Abstract
Water pollution has always been a serious problem across the world; therefore, facile pollutant degradation via light irradiation has been an attractive issue in the field of environmental protection. In this study, a type of Zn-based metal–organic framework (ZIF−8)-wrapped BiVO4 nanorod (BiVO4@ZIF−8) with high efficiency for photocatalytic wastewater treatment was synthesized through a two-step hydrothermal method. The heterojunction structure of BiVO4@ZIF−8 was confirmed by morphology characterization. Due to the introduction of mesoporous ZIF−8, the specific surface area reached up to 304.5 m2/g, which was hundreds of times larger than that of pure BiVO4 nanorods. Furthermore, the band gap of BiVO4@ZIF−8 was narrowed down to 2.35 eV, which enabled its more efficient utilization of visible light. After irradiation under visible light for about 40 min, about 80% of rhodamine B (RhB) was degraded, which was much faster than using pure BiVO4 or other BiVO4-based photocatalysts. The synergistic photocatalysis mechanism of BiVO4@ZIF−8 is also discussed. This study might offer new pathways for effective degradation of wastewater through facile design of novel photocatalysts.
1. Introduction
Recently, water purifying technology has received widespread attention as an emerging field. In particular, photocatalytic degradation is recognized as the most promising way to purify wastewater, due to its low cost and environmentally friendly properties [1,2,3,4]. Photocatalysts can degrade noxious organic pollutants under the irradiation of sunlight without producing any toxic remains [5,6]. The key to realizing this advantage relies on precise design of the photocatalysts.
Metal oxide semiconductors, such as TiO2 (P25), ZnO, Bi2O3, BiFeO3, ZnSnO3 and BiVO4, have all been proved to be efficient photocatalysts [7,8,9,10,11,12,13,14]. Among the many currently studied photocatalysts, BiVO4, as a cost-effective, eco-friendly and chemically stable material has garnered considerable interest recently. As indicated in previous studies, monoclinic scheelite, tetragonal zircon and tetragonal scheelite are the most common forms of BiVO4 existing in nature [15,16]. Compared with tetragonal BiVO4, which mainly responds to UV light, BiVO4 with a monoclinic scheelite structure has better photon harvesting and more sensitive light response properties due to its relatively narrower band gap (2.4 eV); therefore, m-BiVO4 can generate electron–hole pairs under the irradiation of visible light [17,18]. Recently, many efforts have been made to enhance the photocatalytic properties of BiVO4. Guo et al. synthesized V4+ self-doped BiVO4 nanorods with [010] oriented for water purification. In these nanorods, the oxygen vacancies and V4+ ions could act as charge carrier traps and adsorption sites, thus inhibiting the recombination of photogenerated electron–hole pairs, and resulting in an excellent photocatalyst [19].
Photocatalysts’ size and shape can also influence their degradation efficiency. Currently, mainstream photocatalysts are often in a solid bulk shape. However, bulk-shaped photocatalysts always suffer from an increased electron–hole recombination rate, poor electron transportation mobility and worse surface absorbability [20,21]. Therefore, investigating photocatalysts with mesoporous structures has attracted a significant amount of attention. Compared with solid photocatalysts, photocatalysts with mesoporous structures have a relatively larger specific surface area. With the enlarged specific surface area, there will be more contact between organic dyes and the catalyst, thus improving the absorption and photodegradation process [22,23]. Metal–organic frameworks (MOFs), as a type of superior mesoporous material, could be prominent host candidates for water splitting due to their high surface area, mesoporous structure, tunable shape and chemical stability [24]. There are a large number of organic ligands around central metal ions, and the chemical bonds between the organic ligands and metal ions are also flexible. Therefore, MOFs can absorb photons and transfer electrons to the metal ions easily. In addition, the synthesis processes for MOFs are convenient. MOFs are usually synthesized by mixing the aromatic multicarboxylatic ligands and metal ions in organic solutions, which is much more convenient than other synthesis methods. Thus, MOFs can be prominent candidates for photodegradation [25,26,27].
Photocatalysts with a single component readily suffer from rapid electron–hole recombination. Therefore, incorporating metal oxide semiconductors and MOFs together might form new types of photocatalysts with improved photocatalytic efficiency by combining their advantages. In addition, to take advantage of the extremely large surface area of MOFs, which can provide more active sites for dye degradation, a semiconductor–MOF heterojunction can be formed at the interface; this can generate an in-built electric field and inhibit rapid electron–hole recombination, thus enhancing their life time and improving photocatalytic efficiency [28,29]. Moreover, the heterojunction can change the distribution of photogenerated electron–hole pairs in order to adjust the band gap width and promote photocatalytic performance [30].
In this research, ZIF−8-wrapped BiVO4 nanorods with a narrower band gap and larger specific surface area were fabricated through hydrothermal and self-sedimentation processes. The photocatalytic performance of BiVO4@ZIF−8 was evaluated by the degradation of RhB solutions. With the newly designed composites, about 70% of the organic dye could be degraded under irradiation with visible light for nearly 40 min. The mechanism of this synergistic photocatalysis was also investigated.
2. Materials and Methods
2.1. Material Synthesis
2.1.1. Material Preparation
Bismuth nitrate pentahydrate (Bi(NO3)3·5H2O), zinc nitrate hexahydrate (Zn(NO3)2·6H2O), sodium oleate (C17H33CO2Na ≥ 98%), ammonium vanadate (NH4VO3 ≥ 98%), ammonium hydroxide and nitric acid were all bought from Sinopharm Chemical Reagent Co., Ltd. 2-Methylimidazole (C4H6N2 ≥ 98%) was purchased from Macklin Inc.
