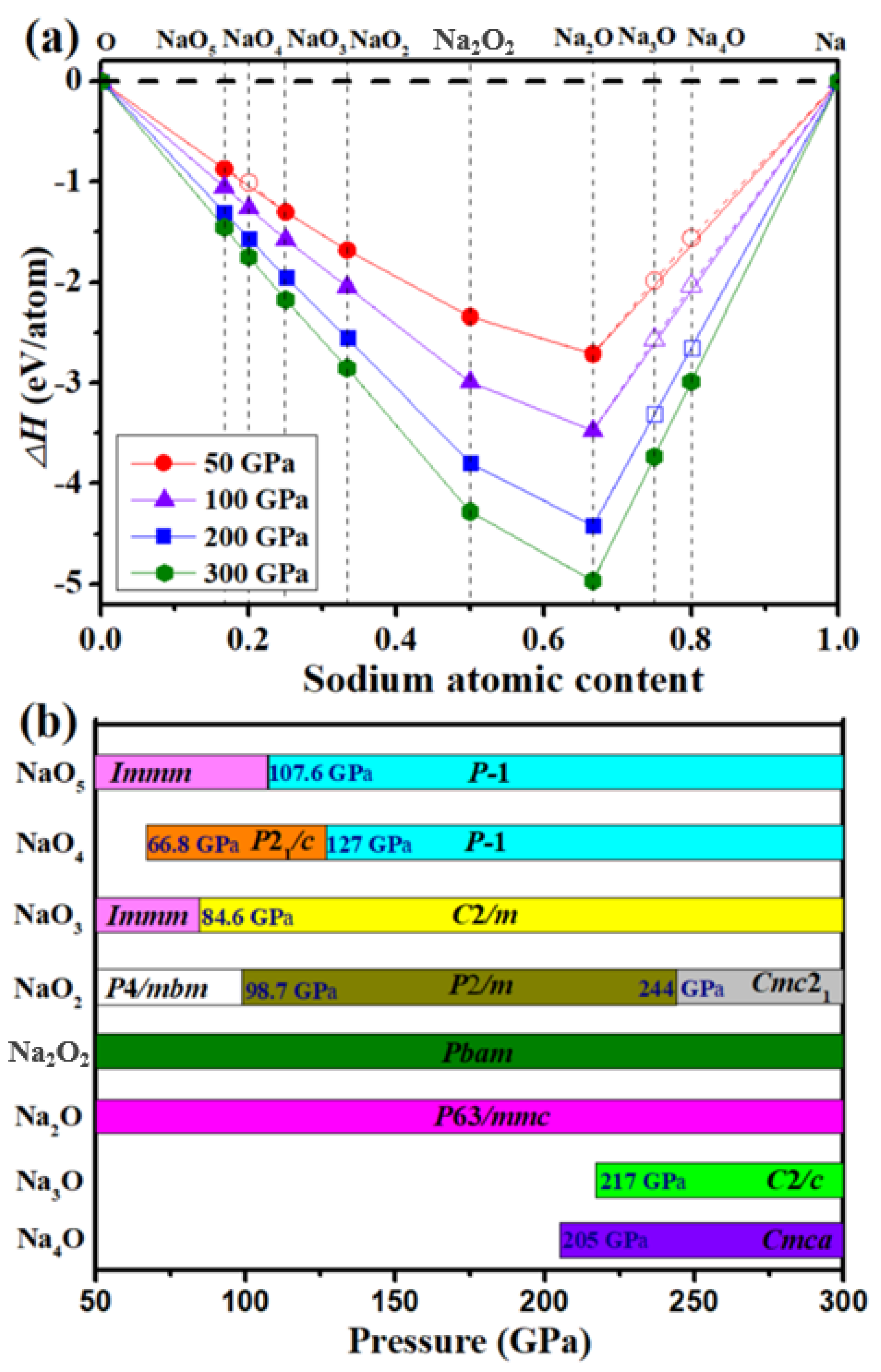
3.1. Stable Na–O Compounds at High Pressure
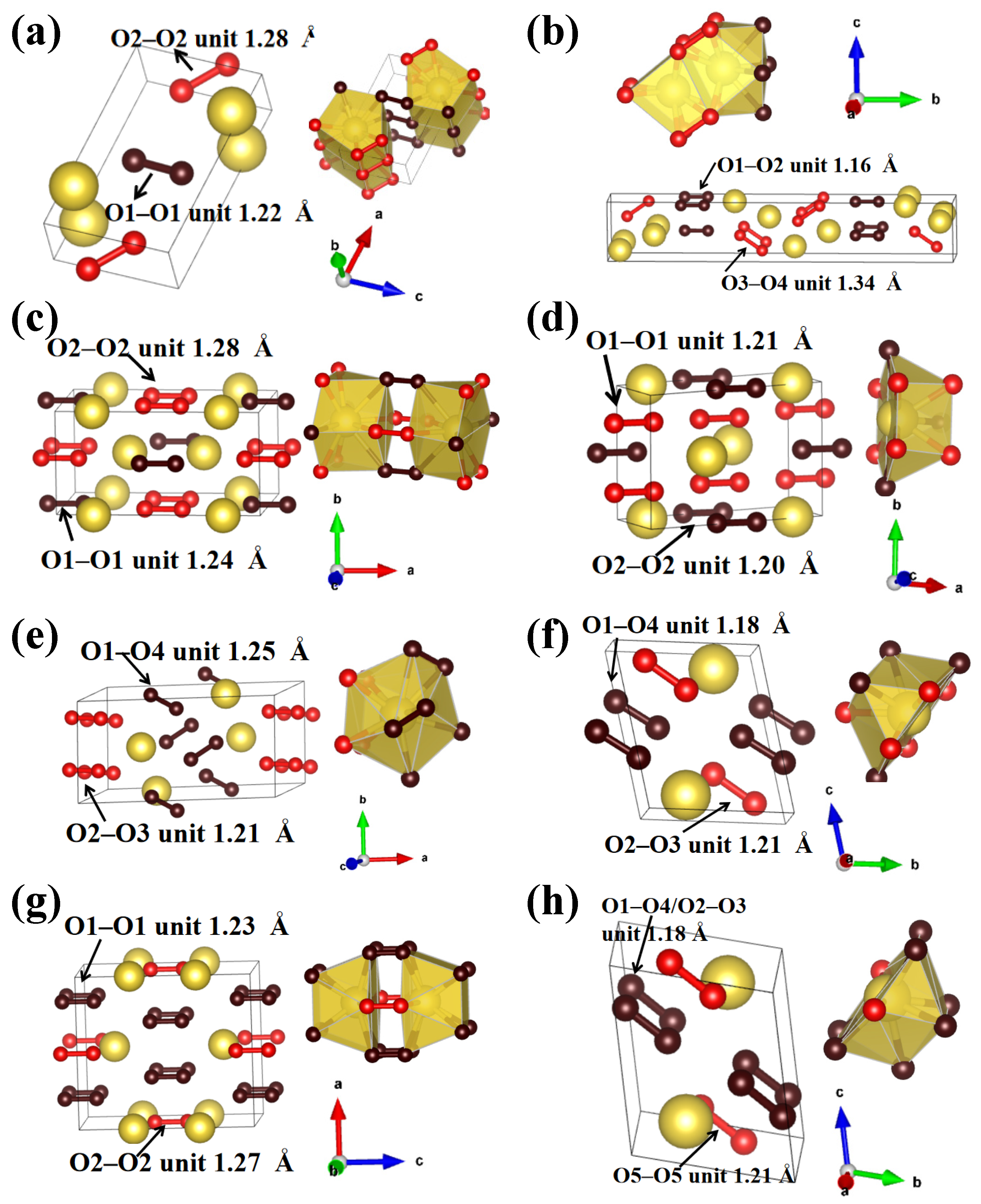
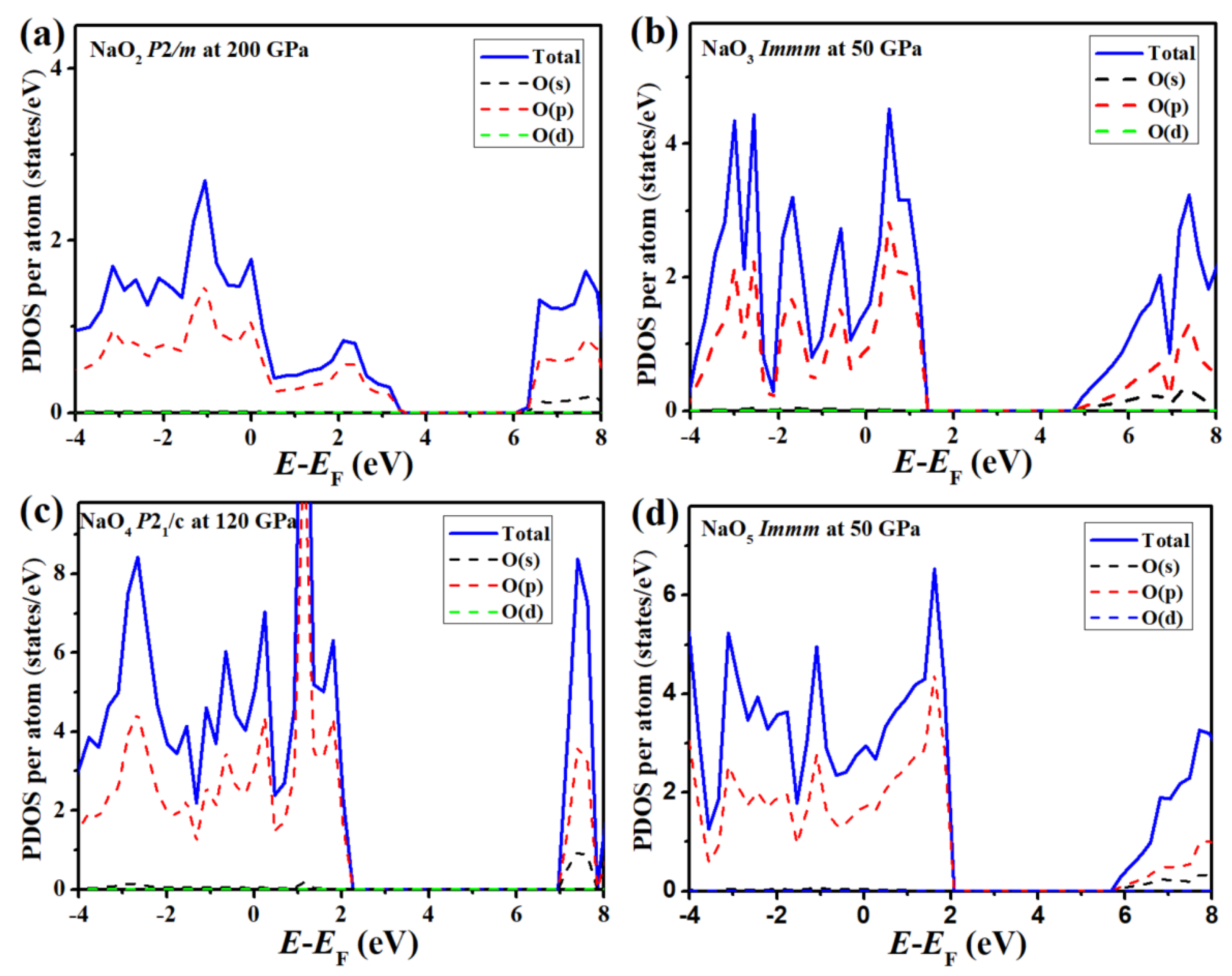
3.2. O-Rich Compounds
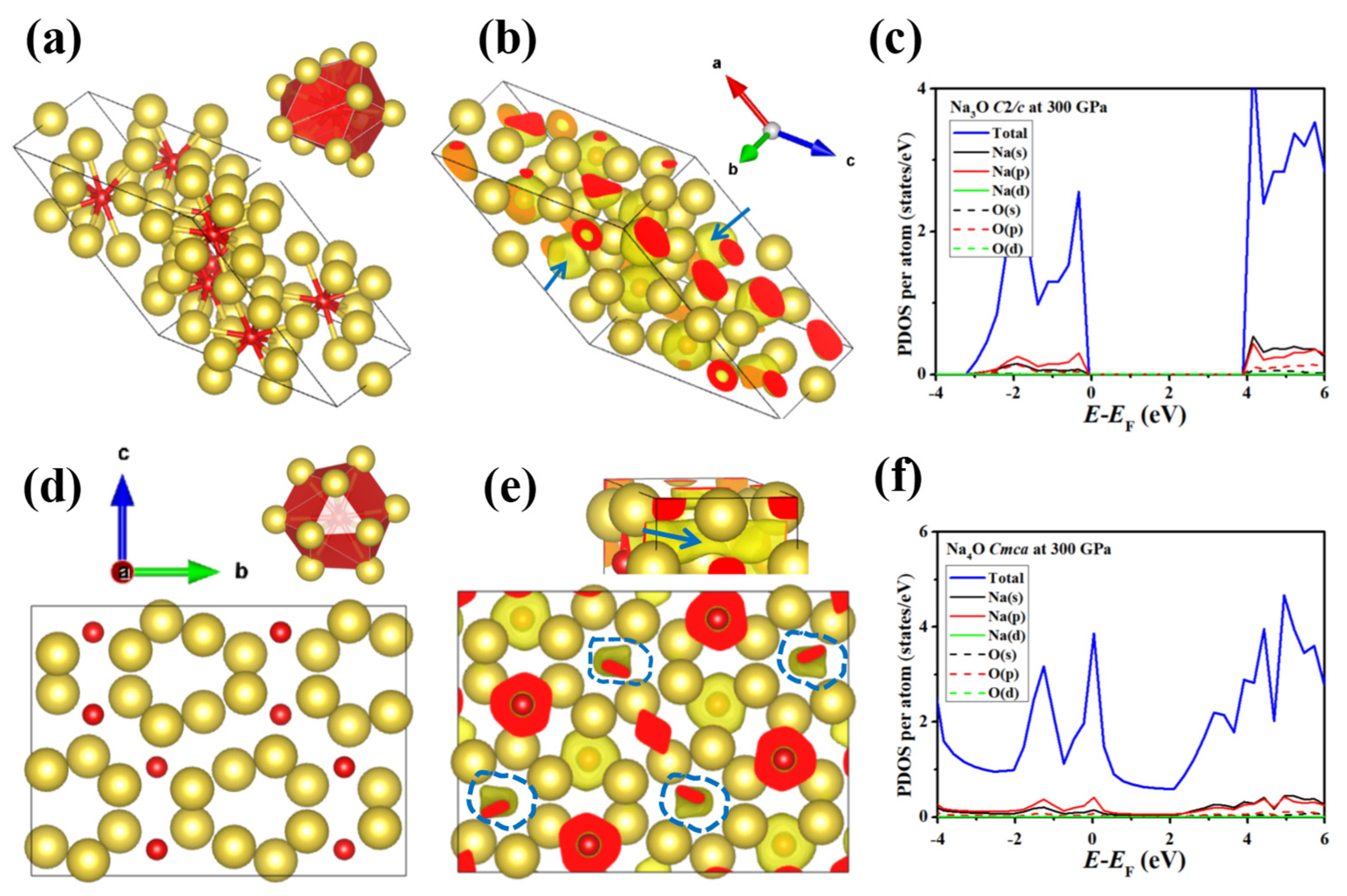
3.3. Na-Rich Compounds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hubbard, W.B. Planetary Interiors; Van Nostrand Reinhold: New York, NY, USA, 1984. [Google Scholar]
- Vol’nov, I.I. Peroxides, Superoxides, and Ozonides of Alkali and Alkaline Earth Metals; Springer: Boston, MA, USA, 1966. [Google Scholar]
- Song, K.; Agyeman, D.A.; Park, M.; Yang, J.; Kang, Y.M. High-Energy-Density Metal–Oxygen Batteries: Lithium–Oxygen Batteries vs Sodium–Oxygen Batteries. Adv. Mater. 2017, 29, 1606572. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, P.; Grübl, D.; Sommer, H.; Janek, J.; Bessler, W.G.; Adelhelm, P. Pressure Dynamics in Metal–Oxygen (Metal–Air) Batteries: A Case Study on Sodium Superoxide Cells. J. Phys. Chem. C 2014, 118, 1461–1471. [Google Scholar] [CrossRef]
- Deng, N.; Yang, G.; Wang, W.; Qiu, Y. Structural transitions and electronic properties of sodium superoxide at high pressures. RSC Adv. 2016, 6, 67910. [Google Scholar] [CrossRef]
- Yang, S.; Siegel, D.J. Intrinsic Conductivity in Sodium–Air Battery Discharge Phases: Sodium Superoxide vs Sodium Peroxide. Chem. Mater. 2015, 27, 3852–3860. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, C.; Wu, X. Research Progresses on Interfaces in Solid-State Sodium Batteries: A Topic Review. Adv. Mater. Interfaces 2020, 7, 2001444. [Google Scholar] [CrossRef]
