Structural, Physical, and Mechanical Analysis of ZnO and TiO2 Nanoparticle-Reinforced Self-Adhesive Coating Restorative Material
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Fourier Transform Infrared (FTIR) Spectroscopy
2.3. X-ray Diffraction (XRD) Analysis
2.4. Scanning Electron Microscopy (SEM)
2.5. Thermal Gravimetric Analysis/Differential Scanning Calorimetric Analysis (TGA/DSC)
2.6. Vickers Micro-Hardness (VH) Test
2.7. Water Sorption Analysis
2.8. Statistical Analysis
3. Results
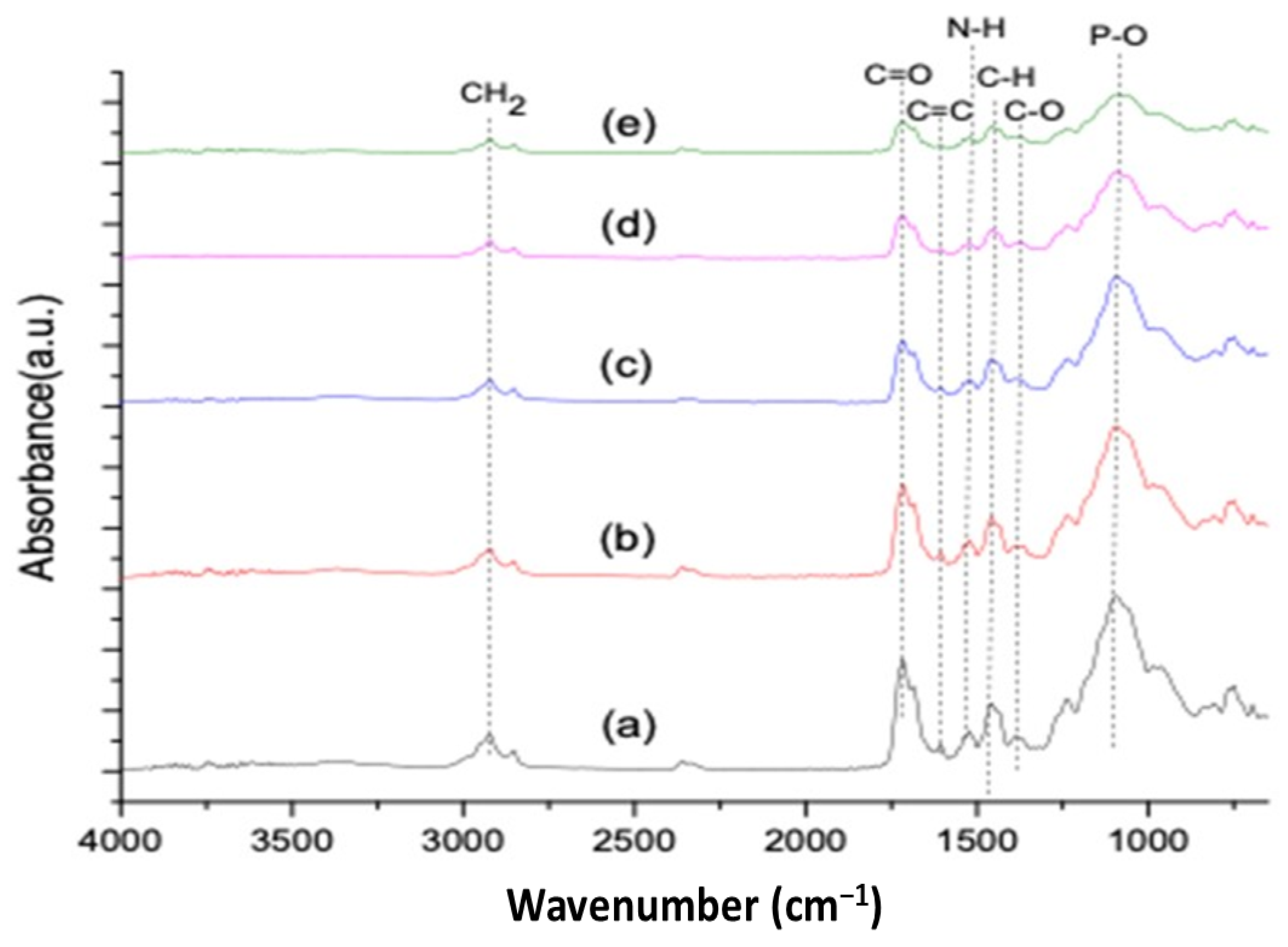
3.1. Fourier Transform Infrared Spectroscopy
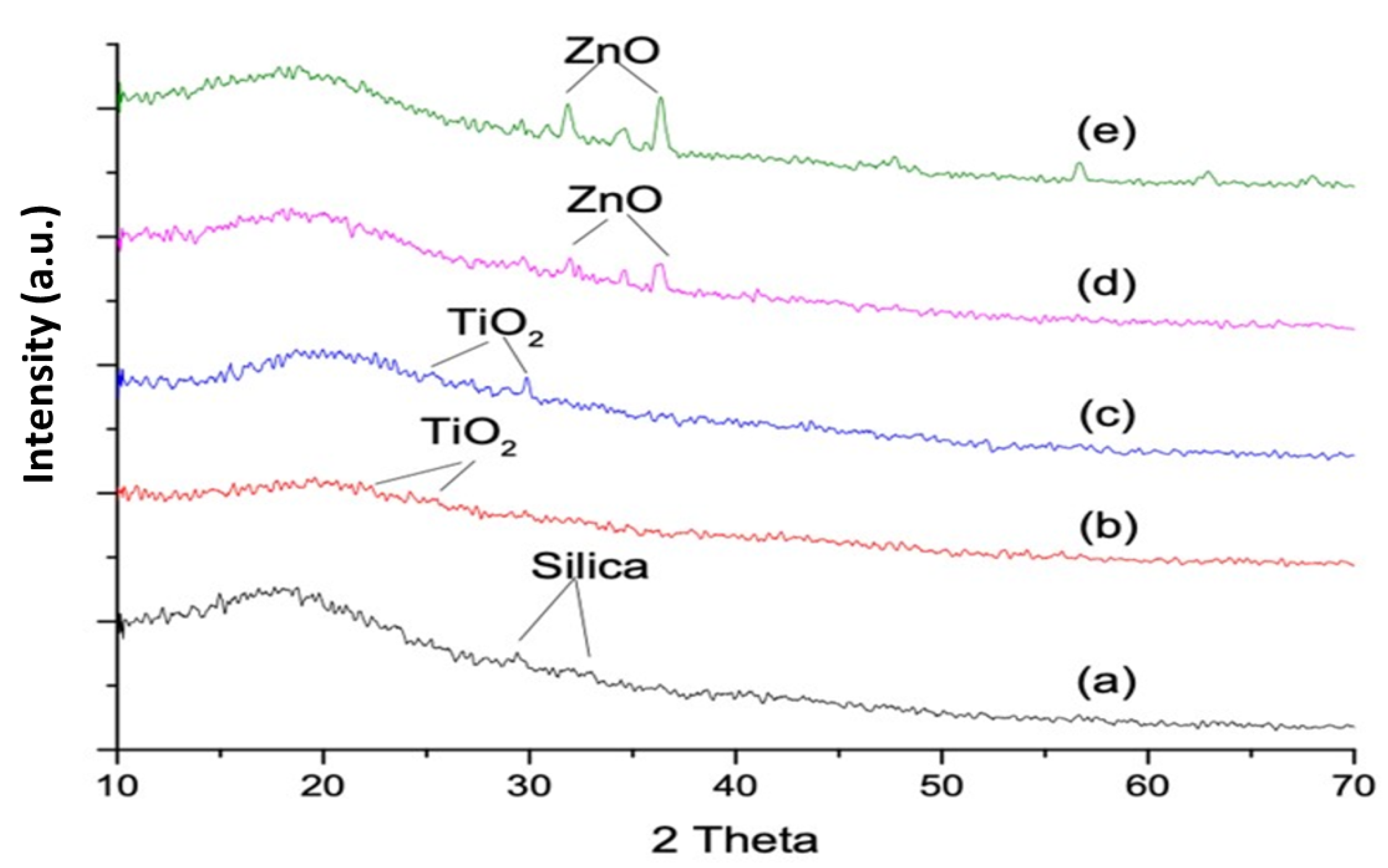
3.2. X-ray Diffraction Analysis
3.3. Scanning Electron Microscopy
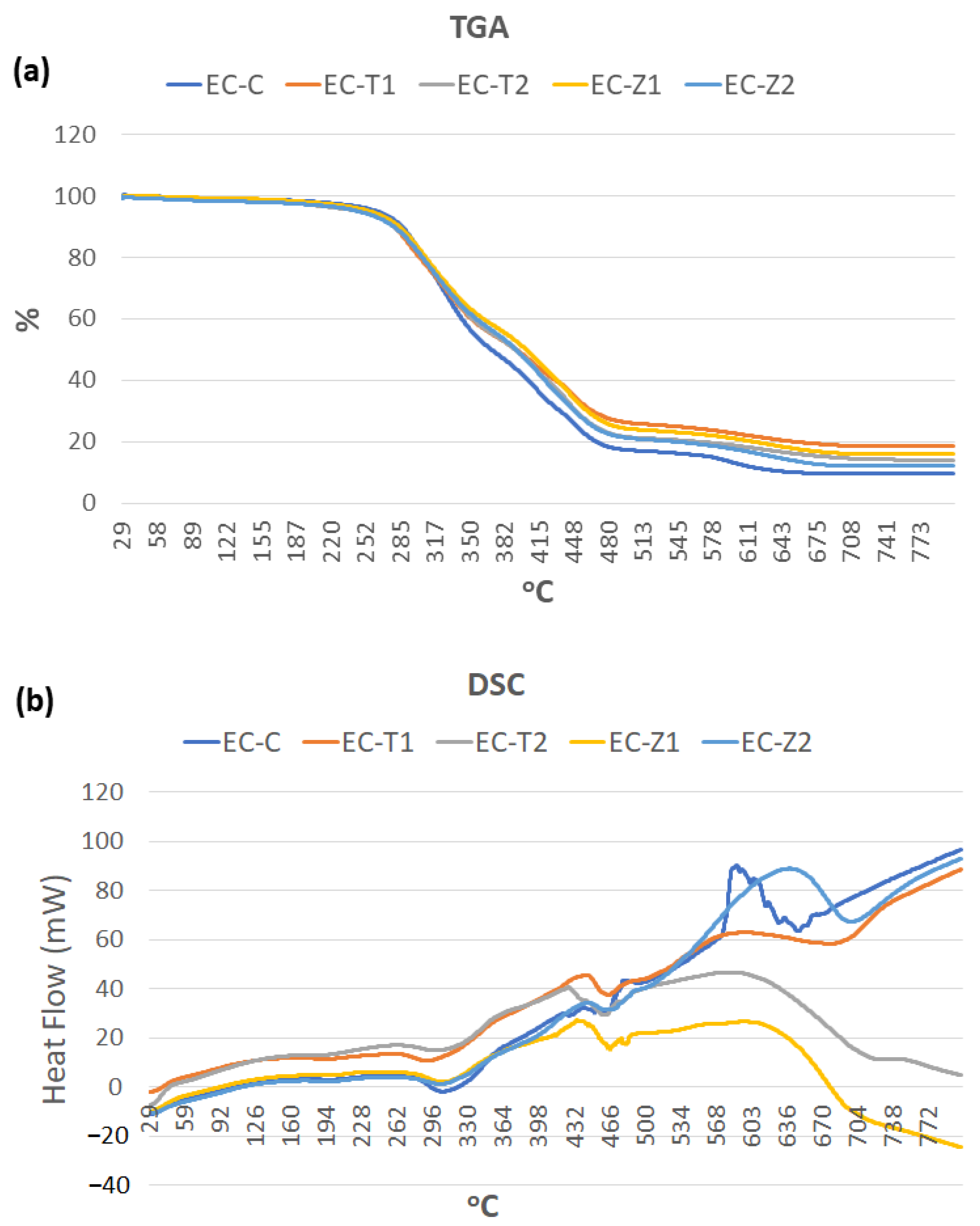
3.4. Thermogravimetric Analysis and Differential Scanning Calorimetry
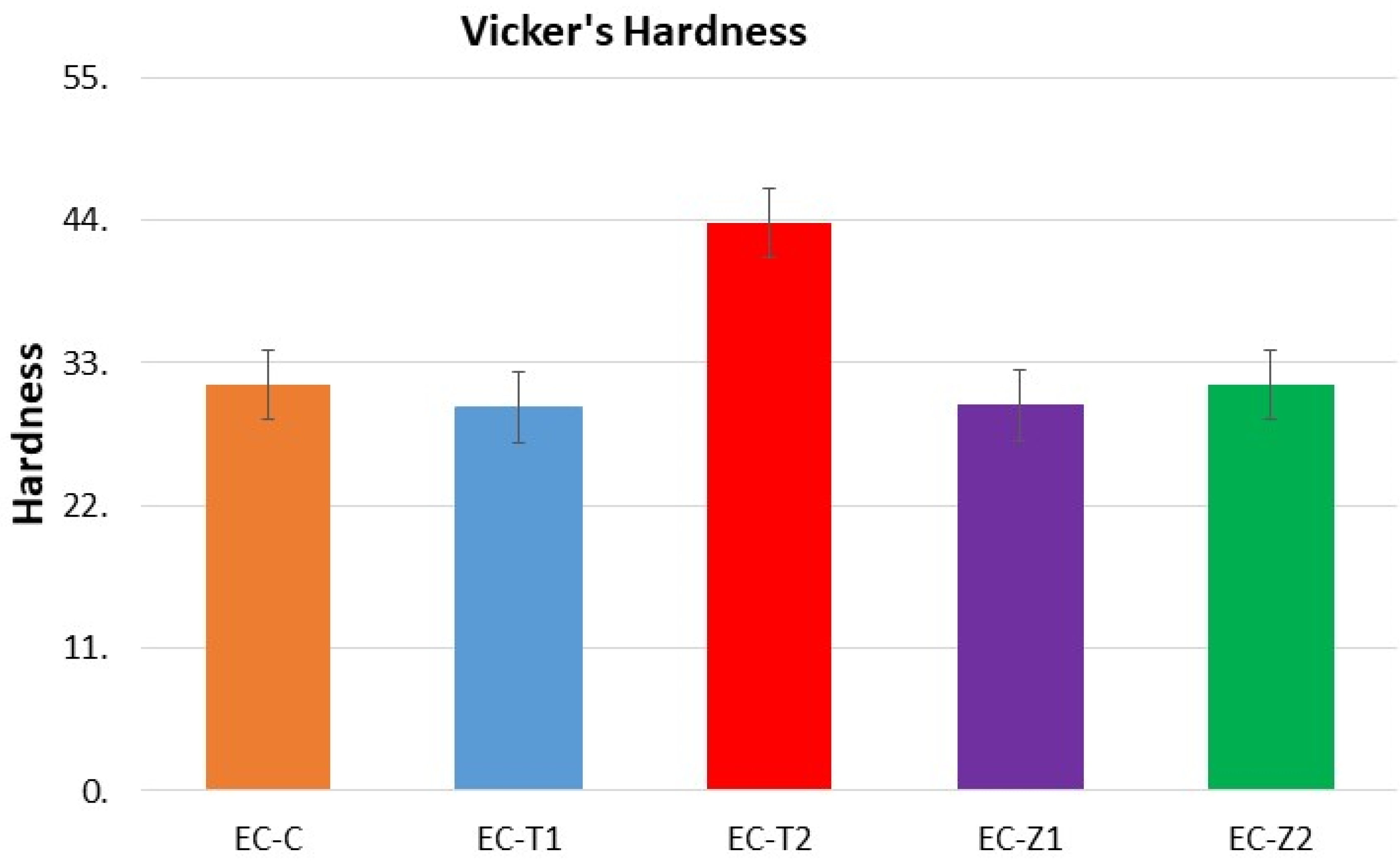
3.5. Vickers Micro-Hardness Test
3.6. Water Sorption Analysis
4. Discussion
5. Conclusions
- The spectral changes were observed at the carbonyl region (1711 cm−1), and the deformation of the N–H peak was observed at 1521 cm−1. The change in peak intensity was more so in the ZnO-based groups compared to the TiO2-based samples;
- The SEM/EDS confirmed the uniform distribution of samples, whereby the XRD pattern of the ZnO-based samples showed characteristic peaks corresponding to the hexagonal phase with a wurtzite structure, and the anatase phase was confirmed for the TiO2-based samples;
- The TGA and DSC curves showed that the thermal decomposition and glass transition temperature increased with higher concentrations of nanoparticles, indicating a strong molecular interaction of organic–inorganic components. The sequence of weight loss (%) was EC-C > EC-T1 > EC-Z1 > EC-Z2 > EC-T2, whereby the initial degradation temperature was in the order: EC-Z2 > EC-Z1 > EC-T1 > EC-T2 > EC-C. The observed sequence for Tg was above the range of the intraoral temperature;
- The increase in the concentration of spherical TiO2 nanoparticles resulted in increased micro-hardness values contrary to the micro-hardness of the ZnO-nanoparticle-reinforced material. Experimental adhesive with a 2 wt.% of TiO2 showed the highest hardness values compared to all other groups, and the 1 wt.% of the TiO2-based samples showed the lowest hardness values;
- The control group (i.e., EQUIA coat) showed the maximum water absorption after 7 days. The ZnO 2 wt.%-based samples showed the lowest water absorption value;
- Future studies may also evaluate the in vitro biological (microbiological and cytotoxicity) performances of these experimental materials in addition to the detailed mechanical testing and elution analysis of organic/inorganic components.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmed, K.E.; Murbay, S. Survival rates of anterior composites in managing tooth wear: Systematic review. J. Oral Rehabil. 2016, 43, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Fuhrmann, D.; Murchison, D.; Whipple, S.; Vandewalle, K. Properties of new glass-ionomer restorative systems marketed for stress-bearing areas. Oper. Dent. 2020, 45, 104–110. [Google Scholar] [CrossRef]
- Nicholson, J.W.; Sidhu, S.K.; Czarnecka, B. Enhancing the mechanical properties of glass-ionomer dental cements: A review. Materials 2020, 13, 2510. [Google Scholar] [CrossRef] [PubMed]
- Moshaverinia, M.; Navas, A.; Jahedmanesh, N.; Shah, K.C.; Moshaverinia, A.; Ansari, S. Comparative evaluation of the physical properties of a reinforced glass ionomer dental restorative material. J. Prosthet. Dent. 2019, 122, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Chadwick, B.L.; Evans, D.J.P. Restoration of class II cavities in primary molar teeth with conventional and resin modified glass ionomer cements: A systematic review of the literature. Eur. Arch. Paediatr. Dent. 2007, 8, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Francisconi, L.F.; Scaffa, P.M.C.; de Barros, V.R.D.S.P.; Coutinho, M.; Francisconi, P.A.S. Glass ionomer cements and their role in the restoration of non-carious cervical lesions. J. Appl. Oral Sci. 2009, 17, 364–369. [Google Scholar] [CrossRef]
- Brkanović, S.; Ivanišević, A.; Miletić, I.; Mezdić, D.; Jukić Krmek, S. Effect of Nano-Filled Protective Coating and Different pH Enviroment on Wear Resistance of New Glass Hybrid Restorative Material. Materials 2021, 14, 755. [Google Scholar] [CrossRef] [PubMed]
- Hutchison, C.; Cave, V. 10 year comparison of glass ionomer and composite resin restoration materials in class 1 and 2 cavities. Evid. Based Dent. 2019, 20, 113–114. [Google Scholar] [CrossRef]
- Tyagi, S.; Thomas, A.M.; Sinnappah-Kang, N.D. A comparative evaluation of resin-and varnish-based surface protective agents on glass ionomer cement—A spectrophotometric analysis. Biomater. Investig. Dent. 2020, 7, 25–30. [Google Scholar] [CrossRef]
- Ong, J.E.-X.; Yap, A.; Hong, J.Y.; Eweis, A.H.; Yahya, N.A. Viscoelastic properties of contemporary bulk-fill restoratives: A dynamic-mechanical analysis. Oper. Dent. 2018, 43, 307–314. [Google Scholar] [CrossRef]
- Najeeb, S.; Khurshid, Z.; Zafar, M.S.; Khan, A.S.; Zohaib, S.; Martí, J.M.N.; Sauro, S.; Matinlinna, J.P.; Rehman, I.U. Modifications in glass ionomer cements: Nano-sized fillers and bioactive nanoceramics. Int. J. Mol. Sci. 2016, 17, 1134. [Google Scholar] [CrossRef] [PubMed]
- Brito, C.R.; Velasco, L.G.; Bonini, G.A.; Imparato, J.C.P.; Raggio, D.P. Glass ionomer cement hardness after different materials for surface protection. J. Biomed. Mater. Res. Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2010, 93, 243–246. [Google Scholar] [CrossRef]
- Gurgan, S.; Kutuk, Z.B.; Ergin, E.; Oztas, S.S.; Cakir, F.Y. Clinical performance of a glass ionomer restorative system: A 6-year evaluation. Clin. Oral Investig. 2017, 21, 2335–2343. [Google Scholar] [CrossRef] [PubMed]
- Gurgan, S.; Kutuk, Z.; Ergin, E.; Oztas, S.; Cakir, F. Four-year randomized clinical trial to evaluate the clinical performance of a glass ionomer restorative system. Oper. Dent. 2015, 40, 134–143. [Google Scholar] [CrossRef]
- Kielbassa, A.M.; Oehme, E.P.; Shakavets, N.; Wolgin, M. In vitro wear of (resin-coated) high-viscosity glass ionomer cements and glass hybrid restorative systems. J. Dent. 2021, 105, 103554. [Google Scholar] [CrossRef] [PubMed]
- Kanik, Ö.; Turkun, L.S.; Dasch, W. In vitro abrasion of resin-coated highly viscous glass ionomer cements: A confocal laser scanning microscopy study. Clin. Oral Investig. 2017, 21, 821–829. [Google Scholar] [CrossRef] [PubMed]
- Hesse, D.; Bonifácio, C.C.; Kleverlaan, C.J.; Raggio, D.P. Clinical wear of approximal glass ionomer restorations protected with a nanofilled self-adhesive light-cured protective coating. J. Appl. Oral Sci. 2018, 26, e20180094. [Google Scholar] [CrossRef]
- Molina, G.F.; Cabral, R.J.; Mazzola, I.; Lascano, L.B.; Frencken, J.E. Mechanical performance of encapsulated restorative glass-ionomer cements for use with Atraumatic Restorative Treatment (ART). J. Appl. Oral Sci. 2013, 21, 243–249. [Google Scholar] [CrossRef]
- Friedl, K.; Hiller, K.-A.; Friedl, K.-H. Clinical performance of a new glass ionomer based restoration system: A retrospective cohort study. Dent. Mater. 2011, 27, 1031–1037. [Google Scholar] [CrossRef]
- Bagheri, R.; Taha, N.; Azar, M.; Burrow, M. Effect of G-Coat Plus on the mechanical properties of glass-ionomer cements. Aust. Dent. J. 2013, 58, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Hamouda, I.M. Current perspectives of nanoparticles in medical and dental biomaterials. J. Biomed. Res. 2012, 26, 143–151. [Google Scholar] [CrossRef]
- Farrugia, C.; Camilleri, J. Antimicrobial properties of conventional restorative filling materials and advances in antimicrobial properties of composite resins and glass ionomer cements—A literature review. Dent. Mater. 2015, 31, e89–e99. [Google Scholar] [CrossRef]
- Lee, J.K.; Choi, J.Y.; Lim, B.S.; Lee, Y.K.; Sakaguchi, R.L. Change of properties during storage of a UDMA/TEGDMA dental resin. J. Biomed. Mater. Res. Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2004, 68, 216–221. [Google Scholar] [CrossRef]
- Garcia-Contreras, R.; Scougall-Vilchis, R.J.; Contreras-Bulnes, R.; Sakagami, H.; Morales-Luckie, R.A.; Nakajima, H. Mechanical, antibacterial and bond strength properties of nano-titanium-enriched glass ionomer cement. J. Appl. Oral Sci. 2015, 23, 321–328. [Google Scholar] [CrossRef]
- Vanajassun, P.P.; Nivedhitha, M.; Nishad, N.; Soman, D. Effects of zinc oxide nanoparticles in combination with conventional glass ionomer cement: In vitro study. Adv. Hum. Biol. 2014, 4, 31. [Google Scholar]
- Nasir, M.; Rauf, S.; Muhammad, N.; Nawaz, M.H.; Chaudhry, A.A.; Malik, M.H.; Shahid, S.A.; Hayat, A. Biomimetic nitrogen doped titania nanoparticles as a colorimetric platform for hydrogen peroxide detection. J. Colloid Interface Sci. 2017, 505, 1147–1157. [Google Scholar] [CrossRef]
- Ahtzaz, S.; Nasir, M.; Shahzadi, L.; Amir, W.; Anjum, A.; Arshad, R.; Iqbal, F.; Chaudhry, A.A.; Yar, M.; ur Rehman, I. A study on the effect of zinc oxide and zinc peroxide nanoparticles to enhance angiogenesis-pro-angiogenic grafts for tissue regeneration applications. Mater. Des. 2017, 132, 409–418. [Google Scholar] [CrossRef]
- Shatnawi, M.; Alsmadi, A.; Bsoul, I.; Salameh, B.; Mathai, M.; Alnawashi, G.; Alzoubi, G.M.; Al-Dweri, F.; Bawa’aneh, M. Influence of Mn doping on the magnetic and optical properties of ZnO nanocrystalline particles. Results Phys. 2016, 6, 1064–1071. [Google Scholar] [CrossRef]
- Main, K. Development of Composites for Bone Repair. Ph.D. Thesis, University College London, London, UK, 2017. [Google Scholar]
- Buruiana, T.; Melinte, V.; Aldea, H.; Pelin, I.M.; Buruiana, E.C. A new fluorinated urethane dimethacrylate with carboxylic groups for use in dental adhesive compositions. Mater. Sci. Eng. C 2016, 62, 96–104. [Google Scholar] [CrossRef]
- Soares, R.; Carone, C.; Einloft, S.; Ligabue, R.; Monteiro, W. Synthesis and characterization of waterborne polyurethane/ZnO composites. Polym. Bull. 2014, 71, 829–838. [Google Scholar] [CrossRef]
- Nguyen, T.V.; Nguyen, T.A.; Dao, P.H.; Nguyen, A.H.; Do, M.T. Effect of rutile titania dioxide nanoparticles on the mechanical property, thermal stability, weathering resistance and antibacterial property of styrene acrylic polyurethane coating. Adv. Nat. Sci. Nanosci. Nanotechnol. 2016, 7, 045015. [Google Scholar] [CrossRef]
- Quiñones-Galván, J.; Sandoval-Jiménez, I.; Tototzintle-Huitle, H.; Hernández-Hernández, L.; de Moure-Flores, F.; Hernández-Hernández, A.; Campos-González, E.; Guillén-Cervantes, A.; Zelaya-Angel, O.; Araiza-Ibarra, J. Effect of precursor solution and annealing temperature on the physical properties of Sol–Gel-deposited ZnO thin films. Results Phys. 2013, 3, 248–253. [Google Scholar] [CrossRef]
- Talam, S.; Karumuri, S.R.; Gunnam, N. Synthesis, characterization, and spectroscopic properties of ZnO nanoparticles. Int. Sch. Res. Not. 2012, 2012, 372505. [Google Scholar] [CrossRef]
- Onwubu, S.C.; Mdluli, P.S.; Singh, S.; Bharuth, V. Remineralization Potential of a Modified Eggshell–Titanium Composite-Scanning Electron Microscope Study. Eur. J. Dent. 2019, 13, 187–192. [Google Scholar] [CrossRef]
- Senthilkumar, S.; Sivakumar, T. Green tea (Camellia sinensis) mediated synthesis of zinc oxide (ZnO) nanoparticles and studies on their antimicrobial activities. Int. J. Pharm. Pharm. Sci. 2014, 6, 461–465. [Google Scholar]
- Aydin Sevinç, B.; Hanley, L. Antibacterial activity of dental composites containing zinc oxide nanoparticles. J. Biomed. Mater. Res. Part B Appl. Biomater. J. 2010, 94, 22–31. [Google Scholar] [CrossRef]
