hBN Flake Embedded Al2O3 Thin Film for Flexible Moisture Barrier
Abstract
:1. Introduction
2. Materials and Methods
2.1. The hBN Flake Exfoliation
2.2. Fabrication of LBL Template & Al2O3 Composite
2.3. Characterizations
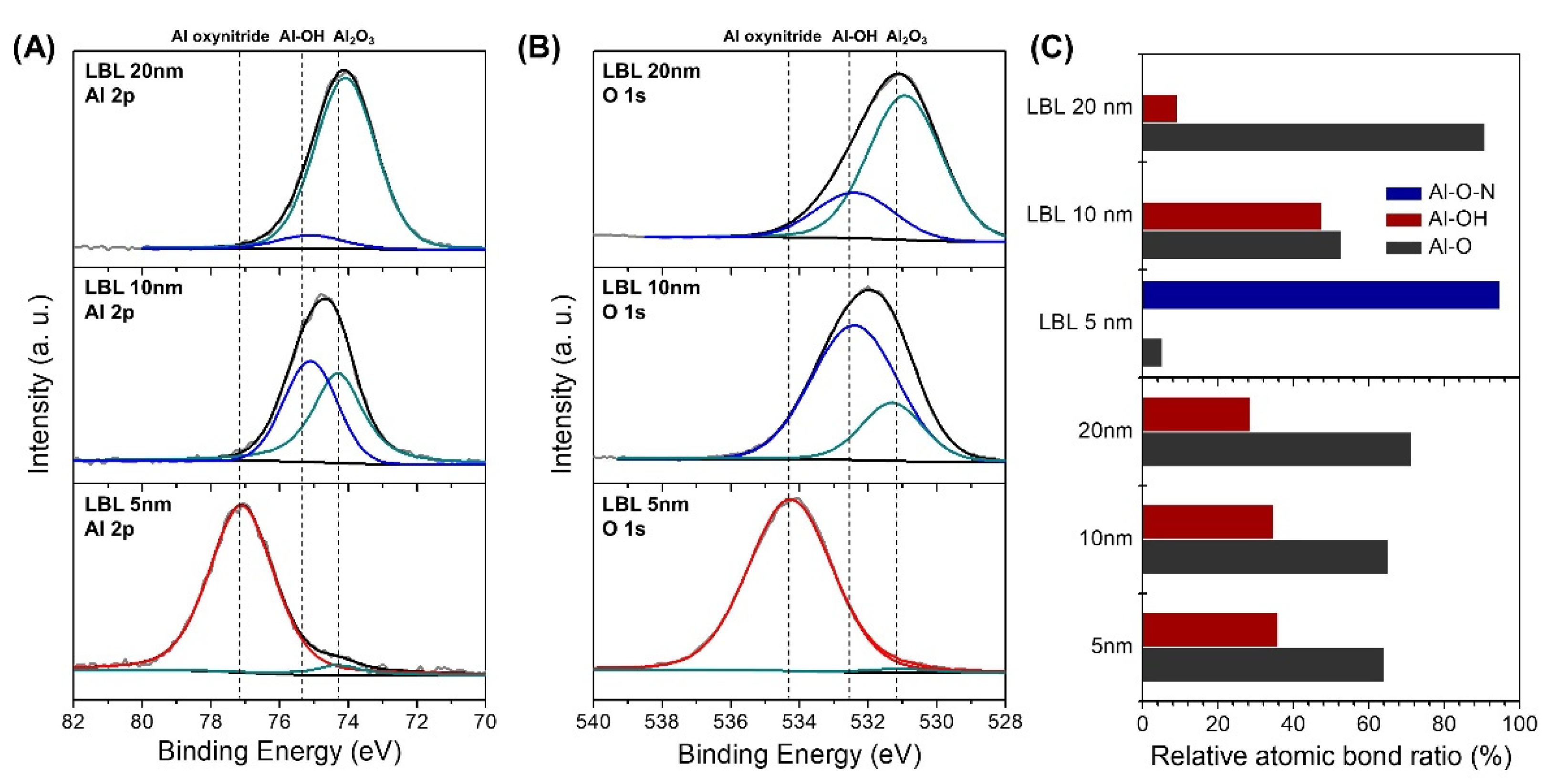
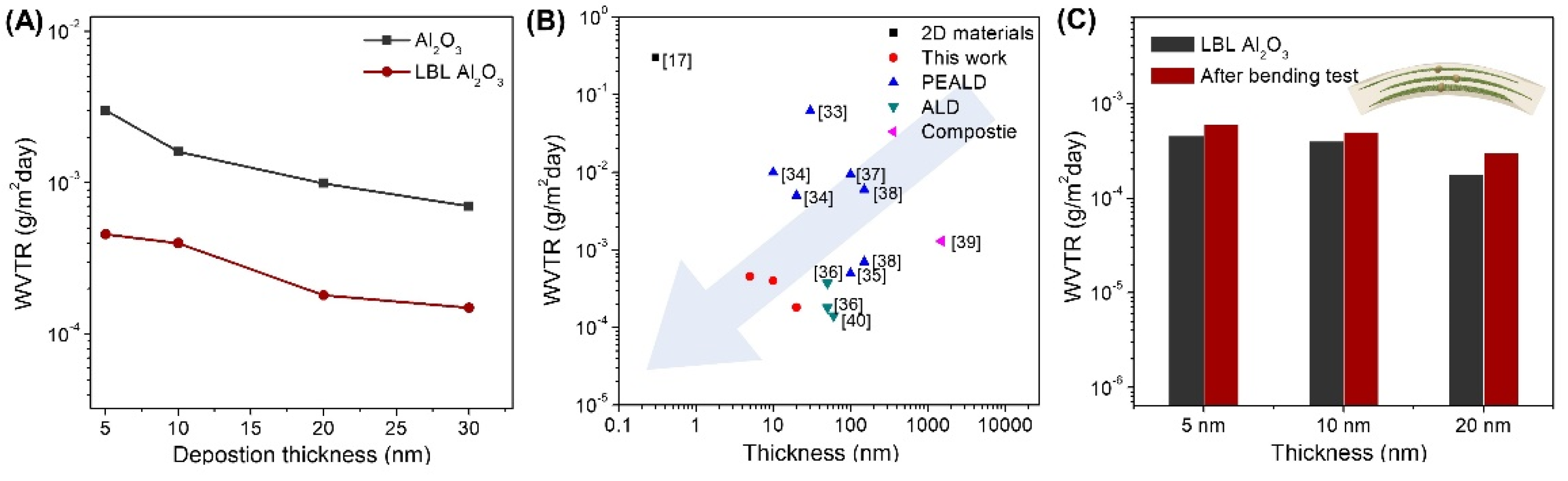
3. Results & Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ostroverkhova, O. Organic Optoelectronic Materials: Mechanisms and Applications. Chem. Rev. 2016, 116, 13279–13412. [Google Scholar] [CrossRef]
- Wei, Q.; Fei, N.N.; Islam, A.; Lei, T.; Hong, L.; Peng, R.X.; Fan, X.; Chen, L.; Gao, P.Q.; Ge, Z.Y. Small-Molecule Emitters with High Quantum Efficiency: Mechanisms, Structures, and Applications in OLED Devices. Adv. Opt. Mater. 2018, 6, 1800512. [Google Scholar] [CrossRef]
- Lin, Y.Z.; Li, Y.F.; Zhan, X.W. Small molecule semiconductors for high-efficiency organic photovoltaics. Chem. Soc. Rev. 2012, 41, 4245–4272. [Google Scholar] [CrossRef]
- Lim, S.F.; Wang, W.; Chua, S.J. Degradation of organic light-emitting devices due to formation and growth of dark spots. Mat. Sci. Eng. B-Solid. 2001, 85, 154–159. [Google Scholar] [CrossRef]
- Boyd, C.C.; Cheacharoen, R.; Leijtens, T.; McGehee, M.D. Understanding Degradation Mechanisms and Improving Stability of Perovskite Photovoltaics. Chem. Rev. 2019, 119, 3418–3451. [Google Scholar] [CrossRef]
- Zhao, O.; Ding, Y.; Pan, Z.; Rolston, N.; Zhang, J.; Dauskardt, R.H. Open-Air Plasma-Deposited Multilayer Thin-Film Moisture Barriers. ACS Appl. Mater. Interfaces 2020, 12, 26405–26412. [Google Scholar] [CrossRef]
- Cheacharoen, R.; Rolston, N.; Harwood, D.; Bush, K.A.; Dauskardt, R.H.; McGehee, M.D. Design and understanding of encapsulated perovskite solar cells to withstand temperature cycling. Energ. Environ. Sci. 2018, 11, 144–150. [Google Scholar] [CrossRef]
- Majee, S.; Geffroy, B.; Bonnassieux, Y.; Bouree, J.E. Interface effects on the moisture barrier properties of SiNx/PMMA/SiNx hybrid structure. Surf. Coat. Tech. 2014, 254, 429–432. [Google Scholar] [CrossRef]
- Nehm, F.; Dollinger, F.; Fahlteich, J.; Klumbies, H.; Leo, K.; Muller-Meskamp, L. Importance of Interface Diffusion and Climate in Defect Dominated Moisture Ultrabarrier Applications. ACS Appl. Mater. Interfaces 2016, 8, 19807–19812. [Google Scholar] [CrossRef]
- Nam, T.; Lee, H.; Seo, S.; Cho, S.M.; Shong, B.; Lee, H.-B.-R.; Kim, H. Moisture barrier properties of low-temperature atomic layer deposited Al2O3 using various oxidants. Ceram. Int. 2019, 45, 19105–19112. [Google Scholar] [CrossRef]
- Leterrier, Y.; Andersons, J.; Pitton, Y.; Manson, J.A.E. Adhesion of silicon oxide layers on poly(ethylene terephthalate). 2. Effect of coating thickness on adhesive and cohesive strengths. J. Polym. Sci. Pol. Phys. 1997, 35, 1463–1472. [Google Scholar] [CrossRef]
- Li, Y.; Xiong, Y.; Yang, H.; Cao, K.; Chen, R. Thin film encapsulation for the organic light-emitting diodes display via atomic layer deposition. J. Mater. Res. 2019, 35, 681–700. [Google Scholar] [CrossRef]
- Seo, T.H.; Lee, S.; Cho, H.; Chandramohan, S.; Suh, E.K.; Lee, H.S.; Bae, S.K.; Kim, S.M.; Park, M.; Lee, J.K.; et al. Tailored CVD graphene coating as a transparent and flexible gas barrier. Sci. Rep. 2016, 6, 24143. [Google Scholar] [CrossRef]
- Sagade, A.A.; Aria, A.I.; Edge, S.; Melgari, P.; Gieseking, B.; Bayer, B.C.; Meyer, J.C.; Bird, D.; Brewer, P.; Hofmann, S. Graphene-based nanolaminates as ultra-high permeation barriers. NPJ 2D Mater. Appl. 2017, 1, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Luo, D.; Wang, M.H.; Li, Y.Q.; Kim, C.; Yu, K.M.; Kim, Y.H.; Han, H.J.; Biswal, M.; Huang, M.; Kwon, Y.; et al. Adlayer-Free Large-Area Single Crystal Graphene Grown on a Cu(111) Foil. Adv. Mater. 2019, 31, 1903615. [Google Scholar] [CrossRef]
- Kim, K.S.; Zhao, Y.; Jang, H.; Lee, S.Y.; Kim, J.M.; Kim, K.S.; Ahn, J.H.; Kim, P.; Choi, J.Y.; Hong, B.H. Large-scale pattern growth of graphene films for stretchable transparent electrodes. Nature 2009, 457, 706–710. [Google Scholar] [CrossRef]
- Lee, J.S.; Choi, S.H.; Yun, S.J.; Kim, Y.I.; Boandoh, S.; Park, J.H.; Shin, B.G.; Ko, H.; Lee, S.H.; Kim, Y.M.; et al. Wafer-scale single-crystal hexagonal boron nitride film via self-collimated grain formation. Science 2018, 362, 817–821. [Google Scholar] [CrossRef] [Green Version]
- Nam, T.; Park, Y.J.; Lee, H.; Oh, I.-K.; Ahn, J.-H.; Cho, S.M.; Kim, H.; Lee, H.-B.-R. A composite layer of atomic-layer-deposited Al2O3 and graphene for flexible moisture barrier. Carbon 2017, 116, 553–561. [Google Scholar] [CrossRef]
- Zhang, B.W.; Wu, Q.; Yu, H.T.; Bulin, C.K.; Sun, H.; Li, R.H.; Ge, X.; Xing, R.G. High-Efficient Liquid Exfoliation of Boron Nitride Nanosheets Using Aqueous Solution of Alkanolamine. Nanoscale Res. Lett. 2017, 12, 1–7. [Google Scholar] [CrossRef]
- Lei, W.; Mochalin, V.N.; Liu, D.; Qin, S.; Gogotsi, Y.; Chen, Y. Boron nitride colloidal solutions, ultralight aerogels and freestanding membranes through one-step exfoliation and functionalization. Nat. Commun. 2015, 6, 8849. [Google Scholar] [CrossRef] [Green Version]
- Song, E.H.; Kang, B.H.; Kim, T.Y.; Lee, H.J.; Park, Y.W.; Kim, Y.C.; Ju, B.K. Highly oriented gold/nanoclay-polymer nanocomposites for flexible gas barrier films. ACS Appl. Mater. