Control of Specific/Nonspecific Protein Adsorption: Functionalization of Polyelectrolyte Multilayer Films as a Potential Coating for Biosensors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Production and Biochemical Modification of Anti-CEA Antibody. Animals and Cells Used in Studies
2.3. Reagents Used for Antibody Production
2.4. Production, Purification, and Biotinylation of a Murine Monoclonal Antibody Specific to CEA
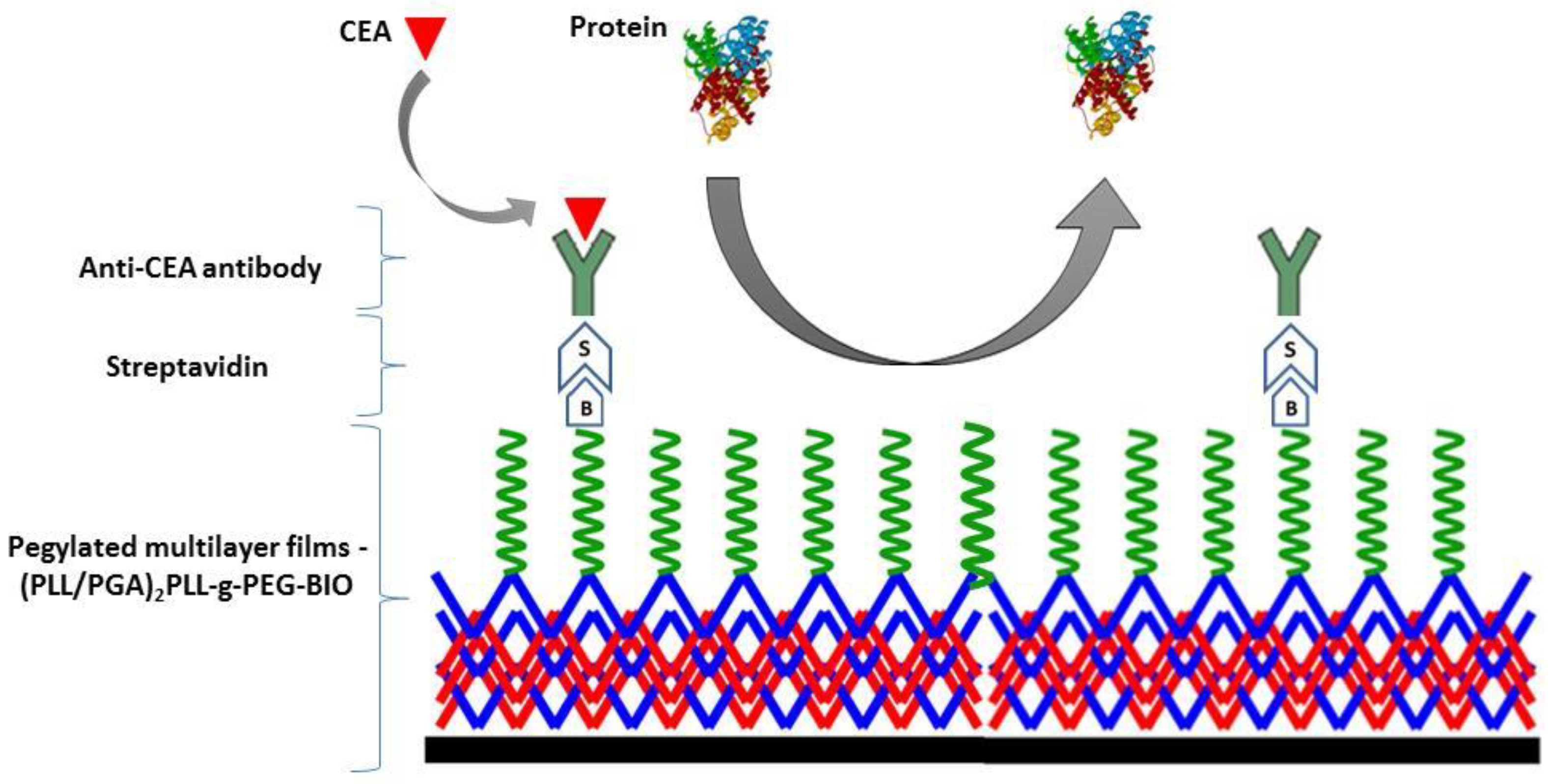
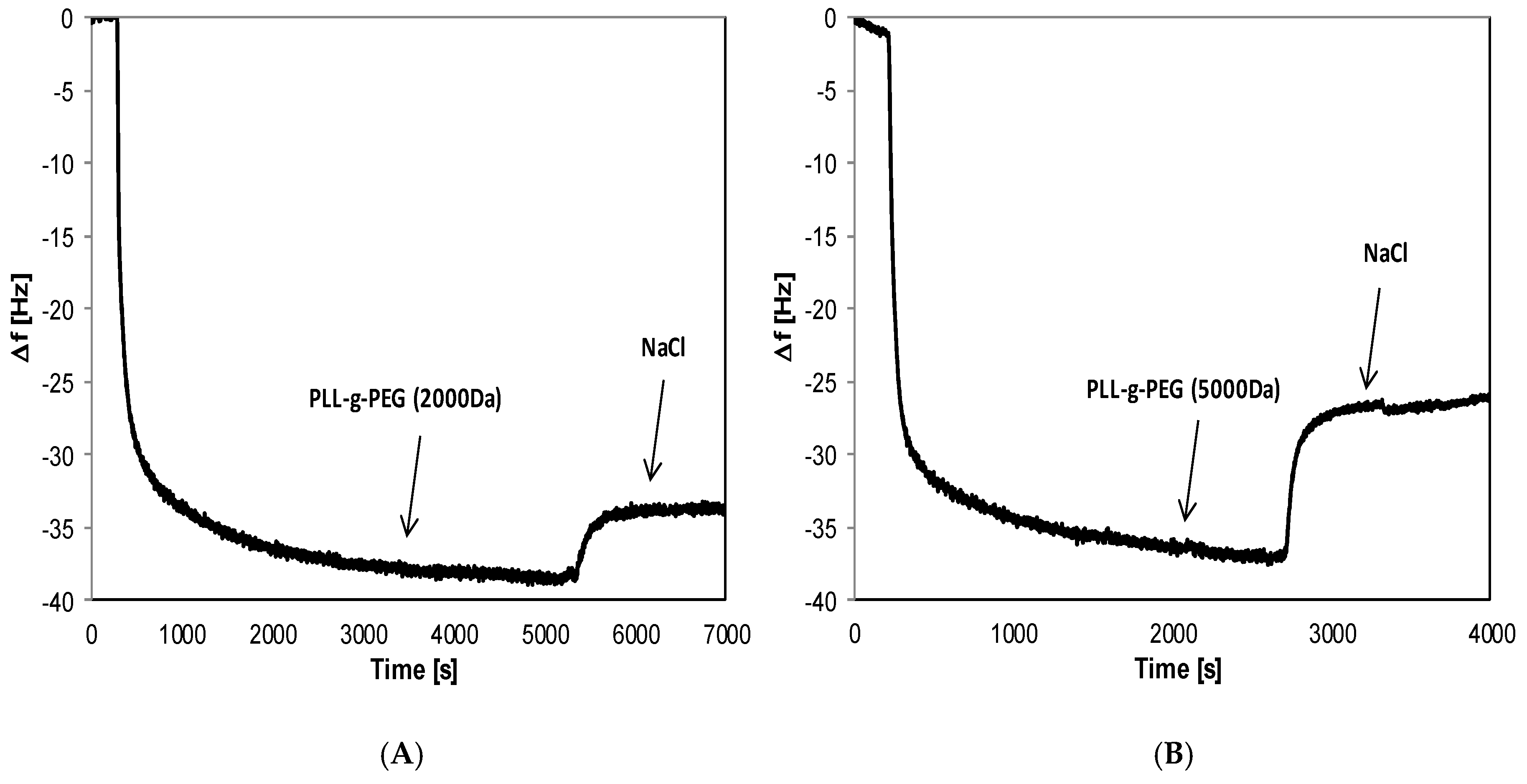
2.5. Formation of Functional Polyelectrolyte Films
2.6. Specific/Nonspecific Protein Adsorption Tests
2.7. QCM-D Studies
2.8. Atomic Force Microscopy Studies
3. Results
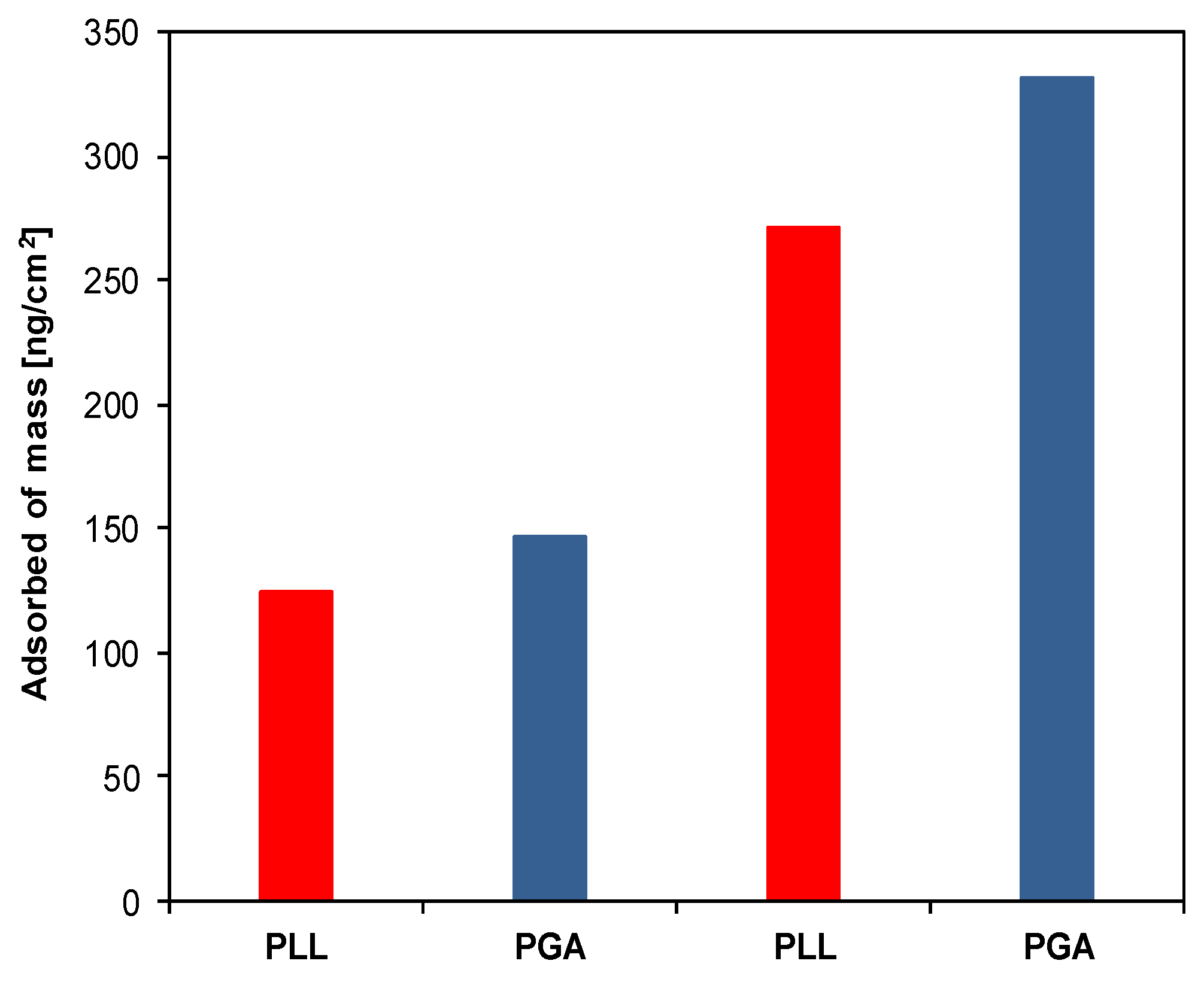
3.1. Elimination of Nonspecific Protein Adsorption
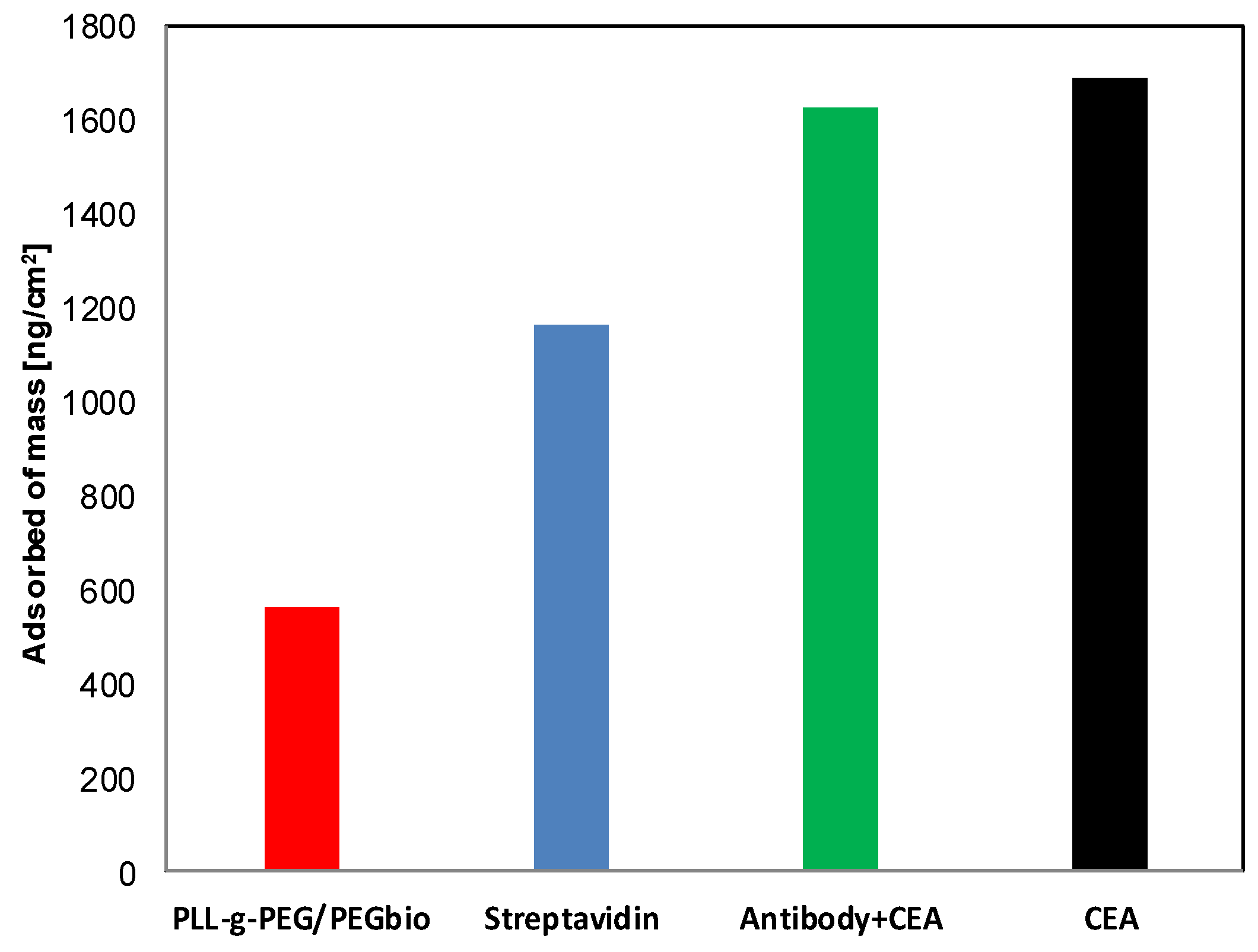
3.2. Specific Protein Interaction
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blaszykowski, C.; Sheikh, S.; Thompson, M. Surface chemistry to minimize fouling from blood-based fluids. Chem. Soc. Rev. 2012, 41, 5599–5612. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Geryak, R.; Geldmeier, J.; Kim, S.; Tsukruk, V.V. Synthesis, Assembly, and Applications of Hybrid Nanostructures for Biosensing. Chem. Rev. 2017, 117, 12942–13038. [Google Scholar] [CrossRef] [PubMed]
- Malmsten, M. Protein Adsorption at Phospholipid Surfaces. J. Colloid Interface Sci. 1995, 172, 106–115. [Google Scholar] [CrossRef]
- Ramanavicius, S.; Ramanavicius, A. Conducting Polymers in the Design of Biosensors and Biofuel Cells. Polymers 2021, 13, 49. [Google Scholar] [CrossRef]
- Ramanavicius, S.; Jagminas, A.; Ramanavicius, A. Advances in Molecularly Imprinted Polymers Based Affinity Sensors. Polymers 2021, 13, 974. [Google Scholar] [CrossRef] [PubMed]