- Jimlim, P.; Tsuppayakorn-aek, P. Theoretical predictions for low–temperature phases, softening of phonons and elastic stiffnesses, and electronic properties of sodium peroxide under high pressure. RSC Adv. 2019, 9, 30964–30975. [Google Scholar] [CrossRef] [Green Version]
- Deng, N.; Wang, W.; Yang, G.; Qiu, Y. Structural and electronic properties of alkali metal peroxides at high pressures. RSC Adv. 2015, 5, 104337–104342. [Google Scholar] [CrossRef]
- Čančarević, Ž.; Schön, J.C.; Jansen, M. Stability of alkali–metal oxides as a function of pressure: Theoretical calculations. Phys. Rev. B. 2006, 73, 224114. [Google Scholar] [CrossRef]
- Lein, W.K.; Armbruster, K.; Jansen, M. Synthesis and crystal structure determination of sodium ozonide. Chem. Commun. 1998, 6, 707–708. [Google Scholar]
- Carter, G.F.; Templeton, D.H. Polymorphism of Sodium Superoxide. J. Am. Chem. Soc. 1953, 75, 5247–5249. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Lv, J.; Ma, Y. Materials discovery at high pressures. Nat. Rev. Mater. 2017, 2, 17005. [Google Scholar] [CrossRef]
- Li, Y.; Hao, J.; Liu, H.; Li, Y.; Ma, Y. The metallization and superconductivity of dense hydrogen sulfifide. J. Chem. Phys. 2014, 140, 174712. [Google Scholar] [CrossRef] [Green Version]
- Miao, M.-S.; Hoffmann, R. High–pressure electrides: The chemical nature of interstitial quasiatoms. J. Am. Chem. Soc. 2015, 137, 3631–3637. [Google Scholar] [CrossRef] [PubMed]
- Pepin, C.M.; Geneste, G.; Dewaele, A.; Mezouar, M.; Loubeyre, P. Synthesis of FeH5: A layered structure with atomic hydrogen slabs. Science 2017, 357, 382. [Google Scholar] [CrossRef] [Green Version]
