The Influence of Pd and Zr Co-Doping on the Microstructure and Oxidation Resistance of Aluminide Coatings on the CMSX-4 Nickel Superalloy
Abstract
:1. Introduction
2. Experimental Procedure
- 1.
- Nickel Electroplating—t = 6 min, T = 20 °C, Current Density 3A dm−2 in the Bath of the following Chemical Composition [26].
| Constituent | Concentration |
|---|---|
| Nickel(II) chloride (NiCl2∙6H2O) | 240 g∙dm−3 |
| Hydrochloric acid (35% wt.) | 31 cm3∙dm−3 |
| Deionised water | to 1 dm3 |
- 2.
- Acidic Palladium Strike Process—t = 90s, T = 40 °C, Current Density 5mAcm−2 in the Bath of the following Chemical Composition [26].
| Constituent | Concentration |
|---|---|
| Palladium(II) chloride | 1.6 g∙dm−3 |
| 1,2-diaminopropane | 5.4 cm3∙dm−3 |
| Glacial acetic acid | 23.3 cm3∙dm−3 |
| Sodium chloride | 60 g∙dm−3 |
| Deionised water | to 1 dm3 |
- 3.
- Palladium Electrodeposition—t = 11 min, T = 55 °C, Current Density 10 mAcm−2 in the Bath of the following Chemical Composition [27].
| Constituent | Concentration |
|---|---|
| Palladium(II) chloride | 13.3 g∙dm−3 |
| diethylenetriamine | 16.2 cm3∙dm−3 |