2.1.2. Synthesis of BiVO4 Nanorods
BiVO4@ZIF−8 was synthesized via a two-step method. Typically, the BiVO4 precursor was synthesized via hydrothermal reaction first, and then the final BiVO4@ZIF−8 product was obtained via a self-sedimentation method. In detail, about 0.4 mmol (0.388 g) of bismuth nitrate pentahydrate was dispersed into 20 mL of deionized water under constant stirring. In order to obtain a homogeneous transparent solution, several drops of HNO3 were added to the mixture. We defined this solution as A. Likewise, 0.4 mmol (0.096 g) NH4VO3 and 1 mmol (0.36 g) sodium oleate were dissolved in deionized water, giving solutions which were defined as B and C, respectively. After 30 min magnetic stirring, we mixed solutions A, B and C together to form a yellow homogeneous suspension. Several drops of ammonium hydroxide were then dropped into the suspension to keep it weakly alkaline (pH ≈ 9). Afterwards, the suspension was poured into a PTFE-made hydrothermal reactor (Yu hua Tech Co., Ltd. Shanghai, China), to be kept at 180 °C for 24 h. After the reaction, the brown powder was collected by centrifugation and dried at 80 °C. To enhance its crystallinity, the BiVO4 powder was further annealed in a furnace at 450 °C for 2 h.
The second step was to obtain ZIF−8-wrapped BiVO4, named BiVO4@ZIF−8, via a self-sedimentation method. Typically, we dissolved 0.5 g Zn(NO3)2·6H2O and 0.8 g 2-methylimidazole separately into absolute methanol to form homogeneous solutions. Then, 1 g as-prepared BiVO4 was dispersed into Zn(NO3)2 solution under vigorous stirring for half an hour. Afterwards, we mixed them together to form a yellow homogeneous suspension and let it stand for half a day. After the reaction, the light yellow powder was collected by centrifugation and dried at 80 °C. The final product was a light yellow BiVO4@ZIF−8 composite.
2.2. Material Characterization
A JSM-IT500HR/LA instrument (JEOL, Tokyo, Japan) was used for scanning electron microscopy (SEM); a JEM-2100Plus (JEOL, Tokyo, Japan) was used as a transmission electron microscope (TEM); and X-ray diffraction (XRD, D2 Advance, BRUKER, Karlsruhe, Germany) and X-ray photoelectron spectroscopy (XPS, ESCALAB 250Xi, Thermo Fisher Scientific, London, UK) were used to investigate the phase and element information of the final products. Belsorp-MAXII (MicrotracBEL, Osaka, Japan) was used to measure the BET surface area. In this study, the photoluminescence spectra were acquired using a photoluminescence spectrometer (Fluorolog-3 Horiba Scientific, NJ, USA), with 360 nm excitation light. Ultraviolet–visible (UV–Vis) spectra were recorded by a Cary 5000 spectrophotometer (Agilent Technologies, Penang, Malaysia).
2.3. Photocatalytic Activity Evaluation
Rhodamine B (RhB) was used to assess the photocatalytic activity of the as-prepared catalyst. Typically, we dispersed 100 mg catalyst (pure BiVO4 or BiVO4@ZIF in this work) into RhB solution with a concentration of 0.5 mg/50 mL. Before the degradation reaction, the suspension was kept in a dark environment for 30 min to reach a photoequilibrium state, using ultrasonic vibration. In the catalysis process, we collected the suspension every 10 min via centrifugation. As the characteristic absorption peak for RhB is at 550 nm, the intensity of the absorption peak for RhB was measured by UV–Vis absorption spectra. Equation (1) below was applied to determine the RhB degradation efficiency (the ratio of the remaining RhB concentration and the initial RhB concentration) in the degradation process:
where ηeff is the degradation efficiency of RhB, and A0 and At are the intensities of absorption peaks before and after degradation for a certain time interval, respectively.
3. Results and Discussion
The phase and crystal structure information of the as-synthesized BiVO4 nanorods and BiVO4@ZIF-8 composites was examined by XRD analysis, as shown in Figure S1 (which can be found in the Supplementary Materials) and Figure 1, respectively. In Figure S1, all of the diffraction peaks were assigned to the standard monoclinic-type BiVO4 (JCPDS card no. 14-0688; a = 5.195 Å, b = 11.704 Å, c = 5.092 Å; space group: I2/a) [31]. There was a minor diffraction peak at 15.11° in the XRD pattern of BiVO4, which could distinguish m-BiVO4 from tetragonal scheelite BiVO4 (t-BiVO4) [32]. It can also be seen in Figure S1 that the intensities of the diffraction peaks of m-BiVO4 were very strong, suggesting that the defect intensity of BiVO4 nanorods was notably reduced via calcination. The lower number of defects and high crystallinity of BiVO4 are conducive to its enhanced catalytic performance, since defects in crystal lattices can play the role of combining centers for photogenerated electrons and holes [33,34,35]. In addition, there were two diffraction peaks of BiVO4 assigned to (200) and (002) at about 35°, which are characteristic of monoclinic scheelite-type BiVO4. The curve in Figure 1 shows the XRD pattern of BiVO4@ZIF−8. The majority of diffraction peaks of ZIF−8 were clearly seen between 10° and 30°, indicating the co-existence of BiVO4 and ZIF−8. We conclude that the BiVO4@ZIF−8 composites were formed simply by the attachment of ZIF−8 nanoparticles to the surface of BiVO4 nanorods, and that no compound was formed.
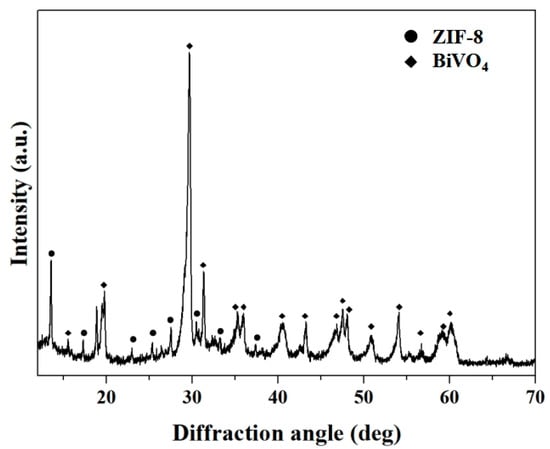
Figure 1.