- Yang, G.; Wang, Y.; Peng, F.; Bergara, A.; Ma, Y. Gold as a 6p–element in dense lithium aurides. J. Am. Chem. Soc. 2016, 138, 4046–4052. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, S.; Yu, T.; Xu, H.; Bergara, A.; Yang, G. Predicted pressure–induced superconducting transition in electride Li6P. Phys. Rev. Lett. 2019, 122, 097002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, D.; Liu, Y.; Tian, F.; Li, D.; Huang, X.; Zhao, Z.; Yu, H.; Liu, B.; Tian, W.; Cui, T. Pressure–induced metallization of dense (H2S)2 H2 with high–Tc superconductivity. Sci. Rep. 2014, 4, 6968. [Google Scholar] [CrossRef] [Green Version]
- Gao, G.; Wang, L.; Li, M.; Zhang, J.; Howie, R.T.; Gregoryanz, E.; Struzhkin, V.V.; Wang, L.; Tse, J.S. Superconducting binary hydrides: Theoretical predictions and experimental progresses. Mater. Today Phys. 2021, 21, 100546. [Google Scholar] [CrossRef]
- Einaga, M.; Sakata, M.; Ishikawa, T.; Shimizu, K.; Eremets, M.I.; Drozdov, A.P.; Troyan, I.A.; Hirao, N.; Ohishi, Y. Crystal structure of the superconducting phase of sulfur hydride. Nat. Phys. 2016, 12, 835–838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Oganov, A.R.; Goncharov, A.F.; Zhu, Q.; Boulfelfel, S.E.; Lyakhov, A.O.; Stavrou, E.; Somayazulu, M.; Prakapenka, V.B.; Konôpková, Z. Unexpected stable stoichiometries of sodium chlorides. Science 2013, 342, 1502. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.; Liu, H.; Pickard, C.J.; Zou, G.; Ma, Y. Reactions of xenon with iron and nickel are predicted in the Earth’s inner core. Nat. Chem. 2014, 6, 644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dewaele, A.; Worth, N.; Pickard, C.J.; Needs, N.W.R.J.; Pascarelli, S.; Mathon, O.; Mezouar, S.P.O.M.M.; Irifune, T. Synthesis and stability of xenon oxides Xe2O5 and Xe3O2 under pressure. Nat. Chem. 2016, 8, 784. [Google Scholar] [CrossRef] [Green Version]
- Peng, F.; Sun, Y.; Pickard, C.J.; Needs, R.J.; Wu, Q.; Ma, Y. Hydrogen clathrate structures in rare earth hydrides at high pressures: Possible route to room–temperature superconductivity. Phys. Rev. Lett. 2017, 119, 1. [Google Scholar] [CrossRef]
- Liu, H.; Naumov, I.I.; Hoffmann, R.; Ashcroft, N.W.; Hemley, R.J. Potential high-Tc superconducting lanthanum and yttrium hydrides at high pressures. Proc. Natl. Acad. Sci. USA 2017, 114, 6990. [Google Scholar] [CrossRef] [Green Version]
- Somayazulu, M.; Ahart, M.; Mishra, A.K.; Geballe, Z.M.; Baldini, M.; Meng, Y.; Struzhkin, V.V.; Hemley, R.J. Evidence for superconductivity above 260 K in Lanthanum superhydride at megabar pressures. Phys. Rev. Lett. 2019, 122, 27001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drozdov, A.P.; Eremets, M.I.; Troyan, I.A.; Ksenofontov, V.; Shylin, S.I. Conventional superconductivity at 203 kelvin at high pressures in the sulfur hydride system. Nature 2015, 525, 73–76. [Google Scholar] [CrossRef]
- Ma, Y.; Eremets, M.; Oganov, A.R.; Xie, Y.; Trojan, I.; Medvedev, S.; Lyakhov, A.O.; Valle, M.; Prakapenka, V. Transparent dense sodium. Nature 2009, 458, 182–185. [Google Scholar] [CrossRef]
- Dong, X.; Oganov, A.R.; Goncharov, A.F.; Stavrou, E.; Lobanov, S.; Saleh, G.; Qian, G.-R.; Zhu, Q.; Gatti, C.; Deringer, V.L.; et al. A stable compound of helium and sodium at high pressure. Nat. Chem. 2017, 9, 440–445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Xu, M.; Yang, L.; Yan, B.; Qin, Q.; Shao, X.; Zhang, Y.; Huang, D.; Lin, X.; Lv, J.; et al. Pressure–stabilized divalent ozonide CaO3 and its impact on Earth’s oxygen cycles. Nat. Commun. 2020, 11, 4702. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Kim, D.Y.; Yang, W.; Yang, L.; Meng, Y.; Zhang, L.; Mao, H. FeO2 and FeOOH under deep lower–mantle conditions and Earth’s oxygen–hydrogen cycles. Nature 2016, 534, 241. [Google Scholar] [CrossRef]
- Bykova, E.; Dubrovinsky, L.; Dubrovinskaia, N.; Bykov, E.B.N.D.M.; McCammon, C.; Ovsyannikov, S.V.; Liermann, H.-P.; Kupenko, I.; Chumakov, A.I.; Rüffer, R.; et al. Structural complexity of simple Fe2O3 at high pressures and temperatures. Nat. Commun. 2016, 7, 10661. [Google Scholar] [CrossRef] [PubMed]
- Lavina, B.; Meng, Y. Unraveling the complexity of iron oxides at high pressure and temperature: Synthesis of Fe5O6. Sci. Adv. 2015, 1, e1400260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Lv, J.; Ma, Y.; Cui, T.; Zou, G. Superconductivity of MgB2 under Ultra high Pressure: A First–Principles Study. Phys. Rev. B 2009, 80, 092505. [Google Scholar] [CrossRef]
- Wang, Y.; Lv, J.; Zhu, L.; Ma, Y. CALYPSO: A Method for Crystal Structure Prediction. Comput. Phys. Commun. 2012, 183, 2063–2070. [Google Scholar] [CrossRef] [Green Version]
- Lv, J.; Wang, Y.; Zhu, L.; Ma, Y. Particle–swarm structure prediction on clusters. J. Chem. Phys. 2012, 137, 084104. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, H.; Wang, Y.; Lv, J.; Ma, Y.; Cui, Q.; Ma, Y.; Zou, G. Substitutional Alloy of Bi and Te at High Pressure. Phys. Rev. Lett. 2011, 106, 18–21. [Google Scholar] [CrossRef]
- Lv, J.; Wang, Y.; Zhu, L.; Ma, Y. Predicted Novel High–Pressure Phases of Lithium. Phys. Rev. Lett. 2011, 106, 015503. [Google Scholar] [CrossRef]