- Allaker, R.P.; Memarzadeh, K. Nanoparticles and the control of oral infections. Int. J. Antimicrob. Agents 2014, 43, 95–104. [Google Scholar] [CrossRef]
- Bukhari, J.H.; Khan, A.S.; Ijaz, K.; Zahid, S.; Chaudhry, A.A.; Kaleem, M. Low-temperature flow-synthesis-assisted urethane-grafted zinc oxide-based dental composites: Physical, mechanical, and antibacterial responses. J. Mater. Sci. Mater. Med. 2021, 32, 87. [Google Scholar] [CrossRef]
- Anaya-Esparza, L.M.; Villagrán-de la Mora, Z.; Ruvalcaba-Gómez, J.M.; Romero-Toledo, R.; Sandoval-Contreras, T.; Aguilera-Aguirre, S.; Montalvo-González, E.; Pérez-Larios, A. Use of Titanium Dioxide (TiO2) Nanoparticles as Reinforcement Agent of Polysaccharide-Based Materials. Process 2020, 8, 1395. [Google Scholar] [CrossRef]
- Sodagar, A.; Akhoundi, M.S.A.; Bahador, A.; Jalali, Y.F.; Behzadi, Z.; Elhaminejad, F.; Mirhashemi, A.H. Effect of TiO2 nanoparticles incorporation on antibacterial properties and shear bond strength of dental composite used in Orthodontics. Dent. Press J. Orthod. 2017, 22, 67–74. [Google Scholar] [CrossRef]
- Poomalai, P.; Varghese, T.O.; Siddaramaiah. Thermomechanical Behaviour of Poly(methyl methacrylate)/Copoly(ether-ester) Blends. ISRN Mater. Sci. 2011, 2011, 921293. [Google Scholar] [CrossRef][Green Version]
- Sideridou, I.D.; Karabela, M.M.; Vouvoudi, E. Physical properties of current dental nanohybrid and nanofill light-cured resin composites. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2011, 27, 598–607. [Google Scholar] [CrossRef]
- Gilman, J.W.; Awad, W.H.; Davis, R.D.; Shields, J.; Harris, R.H.; Davis, C.; Morgan, A.B.; Sutto, T.E.; Callahan, J.; Trulove, P.C.; et al. Polymer/Layered Silicate Nanocomposites from Thermally Stable Trialkylimidazolium-Treated Montmorillonite. Chem. Mater. 2002, 14, 3776–3785. [Google Scholar] [CrossRef]
- Khan, A.S.; Khan, R.S.; Khan, M.; Rehman, I.U. Incorporation of nanoparticles in glass ionomer cements: Clinical applications, properties, and future perspectives. In Nanobiomaterials in Clinical Dentistry, 2nd ed.; Subramani, K., Ahmed, W., Eds.; Elsevier: London, UK, 2019; pp. 113–138. [Google Scholar]
- Erickson, K.L. Thermal Decomposition of Polymers in Nitrogen and in Air; No. SAND2007-5405C; Sandia National Lab (SNL-NM): Albuquerque, NM, USA, 2007. [Google Scholar]
- Barclay, C.W.; Spence, D.; Laird, W.R. Intra-oral temperatures during function. J. Oral Rehabil. 2005, 32, 886–894. [Google Scholar] [CrossRef]
- Younas, B.; Khan, A.S.; Muzaffar, D.; Hussain, I.; Chaudhry, A.A.; Rehman, I.U. In situ reaction kinetic analysis of dental restorative materials. EPJ Appl. 2013, 64, 30701. [Google Scholar] [CrossRef]
- Lohbauer, U.; Zinelis, S.; Rahiotis, C.; Petschelt, A.; Eliades, G. The effect of resin composite pre-heating on monomer conversion and polymerization shrinkage. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2009, 25, 514–519. [Google Scholar] [CrossRef]
- Nayyer, M.; Zahid, S.; Hassan, S.H.; Mian, S.A.; Mehmood, S.; Khan, H.A.; Kaleem, M.; Zafar, M.S.; Khan, A.S. Comparative abrasive wear resistance and surface analysis of dental resin-based materials. Eur. J. Dent. 2018, 12, 57–66. [Google Scholar] [CrossRef]
- Bociong, K.; Szczesio, A.; Sokolowski, K.; Domarecka, M.; Sokolowski, J.; Krasowski, M.; Lukomska-Szymanska, M. The Influence of Water Sorption of Dental Light-Cured Composites on Shrinkage Stress. Materials 2017, 10, 1142. [Google Scholar] [CrossRef]
- Kasraei, S.; Azarsina, M. Addition of silver nanoparticles reduces the wettability of methacrylate and silorane-based composites. Braz. Oral Res. 2012, 26, 505–510. [Google Scholar] [CrossRef]
- Acosta-Torres, L.S.; Lopez-Marin, L.M.; Nunez-Anita, R.E.; Hernandez-Padron, G.; Castano, V.M. Biocompatible metal-oxide nanoparticles: Nanotechnology improvement of conventional prosthetic acrylic resins. J. Nanomater. 2011, 2011, 941561. [Google Scholar] [CrossRef]


| Sr. | Groups | wt.% of Nanoparticles |
|---|---|---|
| 1 | EC-C | EQUIA coat-Control |
| 2 | EC-T1 | TiO2 1 wt.% + EC |
| 3 | EC-T2 | TiO2 2 wt.% + EC |
| 4 | EC-Z1 | ZnO 1 wt.% + EC |
| 5 | EC-Z2 | ZnO 2 wt.% + EC |
| Wavenumber (cm−1) | Assigned Groups | Absorbance (Intensity) | ||||
|---|---|---|---|---|---|---|
| EC-C | EC-T1 | EC-T2 | EC-Z1 | EC-Z2 | ||
| 2923 | CH2 | 0.12 | 0.11 | 0.10 | 0.06 | 0.03 |
| 1716 | C=O | 0.38 | 0.31 | 0.22 | 0.22 | 0.11 |
| 1606 | C=C | 0.09 | 0.08 | 0.08 | 0.03 | 0.03 |
| 1521 | N–H | 0.14 | 0.13 | 0.10 | 0.05 | 0.04 |
| 1457 | C–H | 0.22 | 0.21 | 0.18 | 0.09 | 0.09 |
| 1386 | C–O | 0.11 | 0.11 | 0.11 | 0.09 | 0.05 |
| 1050 | C–O–C | 0.58 | 0.49 | 0.44 | 0.29 | 0.19 |
| Groups | TGA (Weight Loss Percentage) | DSC (Thermal Profile) | |||||
|---|---|---|---|---|---|---|---|
| 100 °C | 300 °C | 350 °C | 450 °C | 600 °C | Tg | IDT | |
| EC-C | 1% | 22% | 42% | 77.6% | 90% | 90 °C | 276.5 °C |
| EC-T 1 | 1% | 19% | 40% | 73% | 83% | 102 °C | 278.3 °C |
| EC-T 2 | 1% | 18% | 40% | 66% | 78% | 111 °C | 277 °C |
| EC-Z 1 | 1% | 17.3% | 36% | 69% | 81% | 92 °C | 280.5 °C |
| EC-Z 2 | 0.8% | 16.8% | 36.8% | 68% | 80% | 98 °C | 282.8 °C |
| EC-T1 | EC-T2 | EC-Z1 | EC-Z2 | EC | |
|---|---|---|---|---|---|
| Tonset | 365.19 °C | 342.65 °C | 328.21 °C | 346.28 °C | 365 °C |
| Tmax | 443.63 °C | 450.73 °C | 452.09 °C | 450.85 °C | 446.43 °C |
| Residue at Tmax | 17% | 20.50% | 16.90% | 18.20% | 15.60% |
| Final Residue | 1.55% | 4.10% | 1.93% | 2.01% | 0.05% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Idrees, Q.T.A.; Gul, N.; Fareed, M.A.; Mian, S.A.; Muzaffar, D.; Nasir, M.; Chaudhry, A.A.; Akhtar, S.; Ahmed, S.Z.; Khan, A.S. Structural, Physical, and Mechanical Analysis of ZnO and TiO2 Nanoparticle-Reinforced Self-Adhesive Coating Restorative Material. Materials 2021, 14, 7507. https://doi.org/10.3390/ma14247507
Idrees QTA, Gul N, Fareed MA, Mian SA, Muzaffar D, Nasir M, Chaudhry AA, Akhtar S, Ahmed SZ, Khan AS. Structural, Physical, and Mechanical Analysis of ZnO and TiO2 Nanoparticle-Reinforced Self-Adhesive Coating Restorative Material. Materials. 2021; 14(24):7507. https://doi.org/10.3390/ma14247507
Chicago/Turabian StyleIdrees, Qura Tul Ain, Nazish Gul, Muhammad Amber Fareed, Salman Aziz Mian, Danish Muzaffar, Muhammad Nasir, Aqif Anwar Chaudhry, Sultan Akhtar, Syed Zubairuddin Ahmed, and Abdul Samad Khan. 2021. "Structural, Physical, and Mechanical Analysis of ZnO and TiO2 Nanoparticle-Reinforced Self-Adhesive Coating Restorative Material" Materials 14, no. 24: 7507. https://doi.org/10.3390/ma14247507
APA StyleIdrees, Q. T. A., Gul, N., Fareed, M. A., Mian, S. A., Muzaffar, D., Nasir, M., Chaudhry, A. A., Akhtar, S., Ahmed, S. Z., & Khan, A. S. (2021). Structural, Physical, and Mechanical Analysis of ZnO and TiO2 Nanoparticle-Reinforced Self-Adhesive Coating Restorative Material. Materials, 14(24), 7507. https://doi.org/10.3390/ma14247507