| Phosphate buffer | to 1 dm3 |
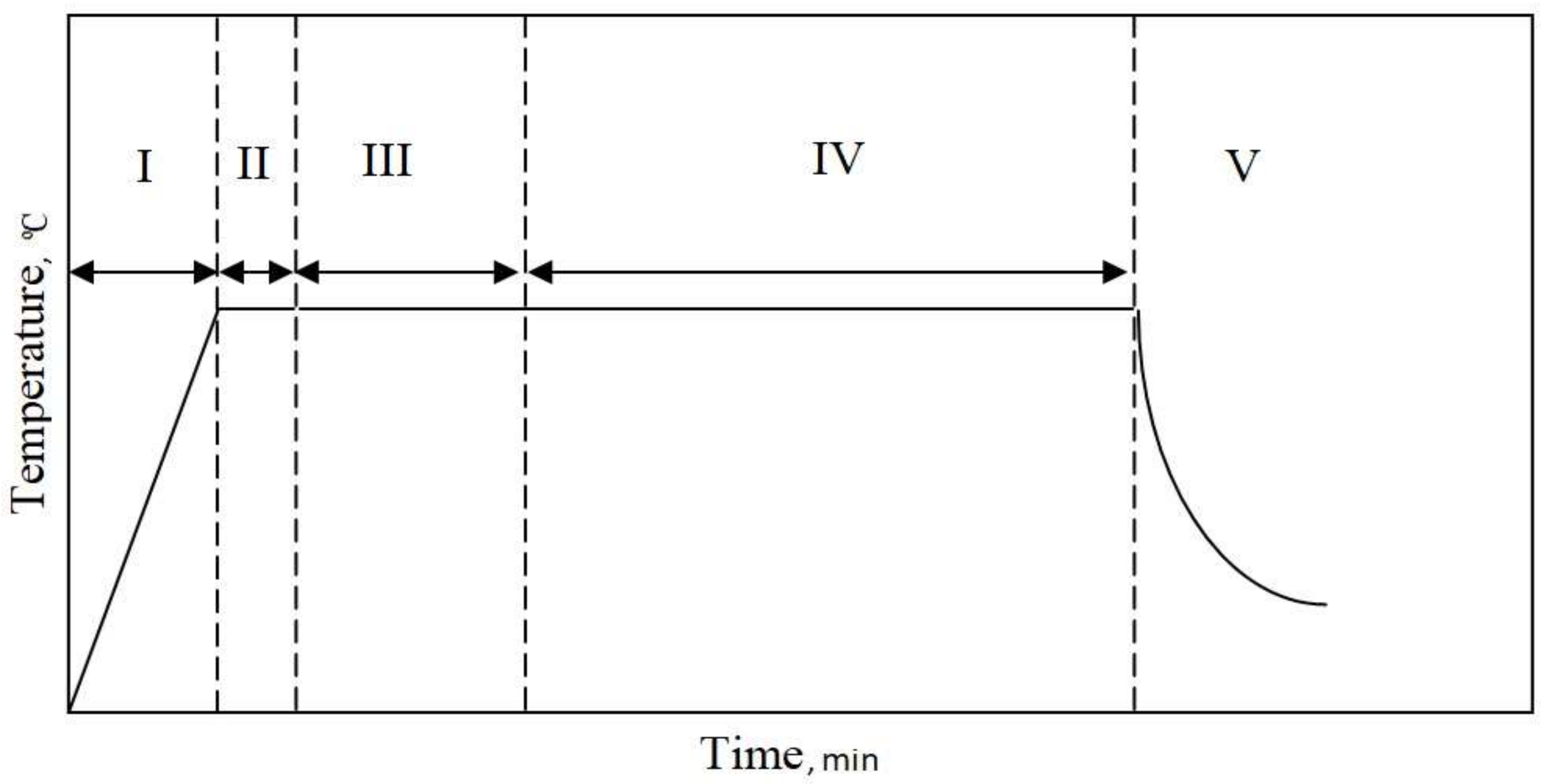
- Heating from room temperature up to 1050 °C
- Aluminizing at 1050 °C for 30 min
- Aluminizing and zirconizing at 1050 °C for 90 min
- Aluminizing at 1050 °C for 240 min
- Cooling to room temperature
3. Results and Discussion
3.1. Palladium and Zirconium Co-Doped Aluminide Coating
