Effect of Hydroxyapatite Microspheres, Amoxicillin–Hydroxyapatite and Collagen–Hydroxyapatite Composites on Human Dental Pulp-Derived Mesenchymal Stem Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis and Characterization of HAp-Based Materials
2.1.1. Synthesis of HAp-Based Materials
2.1.2. Characterization of HAp-Based Materials
2.2. Collection and Characterization of Human Dental Pulp-Derived Mesenchymal Stem Cells (hDPSCs)
Characterization of Human Dental Pulp-Derived Mesenchymal Stem Cells (hDPSCs)
2.3. In Vitro Cell Culture-Based Assays
2.3.1. Experiment of Osteogenic Differentiation
2.3.2. Experiment of Cytotoxicity Analysis
2.3.3. Experiment of Migration Assay
2.4. Statistical Analysis
3. Results
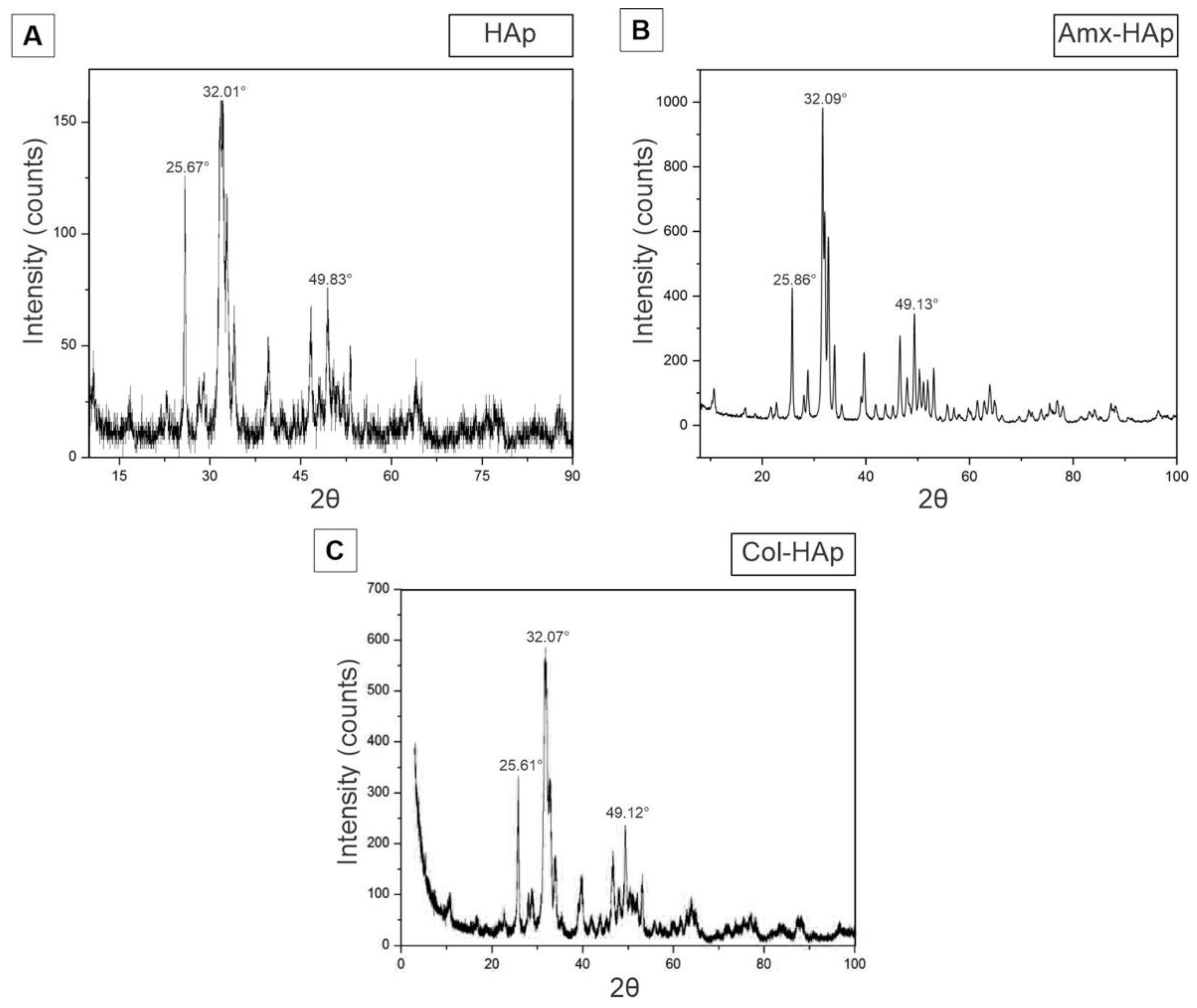
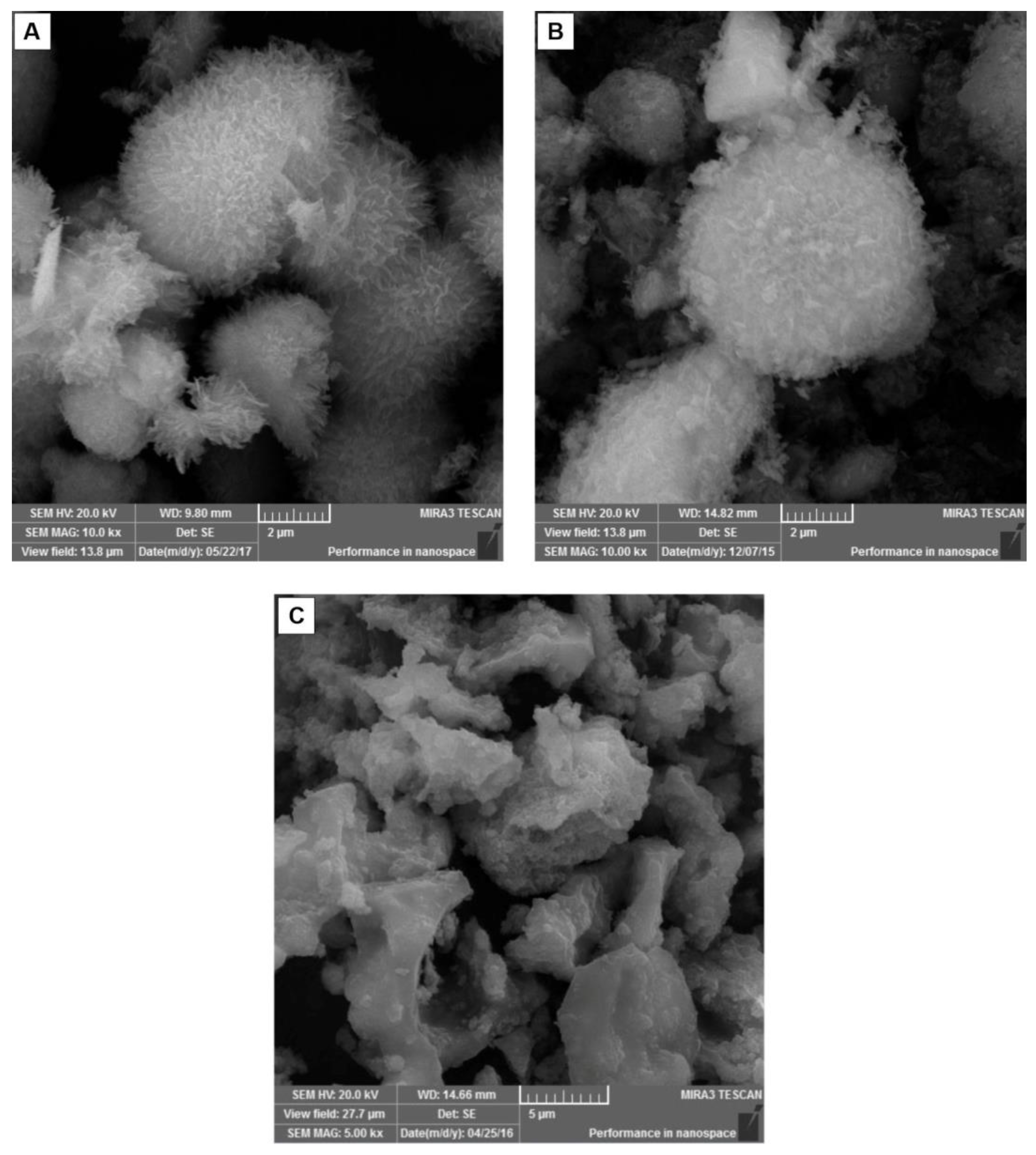
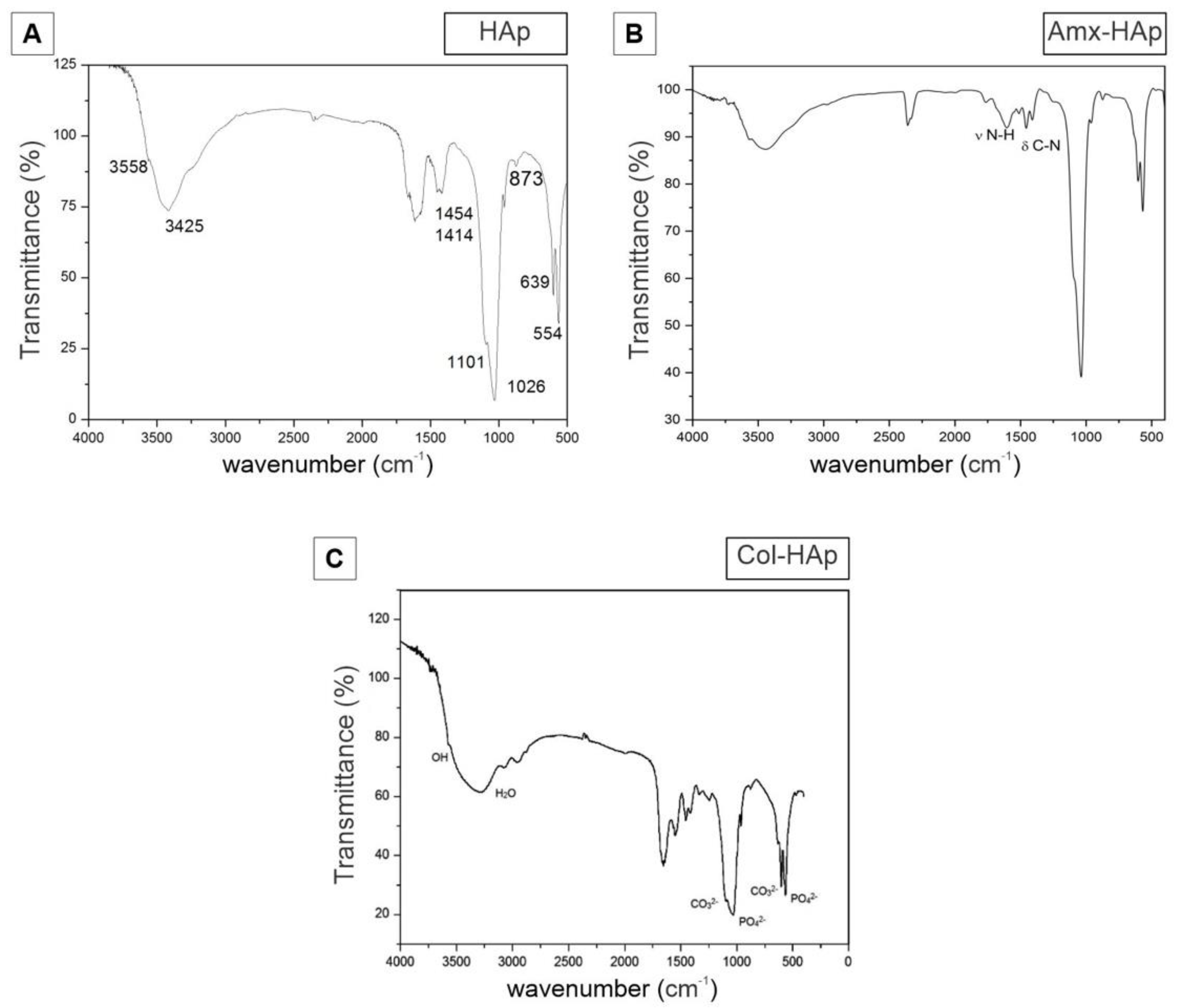
3.1. Results of Synthesis and Characterization of HAp-Based Materials
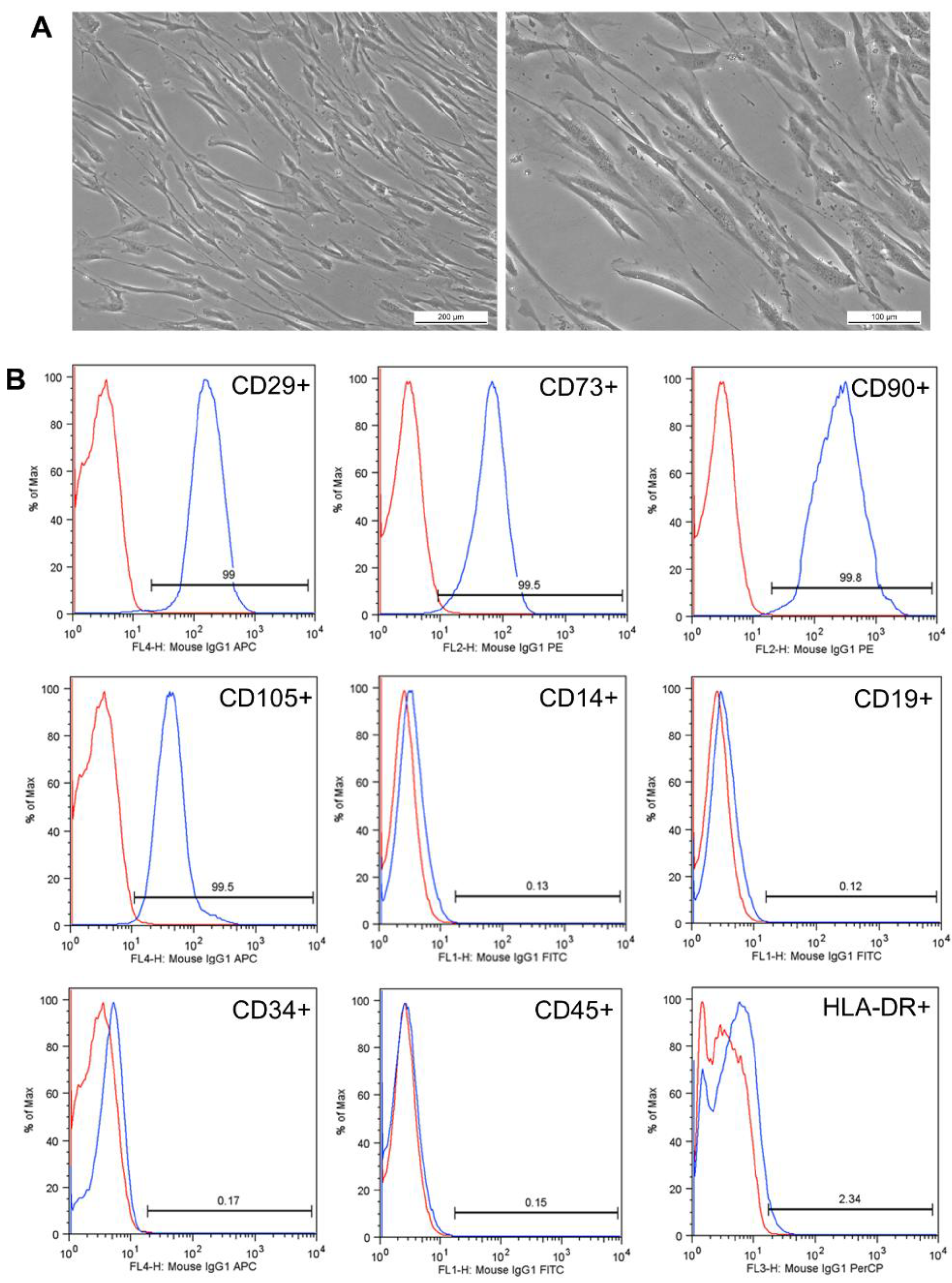
3.2. Results of Collection and Characterization of Human Dental Pulp-Derived Mesenchymal Stem Cells (hDPSCs)
Results of Characterization of Human Dental Pulp-Derived Mesenchymal Stem Cells (hDPSCs)
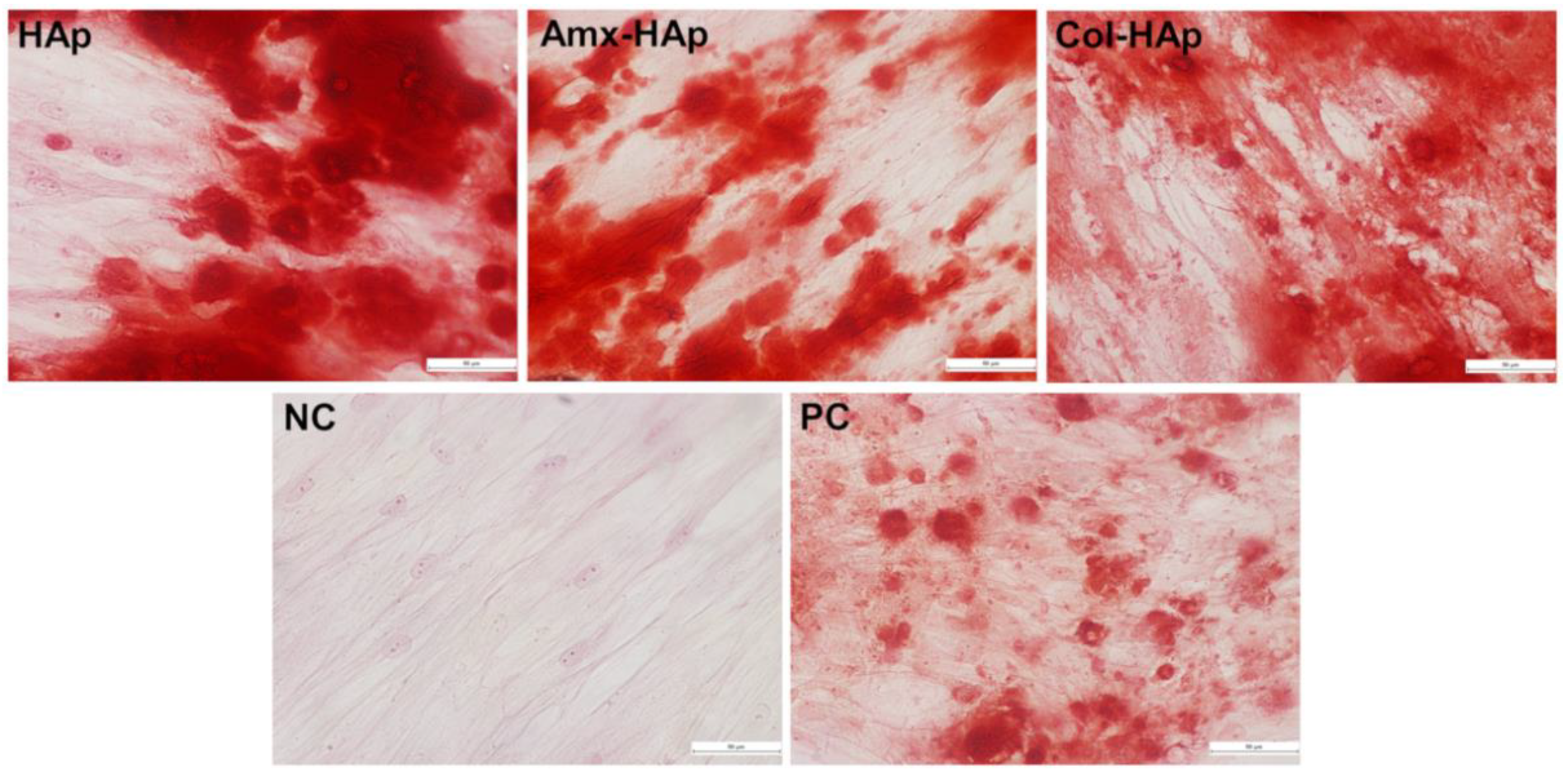
3.3. Results of Osteogenic Differentiation
3.4. Results of Cytotoxicity Analysis
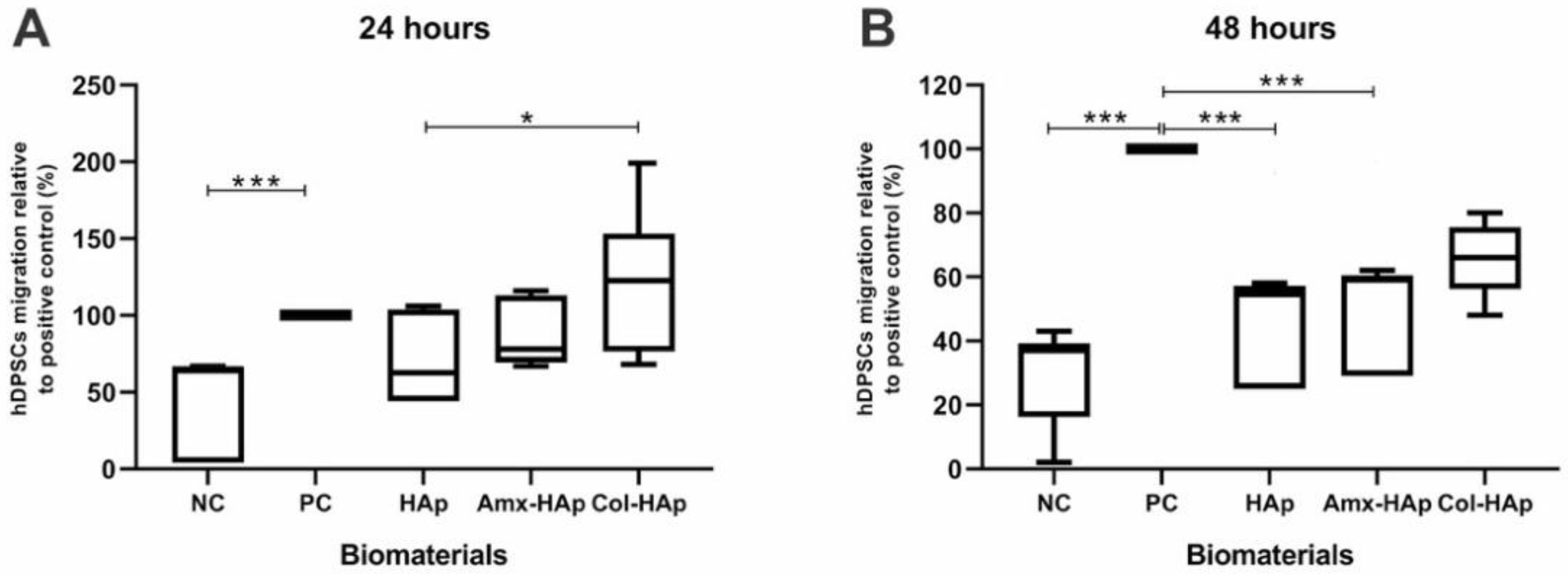
3.5. Results of Migration Assay
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pu′ad, N.M.; Koshy, P.; Abdullah, H.; Idris, M.I.; Lee, T. Syntheses of hydroxyapatite from natural sources. Heliyon 2019, 5, e01588. [Google Scholar] [CrossRef]
- Sadat-Shojai, M.; Khorasani, M.-T.; Dinpanah-Khoshdargi, E.; Jamshidi, A. Synthesis methods for nanosized hydroxyapatite with diverse structures. Acta Biomater. 2013, 9, 7591–7621. [Google Scholar] [CrossRef] [PubMed]