- Li, Y.; Wang, L.; Liu, H.; Zhang, Y.; Hao, J.; Pickard, C.J.; Nelson, J.R.; Needs, R.J.; Li, W.; Huang, Y.; et al. Dissociation Products and Structures of Solid H2S at Strong Compression. Phys. Rev. B 2016, 93, 020103. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Lv, J.; Li, H.; Feng, X.; Lu, C.; Redfern, S.A.T.; Liu, H.; Chen, C.; Ma, Y. Rare Helium–Bearing Compound FeO2He Stabilized at Deep–Earth Conditions. Phys. Rev. Lett. 2018, 121, 255703. [Google Scholar] [CrossRef] [Green Version]
- Kresse, G.; Furthmuller, J. Efficient Iterative Schemes for Ab Initio Total–Energy Calculations Using a Plane–Wave Basis Set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [Green Version]
- Blochl, P.E. Projector Augmented–Wave Method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [Green Version]
- Togo, A.; Oba, F.; Tanaka, I. First–Principles Calculations of the Ferroelastic Transition between Rutile–Type and CaCl2–Type SiO2. Phys. Rev. B 2008, 78, 134106. [Google Scholar] [CrossRef] [Green Version]
- Momma, K.; Izumi, F. VESTA 3 for Three–Dimensional Visualization of Crystal. Volumetric and Morphology Data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Bader, R.F.W. Atoms in Molecules. Acc. Chem. Res. 1985, 18, 9–15. [Google Scholar] [CrossRef]
- Ma, Y.; Oganov, A.R.; Xie, Y. High–Pressure Structures of Lithium, Potassium, Rubidium Predicted by an Ab Initio Evolutionary Algorithm. Phys. Rev. B 2008, 78, 014102. [Google Scholar] [CrossRef] [Green Version]
- Gregoryanz, E.; Lundegaard, L.F.; McMahon, M.I.; Guillaume, C.; Nelmes, R.J.; Mezouar, M. Structural Diversity of Sodium. Science 2008, 320, 1054–1057. [Google Scholar] [CrossRef]
- Hanfland, M.; Loa, I.; Syassen, K. Sodium under Pressure: Bcc to Fcc Structural Transition and Pressure–Volume Relation to 100 GPa. Phys. Rev. B 2002, 65, 184109. [Google Scholar] [CrossRef]