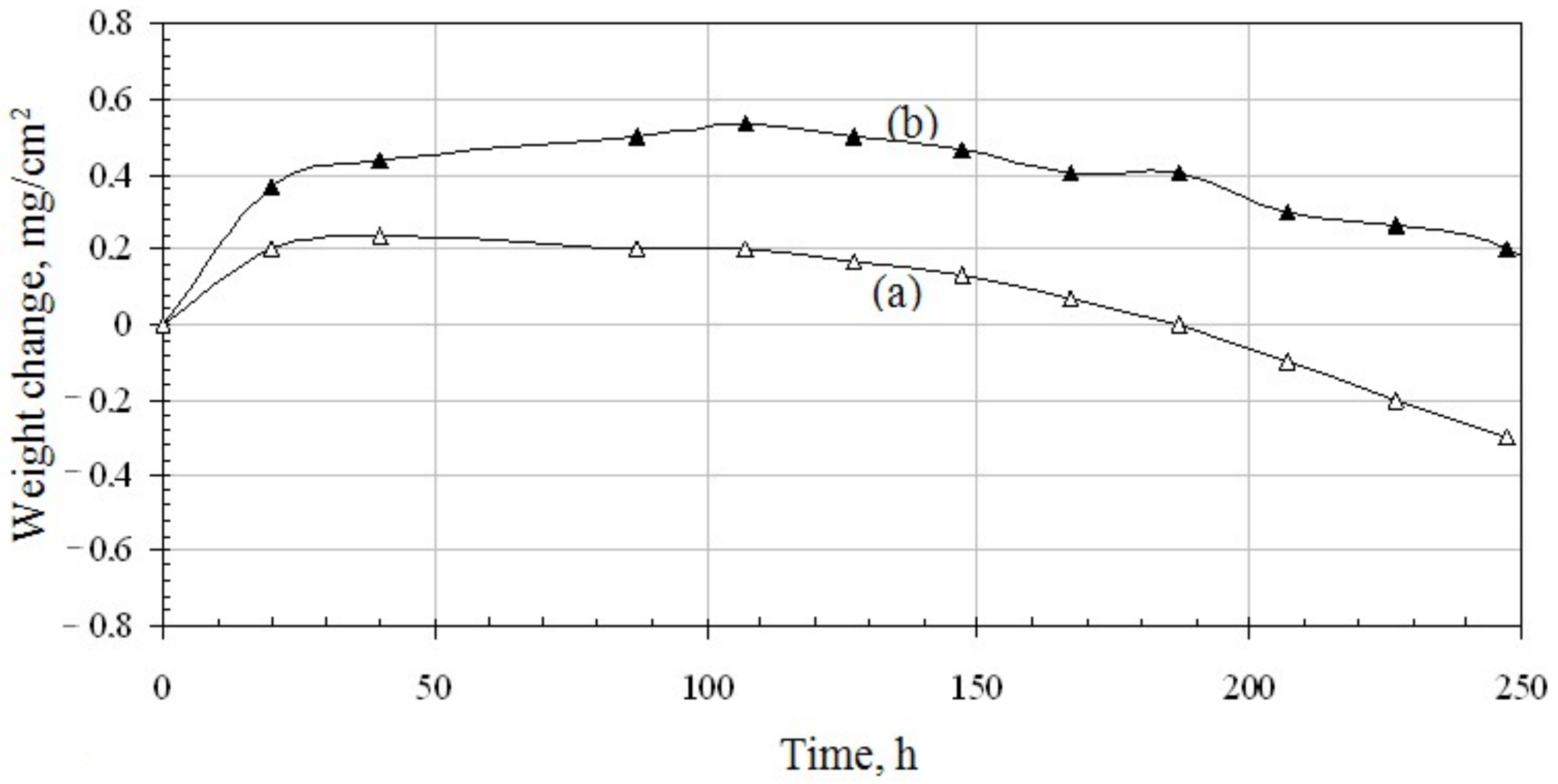
3.2. Oxidation Behavior of Palladium versus Palladium and Zirconium Co-Doped Aluminide Coating
4. Conclusions
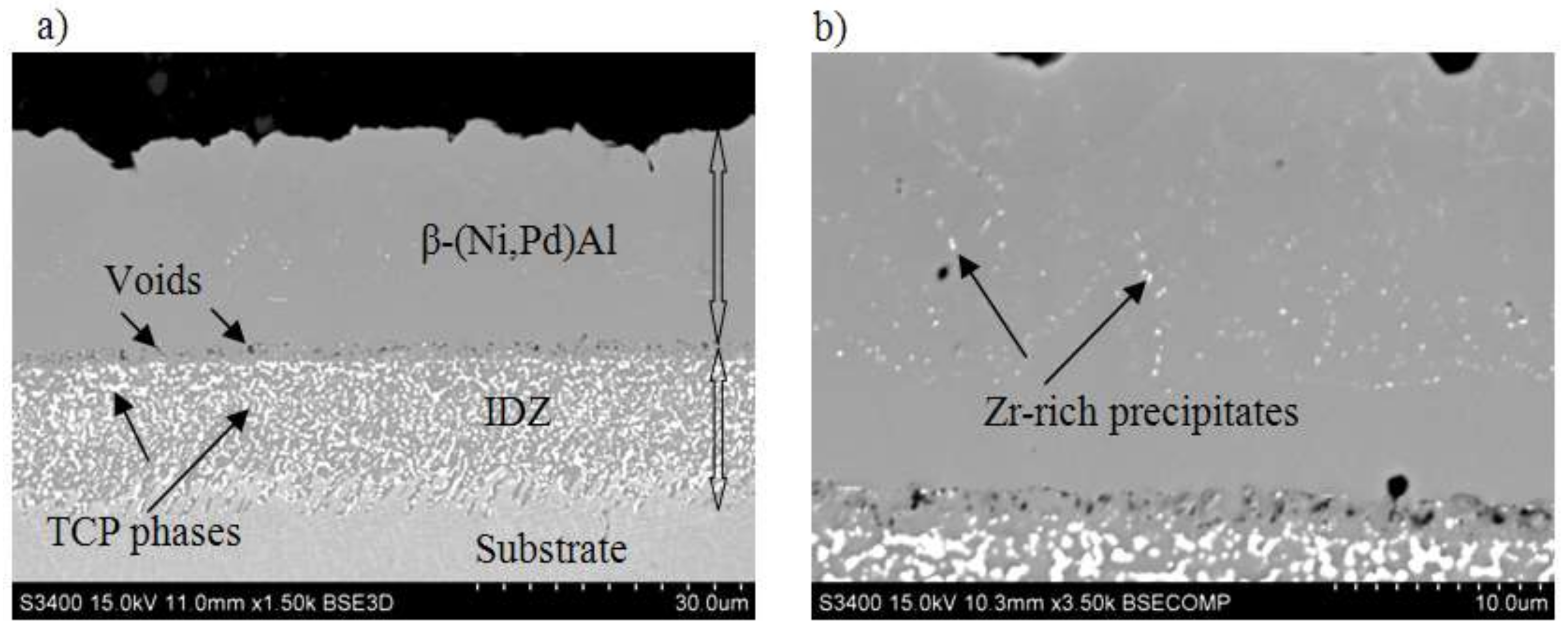
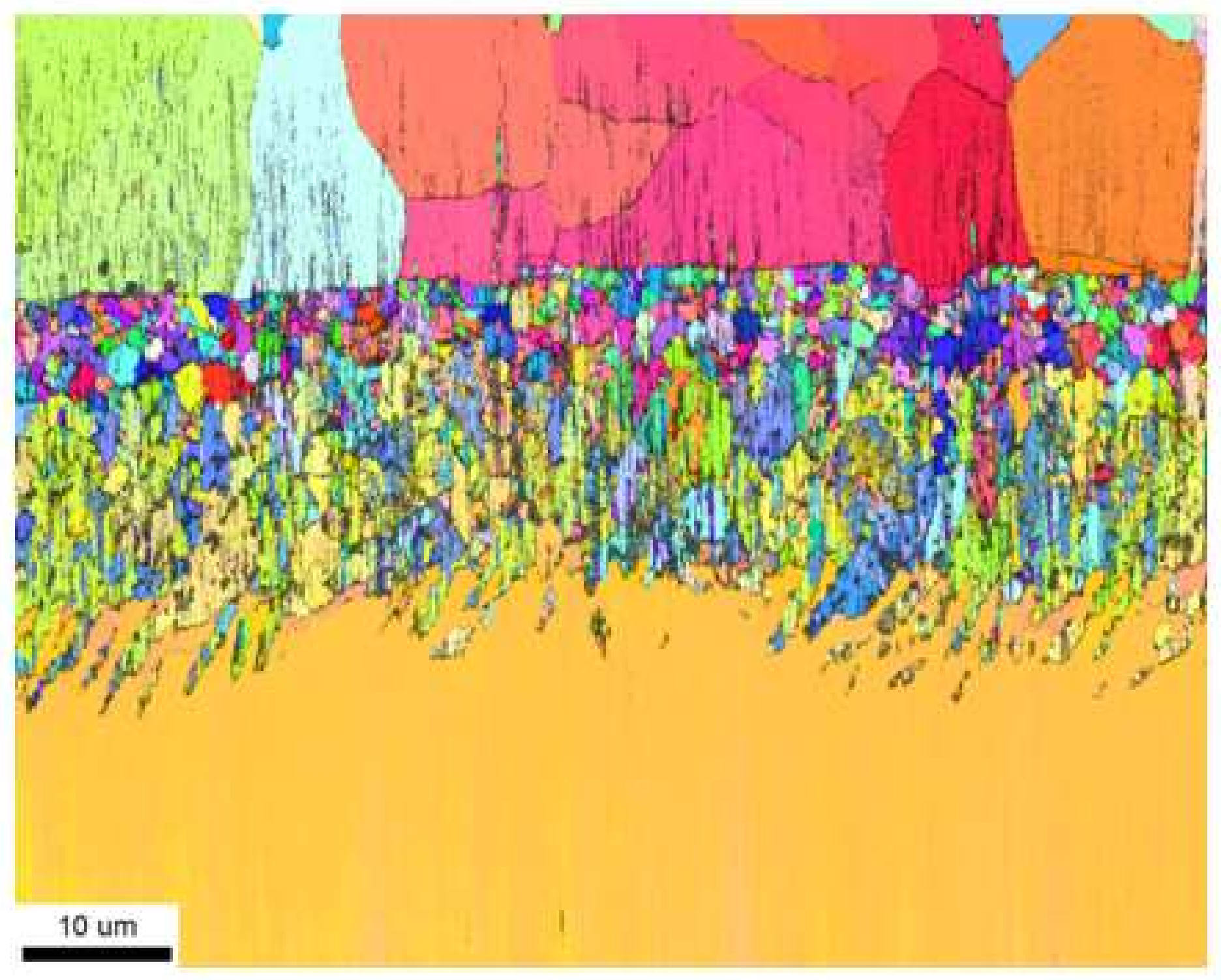
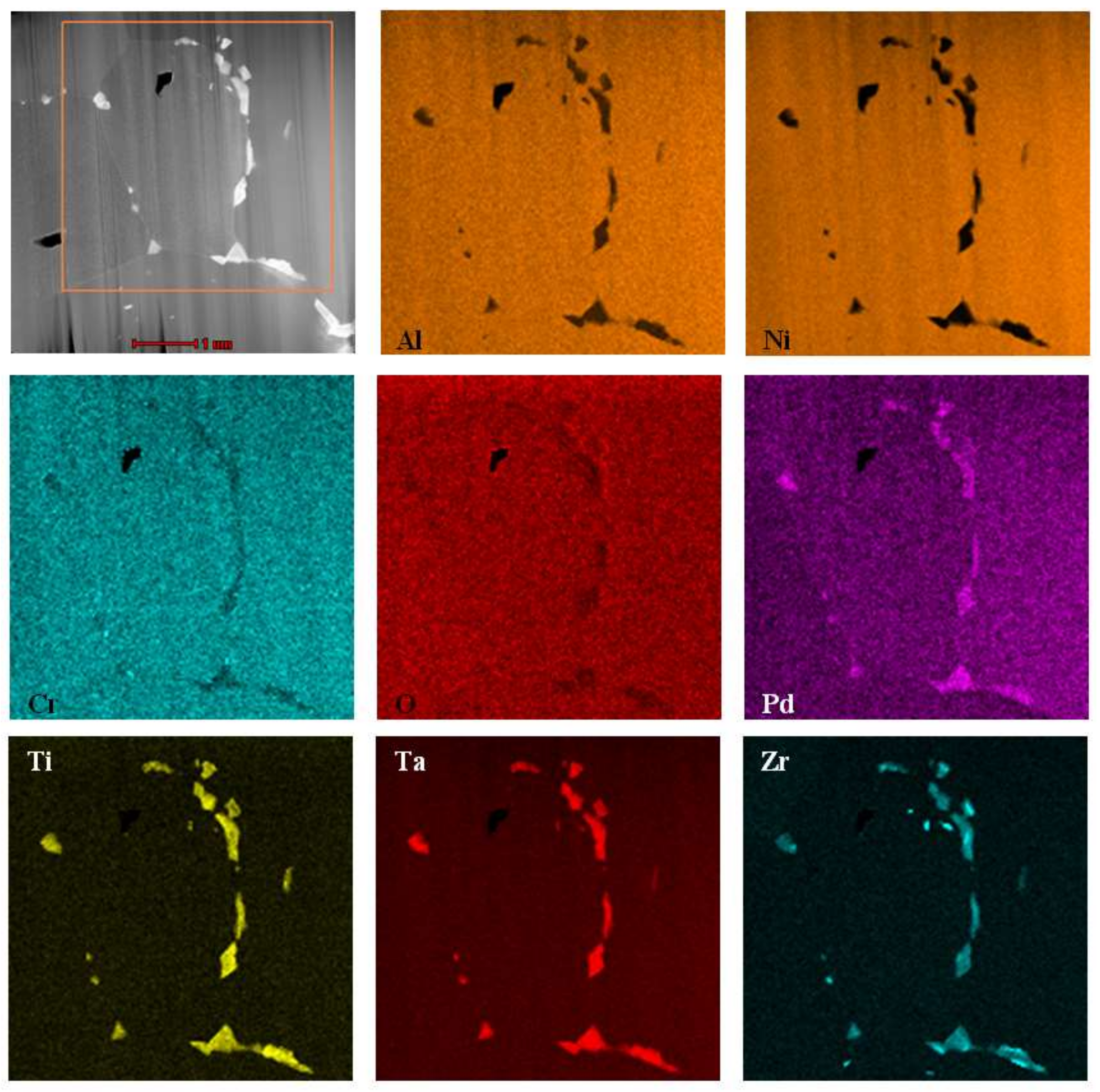
- The Pd and Zr co-doped coating consists of two zones, the additive one (above) and the interdiffusion one (below).
- Both zones are built of the β–(Ni,Pd)Al phase, which proves that palladium has dissolved uniformly in the whole coating.
- A great number of TPC inclusions in the interdiffusion zone prevented crystallites coarsening
- Zirconium rich inclusions are situated in the additive zone, close to the coating’s surface
- The oxidation resistance of the palladium and zirconium co-doped aluminide coating is better than that of the palladium doped one. Zirconium could retard θ-Al2O3 to α-Al2O3 transformation and improve spallation resistance and scale adherence by blocking Al ions diffusion.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pollock, T.M.; Tin, S. Nickel-based superalloys for advanced turbine engines: Chemistry, microstructure and properties. J. Propuls. Power 2006, 22, 210–217. Available online: http://hdl.handle.net/2027.42/77223 (accessed on 1 March 2006). [CrossRef]
- Goward, G.W. Progress in coatings for gas turbine airfoils. Surf. Coat. Technol. 1998, 108–109, 73–79. [Google Scholar] [CrossRef]
- Stacy, J.; Zhang, Y.; Pint, B.; Haynes, J.; Hazel, B.; Nagaraj, B. Synthesis and oxidation performance of Al-enriched γ + γ′ coatings on Ni-based superalloys via secondary aluminizing. Surf. Coat. Technol. 2007, 202, 632–636. [Google Scholar] [CrossRef]
- Li, Y.; Li, S.; Zhang, C.; Xu, N.; Bao, Z. Oxidation Behavior and Oxide Transformation of a Pt-Modified Aluminide Coating at Moderate High Temperature. Crystals 2021, 11, 972. [Google Scholar] [CrossRef]
- Viswanathan, V.; Dwivedi, G.; Sampath, S. Multilayer, Multimaterial Thermal Barrier Coating Systems: Design, Synthesis, and Performance Assessment. J. Am. Ceram. Soc. 2015, 98, 1769–1777. [Google Scholar] [CrossRef]
- Leckie, R.; Krämer, S.; Rühle, M.; Levi, C. Thermochemical compatibility between alumina and ZrO2–GdO3/2 thermal barrier coatings. Acta Mater. 2005, 53, 3281–3292. [Google Scholar] [CrossRef]
- Dwivedi, G.; Tan, Y.; Viswanathan, V.; Sampath, S. Process-Property Relationship for Air Plasma-Sprayed Gadolinium Zirconate Coatings. J. Therm. Spray Technol. 2015, 24, 454–466. [Google Scholar] [CrossRef]
- Góral, M.; Swadźba, R.; Kubaszek, T. TEM investigations of TGO formation during cyclic oxidation in two- and three-layered Thermal Barrier Coatings produced using LPPS, CVD and PS-PVD methods. Surf. Coat. Technol. 2020, 394, 125875. [Google Scholar] [CrossRef]
- Haynes, J.; Zhang, Y.; Cooley, K.; Walker, L.; Reeves, K.; Pint, B. High-temperature diffusion barriers for protective coatings. Surf. Coat. Technol. 2004, 188–189, 153–157. [Google Scholar] [CrossRef]
- Pytel, M.; Góral, M.; Maliniak, M. The influence of production method on oxidation resistance of the aluminide coatings obtained on IN 100 alloy. Arch. Mater. Sci. Eng. 2012, 53, 102–108. [Google Scholar]
- Zagula-Yavorska, M.; Sieniawski, J. Cyclic oxidation of palladium modified and nonmodified aluminide coatings deposited on nickel base superalloys. Arch. Civ. Mech. Eng. 2018, 18, 130–139. [Google Scholar] [CrossRef]
- Texier, D.; Monceau, D.; Selezneff, S.; Longuet, A.; Andrieu, E. High Temperature Micromechanical Behavior of a Pt-Modified Nickel Aluminide Bond-Coating and of Its Interdiffusion Zone with the Superalloy Substrate. Met. Mater. Trans. A 2020, 51, 1475–1480. [Google Scholar] [CrossRef]
- Unocic, K.; Parish, C.; Pint, B. Characterization of the alumina scale formed on coated and uncoated doped superalloys. Surf. Coat. Technol. 2011, 206, 1522–1528. [Google Scholar] [CrossRef]
- Li, D.; Guo, H.; Wang, D.; Zhang, T.; Gong, S.; Xu, H. Cyclic oxidation of β–NiAl with various reactive element dopants at 1200 °C. Corros. Sci. 2013, 66, 125–135. [Google Scholar] [CrossRef]
- Li, S.; Xu, M.; Zhang, C.; Niu, Y.; Bao, Z.; Zhu, S.; Wang, F. Co-doping effect of Hf and Y on improving cyclic oxidation behavior of (Ni,Pt)Al coating at 1150 °C. Corros. Sci. 2021, 178, 109093. [Google Scholar] [CrossRef]
- Qian, L.-Y.; Wang, J.; Guo, Y.-S.; Liu, H.; Bao, Z.-B. Influences of Iridium and Palladium on Oxidation Resistance of PtAl Coating. Acta Met. Sin. Engl. Lett. 2021, 34, 1120–1130. [Google Scholar] [CrossRef]
- Hamadi, S.; Bacos, M.-P.; Poulain, M.; Seyeux, A.; Maurice, V.; Marcus, P. Oxidation resistance of a Zr-doped NiAl coating thermochemically deposited on a nickel-based superalloy. Surf. Coat. Technol. 2009, 204, 756–760. [Google Scholar] [CrossRef]
- Sitek, R.; Kwaśniak, P.; Sopicka-Lizer, M.; Borysiuk, J.; Kaminski, J.; Mizera, J.; Kurzydłowski, K. Experimental and ab-initio study of the Zr- and Cr-enriched aluminide layer produced on an IN 713C Inconel substrate by CVD; investigations of the layer morphology, structural stability, mechanical properties, and corrosion resistance. Intermetallics 2016, 74, 15–24. [Google Scholar] [CrossRef]
- Cho, J.; Wang, C.; Chan, H.; Rickman, J.; Harmer, M. Role of segregating dopants on the improved creep resistance of aluminum oxide. Acta Mater. 1999, 47, 4197–4207. [Google Scholar] [CrossRef]
- Unocic, K.A.; Pint, B.A. Oxidation behavior of co-doped NiCrAl alloys in dry and wet air. Surf. Coat. Technol. 2013, 237, 8–15. [Google Scholar] [CrossRef]
- Swadźba, R.; Hetmańczyk, M.; Wiedermann, J.; Swadźba, L.; Moskal, G.; Witala, B.; Radwański, K. Microstructure degradation of simple, Pt- and Pt + Pd-modified aluminide coatings on CMSX-4 superalloy under cyclic oxidation conditions. Surf. Coat. Technol. 2013, 215, 16–23. [Google Scholar] [CrossRef]
- Fan, Q.; Yu, H.; Wang, T.; Wu, Z.; Liu, Y. Preparation and Isothermal Oxidation Behavior of Zr-Doped, Pt-Modified Aluminide Coating Prepared by a Hybrid Process. Coatings 2017, 8, 1. [Google Scholar] [CrossRef] [Green Version]
- Hong, S.; Hwang, G.; Han, W.; Lee, K.; Kang, S. Effect of zirconium addition on cyclic oxidation behavior of platinum-modified aluminide coating on nickel-based superalloy. Intermetallic 2010, 18, 864–870. [Google Scholar] [CrossRef]
- Romanowska, J.; Morgiel, J.; Kolek, Ł.; Kwolek, P.; Zagula-Yavorska, M. Effect of Pd and Hf co-doping of aluminide coatings on pure nickel and CMSX-4 nickel superalloy. Arch. Civ. Mech. Eng. 2018, 18, 1421–1429. [Google Scholar] [CrossRef]
- Romanowska, J.; Morgiel, J.; Zagula-Yavorska, M.; Sieniawski, J. Nanoparticles in hafnium-doped aluminide coatings. Mater. Lett. 2015, 145, 162–166. [Google Scholar] [CrossRef]
- Abys, J.A.; Straschil, H.K. Acidic Palladium Strike Bath. U.S. Patent 5,178,745, 12 January 1993. [Google Scholar]
- Abys, J.A.; Trop, H.S. Palladium Plating Prodedure. U.S. Patent 4,486,274, 5 October 1985. [Google Scholar]
- Nowotnik, A.; Góral, M.; Pytel, M.; Dychtoń, K. Influence of Coatings Deposition Parameters on Microstructure of Aluminide Coatings Deposited by CVD Method on Ni-Superalloys. Solid State Phenom. 2013, 197, 95–100. [Google Scholar] [CrossRef]
- Zagula-Yavorska, M.; Morgiel, J.; Romanowska, J.; Sieniawski, J. Microstructure and oxidation behaviour investigation of rhodium modified aluminide coating deposited on CMSX 4 superalloy. J. Microsc. 2015, 261, 320–325. [Google Scholar] [CrossRef]
- Jiang, C.; Qian, L.; Feng, M.; Liu, H.; Bao, Z.; Chen, M.; Zhu, S.; Wang, F. Benefits of Zr addition to oxidation resistance of a single-phase (Ni,Pt)Al coating at 1373 K. J. Mater. Sci. Technol. 2019, 35, 1334–1344. [Google Scholar] [CrossRef]
- Pint, B.A. Experimental observations in support of the dynamic-segregation theory to explain the reactive-element effect. Oxid. Met. 1996, 45, 1–37. [Google Scholar] [CrossRef]
- Lamesle, P.; Steinmetz, P.; Alpérine, S. Palladium-Modified Aluminide Coatings: Mechanisms of Formation. J. Electrochem. Soc. 1995, 142, 497–505. [Google Scholar] [CrossRef]
- Wei, L.; Peng, H.; Jia, F.; Zheng, L.; Gong, S.; Guo, H. Cyclic oxidation behavior of Hf/Zr co-doped EB-PVD β–NiAl coatings at 1200 °C. Surf. Coat. Technol. 2015, 276, 721–725. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romanowska, J.; Morgiel, J.; Zagula-Yavorska, M. The Influence of Pd and Zr Co-Doping on the Microstructure and Oxidation Resistance of Aluminide Coatings on the CMSX-4 Nickel Superalloy. Materials 2021, 14, 7579. https://doi.org/10.3390/ma14247579
Romanowska J, Morgiel J, Zagula-Yavorska M. The Influence of Pd and Zr Co-Doping on the Microstructure and Oxidation Resistance of Aluminide Coatings on the CMSX-4 Nickel Superalloy. Materials. 2021; 14(24):7579. https://doi.org/10.3390/ma14247579
Chicago/Turabian StyleRomanowska, Jolanta, Jerzy Morgiel, and Maryana Zagula-Yavorska. 2021. "The Influence of Pd and Zr Co-Doping on the Microstructure and Oxidation Resistance of Aluminide Coatings on the CMSX-4 Nickel Superalloy" Materials 14, no. 24: 7579. https://doi.org/10.3390/ma14247579
APA StyleRomanowska, J., Morgiel, J., & Zagula-Yavorska, M. (2021). The Influence of Pd and Zr Co-Doping on the Microstructure and Oxidation Resistance of Aluminide Coatings on the CMSX-4 Nickel Superalloy. Materials, 14(24), 7579. https://doi.org/10.3390/ma14247579