- Spychalska, K.; Zając, D.; Baluta, S.; Halicka, K.; Cabaj, J. Functional Polymers Structures for (Bio)Sensing Application—A Review. Polymers 2020, 12, 1154. [Google Scholar] [CrossRef]
- Ramanavicius, S.; Ramanavicius, A. Charge Transfer and Biocompatibility Aspects in Conducting Polymer-Based Enzymatic Biosensors and Biofuel Cells. Nanomaterials 2021, 11, 371. [Google Scholar] [CrossRef]
- Plausinaitis, D.; Sinkevicius, L.; Samukaite-Bubniene, U.; Ratautaite, V.; Ramanavicius, A. Evaluation of electrochemical quartz crystal microbalance based sensor modified by uric acid-imprinted polypyrrole. Talanta 2020, 220, 121414. [Google Scholar] [CrossRef]
- Lakard, B. Electrochemical Biosensors Based on Conducting Polymers: A Review. Appl. Sci. 2020, 10, 6614. [Google Scholar] [CrossRef]
- Ramanavicius, S.; Ramanavicius, A. Progress and Insights in the Application of MXenes as New 2D Nano-Materials Suitable for Biosensors and Biofuel Cell Design. Int. J. Mol. Sci. 2020, 21, 9224. [Google Scholar] [CrossRef]
- Harris, J.M. Poly(Ethylene Glycol) Chemistry: Biotechnical and Biomedical Applications; Plenum Press: New York, NY, USA, 1992; p. 485. [Google Scholar]
- Thiele, L.; Rothen-Rutishauser, B.; Jilek, S.; Wunderli-Allenspach, H.; Merkle, H.P.; Walter, E. Evaluation of particle uptake in human blood monocyte-derived cells in vitro. Does phagocytosis activity of dendritic cells measure up with macrophages? J. Control. Release 2001, 76, 59–71. [Google Scholar] [CrossRef]
- Milton Harris, J.; Chess, R.B. Effect of pegylation on pharmaceuticals. Nat. Rev. Drug Discov. 2003, 2, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Vermette, P.; Meagher, L. Interactions of phospholipid- and poly(ethylene glycol)-modified surfaces with biological systems: Relation to physico-chemical properties and mechanisms. Colloids Surf. B Biointerfaces 2003, 28, 153–198. [Google Scholar] [CrossRef]
- Halperin, A. Polymer brushes that resist adsorption of model proteins: Design parameters. Langmuir 1999, 15, 2525–2533. [Google Scholar] [CrossRef]
- Zhu, B.; Eurell, T.; Gunawan, R.; Leckband, D. Chain-length dependence of the protein and cell resistance of oligo(ethylene glycol)-terminated self-assembled monolayers on gold. J. Biomed. Mater. Res. 2001, 56, 406–416. [Google Scholar] [CrossRef]
- Prime, K.L.; Whitesides, G.M. Adsorption of proteins onto surfaces containing end-attached oligo(ethylene oxide): A model system using self-assembled monolayers. J. Am. Chem. Soc. 1993, 115, 10714–10721. [Google Scholar] [CrossRef]
- Michel, R.; Pasche, S.; Textor, M.; Castner, D.G. Influence of PEG architecture on protein adsorption and conformation. Langmuir 2005, 21, 12327–12332. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.; Zhang, Z.; Wang, D.; Hao, J.; Cui, J. Monodispersity of Poly(ethylene glycol) Matters for Low-Fouling Coatings. ACS Macro Lett. 2020, 9, 1478–1482. [Google Scholar] [CrossRef]
- Pasche, S.; Voros, J.; Griesser, H.J.; Spencer, N.D.; Textor, M. Effects of ionic strength and surface charge on protein adsorption at PEGylated surfaces. J. Phys. Chem. B 2005, 109, 17545. [Google Scholar] [CrossRef]
- Huang, N.; Michel, R.; Voros, J.; Textor, M.; Hofer, R.; Rossi, A.; Elbert, D.L.; Hubbell, J.A.; Spencer, N.D. Poly (L-lysine)-g-poly (ethylene glycol) layers on metal oxide surfaces: Surface-analytical characterization and resistance to serum and fibrinogen adsorption. Langmuir 2001, 17, 489–498. [Google Scholar] [CrossRef]
- Boulmedais, F.; Frisch, B.; Etienne, O.; Lavalle, P.; Picart, C.; Ogier, J.; Voegel, J.; Schaaf, P.; Egles, C. Polyelectrolyte multilayer films with pegylated polypeptides as a new type of anti-microbial protection for biomaterials. Biomaterials 2004, 25, 2003–2011. [Google Scholar] [CrossRef] [Green Version]
- Schmolke, H.; Hartwig, S.; Klages, C. Poly (acrylic acid)-graft-poly (ethylene glycol) preparation and adsorption on polyelectrolyte multilayers (PEMs) for custom-made antiadhesive surfaces. Phys. Status Solidi 2011, 208, 1290–1300. [Google Scholar] [CrossRef]
- Klages, C.; Hartwig, S.; Schmolke, H. Adsorption of Poly (Acrylic Acid)-Graft-Poly (Ethylene Glycol) on Polyelectrolyte Multilayers. In Anonymous Trends in Colloid and Interface Science XXIV; Springer: Berlin/Heidelberg, Germany, 2011; pp. 95–101. [Google Scholar]
- Wattendorf, U.; Merkle, H.P. PEGylation as a tool for the biomedical engineering of surface modified microparticles. J. Pharm. Sci. 2008, 97, 4655–4669. [Google Scholar] [CrossRef] [PubMed]
- Seyrek, E.; Decher, G. Layer-by-Layer Assembly of Multifunctional Hybrid Materials and Nanoscale Devices. In Polymer Science: A Comprehensive Reference; Matyjaszewski, K., Müller, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; Volume 7, pp. 159–185. [Google Scholar]
- Decher, G.; Hong, J. Buildup of ultrathin multilayer films by a self-assembly process, 1 consecutive adsorption of anionic and cationic bipolar amphiphiles on charged surfaces. Makromol. Chem. Macromol. Symp. 1991, 46, 321–327. [Google Scholar] [CrossRef]
- Zhao, S.; Caruso, F.; Dähne, L.; Decher, G.; De Geest, B.G.; Fan, J.; Feliu, N.; Gogotsi, Y.; Hammond, P.T.; Hersam, M.C.; et al. The Future of Layer-by-Layer Assembly: A Tribute to ACS Nano Associate Editor Helmuth Möhwald. ACS Nano 2019, 13, 6151–6169. [Google Scholar] [CrossRef] [Green Version]
- Iler, R.K. Multilayers of colloidal particles. J. Colloid Interface Sci. 1966, 21, 569–594. [Google Scholar] [CrossRef]
- Decher, G.; Hong, J.; Schmitt, J. Buildup of ultrathin multilayer films by a self-assembly process: III. Consecutively alternating adsorption of anionic and cationic polyelectrolytes on charged surfaces. Thin Solid Film. 1992, 210, 831–835. [Google Scholar] [CrossRef]
- Kruk, T.; Szczepanowicz, K.; Kręgiel, D.; Szyk-Warszynska, L.; Warszynski, P. Nanostructured multilayer polyelectrolyte films with silver nanoparticles as antibacterial coatings. Colloids Surf. B Biointerfaces 2016, 137, 158–166. [Google Scholar] [CrossRef]
- Decher, G.; Schlenoff, J.B. Multilayer Thin Films: Sequential Assembly of Nanocomposite Materials; John Wiley & Son: Weinheim, Germany, 2006. [Google Scholar]
- Richardson, J.J.; Bjornmalm, M.; Caruso, F. Multilayer assembly. Technology-driven layer-by-layer assembly of nanofilms. Science 2015, 348, 2491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sukhorukov, G.B.; Donath, E.; Lichtenfeld, H.; Knippel, E.; Knippel, M.; Budde, A.; Möhwald, H. Layer-by-layer self assembly of polyelectrolytes on colloidal particles. Colloids Surf. Physicochem. Eng. Asp. 1998, 137, 253–266. [Google Scholar] [CrossRef]
- Karabasz, A.; Bzowska, M.; Łukasiewicz, S.; Bereta, J.; Szczepanowicz, K. Cytotoxic activity of paclitaxel incorporated into polyelectrolyte nanocapsules. J. Nanoparticle Res. 2014, 16, 2340. [Google Scholar] [CrossRef]
- Bazylinska, U.; Skrzela, R.; Piotrowski, M.; Szczepanowicz, K.; Warszynski, P.; Wilk, K.A. Influence of dicephalic ionic surfactant interactions with oppositely charged polyelectrolyte upon the in vitro dye release from oil core nanocapsules. Bioelectrochemistry 2012, 87, 147–153. [Google Scholar] [CrossRef]
- Kenausis, G.L.; Vörös, J.; Elbert, D.L.; Huang, N.; Hofer, R.; Ruiz-Taylor, L.; Textor, M.; Hubbell, J.A.; Spencer, N.D. Poly (L-lysine)-g-poly (ethylene glycol) layers on metal oxide surfaces: Attachment mechanism and effects of polymer architecture on resistance to protein adsorption. J. Phys. Chem. B 2000, 104, 3298–3309. [Google Scholar] [CrossRef]
- Yang, J.M.; Tsai, R.; Hsu, C. Protein adsorption on polyanion/polycation layer-by-layer assembled polyelectrolyte films. Colloids Surf. B Biointerfaces 2016, 142, 98–104. [Google Scholar] [CrossRef]
- Baggerman, J.; Smulders, M.M.J.; Zuilhof, H. Romantic Surfaces: A Systematic Overview of Stable, Biospecific, and Antifouling Zwitterionic Surfaces. Langmuir 2019, 35, 1072–1084. [Google Scholar] [CrossRef] [Green Version]
- Gnanasampanthan, T.; Beyer, C.D.; Yu, W.; Karthäuser, J.F.; Wanka, R.; Spöllmann, S.; Becker, H.W.; Aldred, N.; Clare, A.S.; Rosenhahn, A. Effect of Multilayer Termination on Nonspecific Protein Adsorption and Antifouling Activity of Alginate-Based Layer-by-Layer Coatings. Langmuir 2021, 37, 5950–5963. [Google Scholar] [CrossRef] [PubMed]
- Ladam, G.; Schaaf, P.; Decher, G.; Voegel, J.; Cuisinier, F.J. Protein adsorption onto auto-assembled polyelectrolyte films. Biomol. Eng. 2002, 19, 273–280. [Google Scholar] [CrossRef]
- Heuberger, R.; Sukhorukov, G.; Vörös, J.; Textor, M.; Möhwald, H. Biofunctional polyelectrolyte multilayers and microcapsules: Control of nonspecific and bio-specific protein adsorption. Adv. Funct. Mater. 2005, 15, 357–366. [Google Scholar] [CrossRef]
- Szczepanowicz, K.; Kruk, T.; Światek, W.; Bouzga, A.M.; Simon, C.R.; Warszyński, P. Poly(l-glutamic acid)-g-poly(ethylene glycol) external layer in polyelectrolyte multilayer films: Characterization and resistance to serum protein adsorption. Colloids Surf. B Biointerfaces 2018, 166, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Pasche, S.; De Paul, S.M.; Vörös, J.; Spencer, N.D.; Textor, M. Poly (l-lysine)-g raft-poly (ethylene glycol) Assembled Monolayers on Niobium Oxide Surfaces: A Quantitative Study of the Influence of Polymer Interfacial Architecture on Resistance to Protein Adsorption by ToF-SIMS and in Situ OWLS. Langmuir 2003, 19, 9216–9225. [Google Scholar] [CrossRef]
- Sauerbrey, G. Verwendung von Schwingquarzen zur Wägung dünner Schichten und zur Mikrowägung. Z. Phys. 1959, 155, 206–222. [Google Scholar] [CrossRef]
- Giesbers, M.; Kleijn, J.M.; Stuart, M.A.C. The electrical double layer on gold probed by electrokinetic and surface force measurements. J. Colloid Interface Sci. 2002, 248, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Gergely, C.; Bahi, S.; Szalontai, B.; Flores, H.; Schaaf, P.; Voegel, J.; Cuisinier, F.J. Human serum albumin self-assembly on weak polyelectrolyte multilayer films structurally modified by pH changes. Langmuir 2004, 20, 5575–5582. [Google Scholar] [CrossRef] [PubMed]
- Greg, T. Hermanson. In Bioconjugate Techniques, 3rd ed.; Academic Press: Waltham, MA, USA, 2013. [Google Scholar]


| Multilayer Structure | Pegylated Polyelectrolyte | Areal Density |
|---|---|---|
| (PLL/PGA)2PLL-gPEG2k | PLL(20 kDa)-g(3.5)-PEG(2 kDa) | 700 ng/cm2 |
| (PLL/PGA)2PLL-gPEG5k | PLL(20 kDa)-g(3.5)-PEG(5 kDa) | 542 ng/cm2 |
| Surface | Mass of Proteins (ng/cm2) | Mass of Proteins (ng/cm2) |
|---|---|---|
| HSA | CEA | |
| (PLL/PGA)2PLL-g-PEGBIO | 70 | 180 |
| (PLL/PGA)2PLL-g-PEGBIO/ STREPT/BIO-Anti-CEA | ~0 | 60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kruk, T.; Bzowska, M.; Hinz, A.; Szuwarzyński, M.; Szczepanowicz, K. Control of Specific/Nonspecific Protein Adsorption: Functionalization of Polyelectrolyte Multilayer Films as a Potential Coating for Biosensors. Materials 2021, 14, 7629. https://doi.org/10.3390/ma14247629
Kruk T, Bzowska M, Hinz A, Szuwarzyński M, Szczepanowicz K. Control of Specific/Nonspecific Protein Adsorption: Functionalization of Polyelectrolyte Multilayer Films as a Potential Coating for Biosensors. Materials. 2021; 14(24):7629. https://doi.org/10.3390/ma14247629
Chicago/Turabian StyleKruk, Tomasz, Monika Bzowska, Alicja Hinz, Michał Szuwarzyński, and Krzysztof Szczepanowicz. 2021. "Control of Specific/Nonspecific Protein Adsorption: Functionalization of Polyelectrolyte Multilayer Films as a Potential Coating for Biosensors" Materials 14, no. 24: 7629. https://doi.org/10.3390/ma14247629
APA StyleKruk, T., Bzowska, M., Hinz, A., Szuwarzyński, M., & Szczepanowicz, K. (2021). Control of Specific/Nonspecific Protein Adsorption: Functionalization of Polyelectrolyte Multilayer Films as a Potential Coating for Biosensors. Materials, 14(24), 7629. https://doi.org/10.3390/ma14247629






