Tribo-oxide Competition and Oxide Layer Formation of Ti3SiC2/CaF2 Self-Lubricating Composites during the Friction Process in a Wide Temperature Range
Abstract
:1. Introduction
2. Experiment
2.1. Preparation of Samples
2.2. Mechanical Properties
2.3. Friction and Wear Test
2.4. Analysis
3. Results
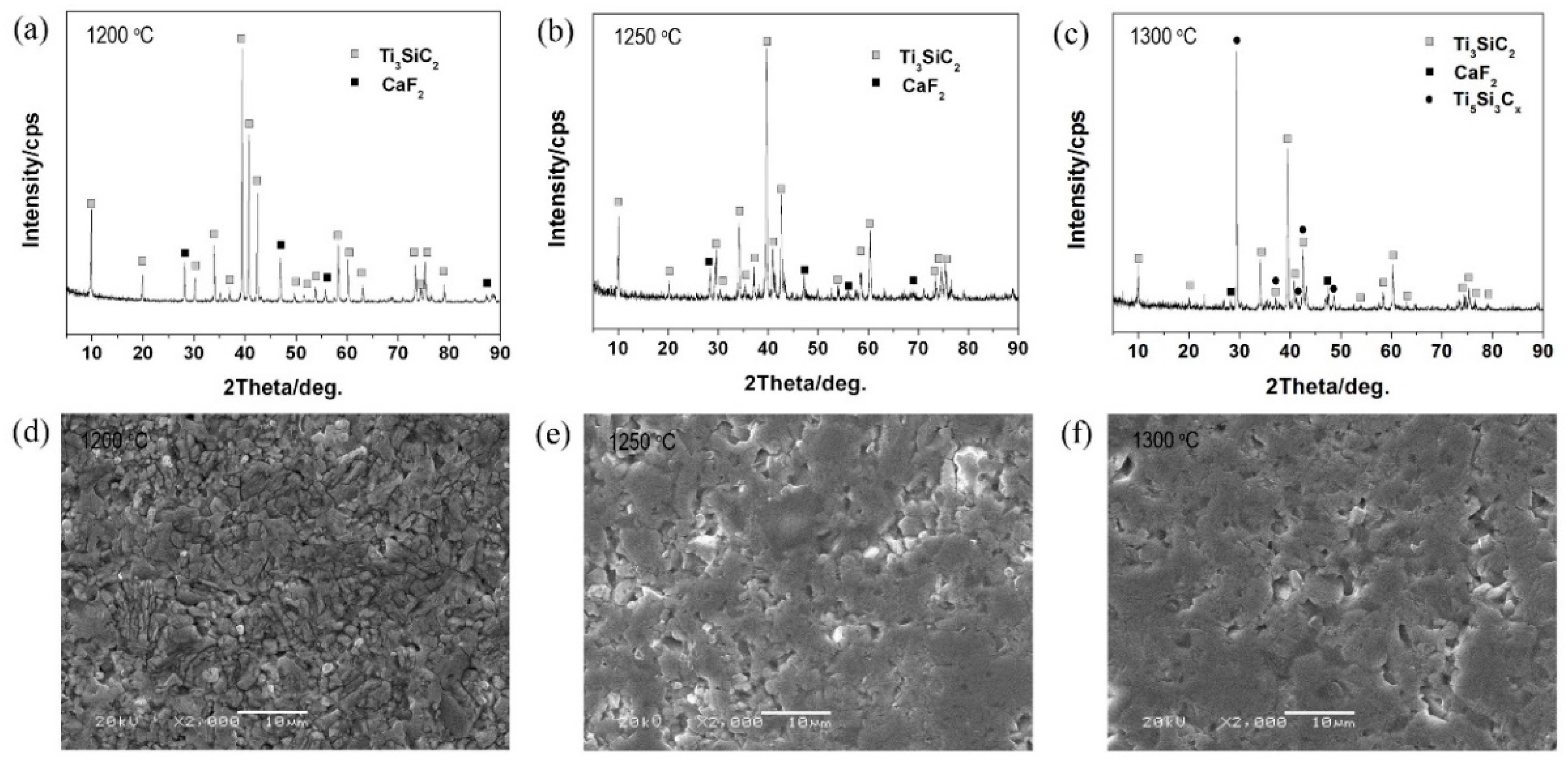
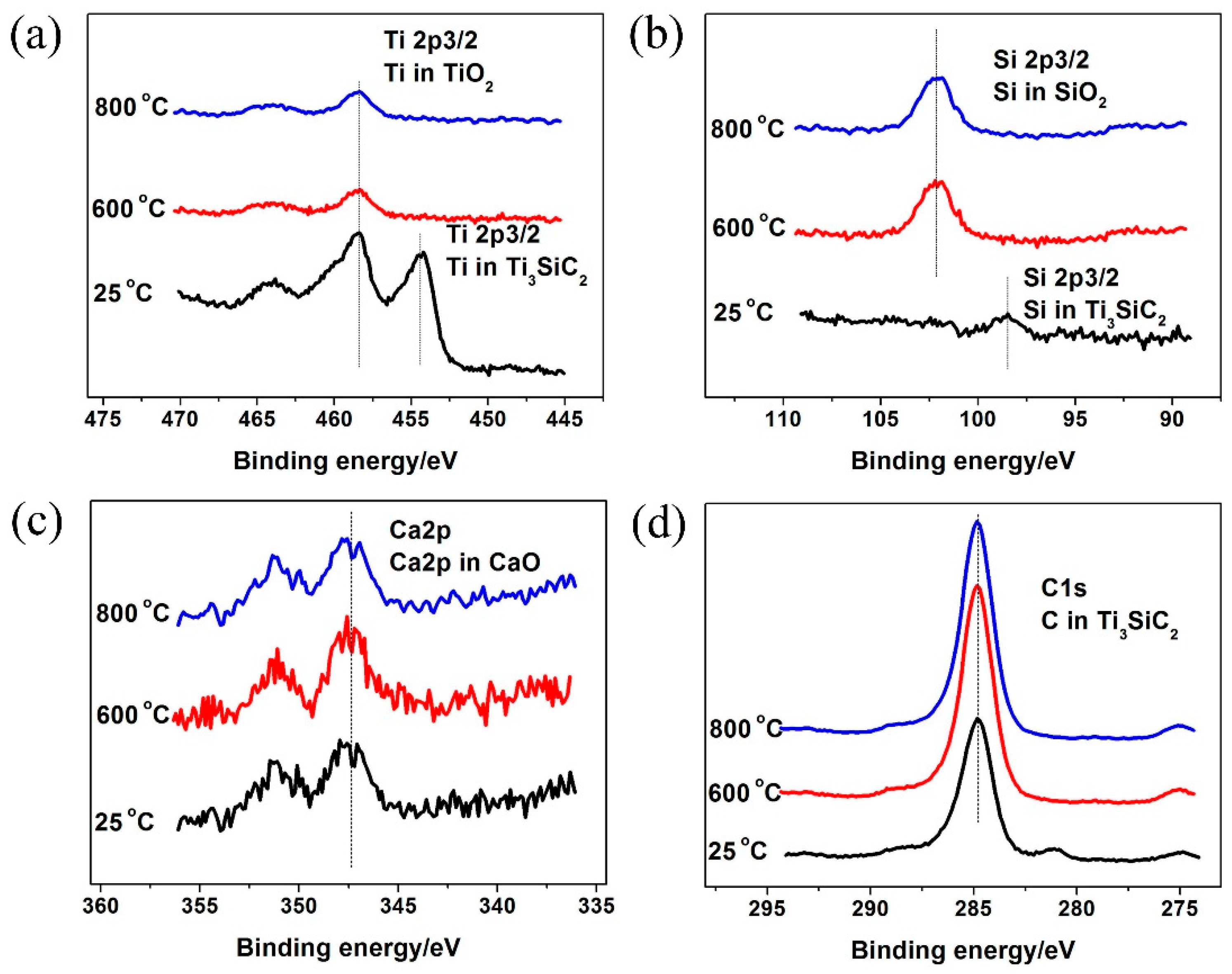
3.1. Phase Composition and Microstructure
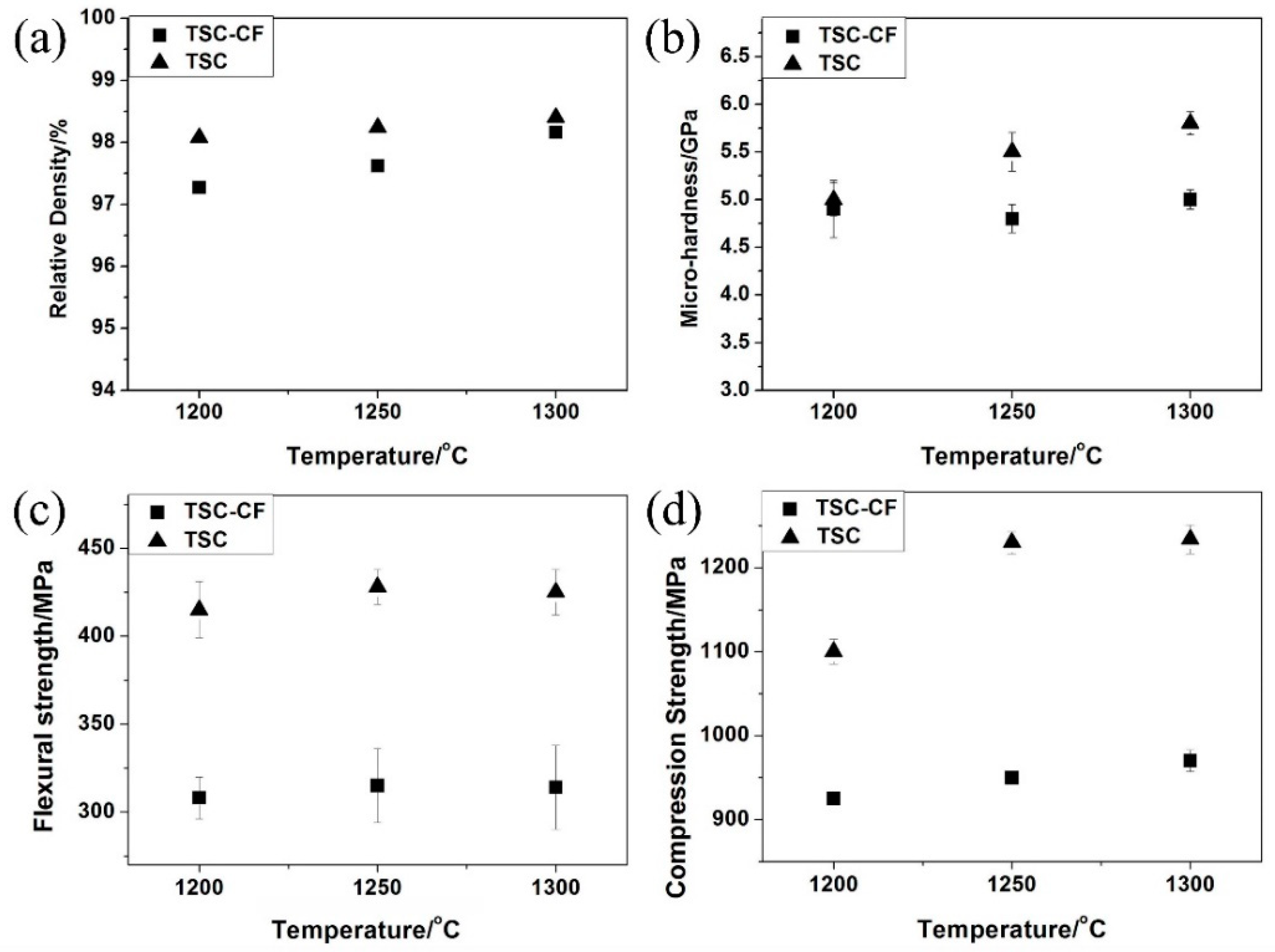
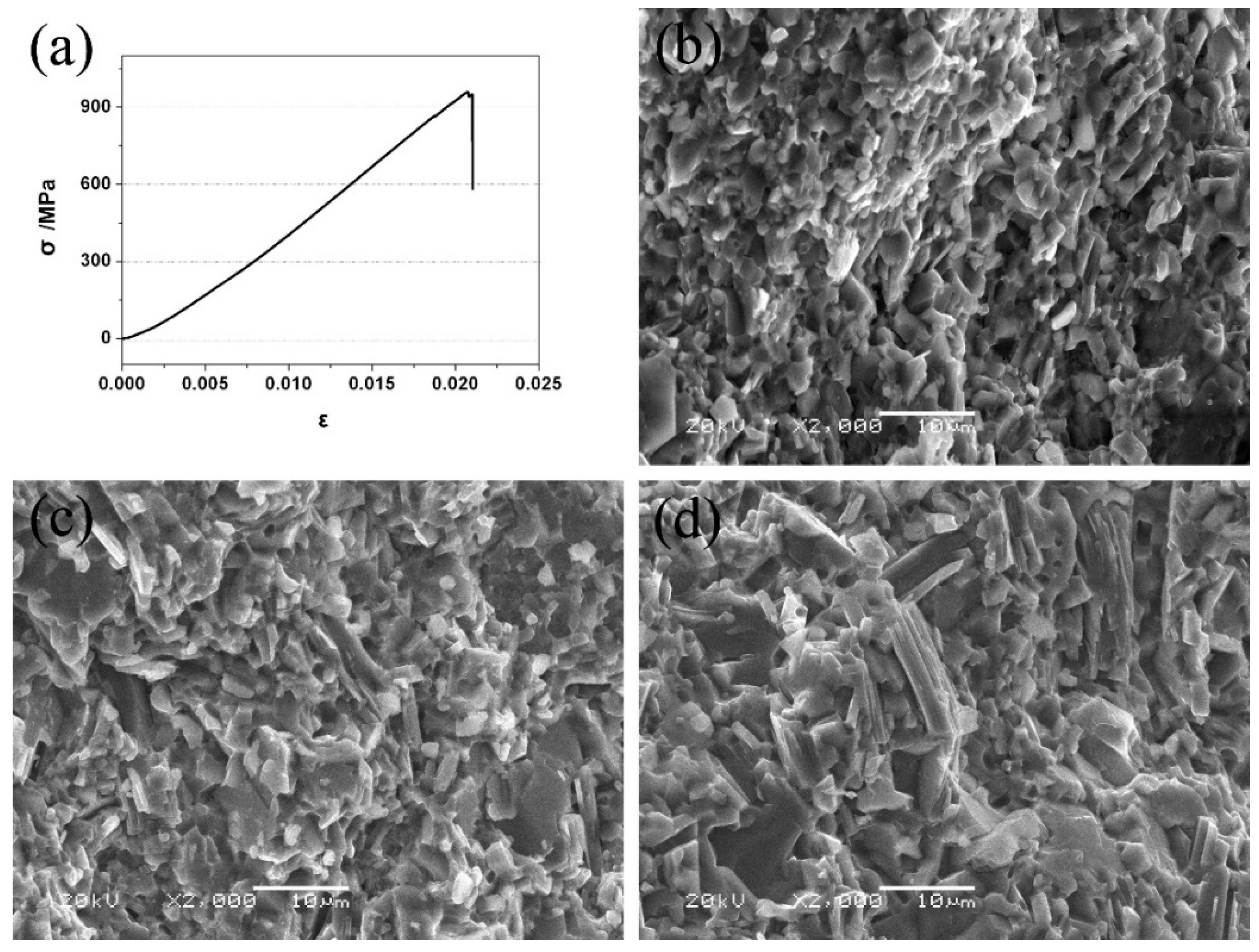
3.2. Mechanical Properties
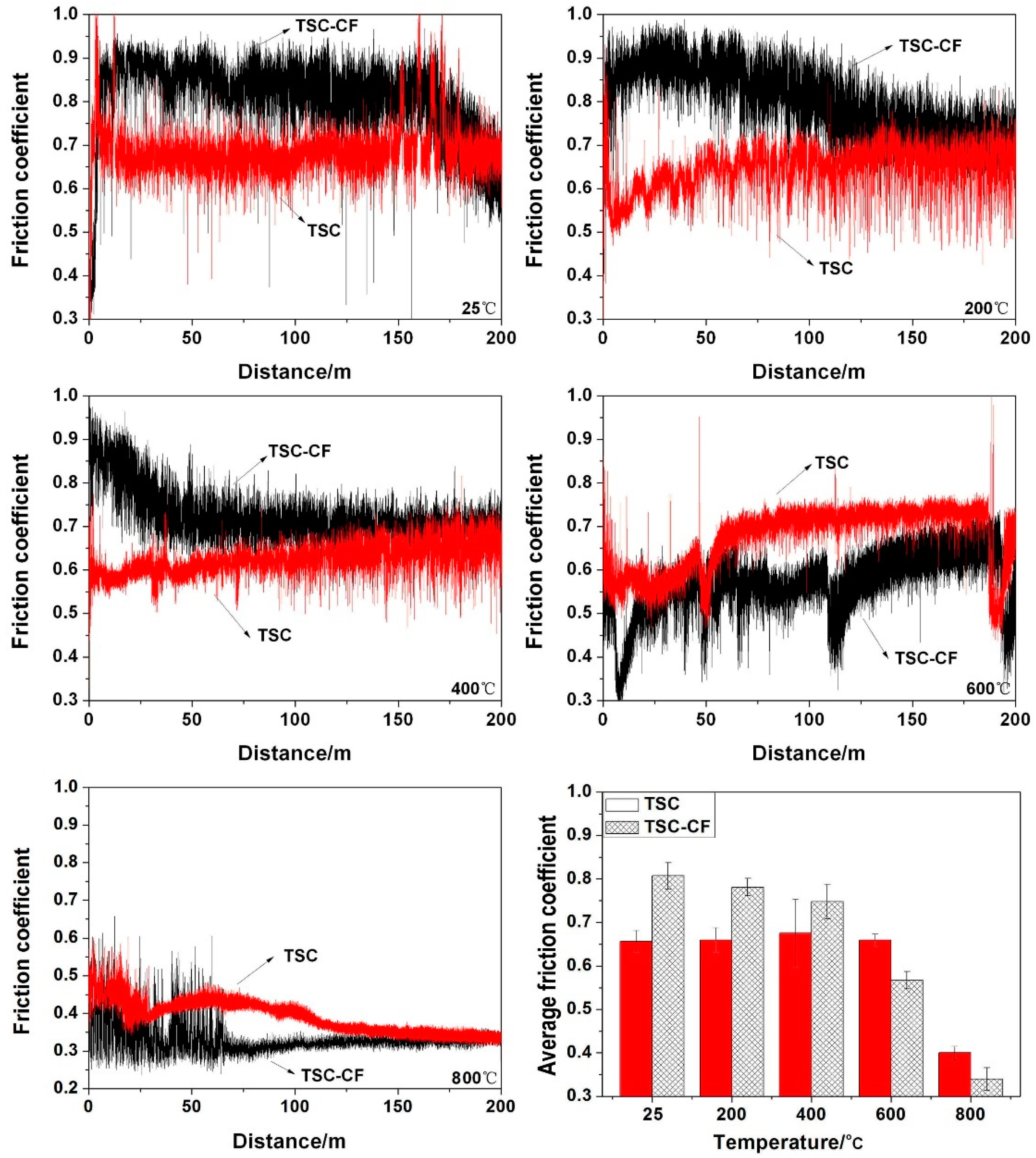
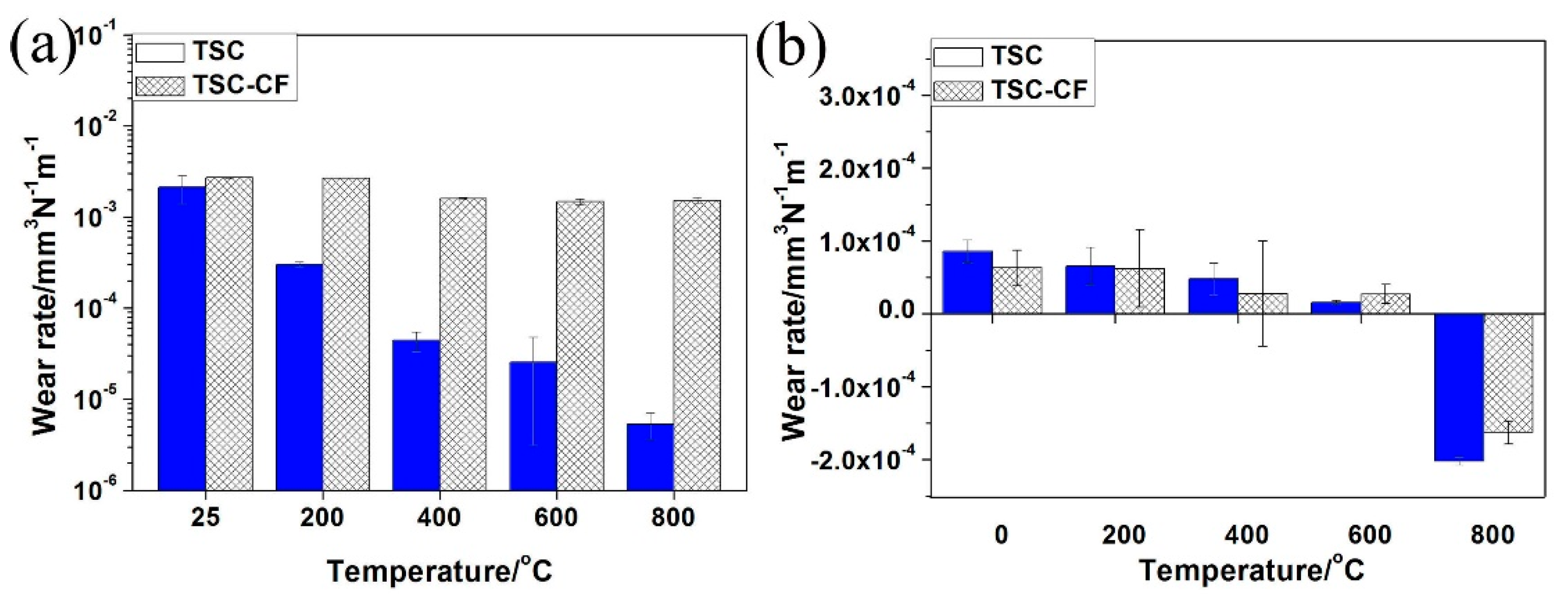
3.3. Friction Coefficients and Wear Rates
3.4. Morphologies and Compositions of Worn Surfaces
4. Discussion
4.1. The Temperature’s Effects on the Tribological Properties of Ti3SiC2/CaF2
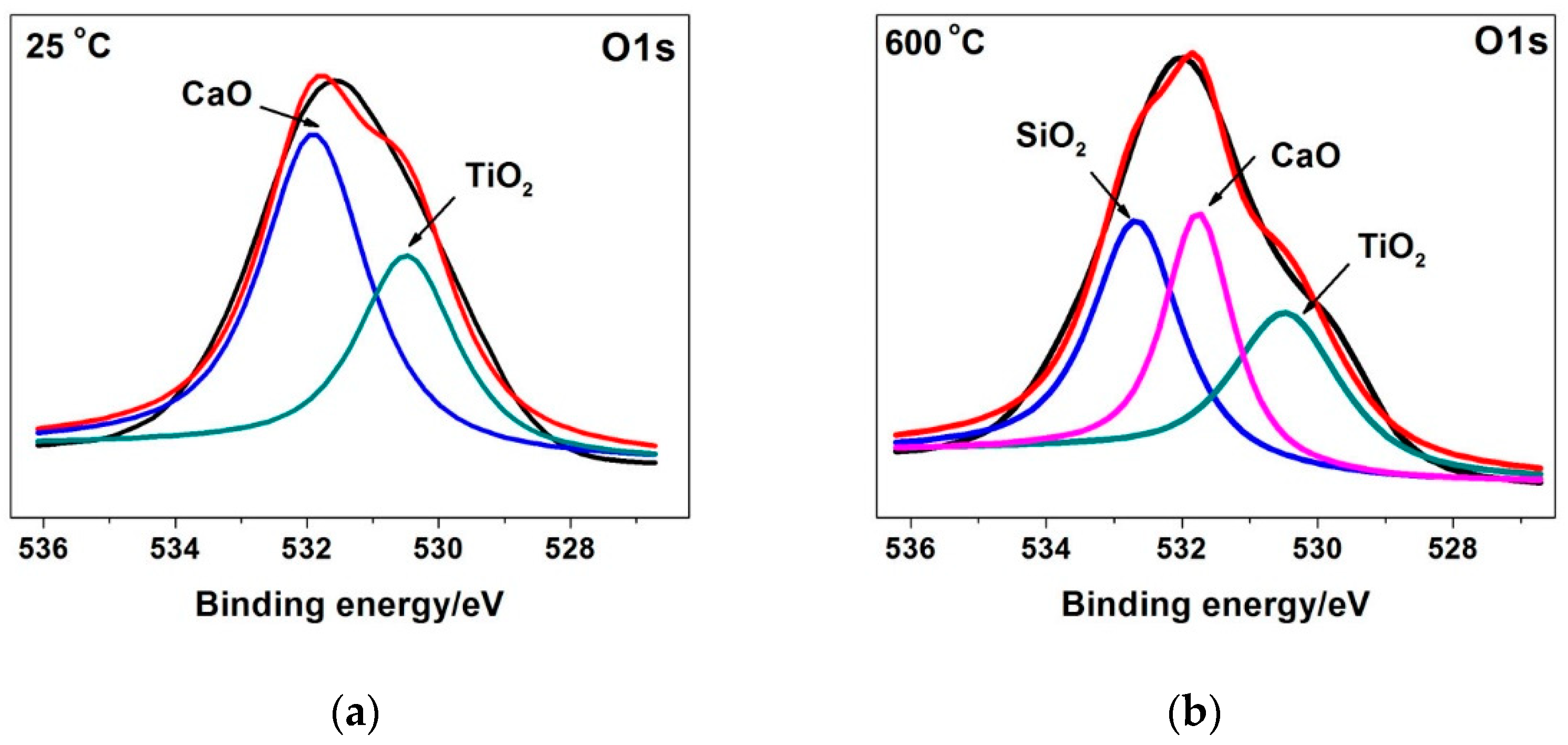
4.2. Competition of Tribo-oxides
4.3. Formation of Oxide Layer
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barsoum, M.W.; El-Raghy, T. Synthesis and Characterization of a Remarkable Ceramic: Ti3SiC2. J. Am. Ceram. Soc. 1996, 79, 1953–1956. [Google Scholar] [CrossRef]
- Barsoum, M.W. The Mn+1AXn phases: A new class of solids: Thernodynamically stable nanolaminates. Prog. Solid State Chem. 2000, 28, 201–281. [Google Scholar] [CrossRef]
- Barsoum, M.; El-Raghy, T.; Radovic, M. High-performance ceramics Ti3SiC2: A layered machinable ductile carbide. Interceram 2000, 49, 226–233. [Google Scholar]
- Gupta, S.; Barsoum, M. On the tribology of the MAX phases and their composites during dry sliding: A review. Wear 2011, 271, 1878–1894. [Google Scholar] [CrossRef]
- Braic, M.; Balaceanu, M.; Parau, A.C.; Dinu, M.; Vladescu, A. Investigation of multilayered TiSiC/NiC protective coatings. Vacuum 2015, 120, 60–66. [Google Scholar] [CrossRef]
- Chen, H.; Peng, J.; Fu, L. Effects of interfacial reaction and atomic diffusion on the mechanical property of Ti3SiC2 ceramic to Cu brazing joints. Vacuum 2016, 130, 56–62. [Google Scholar] [CrossRef]
- Vishnyakov, V.; Lu, J.; Eklund, P.; Hultman, L.; Colligon, J. Ti3SiC2-formation during Ti–C–Si multilayer deposition by magnetron sputtering at 650 °C. Vacuum 2013, 93, 56–59. [Google Scholar] [CrossRef] [Green Version]
- Sarkar, D.; Basu, B.; Cho, S.J.; Chu, M.C.; Hwang, S.S.; Park, S.W. Tribological Properties of Ti3SiC2. J. Am. Ceram. Soc. 2005, 88, 3245–3248. [Google Scholar] [CrossRef]
- Ren, S.; Meng, J.; Wang, J.; Lu, J.; Yang, S. Friction and Wear of Thermal Oxidation-Treated Ti3SiC2. Tribol. Lett. 2009, 37, 59–67. [Google Scholar] [CrossRef]
- Gupta, S.; Filimonov, D.; Palanisamy, T.; Barsoum, M. Tribological behavior of select MAX phases against Al2O3 at elevated temperatures. Wear 2008, 265, 560–565. [Google Scholar] [CrossRef]
- Wang, X.Y.; Shi, G.P.; Li, Q.G.; Wu, J.Y.; Wu, H.; Zhang, L.; Wang, Z. Effect of Ge on microstructure and mechanical properties of Ti3SiC2/Al2O3 composites. Ceram. Int. 2021, 47, 2280–2287. [Google Scholar] [CrossRef]
- Hou, Q.; Yin, H.-F.; Yuan, H.-D.; Tang, Y.; Cai, Y.-Z. Fabrication of TiO2 Nanostructures on Ti3SiC2 Substrate by Anodic Oxidation. J. Nanosci. Nanotechnol. 2018, 18, 1983–1988. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Chen, K.X.; Zhou, H.P.; Ning, X.S. Combustion Synthesis of Ti3SiC2/TiC Composites from Elemental Powders under High-Gravity Conditions. J. Am. Ceram. Soc. 2010, 93, 2182–2187. [Google Scholar] [CrossRef]
- Yin, X.H.; Li, M.S.; Zhou, Y.C. Microstructure and mechanical strength of diffusion-bonded Ti3SiC2/Ni joints. J. Mater. Res. 2006, 21, 2415–2421. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, G.-Q.; Wang, K.; Fu, X.-S.; Zhou, W.-L. Effects of doping Ti3SiC2 with Al on interfacial microstructural evolution, growth kinetics and mechanical properties of Ti3SiC2/TiAl joints. J. Mater. Res. Technol. 2020, 9, 13206–13215. [Google Scholar] [CrossRef]
- Sonoda, T.; Nakao, S.; Ikeyama, M. Deposition and characterization of MAX-phase containing Ti–Si–C thin films by sputtering using elemental targets. Vacuum 2013, 92, 95–99. [Google Scholar] [CrossRef]
- Ahmadifard, S.; Momeni, A.; Bahmanzadeh, S.; Kazemi, S. Microstructure, tribological and mechanical properties of Al7075/Ti3AlC2 MAX-phase surface composite produced by friction stir processing. Vacuum 2018, 155, 134–141. [Google Scholar] [CrossRef]
- Luo, B.; Zhou, H.; Liu, D.; Luo, F.; Tian, Y.; Chen, D.; Wei, W. One-step in-situ reaction synthesis of TiC/graphene composite thin film for titanium foil surface reinforcement. Vacuum 2019, 160, 472–477. [Google Scholar] [CrossRef]
- Lisitsyn, V.M.; Lisitsyna, L.A.; Popov, A.I.; Kotomin, E.A.; Abuova, F.U.; Akilbekov, A.; Maier, J. Stabilization of primary mobile radiation defects in MgF2 crystals. Nucl. Instrum. Methods Phys. Res. Sect. B 2016, 374, 24–28. [Google Scholar] [CrossRef]
- Kotomin, E.A. Nuclear Instruments and Methods in Physics Research section B: Beam interactions with materials and atoms. Nucl. Instrum. Methods Phys. Res. Sect. B 2022, 511, 17. [Google Scholar] [CrossRef]
- Ochs, D.; Brause, M.; Krischok, S.; Stracke, P.; Maus-Friedrichs, W.; Puchin, V.; Popov, A.; Kempter, V. Characterization of LiF and CaF2 surfaces using MIES and UPS (HeI). J. Electron Spectrosc. Relat. Phenom. 1998, 88–91, 725–732. [Google Scholar] [CrossRef]
- Zhu, S.; Bi, Q.; Yang, J.; Liu, W. Effect of Fluoride Content on Friction and Wear Performance of Ni3Al Matrix High-Temperature Self-Lubricating Composites. Tribol. Lett. 2011, 43, 341–349. [Google Scholar] [CrossRef]
- Benamor, A.; Hadji, Y.; Chiker, N.; Haddad, A.; Guedouar, B.; Labaiz, M.; Hakem, M.; Tricoteaux, A.; Nivot, C.; Erauw, J.P.; et al. Spark Plasma Sintering and tribological behavior of Ti3SiC2–Ti5Si3–TiC composites. Ceram. Int. 2019, 45, 21781–21792. [Google Scholar] [CrossRef]
- Ouyang, J.H.; Sasaki, S.; Murakami, T.; Umeda, K. The synergistic effects of CaF2 and Au lubricants on tribological properties of spark-plasma-sintered ZrO2(Y2O3) matrix composites. Mat. Sci. Eng. A 2004, 386, 234–243. [Google Scholar] [CrossRef]
- Ouyang, J.; Sasaki, S.; Murakami, T.; Umeda, K. Tribological properties of spark-plasma-sintered ZrO2(Y2O3)–CaF2–Ag composites at elevated temperatures. Wear 2005, 258, 1444–1454. [Google Scholar] [CrossRef]
- Zhang, R.; Feng, K.; Meng, J.; Su, B.; Ren, S.; Hai, W. Synthesis and characterization of spark plasma sintered Ti3SiC2/Pb composites. Ceram. Int. 2015, 41, 10380–10386. [Google Scholar] [CrossRef]
- Wu, E.; Kisi, E.H.; Kennedy, S.J.; Studer, A.J. In Situ Neutron Powder Diffraction Study of Ti3SiC2 Synthesis. J. Am. Ceram. Soc. 2004, 84, 2281–2288. [Google Scholar] [CrossRef]
- Huang, Z.; Zhai, H.; Guan, M.; Liu, X.; Ai, M.; Zhou, Y. Oxide-film-dependent tribological behaviors of Ti3SiC2. Wear 2007, 262, 1079–1085. [Google Scholar] [CrossRef]
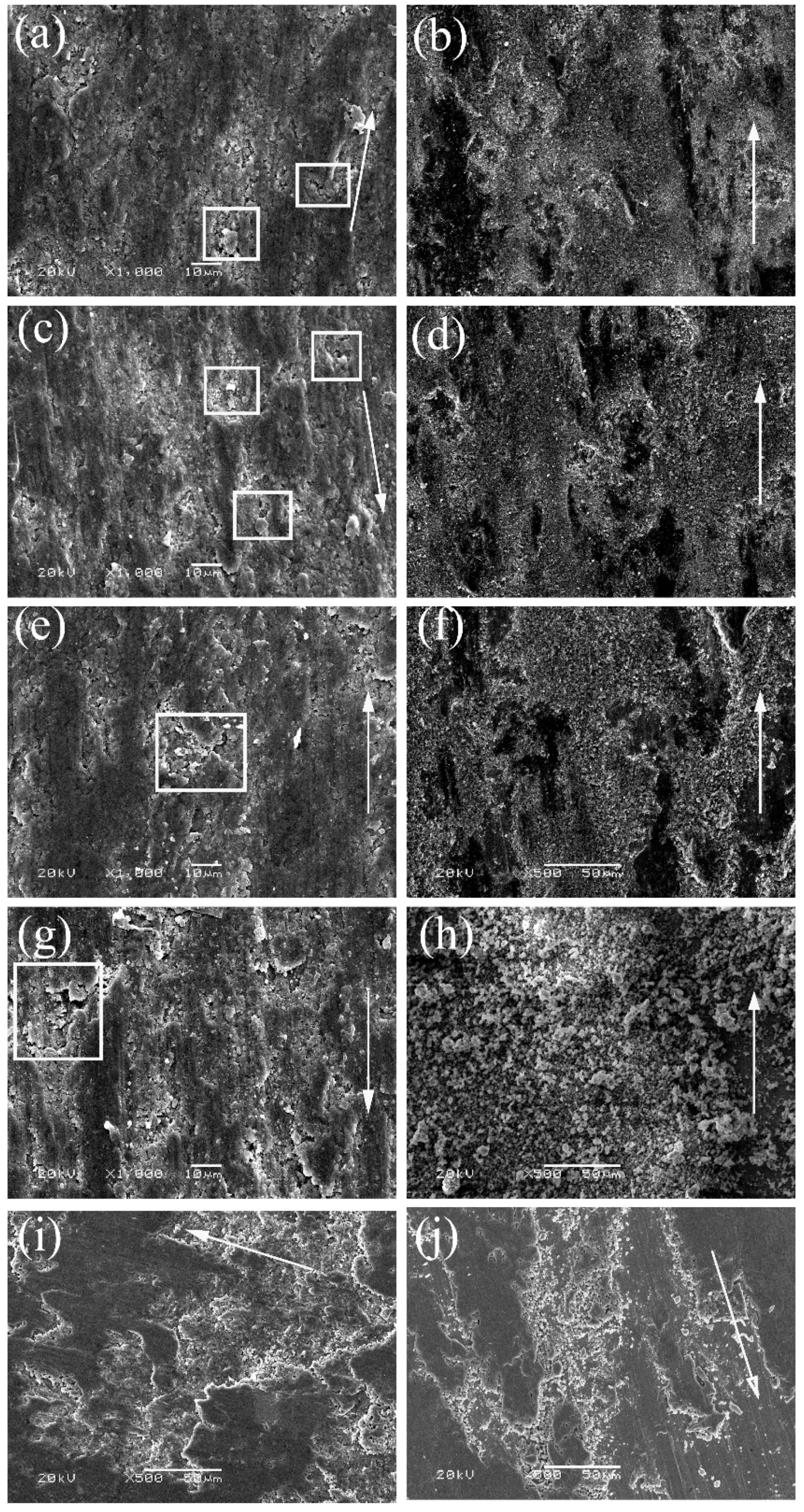
| Position | Sample | Temperature/°C | Atomic Percentages |
|---|---|---|---|
| a | TSC-CF pin | 25 | 30.7%Ti, 13.2%Si, 41.3%C, 3.4%Al, 4.1%Ca, 7.3%F |
| c | TSC-CF pin | 200 | 27.0%Ti, 11.2%Si, 44.1%C, 3.3%Al, 4.6%Ca, 9.8%F |
| e | TSC-CF pin | 400 | 27.4%Ti, 10.1%Si, 47.0%C, 3.0%Al, 4.3%Ca, 8.2%F |
| g | TSC-CF pin | 600 | 32.3%Ti, 9.5%Si, 42.9%C, 2.5%Al, 3.4%Ca, 9.4%F |
| i | TSC-CF pin | 800 | 34.3%Ti, 8.9%Si, 41.3%C, 3.3%Al, 4.1%Ca, 8.1%F |
| b | Inconel 718 disk | 25 | 15.6%Ti, 7.0%Si, 33.8%C, 1.6%Al, 2.0%Ca, 20.2%O, 9.3%F, 5.3%Ni, 2.7%Cr, 2.5%Fe |
| d | Inconel 718 disk | 200 | 16.7%Ti, 7.1%Si, 32.8%C, 1.8%Al, 2.0%Ca, 23.1%O, 8.8%F, 3.8%Ni, 2.1%Cr, 1.8%Fe |
| f | Inconel 718 disk | 400 | 13.7%Ti, 6.1%Si, 30.6%C, 2.0%Al, 1.6%Ca, 26.8%O, 7.6%F, 6.1%Ni, 3.0%Cr, 2.5%Fe |
| h | Inconel 718 disk | 600 | 14.3%Ti, 5.2%Si, 12.7%C, 1.7%Al, 1.6%Ca, 59.8%O, 3.4%F, 0.7%Ni, 0.6%Fe |
| j | Inconel 718 disk | 800 | 15.3%Ti, 5.8%Si, 11.2%C, 1.7%Al, 1.6%Ca, 59.6%O, 3.3%F, 0.9%Ni, 0.6%Fe |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, R.; Feng, W.; Liu, F. Tribo-oxide Competition and Oxide Layer Formation of Ti3SiC2/CaF2 Self-Lubricating Composites during the Friction Process in a Wide Temperature Range. Materials 2021, 14, 7466. https://doi.org/10.3390/ma14237466
Zhang R, Feng W, Liu F. Tribo-oxide Competition and Oxide Layer Formation of Ti3SiC2/CaF2 Self-Lubricating Composites during the Friction Process in a Wide Temperature Range. Materials. 2021; 14(23):7466. https://doi.org/10.3390/ma14237466
Chicago/Turabian StyleZhang, Rui, Wei Feng, and Fuyan Liu. 2021. "Tribo-oxide Competition and Oxide Layer Formation of Ti3SiC2/CaF2 Self-Lubricating Composites during the Friction Process in a Wide Temperature Range" Materials 14, no. 23: 7466. https://doi.org/10.3390/ma14237466
APA StyleZhang, R., Feng, W., & Liu, F. (2021). Tribo-oxide Competition and Oxide Layer Formation of Ti3SiC2/CaF2 Self-Lubricating Composites during the Friction Process in a Wide Temperature Range. Materials, 14(23), 7466. https://doi.org/10.3390/ma14237466