The XRD pattern of BiVO4@ZIF−8. The diffraction peaks are assigned to BiVO4 and ZIF−8 as indicated.
Morphologies of as-synthesized BiVO4 nanorods and BiVO4@ZIF−8 composites were obtained by SEM and TEM and are shown in Figure 2a–f. As indicated in Figure 2a–c, the diameter and length of BiVO4 nanorods were about 150 nm and 3000 nm, respectively. In addition, the BiVO4 nanorods were aggregated together to form a flower-like shape. Figure 2c (insert) shows the HR-TEM image of an individual BiVO4 nanorod and its corresponding SAED pattern. As shown in Figure 2c (insert), the diffraction fringes of BiVO4 nanorods with the spacing of 0.582 nm were well assigned to their (020) lattice planes (b = 11.704Å, two times the fringe spacing), indicating that the BiVO4 nanorods were probably growing along the [010] direction. Figure 2c (insert) also shows the diffraction spots along the [102] zone axis in the SAED pattern. There were two spots around the (000) spots, indexed as the (020) and (211) lattice planes. In addition, there was only one set of diffraction spots in our SAED pattern, indicating that the as-synthesized BiVO4 was a pure phase without any impurities. Figure 2d–f show SEM and TEM images of BiVO4@ ZIF−8. The surface of BiVO4@ZIF−8 nanorods was coarser than that of pure BiVO4. In addition, the BiVO4@ZIF−8 nanorods were well dispersed, owing to the existence of the MOF. We could clearly see that ZIF−8 nanoparticles with a size of ~30 nm were tightly attached to the BiVO4 nanorod’s surface and that a heterojunction between the BiVO4 and ZIF−8 was formed, which could enhance the photocatalysis performance of BiVO4@ ZIF−8.
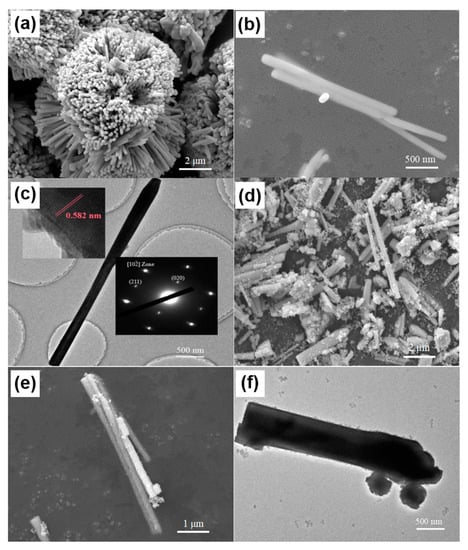
Figure 2.
Morphology and structure characterization of BiVO4 and BiVO4@ZIF−8. Typical SEM images of as-synthesized BiVO4 precursor at (a) low and (b) high magnification; typical (c) TEM image with HR-TEM and SAED images inserted of as-synthesized BiVO4 precursor with the length of ~3000 nm and width of ~150 nm; typical SEM images of as-synthesized BiVO4@ZIF−8 at (d) low and (e) high magnification; (f) typical TEM image of as-synthesized BiVO4@ZIF−8 with the ZIF−8 nanoparticles tightly attached to the surface of the BiVO4 nanorod.
XPS (seen in Figure 3a) showed that only Bi, V, C and O existed in as-prepared BiVO4. Figure 3b,c show the characteristic peaks for Bi3+ and V5+ in BiVO4. The binding energies of Bi 4f7/2 and Bi 4f5/2 were 158.5 and 164.4 eV, respectively, and the binding energies of V 2p3/2 and V 2p1/2 were 516.5 eV and 524.3 eV, respectively, consistent with previous reports [36,37]. The appearance of the C 1s signal at 284.8 eV might be an instrument error during the XPS test. It is noticeable that, in Figure 3d, the O 1s peaks were split into two peaks, which correspond to the O element in the BiVO4 crystal lattice (528.9 eV) and O2 absorbed via BiVO4 (532.5 eV).
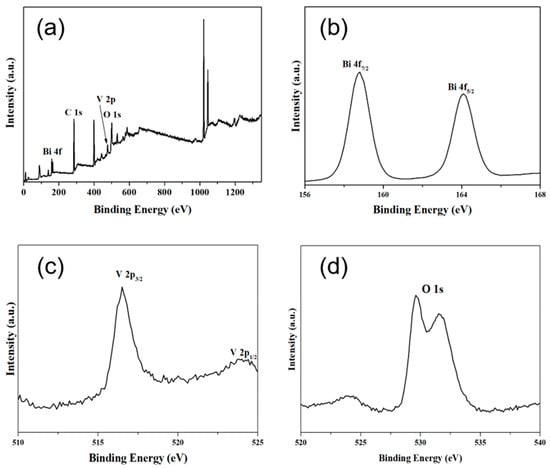
Figure 3.
(a) XPS spectra of BiVO4 showing the presence of Bi, C, O and V. High-resolution XPS spectra showing: (b) Bi 4f at 158.5 and 164.4 eV, (c) V 2p 516.5 eV and 524.3 eV and (d) O 1s at 528.9 and 532.5 eV.
Specific surface areas of BiVO4 and BiVO4@ ZIF−8 were obtained using nitrogen adsorption–desorption tests. Owing to the addition of mesoporous ZIF−8, the as-prepared BiVO4@ZIF−8 composite showed a type IV hysteresis loop, as seen in Figure 4b [20]. Moreover, as calculated via the instrument, the surface area of BiVO4@ZIF−8 was as high as 304.5 m2/g, which was much larger than that of pure BiVO4 (2.53 m2/g). Owing to its larger surface area, BiVO4@ZIF−8 had more active sites to absorb and degrade organic dyes than pure BiVO4; thus, the catalytic performance could be significantly enhanced.
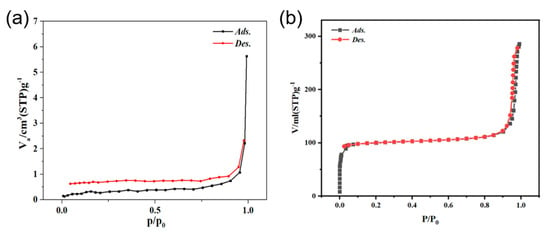
Figure 4.
(a) The N2 adsorption−desorption isotherm of BiVO4 nanorods; (b) the N2 adsorption−desorption isotherm of BiVO4@ZIF−8.
The property of light absorption was another crucial factor that affected the photocatalytic properties of BiVO4 and BiVO4@ZIF−8. Therefore, ultraviolet–visible absorption spectra were applied to characterize the light absorption abilities of BiVO4 and BiVO4@ZIF−8. From the spectra in Figure 5, we can see that the absorption edges of pure BiVO4 and BiVO4@ZIF−8 composites were all around 500 nm. Tauc’s equation, as shown in Equation (2), was applied to determine the band gap value of semiconductors with a direct band gap:
where h, v, A, Eg and α represent Plank’s constant, light frequency, absorbance, band gap value and absorption coefficient, respectively. In Equation (2), the parameters A and α are constant for a specific sample.
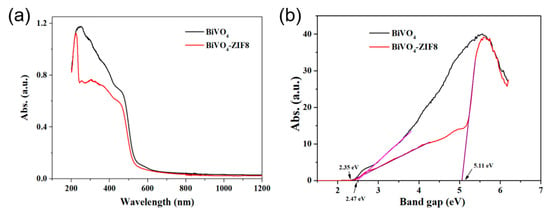
Figure 5.
(a) The UV–Vis absorption curves of pure BiVO4 (black) and BiVO4@ZIF−8 (red); (b) the corresponding Kubelka–Munk (K–M) transformed curves of pure BiVO4 (black) and BiVO4@ZIF−8 (red). The band gaps for BiVO4@ZIF−8 and pure BiVO4 were calculated as 2.35 eV and 2.47 eV, respectively.
Figure 5b shows the K–M transformed reflectance spectra corresponding to Figure 5a. Extrapolating the quasi-straight line to intersect with the X-axis can provide the band gap values of the measured samples. As calculated in Figure 5b, BiVO4 without modification showed a band gap value of 2.47 eV. However, as shown in Figure 5b, after modification with ZIF−8, the band gap value for BiVO4@ZIF−8 was divided into two values: 2.35 eV and 5.11 eV. The latter was assigned to the value for ZIF−8. In addition, the band gap value for BiVO4 after modification with ZIF−8 (2.35 eV) was slightly narrower than that of pure BiVO4 in our research and other papers [31,32,38,39]. Smaller band gaps are typically more favorable for efficient utilization of solar energy to produce photogenerated electron–hole pairs, which could directly enhance the photocatalytic properties.
When the photogenerated electrons and holes are recombining, strong photoluminescence signals will occur and can be detected via PL spectra, as shown in Figure 6. The PL spectra of BiVO4 and BiVO4@ZIF−8 were both excited at 365 nm, which corresponded to a photon energy of 3.39 eV, and was larger than the band gap of both BiVO4 (2.47 eV) and BiVO4@ZIF−8 (2.35 eV). This energy was strong enough for both BiVO4 and BiVO4@ZIF−8 to excite valence electrons to the conduction band. As shown in Figure 6, the specific peak intensities of BiVO4@ZIF−8 at 540 nm were much weaker than those of pure BiVO4. The weaker PL intensity indicated that the recombination of photogenerated electrons and holes was largely suppressed in the BiVO4@ZIF−8 composite, which was conducive to enhancing photocatalytic performance [40].
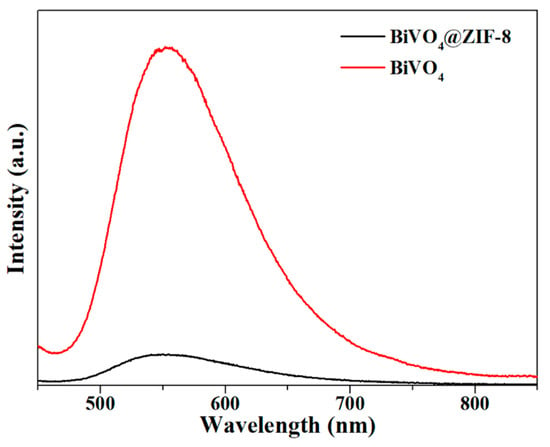
Figure 6.
The PL spectrum of BiVO4 and BiVO4@ZIF−8; the PL intensity of BiVO4@ZIF−8 is much weaker than that for pure BiVO4.
The photocatalytic performance of pure BiVO4 nanorods and BiVO4@ZIF−8 composites was evaluated by RhB photodegradation tests, as shown in Figure 7. Figure 7a shows the RhB degradation efficiencies of pure BiVO4 nanorods and BiVO4@ZIF−8 composites under UV light and visible light at different degradation times. As shown in Equation (1), the degradation efficiency of RhB was determined via UV–Vis absorption spectra, as presented in Figure S2. It is noticeable that about 15% to 23% of RhB was missing before the light irradiation of pure BiVO4 and BiVO4@ZIF−8, respectively, mainly due to the adsorption characteristics of BiVO4 and BiVO4@ZIF−8. Owing to the larger surface area, more RhB will be absorbed by BiVO4@ZIF−8 than by pure BiVO4. When RhB is dissolved in water, it is positively charged. Therefore, RhB cations will be attracted by the O2− or OH− anions at the photocatalyst’s surface. As shown in Figure 7a, after being irradiated under visible light for 40 min, about 80% of RhB was degraded by BiVO4@ZIF−8, while 32% of RhB still remained with the pure BiVO4 nanorods. It can also be seen in Figure 7a that, under full-spectrum irradiation, more than 90% of RhB was degraded by BiVO4@ZIF−8 in 20 min and entirely decomposed after 60 min. In comparison, after being irradiated under UV light for 20 min, less than 70% of RhB was degraded by the pure BiVO4 nanorods, and still 20% of RhB remained after irradiation for 40 min. The photocatalytic property of pure ZIF−8 is also shown in Figure S2e,f, which show that over 60% of RhB was absorbed by the MOF, owing to its large surface area and mesoporous structure. However, only about 80% of RhB was degraded during the following photodegradation.
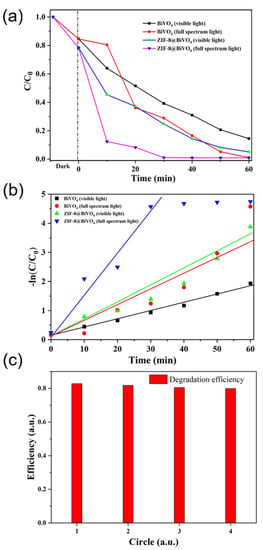
Figure 7.
(a) The photodegradation efficiency of BiVO4 and BiVO4@ZIF−8 under full-spectrum and visible light. In the presence of BiVO4-ZIF 8, more than 90% of RhB is degraded after 60 min of visible light irradiation, while only 80% of RhB is degraded in the presence of pure BiVO4. (b) The value of ln (C0/C) vs. time for RhB degradation; BiVO4@ZIF−8 shows a higher kinetic constant with the value of 0.133 min−1. (c) The reproducibility test of photocatalysis.
At a relatively low concentration (10 mg/L) of RhB solution, the kinetics of the degradation process of RhB could be defined as the pseudo-first-order reaction mode, which means that the values of the logarithm of the concentration ratio (ln(C0/C)) and irradiation time have a linear relationship [41]. Therefore, the kinetic constant of k for RhB degradation was defined as the slope of the quasi-line of the logarithm of the concentration ratio and irradiation time, as illustrated in Figure 7b. After calculation, the value of k for BiVO4 was 0.05 min−1. The k value for BiVO4@ZIF−8 reached 0.133 min−1, which indicates that BiVO4@ZIF−8 showed better photocatalytic activity under solar irradiation than pure BiVO4. Being as important as the catalytic efficiency, excellent reproducibility is also a crucial factor for photocatalysts. In Figure 7c, the reproducibility of BiVO4@ZIF−8 was examined by recycling BiVO4@ZIF−8 for four cycles. The results show that the degradation efficiency of BiVO4@ZIF−8 was stable with no drastic deactivation after recycling, indicating its feasibility for practical applications such as water purification.
Figure 8 shows the photocatalysis mechanism in the presence of pure BiVO4 and BiVO4@ZIF−8. As a type of semiconductor, the valence band (VB) is full of electrons, while the conduction band (CB) lacks electrons. When a semiconductor is irradiated under light with energy no less than the Eg of that semiconductor, the light can excite the electrons at the VB to the CB. Therefore, holes will be left in the VB, and thus photogenerated electron–hole pairs will be formed. The photogenerated electron–hole pairs can oxidize H2O molecules, OH− ions and dissolved O2 in H2O to form ·OH or O2−· radicals, which have strong oxidation properties. Moreover, the organic dye RhB, with its characteristic absorption wavelength of about 550 nm, could be photosensitized under light as well. Therefore, the sensitized RhB dye could be oxidized into water and CO2 by ·OH or O2−· radicals, which is one of the possible degradation mechanisms of an organic dye. We may summarize the set of RhB degradation processes as shown in Equations (3)–(6) below [42]:
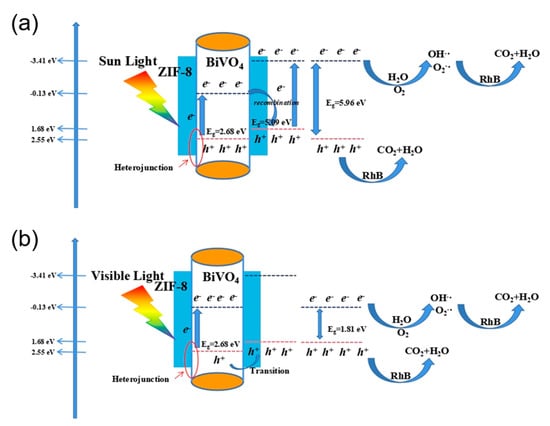
Figure 8.
The working mechanism of RhB photodegradation under (a) sunlight and (b) visible light.
In Equation (6), there are two pathways involved in RhB degradation: the stepwise de-ethylation process, and cleavage of the conjugated hydrocarbon structure [43,44], which can be directly seen in the UV–Vis absorption curve in Figure S2. The cleavage of the conjugated hydrocarbon structure leads to a decreased absorption intensity of the RhB solution, and the de-ethylation process leads to the red shift in the absorption peak of RhB to about 500 nm.
The mechanism of the enhanced photocatalysis performance of BiVO4@ZIF−8 could be further explained by its band gap structure. The positions of the CB and VB for BiVO4 and ZIF−8 are clearly shown in Figure 8. For the BiVO4@ZIF−8 heterojunction structure, the VB and CB positions of BiVO4 are all much more positive than those of ZIF−8 (the CB and VB positions are −0.13 eV and 2.55 eV for BiVO4, respectively, and −3.41 eV and 1.68 eV for ZIF−8, respectively). Hence, we may discuss the enhancing mechanism under both UV light and visible light. When irradiated under UV light, the photogenerated electron–hole pairs can be generated from both BiVO4 and ZIF−8 at the CB and VB, respectively. Owing to the closer position of the CB of BiVO4 and the VB of ZIF−8, the electron at the conduction band of BiVO4 and the hole at the valence band of ZIF−8 tend to recombine with each other more easily. Therefore, the e- and h+ of BiVO4@ZIF−8 only remain at the CB of ZIF−8 and the VB of BiVO4, respectively, which indicates that the band gap width of BiVO4@ZIF−8 is extended to 5.96 eV. The O2−· at the CB of ZIF−8 has higher energy than that of BiVO4; therefore, BiVO4@ZIF−8 is more beneficial for RhB degradation. When irradiated under visible light, due to the larger band gap of ZIF−8, the photogenerated electron–hole pairs can only be excited at the BiVO4 surface. As the VB of ZIF−8 is more negative than that of BiVO4, the covalent Bi-O-Zn at the heterojunction could play a role that transfers the holes at the VB of BiVO4 to the VB of ZIF−8 and hinders the recombination of the photogenerated electron–hole pairs [45]. In addition, the processes of degradation often occur at the surfaces of photocatalysts; in the BiVO4@ZIF−8 composite, ZIF−8, with its mesoporous structure and large surface area, has more active sites for h+ to form free radicals to photodegrade RhB. Thus, BiVO4@ZIF−8, with its larger surface area and heterojunction structures, exhibited better photocatalysis performance.
As a class of multiple functional materials, BiVO4 and its composites have been widely applied in the field of water treatment via photodegradation. Therefore, we investigated the degradation efficiency via photocatalysis of different photocatalysts based on BiVO4 composite materials, as listed in Table S1. In addition, we investigated the performance of different photocatalysts based on ZIF−8 composite materials, as illustrated in Table S2. As shown in Table S2, the BiVO4@ZIF−8 heterojunction had a better catalysis performance than other BiVO4- or ZIF−8-based composite materials.
4. Conclusions
In conclusion, BiVO4@ZIF−8, with its larger surface area and heterojunction structure, was fabricated using a hydrothermal technique first, and then a self-sedimentation technique. Owing to its mesoporous surface and heterojunction structure, the as-synthesized BiVO4@ZIF−8 exhibited a higher degradation efficiency than that of pure BiVO4. As shown in our research, about 90% of RhB was degraded under 60 min of visible light irradiation. The high degradation efficiency and reproducibility demonstrate that BiVO4@ZIF−8, as designed in our work, could be a prominent candidate for wastewater treatment.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/ma14237424/s1, Figure S1: The XRD pattern (a) and the standard PDF card (b) of pure BiVO4. Figure S2: The light absorption spectra of RhB solution after photo-degraded by BiVO4 (a) and BiVO4@ZIF-8 (b) in visible light; BiVO4 (c), BiVO4@ZIF-8 (d) and pure ZIF-8 (e) in UV light; (f) The photo degradation efficiency of pure ZIF-8 under UV light. Table S1: The performances of photocatalysts based on different BiVO4 composite materials. Table S2: The performances of photocatalysts based on different MOF composite materials.
Author Contributions
Conceptualization, R.G. and H.Z.; data curation, R.G., Y.X., M.L. and T.B.; funding acquisition, C.P. and H.Z.; investigation, R.G., C.P. and H.Z.; methodology, R.G. and Y.X.; resources, H.Z.; supervision, C.P. and H.Z.; writing—original draft, R.G.; writing—review and editing, Y.X., M.L., T.B., C.P. and H.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by start-up funding from ShanghaiTech University (for H.Z. and C.P.), the National Natural Science Foundation of China (Grant No. 11902200 for H.Z. and 21902142 for C.P.) and the Shanghai Sailing Program (Grant 19YF1433600).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data supporting reported results of the current study are available from the corresponding authors on reasonable request.
Acknowledgments
The authors acknowledge support from the Centre for High-resolution Electron Microscopy (CℏEM) (#EM02161943), and the Analytical Instrumentation Center (#SPST-AIC10112914), SPST, ShanghaiTech University.
Conflicts of Interest
The authors declare no conflict of interest.
References
- An, J.; Zhou, Q. Degradation of some typical pharmaceuticals and personal care products with copper-plating iron doped Cu2O under visible light irradiation. J. Environ. Sci. 2012, 24, 827–833. [Google Scholar] [CrossRef]
- Kitsiou, V.; Filippidis, N.; Mantzavinos, D.; Poulios, I. Heterogeneous and homogeneous photocatalytic degradation of the insecticide imidacloprid in aqueous solutions. Appl. Catal. B Environ. 2009, 86, 27–35. [Google Scholar] [CrossRef]
- Jiang, J.; Wang, H.; Chen, X.; Li, S.; Xie, T.; Wang, D.; Lin, Y. Enhanced photocatalytic degradation of phenol and photogenerated charges transfer property over BiOI-loaded ZnO composites. J. Colloid Interface Sci. 2017, 494, 130–138. [Google Scholar] [CrossRef]
- Tian, L.; Rui, Y.; Sun, K.; Cui, W.; An, W. Surface Decoration of ZnWO4 Nanorods with Cu2O Nanoparticles to Build Heterostructure with Enhanced Photocatalysis. Nanomaterials 2018, 8, 33. [Google Scholar] [CrossRef] [Green Version]
- Kisch, H. Semiconductor Photocatalysis—Mechanistic and Synthetic Aspects. Angew. Chem. Int. Ed. 2013, 52, 812–847. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.-S.; Zhang, W.-D. Monodispersed Ag3PO4 nanocrystals loaded on the surface of spherical Bi2MoO6 with enhanced photocatalytic performance. Dalton Trans. 2013, 42, 1094–1101. [Google Scholar] [CrossRef] [PubMed]
- Andersson, M.; Österlund, L.; Ljungström, S.; Palmqvist, A. Preparation of Nanosize Anatase and Rutile TiO2 by Hydrothermal Treatment of Microemulsions and Their Activity for Photocatalytic Wet Oxidation of Phenol. J. Phys. Chem. B 2022, 106, 10674–10679. [Google Scholar] [CrossRef]
- Deng, D.; Martin, S.T.; Ramanathan, S. Synthesis and characterization of one-dimensional flat ZnO nanotower arrays as high-efficiency adsorbents for the photocatalytic remediation of water pollutants. Nanoscale 2010, 2, 2685–2691. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Sun, F.; Zhang, Y.; Gu, K.; Chen, W.; Li, W. Photochemical construction of free-standing Sn-filled SnO2 nanotube array on a solution surface for flexible use in photocatalysis. J. Mater. Chem. 2011, 21, 12407–12413. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, H.; Qu, J. Photoelectrocatalytic degradation of organic contaminants at Bi2O3/TiO2 nanotube array electrode. Appl. Surf. Sci. 2011, 257, 4621–4624. [Google Scholar] [CrossRef]
- Mohan, S.; Subramanian, B. A strategy to fabricate bismuth ferrite (BiFeO3) nanotubes from electrospun nanofibers and their solar light-driven photocatalytic properties. RSC Adv. 2013, 3, 23737–23744. [Google Scholar] [CrossRef]
- Monfort, O.; Sfaelou, S.; Satrapinskyy, L.; Plecenik, T.; Roch, T.; Plesch, G.; Lianos, P. Comparative study between pristine and Nb-modified BiVO4 films employed for photoelectrocatalytic production of H2 by water splitting and for photocatalytic degradation of organic pollutants under simulated solar light. Catal. Today 2017, 280, 51–57. [Google Scholar] [CrossRef]
- Zhao, Q.; Ju, D.; Song, X.; Deng, X.; Ding, M.; Xu, X.; Zeng, H. Polyhedral Zn2SnO4: Synthesis, enhanced gas sensing and photocatalytic performance. Sens. Actuators B Chem. 2016, 229, 627–634. [Google Scholar] [CrossRef]
- Di, L.J.; Yang, H.; Hu, G.; Xian, T.; Ma, J.Y.; Jiang, J.L.; Li, R.S.; Wei, Z.Q. Enhanced photocatalytic activity of BiFeO3 particles by surface decoration with Ag nanoparticles. J. Mater. Sci. Mater. Electron. 2014, 25, 2463–2469. [Google Scholar] [CrossRef]
- Obregón, S.; Colón, G. Heterostructured Er3+ doped BiVO4 with exceptional photocatalytic performance by cooperative electronic and luminescence sensitization mechanism. Appl. Catal. B Environ. 2014, 158–159, 242–249. [Google Scholar] [CrossRef]
- Dolić, S.D.; Jovanović, D.J.; Smits, K.; Babić, B.; Marinović-Cincović, M.; Porobić, S.; Dramićanin, M.D. A comparative study of photocatalytically active nanocrystalline tetragonal zyrcon-type and monoclinic scheelite-type bismuth vanadate. Ceram. Int. 2018, 44, 17953–17961. [Google Scholar] [CrossRef]
- Liang, Z.; Cao, Y.; Qin, H.; Jia, D. Low-heating solid-state chemical synthesis of monoclinic scheelite BiVO4 with different morphologies and their enhanced photocatalytic property under visible light. Mater. Res. Bull. 2016, 84, 397–402. [Google Scholar] [CrossRef]
- Tokunaga, S.; Kato, H.; Kudo, A. ChemInform Abstract: Selective Preparation of Monoclinic and Tetragonal BiVO4 with Scheelite Structure and Their Photocatalytic Properties. ChemInform 2002, 33, 4624–4628. [Google Scholar] [CrossRef]
- Guo, L.; Li, J.; Lei, N.; Song, Q.; Liang, Z. Morphological evolution and enhanced photoelectrochemical performance of V4+ self-doped, [010] oriented BiVO4 for water splitting. J. Alloy. Compd. 2019, 771, 914–923. [Google Scholar] [CrossRef]
- Ge, L.; Liu, J. Efficient visible light-induced photocatalytic degradation of methyl orange by QDs sensitized CdS-Bi2WO6. Appl. Catal. B Environ. 2011, 105, 289–297. [Google Scholar] [CrossRef]
- Hong, S.J.; Lee, S.; Jang, J.S.; Lee, J.S. Heterojunction BiVO4/WO3 electrodes for enhanced photoactivity of water oxidation. Energy Environ. Sci. 2011, 4, 1781–1787. [Google Scholar] [CrossRef] [Green Version]
- Xi, G.; Ye, J. Synthesis of bismuth vanadate nanoplates with exposed {001} facets and enhanced visible-light photocatalytic properties. Chem. Commun. 2010, 46, 1893–1895. [Google Scholar] [CrossRef]
- Xia, Y.; Shang, S.-k.; Zeng, X.-r.; Zhou, J.; Li, Y.-y. A Novel Bi2MoO6/ZIF−8 Composite for Enhanced Visible Light Photocatalytic Activity. Nanomaterials 2019, 9, 545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Jiang, L.; Dong, W.; Zhang, E.; Ji, Z. Preparation and characterization of ZnO/ZIF−8 composite with selective photoelectrochemical responses. Mater. Lett. 2017, 201, 165–168. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, J.; Tan, X.; Shao, D.; Shi, J.; Zheng, L.; Zhang, J.; Yang, G.; Han, B. MIL-125-NH2@TiO2 Core–Shell Particles Produced by a Post-Solvothermal Route for High-Performance Photocatalytic H2 Production. ACS Appl. Mater. Interfaces 2018, 10, 16418–16423. [Google Scholar] [CrossRef]
- Li, X.; Pi, Y.; Hou, Q.; Yu, H.; Li, Z.; Li, Y.; Xiao, J. Amorphous TiO2@NH2-MIL-125(Ti) homologous MOF-encapsulated heterostructures with enhanced photocatalytic activity. Chem. Commun. 2018, 54, 1917–1920. [Google Scholar] [CrossRef]
- Xu, J.; Gao, J.; Wang, C.; Yang, Y.; Wang, L. NH2-MIL-125(Ti)/graphitic carbon nitride heterostructure decorated with NiPd co-catalysts for efficient photocatalytic hydrogen production. Appl. Catal. B Environ. 2017, 219, 101–108. [Google Scholar] [CrossRef]
- Liang, P.; Zhang, C.; Sun, H.; Liu, S.; Tadé, M.; Wang, S. Photocatalysis of C, N-doped ZnO derived from ZIF−8 for dye degradation and water oxidation. RSC Adv. 2016, 6, 95903–95909. [Google Scholar] [CrossRef]
- Wang, D.; Li, Z.; Zhou, J.; Fang, H.; He, X.; Jena, P.; Zeng, J.-B.; Wang, W.-N. Simultaneous Detection and Removal of Formaldehyde at Room Temperature: Janus Au@ZnO@ZIF−8 Nanoparticles. Nano-Micro Lett. 2017, 10, 4. [Google Scholar] [CrossRef] [Green Version]
- Shao, C.; Feng, S.; Zhu, G.; Zheng, W.; Sun, J.; Huang, X.; Ni, Z. Synergistic effects in N-K2Ti4O9/ZIF−8 composite and its photocatalysis degradation of Bisphenol A. Mater. Lett. 2020, 268, 127334. [Google Scholar] [CrossRef]
- Lin, Y.; Lu, C.; Wei, C. Microstructure and photocatalytic performance of BiVO4 prepared by hydrothermal method. J. Alloy. Compd. 2019, 781, 56–63. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, Y.; Duan, H.; Li, H.; Sun, C.; Liu, H. Facile synthesis of V4+ self-doped, [010] oriented BiVO4 nanorods with highly efficient visible light-induced photocatalytic activity. Phys. Chem. Chem. Phys. 2014, 16, 24519–24526. [Google Scholar] [CrossRef]
- Joo, J.B.; Zhang, Q.; Dahl, M.; Zaera, F.; Yin, Y. Synthesis, crystallinity control, and photocatalysis of nanostructured titanium dioxide shells. J. Mater. Res. 2013, 28, 362–368. [Google Scholar] [CrossRef]
- Wu, J.; Lü, X.; Zhang, L.; Xia, Y.; Huang, F.; Xu, F. Crystallinity control on photocatalysis and photoluminescence of TiO2-based nanoparticles. J. Alloy. Compd. 2010, 496, 234–240. [Google Scholar] [CrossRef]
- Wong, M.-S.; Hsu, S.-W.; Rao, K.K.; Kumar, C.P. Influence of crystallinity and carbon content on visible light photocatalysis of carbon doped titania thin films. J. Mol. Catal. A Chem. 2008, 279, 20–26. [Google Scholar] [CrossRef]
- Yin, C.; Zhu, S.; Chen, Z.; Zhang, W.; Gu, J.; Zhang, D. One step fabrication of C-doped BiVO4 with hierarchical structures for a high-performance photocatalyst under visible light irradiation. J. Mater. Chem. A 2013, 1, 8367–8378. [Google Scholar] [CrossRef]
- Liu, Y.; Dai, H.; Deng, J.; Zhang, L.; Au, C.T. Three-dimensional ordered macroporous bismuth vanadates: PMMA-templating fabrication and excellent visible light-driven photocatalytic performance for phenol degradation. Nanoscale 2012, 4, 2317–2325. [Google Scholar] [CrossRef]
- Kong, D.; Qi, J.; Liu, D.; Zhang, X.; Pan, L.; Zou, J. Ni-Doped BiVO4 with V4+ Species and Oxygen Vacancies for Efficient Photoelectrochemical Water Splitting. Trans. Tianjin Univ. 2019, 25, 340–347. [Google Scholar] [CrossRef] [Green Version]
- Obregón, S.; Caballero, A.; Colón, G. Hydrothermal synthesis of BiVO4: Structural and morphological influence on the photocatalytic activity. Appl. Catal. B Environ. 2012, 117–118, 59–66. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, Y.; Jiang, S.; Li, B.; Wang, J.; Shao, X.; Wang, D.; Wang, K.; Yan, Z. Bacitracin-assisted synthesis of spherical BiVO4 nanoparticles with C doping for remarkable photocatalytic performance under visible light. CrystEngComm 2020, 22, 1812–1821. [Google Scholar] [CrossRef]
- Ma, D.; Shi, J.-W.; Zou, Y.; Fan, Z.; Ji, X.; Niu, C. Highly Efficient Photocatalyst Based on a CdS Quantum Dots/ZnO Nanosheets 0D/2D Heterojunction for Hydrogen Evolution from Water Splitting. ACS Appl. Mater. Interfaces 2017, 9, 25377–25386. [Google Scholar] [CrossRef]
- Guo, R.; Tian, R.; Shi, D.; Li, H.; Liu, H. S-Doped ZnSnO3 Nanoparticles with Narrow Band Gaps for Photocatalytic Wastewater Treatment. ACS Appl. Nano Mater. 2019, 2, 7755–7765. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, C.; Zhao, D.; Ma, W.; Zhao, J. Change of Adsorption Modes of Dyes on Fluorinated TiO2 and Its Effect on Photocatalytic Degradation of Dyes under Visible Irradiation. Langmuir 2008, 24, 7338–7345. [Google Scholar] [CrossRef] [PubMed]
- Lei, P.; Chen, C.; Yang, J.; Ma, W.; Zhao, J.; Zang, L. Degradation of Dye Pollutants by Immobilized Polyoxometalate with H2O2 under Visible-Light Irradiation. Environ. Sci. Technol. 2005, 39, 8466–8474. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.-H.; Zhang, X.-L.; Zhang, N.; Zhang, J.-Y.; Zhang, R.; Liu, Y.-F.; Fang, Y.-Z. A visible-light driven Bi2S3@ZIF−8 core–shell heterostructure and synergistic photocatalysis mechanism. Dalton Trans. 2018, 47, 684–692. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).