- Szcześ, A.; Holysz, L.; Chibowski, E. Synthesis of hydroxyapatite for biomedical applications. Adv. Colloid Interface Sci. 2017, 249, 321–330. [Google Scholar] [CrossRef]
- Grigoraviciute-Puroniene, I.; Tanaka, Y.; Vegelyte, V.; Nishimoto, Y.; Ishikawa, K.; Kareiva, A. A novel synthetic approach to low-crystallinity calcium deficient hydroxyapatite. Ceram. Int. 2019, 45, 15620–15623. [Google Scholar] [CrossRef]
- Murata, M.; Hino, J.; Kabir, A.; Yokozeki, K.; Sakamoto, M.; Nakajima, T.; Akazawa, T. Osteoinduction in novel micropores of partially dissolved and precipitated hydroxyapatite block in scalp of young rats. Materials 2021, 14, 196. [Google Scholar] [CrossRef]
- Prakash, J.; Kumar, T.S.; Venkataprasanna, K.; Niranjan, R.; Kaushik, M.; Samal, D.B.; Venkatasubbu, G.D. PVA/alginate/hydroxyapatite films for controlled release of amoxicillin for the treatment of periodontal defects. Appl. Surf. Sci. 2019, 495, 143543. [Google Scholar] [CrossRef]
- Prasanna, A.P.S.; Venkatasubbu, G.D. Sustained release of amoxicillin from hydroxyapatite nanocomposite for bone infections. Prog. Biomater. 2018, 7, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Bakhsheshi-Rad, H.R.; Hamzah, E.; Abbasizadeh, N.; Najafinezhad, A.; Kashefian, M. Synthesis of novel nanostructured bredigite–amoxicillin scaffolds for bone defect treatment: Cytocompatibility and antibacterial activity. J. Sol-Gel Sci. Technol. 2018, 86, 83–93. [Google Scholar] [CrossRef]
- Xing, F.; Chi, Z.; Yang, R.; Xu, D.; Cui, J.; Huang, Y.; Zhou, C.; Liu, C. Chitin-hydroxyapatite-collagen composite scaffolds for bone regeneration. Int. J. Biol. Macromol. 2021, 184, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Pennicooke, B.; Hussain, I.; Berlin, C.; Sloan, S.R.; Borde, B.; Moriguchi, Y.; Lang, G.; Navarro-Ramirez, R.; Cheetham, J.; Bonassar, L.J.; et al. Annulus fibrosus repair using high-density collagen gel an in vivo ovine model. Spine 2018, 43, E208–E215. [Google Scholar] [CrossRef]
- Moriguchi, Y.; Borde, B.; Berlin, C.; Wipplinger, C.; Sloan, S.R.; Kirnaz, S.; Pennicooke, B.; Navarro-Ramirez, R.; Khair, T.; Grunert, P.; et al. In vivo annular repair using high-density collagen gel seeded with annulus fibrosus cells. Acta Biomater. 2018, 79, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Mittal, S.; Bansal, R. Current overview on challenges in regenerative endodontics. J. Conserv. Dent. 2015, 18, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Yu, Y.; Zhang, G.; Tang, C.; Yu, J. A journey from dental pulp stem cells to a bio-tooth. Stem Cell Rev. Rep. 2010, 7, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, N.T.; Munévar, J.C.; González, J.M.; Infante, C.; Lara, S.J.P. In vitro response of dental pulp stem cells in 3D scaffolds: A regenerative bone material. Heliyon 2018, 4, e00775. [Google Scholar] [CrossRef]
- Khojasteh, A.; Motamedian, S.R.; Rad, M.R.; Shahriari, M.H.; Nadjmi, N. Polymeric vs hydroxyapatite-based scaffolds on dental pulp stem cell proliferation and differentiation. World J. Stem Cells 2015, 7, 1215–1221. [Google Scholar] [CrossRef]
- Bar, J.K.; Lis-Nawara, A.; Grelewski, P.G. Dental pulp stem cell-derived secretome and its regenerative potential. Int. J. Mol. Sci. 2021, 22, 12018. [Google Scholar] [CrossRef]
- Yanovska, A.; Husak, Y.; Mishchenko, O.; Gudakov, A.; Oleshko, O.; Yusupova, A.; Vielikov, M.; Radwan-Pragłowska, J.; Piątkowski, M.; Janus, Ł.; et al. Cell viability and collagen deposition on hydroxyapatite coatings formed on pretreated substrates. Mater. Chem. Phys. 2020, 258, 123978. [Google Scholar] [CrossRef]
- Simomukay, E.; Souza, E.; Antunes, S.R.M.; Borges, C.P.F.; Michel, M.D.; Antunes, A.C. Biocerâmicas aditivadas com nióbio (V): Avaliação da rota hidrotérmica modificada com ácido cítrico e ureia para obtenção de hidroxiapatitas modificadas. Ceramica 2016, 62, 9–14. [Google Scholar] [CrossRef][Green Version]
- Mendes, J.B.E.; Riekes, M.K.; de Oliveira, V.M.; Michel, M.D.; Stulzer, H.K.; Khalil, N.M.; Zawadzki, S.F.; Mainardes, R.M.; Farago, P.V. PHBV/PCL microparticles for controlled release of resveratrol: Physicochemical characterization, antioxidant potential, and effect on hemolysis of human erythrocytes. Sci. World J. 2012, 2012, 542937. [Google Scholar] [CrossRef]
- Galler, K.M.; Widbiller, M.; Buchalla, W.; Eidt, A.; Hiller, K.A.; Hoffer, P.C.; Schmalz, G.H. EDTA conditioning of dentine promotes adhesion, migration and differentiation of dental pulp stem cells. Int. Endod. J. 2015, 49, 581–590. [Google Scholar] [CrossRef]
- Stanić, V.; Dimitrijevic-Brankovic, S.; Stankovic, J.A.; Mitrić, M.; Jokić, B.; Plećaš, I.B.; Raičević, S. Synthesis, characterization and antimicrobial activity of copper and zinc-doped hydroxyapatite nanopowders. Appl. Surf. Sci. 2010, 256, 6083–6089. [Google Scholar] [CrossRef]
- Pupo, Y.M.; Nadal, J.M.; Maluf, D.F.; De Lara, E.L.; Saito, R.E.; Michél, M.D.; Antunes, S.R.M.; Toledo, M.D.G.; Gomes, J.C.; Farago, P.V. Effect of hydroxyapatite morphology and quaternary ammonium polymer co-inclusion on bond strength, cytotoxicity, and cell morphology of self-etching adhesive. Int. J. Adhes. Adhes. 2019, 92, 7–15. [Google Scholar] [CrossRef]
- Bisson-Boutelliez, C.; Fontanay, S.; Finance, C.; Kedzierewicz, F. Preparation and physicochemical characterization of amoxicillin β-cyclodextrin complexes. AAPS PharmSciTech 2010, 11, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Riaz, T.; Zeeshan, R.; Zarif, F.; Ilyas, K.; Muhammad, N.; Safi, S.Z.; Rahim, A.; Rizvi, S.A.A.; Rehman, I.U. FTIR analysis of natural and synthetic collagen. Appl. Spectrosc. Rev. 2018, 53, 703–746. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Tuna, E.B.; Oshida, Y.; Ozen, B.; Gjorgievska, E.; Tuzuner, T. Biomaterials for dental applications. BioMed Res. Int. 2017, 2017, 2520536. [Google Scholar] [CrossRef]
- Zhao, X.-Y.; Zhu, Y.-J.; Chen, F.; Lu, B.-Q.; Qi, C.; Zhao, J.; Wu, J. Hydrothermal synthesis of hydroxyapatite nanorods and nanowires using riboflavin-5′-phosphate monosodium salt as a new phosphorus source and their application in protein adsorption. CrystEngComm 2013, 15, 7926–7935. [Google Scholar] [CrossRef]
- Xu, Y.-J.; Dong, L.; Lu, Y.; Zhang, L.-C.; An, D.; Gao, H.-L.; Yang, D.-M.; Hu, W.; Sui, C.; Xu, W.-P.; et al. Magnetic hydroxyapatite nanoworms for magnetic resonance diagnosis of acute hepatic injury. Nanoscale 2015, 8, 1684–1690. [Google Scholar] [CrossRef]
- Qi, C.; Zhu, Y.-J.; Wu, C.-T.; Sun, T.-W.; Jiang, Y.-Y.; Zhang, Y.-G.; Wu, J.; Chen, F. Sonochemical synthesis of hydroxyapatite nanoflowers using creatine phosphate disodium salt as an organic phosphorus source and their application in protein adsorption. RSC Adv. 2016, 6, 9686–9692. [Google Scholar] [CrossRef]
- Yang, H.; Hao, L.; Zhao, N.; Du, C.; Wang, Y. Hierarchical porous hydroxyapatite microsphere as drug delivery carrier. CrystEngComm 2013, 15, 5760–5763. [Google Scholar] [CrossRef]
- Duan, H.; Ma, Y.; Liu, X.; Hao, L.; Zhao, N. Hierarchically nanostructured hydroxyapatite microspheres as drug delivery carriers and their effects on cell viability. RSC Adv. 2015, 5, 83522–83529. [Google Scholar] [CrossRef]
- Long, T.; Guo, Y.-P.; Liu, Y.-Z.; Zhu, Z.-A. Hierarchically nanostructured mesoporous carbonated hydroxyapatite microspheres for drug delivery systems with high drug-loading capacity. RSC Adv. 2013, 3, 24169–24176. [Google Scholar] [CrossRef]
- Qi, C.; Zhu, Y.-J.; Lu, B.-Q.; Zhao, X.-Y.; Zhao, J.; Chen, F. Hydroxyapatite nanosheet-assembled porous hollow microspheres: DNA-templated hydrothermal synthesis, drug delivery and protein adsorption. J. Mater. Chem. 2012, 22, 22642–22650. [Google Scholar] [CrossRef]
- Yu, Y.-D.; Zhu, Y.-J.; Qi, C.; Wu, J. Hydroxyapatite nanorod-assembled hierarchical microflowers: Rapid synthesis via microwave hydrothermal transformation of CaHPO4 and their application in protein/drug delivery. Ceram. Int. 2017, 43, 6511–6518. [Google Scholar] [CrossRef]
- He, Q.; Pan, L.; Wang, Y.; Meldrum, F.C. Bioinspired synthesis of large-pore, mesoporous hydroxyapatite nanocrystals for the controlled release of large pharmaceutics. Cryst. Growth Des. 2015, 15, 723–731. [Google Scholar] [CrossRef]
- Lelli, M.; Roveri, N.; Marzano, C.; Hoeschele, J.D.; Curci, A.; Margiotta, N.; Gandin, V.; Natile, G. Hydroxyapatite nanocrystals as a smart, pH sensitive, delivery system for kiteplatin. Dalton Trans. 2016, 45, 13187–13195. [Google Scholar] [CrossRef]
- Yang, P.; Quan, Z.; Li, C.; Kang, X.; Lian, H.; Lin, J. Bioactive, luminescent and mesoporous europium-doped hydroxyapatite as a drug carrier. Biomaterials 2008, 29, 4341–4347. [Google Scholar] [CrossRef]
- Zou, Z.; Liu, X.; Chen, L.; Lin, K.; Chang, J. Dental enamel-like hydroxyapatite transformed directly from monetite. J. Mater. Chem. 2012, 22, 22637–22641. [Google Scholar] [CrossRef]
- Calabrese, G.; Giuffrida, R.; Fabbi, C.; Figallo, E.; Lo Furno, D.; Gulino, R.; Colarossi, C.; Fullone, F.; Giuffrida, R.; Parenti, R.; et al. Collagen-hydroxyapatite scaffolds induce human adipose derived stem cells osteogenic differentiation in vitro. PLoS ONE 2016, 11, e0151181. [Google Scholar] [CrossRef]
- Pasqui, D.; Torricelli, P.; De Cagna, M.; Fini, M.; Barbucci, R. Carboxymethyl cellulose-hydroxyapatite hybrid hydrogel as a composite material for bone tissue engineering applications. J. Biomed. Mater. Res. Part A 2013, 102, 1568–1579. [Google Scholar] [CrossRef]
- Oryan, A.; Alidadi, S.; Moshiri, A.; Maffulli, N. Bone regenerative medicine: Classic options, novel strategies, and future directions. J. Orthop. Surg. Res. 2014, 9, 18. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, L.L.; Resende, C.X.; Tavares, D.S.; Soares, G.A.; Castro, L.O.; Granjeiro, J. Cytocompatibility of chitosan and collagen-chitosan scaffolds for tissue engineering. Polímeros 2011, 21, 1–6. [Google Scholar] [CrossRef]
- Soares, I.M.V.; Fernandes, G.V.D.O.; Cavalcante, L.C.; Leite, Y.K.P.D.C.; Bezerra, D.D.O.; De Carvalho, M.A.M.; Carvalho, C.M.R.S.; Cordeiro, C.L. The influence of Aloe vera with mesenchymal stem cells from dental pulp on bone regeneration: Characterization and treatment of non-critical defects of the tibia in rats. J. Appl. Oral Sci. 2019, 27, e20180103. [Google Scholar] [CrossRef] [PubMed]
- Justus, C.R.; Leffler, N.; Ruiz-Echevarria, M.; Yang, L.V. In vitro cell migration and invasion assays. J. Vis. Exp. 2014, e51046. [Google Scholar] [CrossRef]
- Flocea, P.; Popa, M.; Munteanu, F.; Vereştiuc, L. Collagen-hydroxyapatite composites with applications as bone substitutes: Synthesis and characterisation. Rev. Med. Chir. Soc. Med. Nat. Iasi. 2009, 113, 286–292. [Google Scholar] [PubMed]

Publisher′s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pupo, Y.M.; Leite, L.M.B.; Senegaglia, A.C.; Antunes, L.; Nadal, J.M.; de Lara, E.L.; Saito, R.E.; Antunes, S.R.M.; Lacerda, W.F.; Farago, P.V. Effect of Hydroxyapatite Microspheres, Amoxicillin–Hydroxyapatite and Collagen–Hydroxyapatite Composites on Human Dental Pulp-Derived Mesenchymal Stem Cells. Materials 2021, 14, 7515. https://doi.org/10.3390/ma14247515
Pupo YM, Leite LMB, Senegaglia AC, Antunes L, Nadal JM, de Lara EL, Saito RE, Antunes SRM, Lacerda WF, Farago PV. Effect of Hydroxyapatite Microspheres, Amoxicillin–Hydroxyapatite and Collagen–Hydroxyapatite Composites on Human Dental Pulp-Derived Mesenchymal Stem Cells. Materials. 2021; 14(24):7515. https://doi.org/10.3390/ma14247515
Chicago/Turabian StylePupo, Yasmine Mendes, Lidiane Maria Boldrini Leite, Alexandra Cristina Senegaglia, Liziane Antunes, Jessica Mendes Nadal, Eliane Leal de Lara, Rafael Eiji Saito, Sandra Regina Masetto Antunes, William Fernandes Lacerda, and Paulo Vitor Farago. 2021. "Effect of Hydroxyapatite Microspheres, Amoxicillin–Hydroxyapatite and Collagen–Hydroxyapatite Composites on Human Dental Pulp-Derived Mesenchymal Stem Cells" Materials 14, no. 24: 7515. https://doi.org/10.3390/ma14247515
APA StylePupo, Y. M., Leite, L. M. B., Senegaglia, A. C., Antunes, L., Nadal, J. M., de Lara, E. L., Saito, R. E., Antunes, S. R. M., Lacerda, W. F., & Farago, P. V. (2021). Effect of Hydroxyapatite Microspheres, Amoxicillin–Hydroxyapatite and Collagen–Hydroxyapatite Composites on Human Dental Pulp-Derived Mesenchymal Stem Cells. Materials, 14(24), 7515. https://doi.org/10.3390/ma14247515






