Polyaniline Synthesized by Different Dopants for Fluorene Detection via Photoluminescence Spectroscopy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PANI
2.3. Preparation of Fluorene
2.4. Preparation of Thin Film
2.5. Analytical Methods
3. Result and Discussion
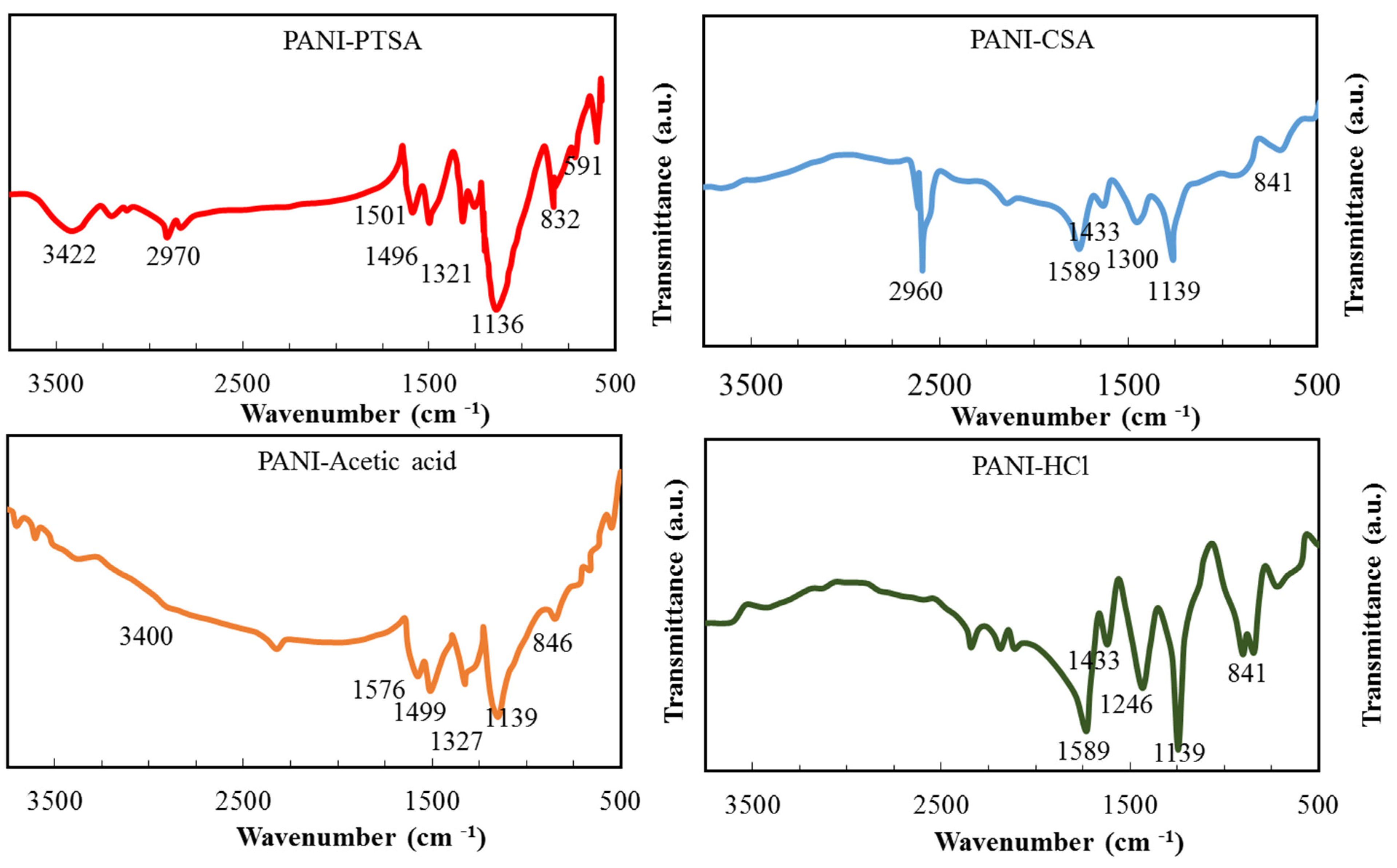
3.1. FT-IR Characterization
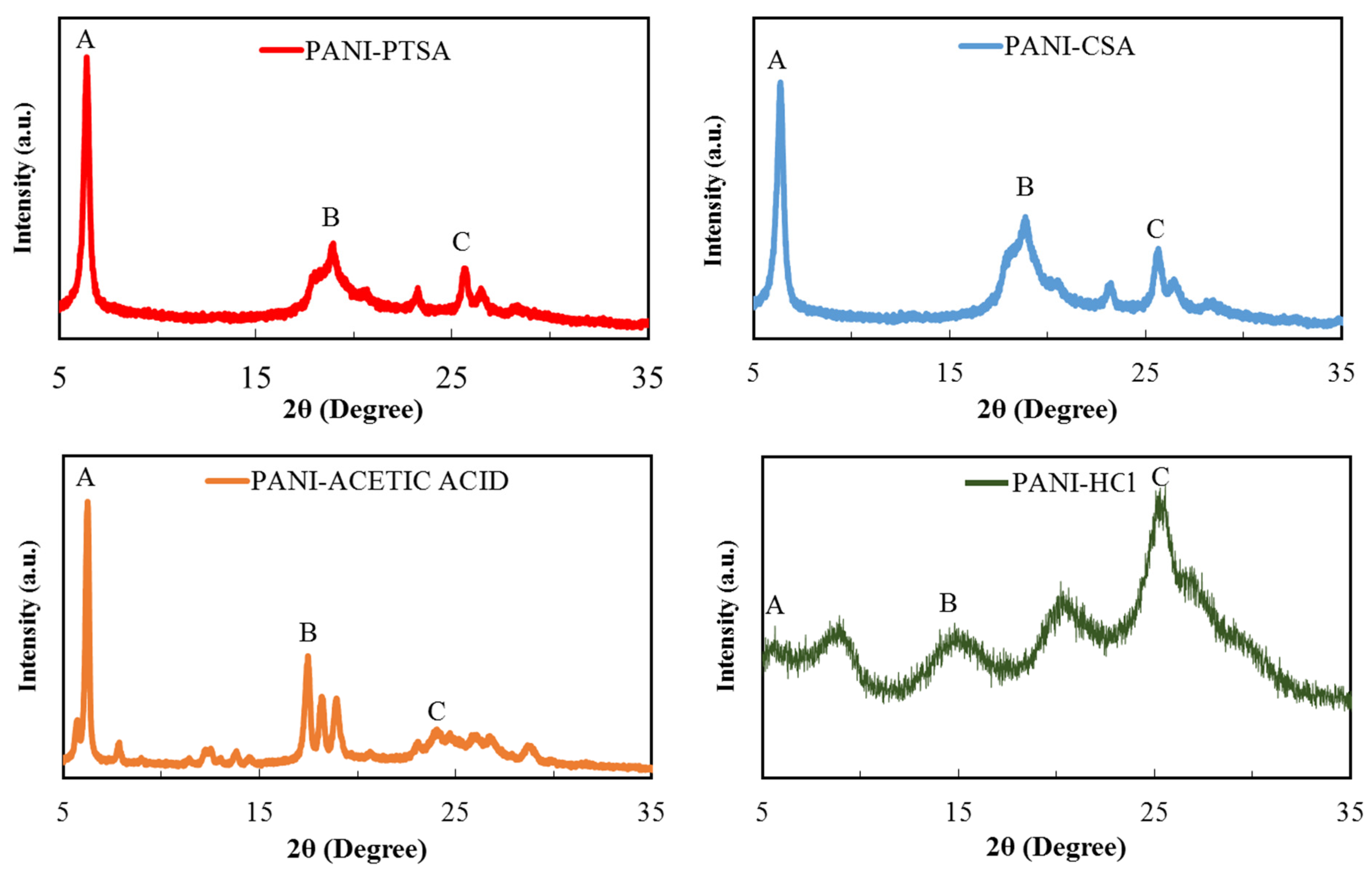
3.2. XRD Characterization
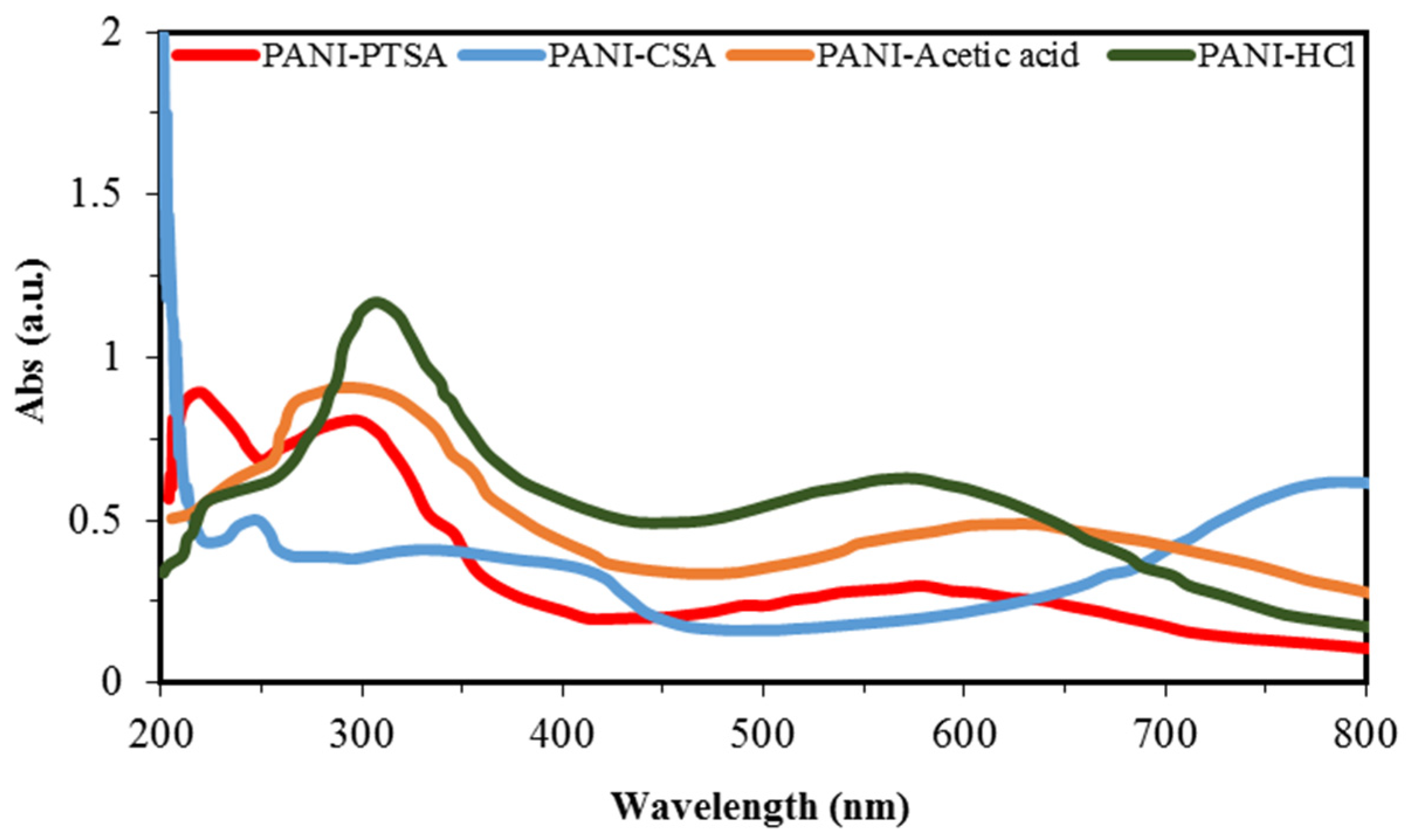
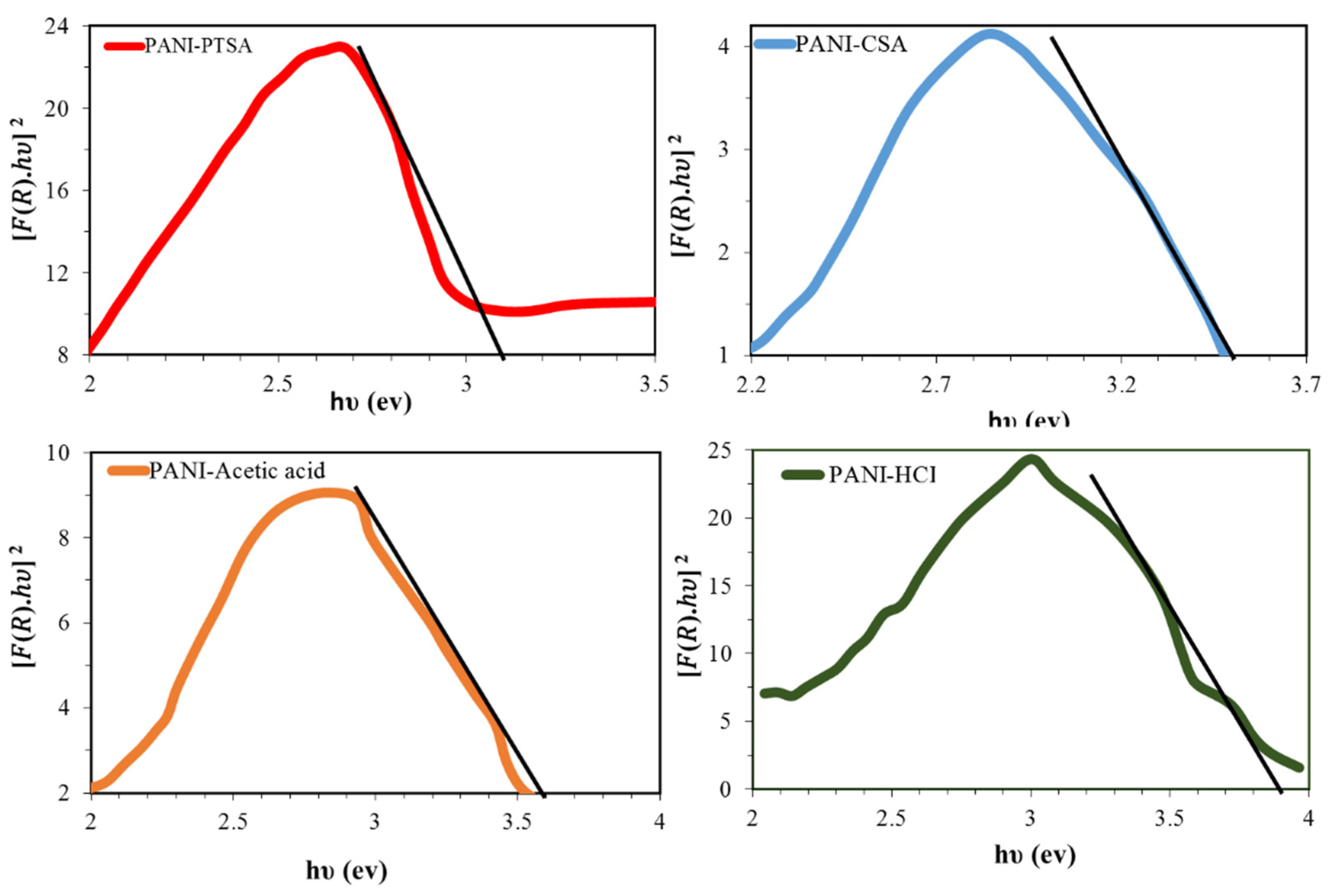
3.3. UV-Vis Characterization
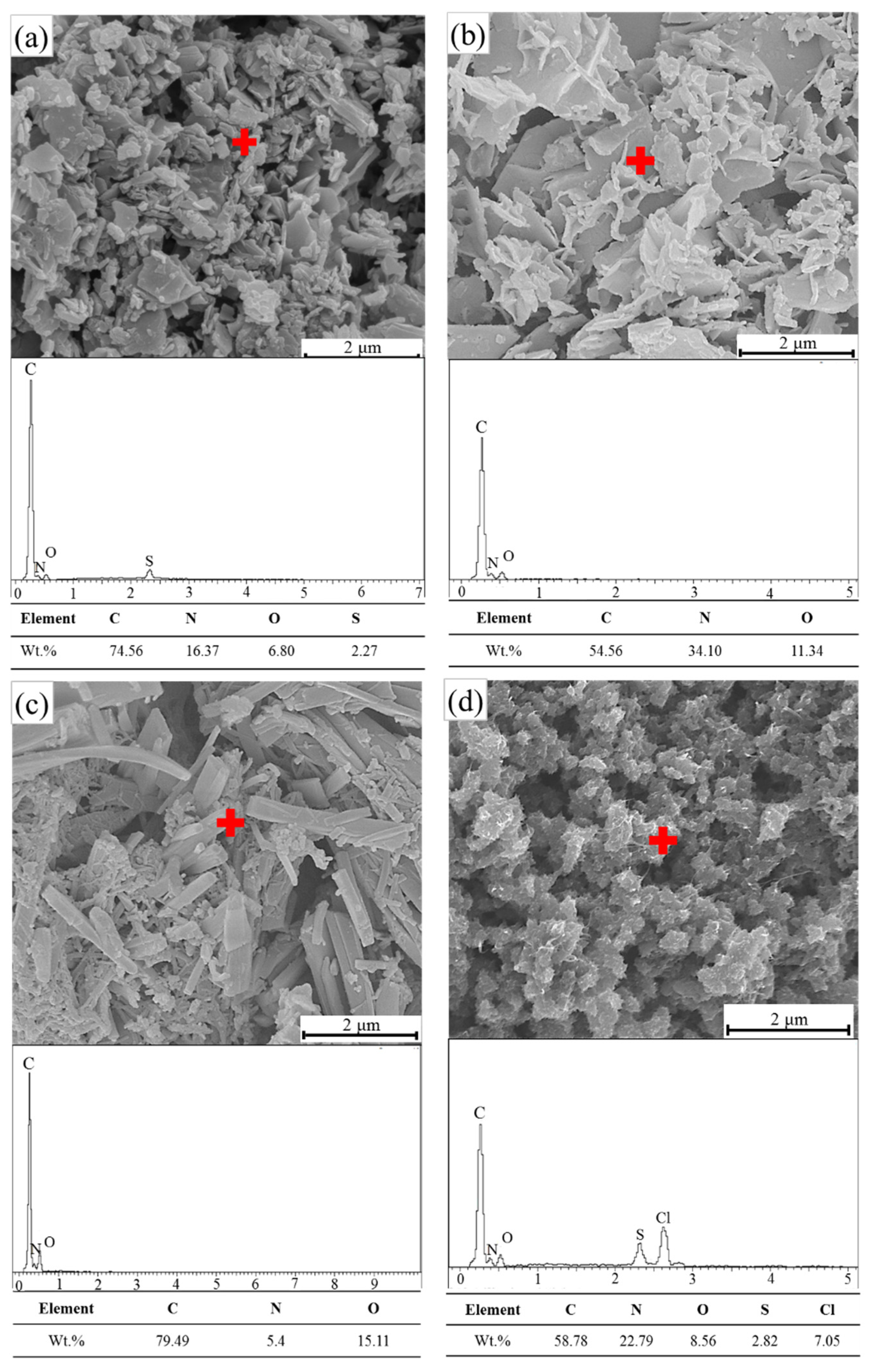
3.4. FE-SEM and EDS Characterization
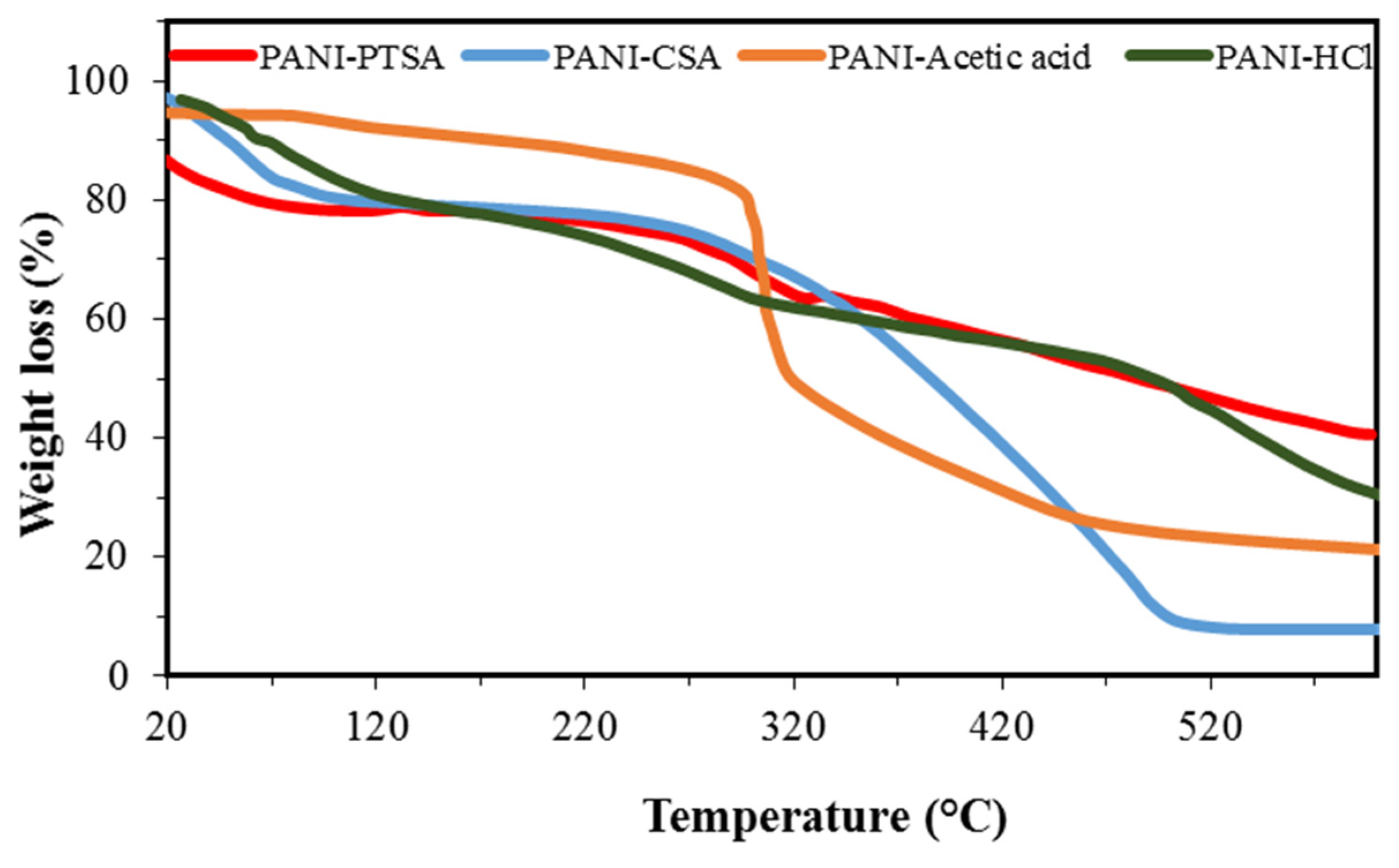
3.5. TGA Characterization
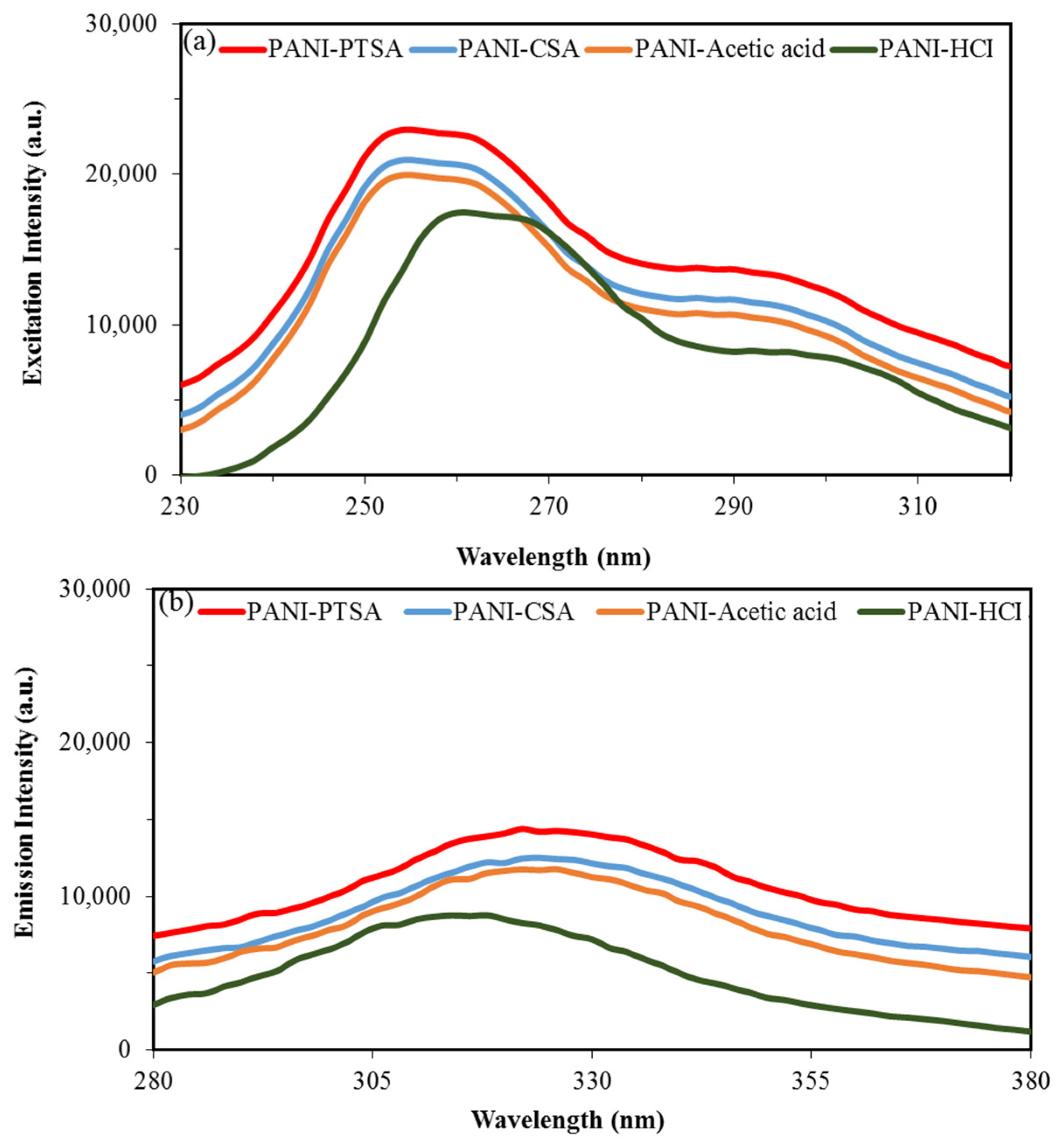
3.6. PL Analysis
3.7. Electrical Analysis
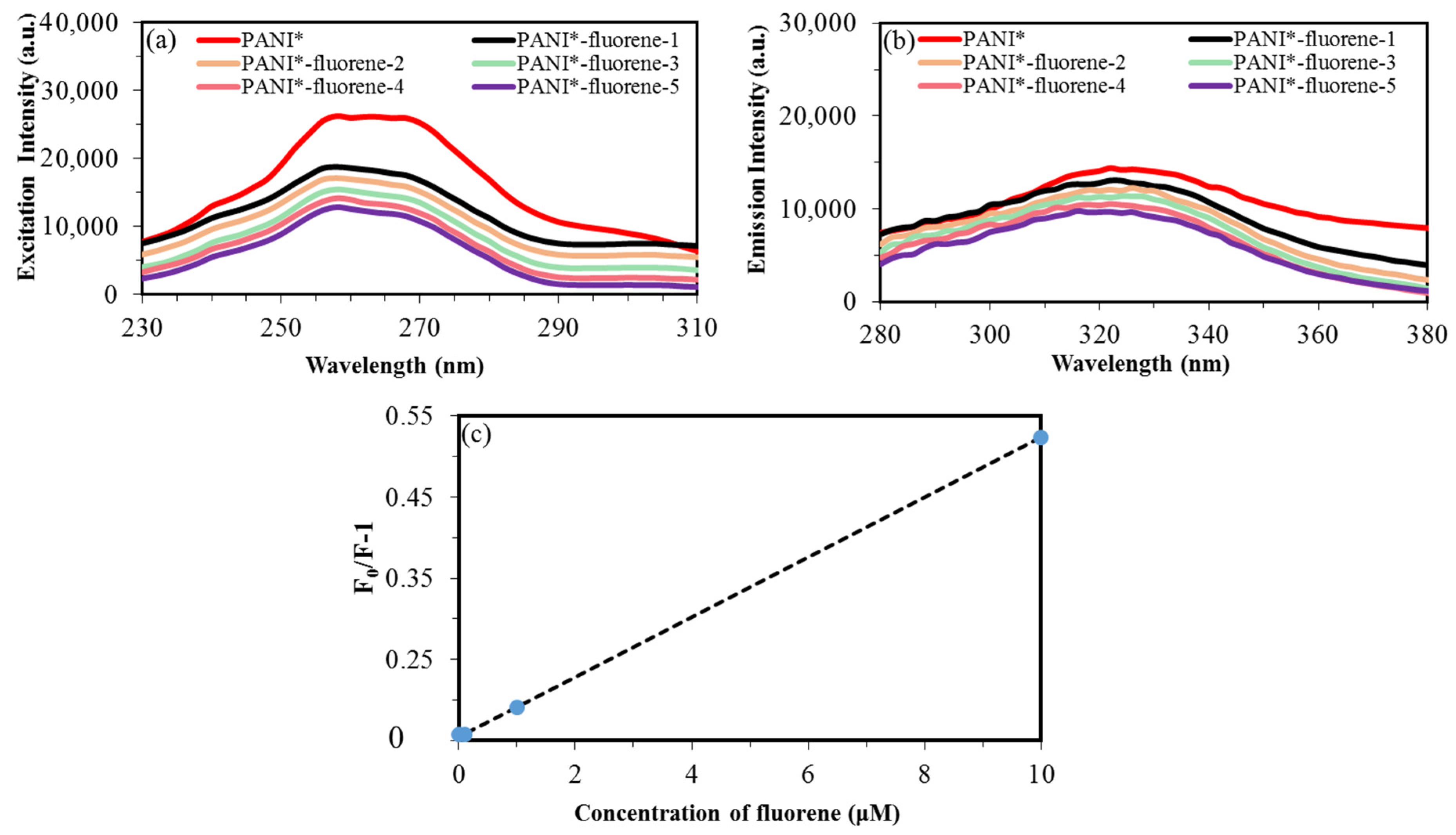
3.8. Fluorescence Detection of Fluorene
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kapp, R. Fluorine. In Encyclopedia of Toxicology; Elsevier: Amsterdam, The Netherlands, 2005; pp. 343–346. [Google Scholar]
- Yang, L.; Watts, D.J. Particle surface characteristics may play an important role in phytotoxicity of alumina nanoparticles. Toxicol. Lett. 2005, 158, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Alfano, B.; Massera, E.; De Maria, A.; De Girolamo, A.; Di Francia, G.; Delli Veneri, P.; Napolitano, T.; Borriello, A. Polyaniline proton doping for sensor application. In Proceedings of the 2015 XVIII AISEM Annual Conference, Trento, Italy, 3–5 February 2015; IEEE: Piscataway, NJ, USA, 2015; pp. 1–4. [Google Scholar]
- Quijada, C. Special Issue: Conductive Polymers: Materials and Applications. Materials 2020, 13, 2344. [Google Scholar] [CrossRef]
- Abdolahi, A.; Hamzah, E.; Ibrahim, Z.; Hashim, S. Synthesis of uniform polyaniline nanofibers through interfacial polymerization. Materials 2012, 5, 1487–1494. [Google Scholar] [CrossRef] [Green Version]
- Namsheer, K.; Rout, C.S. Conducting polymers: A comprehensive review on recent advances in synthesis, properties and applications. RSC Adv. 2021, 11, 5659–5697. [Google Scholar] [CrossRef]
- Chen, S.; Li, B.; Xiao, R.; Luo, H.; Yu, S.; He, J.; Liao, X. Design an Epoxy Coating with TiO2/GO/PANI Nanocomposites for Enhancing Corrosion Resistance of Q235 Carbon Steel. Materials 2021, 14, 2629. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Wu, H.; Bu, X.; Wu, Q.; Wang, X.; Han, C.; Li, X.; Wang, X.; Liu, W. Improving Ammonia Detecting Performance of Polyaniline Decorated rGO Composite Membrane with GO Doping. Materials 2021, 14, 2829. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liu, L.; Meng, F.; Li, Y.; Wang, F. Protective Performance of Polyaniline-Sulfosalicylic Acid/Epoxy Coating for 5083 Aluminum. Materials 2018, 11, 292. [Google Scholar] [CrossRef] [Green Version]
- Beygisangchin, M.; Abdul Rashid, S.; Shafie, S.; Sadrolhosseini, A.R.; Lim, H.N. Preparations, Properties, and Applications of Polyaniline and Polyaniline Thin Films—A Review. Polymers 2021, 13, 2003. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Zhong, X.-X.; Huang, H.-X.; Wang, H.-Q.; Feng, Q.-P.; Li, Q.-Y. Preparation and Application of Polyaniline Doped with Different Sulfonic Acids for Supercapacitor. Acta Phys. Chim. Sin. 2016, 32, 474–480. [Google Scholar] [CrossRef]
- Li, Z.; Gong, L. Research Progress on Applications of Polyaniline (PANI) for Electrochemical Energy Storage and Conversion. Materials 2020, 13, 548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brachetti-Sibaja, S.B.; Palma-Ramírez, D.; Torres-Huerta, A.M.; Domínguez-Crespo, M.A.; Dorantes-Rosales, H.J.; Rodríguez-Salazar, A.E.; Ramírez-Meneses, E. Cvd conditions for mwcnts production and their effects on the optical and electrical properties of ppy/mwcnts, pani/mwcnts nanocomposites by in situ electropolymerization. Polymers 2021, 13, 351. [Google Scholar] [CrossRef] [PubMed]
- Lyu, H. Triple layer tungsten trioxide, graphene, and polyaniline composite films for combined energy storage and electrochromic applications. Polymers 2020, 12, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silakhori, M.; Naghavi, M.; Metselaar, H.; Mahlia, T.; Fauzi, H.; Mehrali, M. Accelerated Thermal Cycling Test of Microencapsulated Paraffin Wax/Polyaniline Made by Simple Preparation Method for Solar Thermal Energy Storage. Materials 2013, 6, 1608–1620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, C.; Park, J.-K.; Yun, G.-H.; Choi, H.H.; Lee, H.-J.; Yook, J.-G. Radio-Frequency/Microwave Gas Sensors Using Conducting Polymer. Materials 2020, 13, 2859. [Google Scholar] [CrossRef] [PubMed]
- Ramanavicius, S.; Ramanavicius, A. Conducting polymers in the design of biosensors and biofuel cells. Polymers 2021, 13, 49. [Google Scholar] [CrossRef]
- Zarach, Z.; Trzciński, K.; Łapiński, M.; Lisowska-Oleksiak, A.; Szkoda, M. Improving the Performance of a Graphite Foil/Polyaniline Electrode Material by a Thin PEDOT:PSS Layer for Application in Flexible, High Power Supercapacitors. Materials 2020, 13, 5791. [Google Scholar] [CrossRef]
- Ghorbani, F.; Zamanian, A.; Aidun, A. Conductive electrospun polyurethane-polyaniline scaffolds coated with poly(vinyl alcohol)-GPTMS under oxygen plasma surface modification. Mater. Today Commun. 2020, 22, 100752. [Google Scholar] [CrossRef]
- Llorens, E.; Armelin, E.; del Mar Pérez-Madrigal, M.; del Valle, L.; Alemán, C.; Puiggalí, J. Nanomembranes and Nanofibers from Biodegradable Conducting Polymers. Polymers 2013, 5, 1115–1157. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, M.; Shahid, M.; Khan, Z.; Ammar, A.; Saboor, A.; Khalid, A.; Hayat, A.; Saeed, A.; Koohgilani, M. Electrochemical Comparison of SAN/PANI/FLG and ZnO/GO Coated Cast Iron Subject to Corrosive Environments. Materials 2018, 11, 2239. [Google Scholar] [CrossRef] [Green Version]
- Mohseni, H.R.; Dehghanipour, M.; Dehghan, N.; Tamaddon, F.; Ahmadi, M.; Sabet, M.; Behjat, A. Enhancement of the photovoltaic performance and the stability of perovskite solar cells via the modification of electron transport layers with reduced graphene oxide/polyaniline composite. Sol. Energy 2021, 213, 59–66. [Google Scholar] [CrossRef]
- Zhou, D.D.; Cui, X.T.; Hines, A.; Greenberg, R.J. Conducting polymers in neural stimulation applications. In Implantable Neural Prostheses 2; Springer: Berlin/Heidelberg, Germany, 2009; pp. 217–252. [Google Scholar]
- Gospodinova, N.; Terlemezyan, L. Conducting polymers prepared by oxidative polymerization: Polyaniline. Prog. Polym. Sci. 1998, 23, 1443–1484. [Google Scholar] [CrossRef]
- Bhadra, S.; Khastgir, D.; Singha, N.K.; Lee, J.H. Progress in preparation, processing and applications of polyaniline. Prog. Polym. Sci. 2009, 34, 783–810. [Google Scholar] [CrossRef]
- Yan, H.; Sada, N.; Toshima, N. Thermal transporting properties of electrically conductive polyaniline films as organic thermoelectric materials. J. Therm. Anal. Calorim. 2002, 69, 881–887. [Google Scholar] [CrossRef]
- Kulkarni, M.V.; Viswanath, A.K.; Marimuthu, R.; Seth, T. Spectroscopic, transport, and morphological studies of polyaniline doped with inorganic acids. Polym. Eng. Sci. 2004, 44, 1676–1681. [Google Scholar] [CrossRef]
- Borole, D.; Kapadi, U.; Kumbhar, P.; Hundiwale, D. Influence of inorganic and organic supporting electrolytes on the electrochemical synthesis of polyaniline, poly(o-toluidine) and their copolymer thin films. Mater. Lett. 2002, 56, 685–691. [Google Scholar] [CrossRef]
- Stafström, S.; Brédas, J.L.; Epstein, A.J.; Woo, H.S.; Tanner, D.B.; Huang, W.S.; MacDiarmid, A.G. Polaron lattice in highly conducting polyaniline: Theoretical and optical studies. Phys. Rev. Lett. 1987, 59, 1464–1467. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Bhadra, S.; Khastgir, D. Effect of dopant type on the properties of polyaniline. J. Appl. Polym. Sci. 2009, 112, 3135–3140. [Google Scholar] [CrossRef]
- Abdiryim, T.; Jamal, R.; Nurulla, I. Doping effect of organic sulphonic acids on the solid-state synthesized polyaniline. J. Appl. Polym. Sci. 2007, 105, 576–584. [Google Scholar] [CrossRef]
- Kulkarni, M.V.; Viswanath, A.K.; Marimuthu, R.; Seth, T. Synthesis and characterization of polyaniline doped with organic acids. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 2043–2049. [Google Scholar] [CrossRef]
- Hatchett, D.W.; Josowicz, M.; Janata, J. Acid Doping of Polyaniline: Spectroscopic and Electrochemical Studies. J. Phys. Chem. B 1999, 103, 10992–10998. [Google Scholar] [CrossRef]
- Bredas, J.L.; Street, G.B. Polarons, bipolarons, and solitons in conducting polymers. Acc. Chem. Res. 1985, 18, 309–315. [Google Scholar] [CrossRef]
- Le, T.-H.; Kim, Y.; Yoon, H. Electrical and Electrochemical Properties of Conducting Polymers. Polymers 2017, 9, 150. [Google Scholar] [CrossRef]
- Cho, S.; Lee, J.S.; Joo, H. Recent Developments of the Solution-Processable and Highly Conductive Polyaniline Composites for Optical and Electrochemical Applications. Polymers 2019, 11, 1965. [Google Scholar] [CrossRef] [Green Version]
- Amiri, A.; Baghayeri, M.; Koshki, M.-S. Electrochemical Deposition of Polyaniline on the Stainless Steel Mesh for the Extraction of Polycyclic Aromatic Hydrocarbons. SSRN Electron. J. 2021, 1, 1–10. [Google Scholar] [CrossRef]
- Na, W.; Liu, Q.; Sui, B.; Hu, T.; Su, X. Highly sensitive detection of acid phosphatase by using a graphene quantum dots-based förster resonance energy transfer. Talanta 2016, 161, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Usman, F.; Dennis, J.O.; Seong, K.C.; Yousif Ahmed, A.; Meriaudeau, F.; Ayodele, O.B.; Tobi, A.R.; Rabih, A.A.S.; Yar, A. Synthesis and characterisation of a ternary composite of polyaniline, reduced graphene-oxide and chitosan with reduced optical band gap and stable aqueous dispersibility. Results Phys. 2019, 15, 102690. [Google Scholar] [CrossRef]
- Saravanan, S.; Joseph Mathai, C.; Anantharaman, M.R.; Venkatachalam, S.; Prabhakaran, P.V. Investigations on the electrical and structural properties of polyaniline doped with camphor sulphonic acid. J. Phys. Chem. Solids 2006, 67, 1496–1501. [Google Scholar] [CrossRef]
- Trchová, M.; Stejskal, J. Polyaniline: The infrared spectroscopy of conducting polymer nanotubes (IUPAC Technical Report). Pure Appl. Chem. 2011, 83, 1803–1817. [Google Scholar] [CrossRef]
- Chen, S.A.; Lee, H.T. Polyaniline plasticized with 1-methyl-2-pyrrolidone: Structure and doping behavior. Macromolecules 1993, 26, 3254–3261. [Google Scholar] [CrossRef]
- Han, D.; Chu, Y.; Yang, L.; Liu, Y.; Lv, Z. Reversed micelle polymerization: A new route for the synthesis of DBSA–polyaniline nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2005, 259, 179–187. [Google Scholar] [CrossRef]
- Cai, L.-T.; Yao, S.-B.; Zhou, S.-M. Effects of the magnetic field on the polyaniline film studied by in situ conductivity measurements and X-ray diffraction. J. Electroanal. Chem. 1997, 421, 45–48. [Google Scholar] [CrossRef]
- Bala, P.; Samantaray, B.K.; Srivastava, S.K.; Nando, G.B. Organomodified montmorillonite as filler in natural and synthetic rubber. J. Appl. Polym. Sci. 2004, 92, 3583–3592. [Google Scholar] [CrossRef]
- Jayakannan, M.; Annu, S.; Ramalekshmi, S. Structural effects of dopants and polymerization methodologies on the solid-state ordering and morphology of polyaniline. J. Polym. Sci. Part B Polym. Phys. 2005, 43, 1321–1331. [Google Scholar] [CrossRef]
- Rathore, B.S.; Chauhan, N.P.S.; Rawal, M.K.; Ameta, S.C.; Ameta, R. Chitosan–polyaniline–copper(II) oxide hybrid composite for the removal of methyl orange dye. Polym. Bull. 2020, 77, 4833–4850. [Google Scholar] [CrossRef]
- Diggikar, R.S.; Deshmukh, S.P.; Thopate, T.S.; Kshirsagar, S.R. Performance of Polyaniline Nanofibers (PANI NFs) as PANI NFs-Silver (Ag) Nanocomposites (NCs) for Energy Storage and Antibacterial Applications. ACS Omega 2019, 4, 5741–5749. [Google Scholar] [CrossRef]
- Ajekwene, K.K.; Kurian, T. Bulk and nano-structured polyaniline: Synthesis, characterization, thermal behaviour and dc conductivity. Int. J. Res. Advent Technol. 2018, 6, 2321–9637. [Google Scholar]
- Bhadra, S.; Singha, N.K.; Khastgir, D. Dual functionality of PTSA as electrolyte and dopant in the electrochemical synthesis of polyaniline, and its effect on electrical properties. Polym. Int. 2007, 56, 919–927. [Google Scholar] [CrossRef]
- Bhadra, S.; Singha, N.K.; Khastgir, D. Polyaniline by new miniemulsion polymerization and the effect of reducing agent on conductivity. Synth. Met. 2006, 156, 1148–1154. [Google Scholar] [CrossRef]
- Geethalakshmi, D.; Muthukumarasamy, N.; Balasundaraprabhu, R. CSA-doped PANI semiconductor nanofilms: Synthesis and characterization. J. Mater. Sci. Mater. Electron. 2015, 26, 7797–7803. [Google Scholar] [CrossRef]
- Bednarczyk, K.; Matysiak, W.; Tański, T.; Janeczek, H.; Schab-Balcerzak, E.; Libera, M. Effect of polyaniline content and protonating dopants on electroconductive composites. Sci. Rep. 2021, 11, 7487. [Google Scholar] [CrossRef]
- Bai, X.L.; Mei, J.T.; Bai, Y.; Mu, Z.G. Synthesis and Characterization of Polyaniline Nanotubes Doped with Amino Acetic Acid. Appl. Mech. Mater. 2012, 184–185, 1285–1288. [Google Scholar] [CrossRef]
- Karaoğlan, N.; Bindal, C. Synthesis and optical characterization of benzene sulfonic acid doped polyaniline. Eng. Sci. Technol. Int. J. 2018, 21, 1152–1158. [Google Scholar] [CrossRef]
- Al-Daghman, A.N.J.; Ibrahim, K.; Ahmed, N.M.; Al-Messiere, M.A. Effect of doping by stronger ions salt on the microstructure of conductive polyaniline-ES: Structure and properties. J. Optoelectron. Biomed. Mater. 2016, 8, 175–183. [Google Scholar]
- Swaruparani, H.; Basavaraja, S.; Basavaraja, C.; Huh, D.S.; Venkataraman, A. A new approach to soluble polyaniline and its copolymers with toluidines. J. Appl. Polym. Sci. 2010, 117, 1350–1360. [Google Scholar] [CrossRef]
- Bhadra, S.; Singha, N.K.; Khastgir, D. Electrochemical synthesis of polyaniline and its comparison with chemically synthesized polyaniline. J. Appl. Polym. Sci. 2007, 104, 1900–1904. [Google Scholar] [CrossRef]
- Li, X.-G.; Li, A.; Huang, M.-R. Facile High-Yield Synthesis of Polyaniline Nanosticks with Intrinsic Stability and Electrical Conductivity. Chem. Eur. J. 2008, 14, 10309–10317. [Google Scholar] [CrossRef]
- Neelgund, G.M.; Oki, A. A facile method for the synthesis of polyaniline nanospheres and the effect of doping on their electrical conductivity. Polym. Int. 2011, 60, 1291–1295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, J.; Virji, S.; Weiller, B.H.; Kaner, R.B. Polyaniline Nanofibers: Facile Synthesis and Chemical Sensors. J. Am. Chem. Soc. 2003, 125, 314–315. [Google Scholar] [CrossRef]
- Casado, U.M.; Quintanilla, R.M.; Aranguren, M.I.; Marcovich, N.E. Composite films based on shape memory polyurethanes and nanostructured polyaniline or cellulose–polyaniline particles. Synth. Met. 2012, 162, 1654–1664. [Google Scholar] [CrossRef]
- Huang, J.; Kaner, R.B. The intrinsic nanofibrillar morphology of polyaniline. Chem. Commun. 2006, 4, 367–376. [Google Scholar] [CrossRef]
- Banerjee, S.; Kumar, A. Dielectric behavior and charge transport in polyaniline nanofiber reinforced PMMA composites. J. Phys. Chem. Solids 2010, 71, 381–388. [Google Scholar] [CrossRef]
- Rahayu, I.; Eddy, D.R.; Novianty, A.R.; Anggreni, A.; Bahti, H.; Hidayat, S. The effect of hydrochloric acid-doped polyaniline to enhance the conductivity. IOP Conf. Ser. Mater. Sci. Eng. 2019, 509, 012051. [Google Scholar] [CrossRef]
- Jing, X.; Wang, Y.; Wu, D.; Qiang, J. Sonochemical synthesis of polyaniline nanofibers. Ultrason. Sonochem. 2007, 14, 75–80. [Google Scholar] [CrossRef]
- Saini, P.; Arora, M.; Arya, S.K.; Tawale, J.S. Effect of controlled doping on electrical properties and permittivity of PTSA doped polyanilines and their EMI shielding performance. Indian J. Pure Appl. Phys. 2014, 52, 175–182. [Google Scholar]
- Yang, L.; Zhang, C. Sen Effect of Dopants on Microstructure and Properties of Polyaniline and Polypyrrole. Adv. Mater. Res. 2011, 328–330, 1576–1579. [Google Scholar] [CrossRef]
- Kumar, N.A.; Choi, H.J.; Shin, Y.R.; Chang, D.W.; Dai, L.; Baek, J.B. Polyaniline-grafted reduced graphene oxide for efficient electrochemical supercapacitors. ACS Nano 2012, 6, 1715–1723. [Google Scholar] [CrossRef] [PubMed]
- Meriga, V.; Valligatla, S.; Sundaresan, S.; Cahill, C.; Dhanak, V.R.; Chakraborty, A.K. Optical, electrical, and electrochemical properties of graphene based water soluble polyaniline composites. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Arora, M.; Arya, S.K.; Barala, S.K.; Saini, P. Magnetic resonance and electrical properties of p-toluene sulphonic acid doped polyaniline. Appl. Phys. Mater. Sci. AIP Conf. Proc. 2013, 1536, 1235–1236. [Google Scholar] [CrossRef]
- Yan, F.; Xue, G. Synthesis and characterization of electrically conducting polyaniline in water–oil microemulsion. J. Mater. Chem. 1999, 9, 3035–3039. [Google Scholar] [CrossRef]
- Babu, V.J.; Vempati, S.; Ramakrishna, S. Conducting Polyaniline-Electrical Charge Transportation. Mater. Sci. Appl. 2013, 4, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Shimano, J.Y.; MacDiarmid, A.G. Polyaniline, a dynamic block copolymer: Key to attaining its intrinsic conductivity? Synth. Met. 2001, 123, 251–262. [Google Scholar] [CrossRef]
- Wohlgenannt, M.; Vardeny, Z. V Spin-dependent exciton formation rates in -conjugated materials. J. Phys. Condens. Matter 2003, 15, R83–R107. [Google Scholar] [CrossRef]
- Chiang, J.C.; MacDiarmid, A.G. “Polyaniline”: Protonic acid doping of the emeraldine form to the metallic regime. Synth. Met. 1986, 13, 193–205. [Google Scholar] [CrossRef]
- Cao, Y.; Andreatta, A.; Heeger, A.J.; Smith, P. Influence of chemical polymerization conditions on the properties of polyaniline. Polymer 1989, 30, 2305–2311. [Google Scholar] [CrossRef]
- Bhadra, S.; Singha, N.K.; Chattopadhyay, S.; Khastgir, D. Effect of different reaction parameters on the conductivity and dielectric properties of polyaniline synthesized electrochemically and modeling of conductivity against reaction parameters through regression analysis. J. Polym. Sci. Part B Polym. Phys. 2007, 45, 2046–2059. [Google Scholar] [CrossRef]
- Sui, B.; Kim, B.; Zhang, Y.; Frazer, A.; Belfield, K.D. Highly Selective Fluorescence Turn-On Sensor for Fluoride Detection. ACS Appl. Mater. Interfaces 2013, 5, 2920–2923. [Google Scholar] [CrossRef]
- Sadrolhosseini, A.R.; Krishnan, G.; Safie, S.; Beygisangchin, M.; Rashid, S.A.; Harun, S.W. Enhancement of the fluorescence property of carbon quantum dots based on laser ablated gold nanoparticles to evaluate pyrene. Opt. Mater. Express 2020, 10, 2227–2241. [Google Scholar] [CrossRef]
- Li, Y.; Chen, J.; Wang, Y.; Li, H.; Yin, J.; Li, M.; Wang, L.; Sun, H.; Chen, L. Large-scale direct pyrolysis synthesis of excitation-independent carbon dots and analysis of ferric (III) ion sensing mechanism. Appl. Surf. Sci. 2021, 538, 148151. [Google Scholar] [CrossRef]
- Abbas, A.M.; Anwar, Z.M.; Soliman, Y.A.; Abdel-Hadi, M.M.; Kamel, R.M. A turn-off luminescent europium probe for efficient and sensitive detection of some low molecular weight polycyclic aromatic hydrocarbons in seawater. Microchem. J. 2020, 159, 105471. [Google Scholar] [CrossRef]
- Rau, A. Basic experiments in thin-layer chromatography-fourier transform Raman spectrometry. J. Raman Spectrosc. 1993, 24, 251–254. [Google Scholar] [CrossRef]
- Kumar, R.; Arora, M.; Jain, A.K.; Babu, J.N. 1,3-Bis(cyanomethoxy)calix[4]arene capped CdSe quantum dots for the fluorogenic sensing of fluorene. RSC Adv. 2017, 7, 14015–14020. [Google Scholar] [CrossRef] [Green Version]
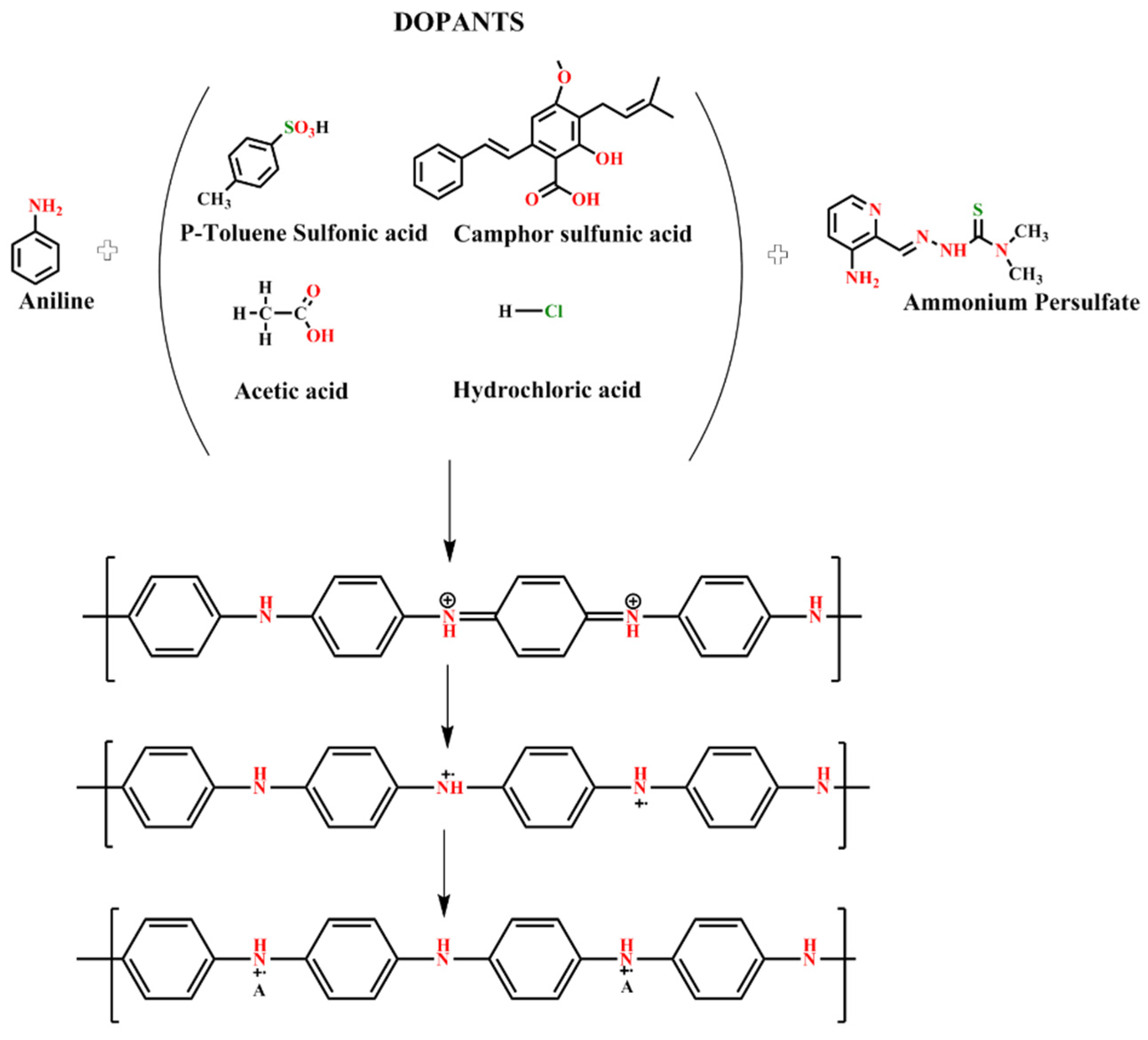
| Sample Name | Aniline Monomer Concentration (mL) | APS as Oxidant Monomer (g) | Different Acids (0.4 M) | Weight of the PANI (g) |
|---|---|---|---|---|
| PANI-PTSA | 2.75 | 1.7 | PTSA | 0.2 |
| PANI-CSA | 2.75 | 1.7 | CSA | 0.18 |
| PANI-Acetic acid | 2.75 | 1.7 | Acetic acid | 0.15 |
| PANI-HCl | 2.75 | 1.7 | HCl | 0.19 |
| Sample Name | Amount of PANI* (μL) | Amount of Fluorene (μL) | Fluorene Concentrations (μM) |
|---|---|---|---|
| PANI-PTSA-fluorene-1 | 2900 | 100 | 0.001 |
| PANI-PTSA-fluorene-2 | 2900 | 100 | 0.01 |
| PANI-PTSA-fluorene-3 | 2900 | 100 | 0.1 |
| PANI-PTSA-fluorene-4 | 2900 | 100 | 1 |
| PANI-PTSA-fluorene-5 | 2900 | 100 | 10 |
| Assignment | PANI-PTSA [39] | PANI-CSA [40] | PANI-Acetic Acid [41,42] | PANI-HCl [40] | |
|---|---|---|---|---|---|
| Symmetric and asymmetric stretching | NH2 and NH | 3422 | - | 3400 | - |
| Aromatic aniline ring | C–H and CH2 | 2970 | 2960 | - | - |
| Quinoid ring stretching | C = C | 1501 | 1589 | 1576 | 1589 |
| Benzonoid ring stretching | N–B–N | 1496 | 1433 | 1499 | 1433 |
| C-N stretching of benzenoid ring | C–N | 1321 | 1300 | 1327 | 1246 |
| In-plane bending vibration of C-H | C–H | 1136 | 1139 | 1139 | 1139 |
| Ortho substitutions, 1,2 disubstitution in benzene ring | C–H | 832,591 | 841 | 846 | 841 |
| Sample Name | Peak Sign | d-Spacing (Å) | FWHM (B) | Crystallite Size (nm) | Inter-Chain Separation (Å) |
|---|---|---|---|---|---|
| PANI-PTSA-1 | A | 15.71429 | 0.006 | 241.750167 | 19.64285714 |
| PANI-PTSA-2 | B | 4.695122 | 0.044 | 33.36713996 | 5.868902439 |
| PANI-PTSA-3 | C | 3.484163 | 0.017 | 87.33634992 | 4.35520362 |
| PANI-CSA-1 | A | 14.25926 | 0.008 | 181.3126253 | 17.82407407 |
| PANI-CSA-2 | B | 4.723926 | 0.051 | 28.78733644 | 5.904907975 |
| PANI-CSA-3 | C | 3.494163 | 0.027 | 59.56613339 | 4.36520362 |
| PANI-Acetic acid-1 | A | 14.5283 | 0.004 | 362.6252505 | 18.16037736 |
| PANI-ACETIC acid-2 | B | 5.099338 | 0.02 | 72.5250501 | 6.374172185 |
| PANI-ACETIC acid-3 | C | 3.615023 | 0.0249 | 54.98955366 | 4.375 |
| PANI-HCl-1 | A | 16.04167 | 0.037 | 39.20272978 | 20.05208333 |
| PANI-HCl-3 | B | 5.968992 | 0.091 | 16.05216176 | 7.46124031 |
| PANI-HCl-5 | C | 3.5 | 0.107 | 13.87586868 | 4.518779343 |
| Sample Name | Crystallite Size (nm) | d-Spacing (Å) | Inter-Chain Separation (A˚) | Bandgap | Conductivity (S·cm−1) | References |
|---|---|---|---|---|---|---|
| PANI-PTSA | 47 | 3.50 | 4.37 | 4.50 | 4.8 × 10−2 | [30] |
| 13.8 | 3.51 | 4.39 | 4.196 | 1 × 10−2 | [50] | |
| 29 | 4.6 | - | - | 12 × 10−2 | [46] | |
| 87 | 3.48 | 4.35 | 3.1 | 3.84 × 101 | Current study | |
| PANI-CSA | - | 3.52 | - | 3.8 | 11 × 10−2 | [52] |
| 65 | 4.9 | - | - | 21 × 10−2 | [46] | |
| 56 | - | - | - | 2.7× 101 | [40] | |
| 59 | 3.49 | 4.36 | 3.5 | 2.92 × 101 | Current study | |
| PANI-Acetic acid | 49 | - | - | - | 6.5× 10−2 | [53] |
| - | - | - | - | 4.21 × 10−2 | [32] | |
| - | - | - | - | 6.5 × 10−5 | [54] | |
| 55 | 3.6 | 4.37 | 3.6 | 2.50 × 10−2 | Current study | |
| PANI-HCl | 59 | 3.49 | 4.36 | 4.48 | 1.6 × 10−1 | [30] |
| - | - | - | 2.40 | 54 × 10−2 | [55] | |
| 38.3 | 3.9 | - | 3.85 | - | [56] | |
| 14 | 3.5 | 4.5 | 3.9 | 2.44 × 10−2 | Current study |
| Π–π* | Polaron to π* | π to Polaron | |||
|---|---|---|---|---|---|
| PANI-Samples | Wavelength (nm) | PANI-Samples | Wavelength (nm) | PANI-Samples | Wavelength (nm) |
| PANI-PTSA | 207 | PANI-PTSA | 321 | PANI-PTSA | 578 |
| PANI-CSA | 253 | PANI-CSA | 338 | PANI-CSA | 785 |
| PANI-Acetic acid | 283 | PANI-Acetic acid | - | PANI-Acetic acid | 630 |
| PANI-HCl | 317 | PANI-HCl | - | PANI-HCl | 579 |
| Sample Name | R (Ω) | Rs (Ω/sq) | Rv (Ω·cm) | Conductivity (Ω·cm)–1 |
|---|---|---|---|---|
| PANI-PTSA | 9.98 × 10−1 | 8.84 | 2.61 × 10−2 | 3.84 × 101 |
| PANI-CSA | 1.31 × 10−1 | 1.16 | 3.42 × 10−2 | 2.92 × 101 |
| PANI-Acetic acid | 1.53 × 103 | 1.36 × 104 | 4.00 × 10−2 | 2.50 × 10−2 |
| PANI-HCl | 1.57 × 103 | 1.39 × 104 | 4.10 × 101 | 2.44 × 10−2 |
| No. | Fluorescent Sensor Methods | Linear Range (μM) | LOD (nM) | References |
|---|---|---|---|---|
| 1 | Eu(III)-(CCA)2 probe | 0.1–1.9 | 0.43 | [82] |
| 2 | Thin-layer chromatography (TLC) | - | 0.5 | [83] |
| 3 | 1,3-bis(cyanomethoxy)-tert-butylcalix[4]arene (CAD) onto CdSe quantum dots (QDs) CAD@CdSe | 0–20 | 0.8 | [84] |
| 4 | PANI doped PTSA | 0.001–10 | 0.26 | Current study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beygisangchin, M.; Abdul Rashid, S.; Shafie, S.; Sadrolhosseini, A.R. Polyaniline Synthesized by Different Dopants for Fluorene Detection via Photoluminescence Spectroscopy. Materials 2021, 14, 7382. https://doi.org/10.3390/ma14237382
Beygisangchin M, Abdul Rashid S, Shafie S, Sadrolhosseini AR. Polyaniline Synthesized by Different Dopants for Fluorene Detection via Photoluminescence Spectroscopy. Materials. 2021; 14(23):7382. https://doi.org/10.3390/ma14237382
Chicago/Turabian StyleBeygisangchin, Mahnoush, Suraya Abdul Rashid, Suhaidi Shafie, and Amir Reza Sadrolhosseini. 2021. "Polyaniline Synthesized by Different Dopants for Fluorene Detection via Photoluminescence Spectroscopy" Materials 14, no. 23: 7382. https://doi.org/10.3390/ma14237382
APA StyleBeygisangchin, M., Abdul Rashid, S., Shafie, S., & Sadrolhosseini, A. R. (2021). Polyaniline Synthesized by Different Dopants for Fluorene Detection via Photoluminescence Spectroscopy. Materials, 14(23), 7382. https://doi.org/10.3390/ma14237382