- Ackland, G.J.; Macleod, I.R. Origin of the Complex Crystal Structures of Elements at Intermediate Pressure. New J. Phys. 2004, 6, 138. [Google Scholar] [CrossRef]
- Ma, Y.; Oganov, A.R.; Glass, C.W. Structure of the metallic ζ–phase of oxygen and isosymmetric nature of the ε−ζ phase transition: Ab initio simulation. Phys. Rev. B 2007, 76, 64101. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Kim, D.Y.; Yang, L.; Li, N.; Tang, L.; Amine, K.; Mao, H. Oxygen–Rich Lithium Oxide Phases Formed at High Pressure for Potential Lithium–Air Battery Electrode. Adv. Sci. 2017, 4, 1600453. [Google Scholar] [CrossRef] [Green Version]
- Dong, X.; Hou, J.; Kong, J.; Cui, H.; Li, Y.; Oganov, A.R.; Li, K.; Zheng, H.; Zhou, X.; Wang, H.-T. Predicted lithium oxide compounds and superconducting low–pressure LiO4. Phys. Rev. B 2019, 100, 144104. [Google Scholar] [CrossRef] [Green Version]
- Yang, Q.; Lin, J.; Li, F.; Zhang, J.; Zurek, E.; Yang, G. Pressure–induced yttrium oxides with unconventional stoichiometries and novel properties. Phys. Rev. Mater. 2021, 5, 044802. [Google Scholar] [CrossRef]
- Zhang, J.; Oganov, A.R.; Li, X.; Esfahani, M.M.D.; Dong, H. First–principles investigation of Zr–O compounds, their crystal structures, and mechanical properties. J. Appl. Phys. 2017, 121, 155104. [Google Scholar] [CrossRef] [Green Version]
- Bouibes, A.; Zaoui, A. Investigating new polyphorms of Zn–O from variable composition. Solid State. Commun. 2015, 220, 36–38. [Google Scholar] [CrossRef]
- Maple, M.B.; Wittig, J.; Kim, K.S. Pressure-Induced Magnetic-Nonmagnetic Transition of Ce Impurities in La. Phys. Rev. Lett. 1969, 23, 1375. [Google Scholar] [CrossRef]
- Hosono, H.; Mishima, Y.; Takezoe, H.; MacKenzie, K.J.D. Nanomaterials: Research Towards Applications; Elsevier Science: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Tsuji, Y.; Dasari, P.L.V.K.; Elatresh, S.F.; Hoffmann, R.; Ashcroft, N.W. Structural Diversity and Electron Confinement in Li4N; the Potential for 0-D, 2-D and 3-D Electrides. J. Am. Chem. Soc. 2016, 138, 14108. [Google Scholar] [CrossRef]
- Miao, M.-S.; Hoffmann, R. High Pressure Electrides: A Predictive Chemical and Physical Theory. Acc. Chem. Res. 2014, 47, 1311–1317. [Google Scholar] [CrossRef]
- Zhu, Q.; Oganov, A.R.; Lyakhov, A.O. Novel stable compounds in the Mg–O system under high pressure. Phys. Chem. Chem. Phys. 2013, 15, 7696. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.; Zhang, Y.; Chen, Y.; Zhong, X.; Wang, D.; Lang, J.; Qu, X.; Yang, J. Unconventional Stoichiometries of Na–O Compounds at High Pressures. Materials 2021, 14, 7650. https://doi.org/10.3390/ma14247650
Yang L, Zhang Y, Chen Y, Zhong X, Wang D, Lang J, Qu X, Yang J. Unconventional Stoichiometries of Na–O Compounds at High Pressures. Materials. 2021; 14(24):7650. https://doi.org/10.3390/ma14247650
Chicago/Turabian StyleYang, Lihua, Yukai Zhang, Yanli Chen, Xin Zhong, Dandan Wang, Jihui Lang, Xin Qu, and Jinghai Yang. 2021. "Unconventional Stoichiometries of Na–O Compounds at High Pressures" Materials 14, no. 24: 7650. https://doi.org/10.3390/ma14247650
APA StyleYang, L., Zhang, Y., Chen, Y., Zhong, X., Wang, D., Lang, J., Qu, X., & Yang, J. (2021). Unconventional Stoichiometries of Na–O Compounds at High Pressures. Materials, 14(24), 7650. https://doi.org/10.3390/ma14247650





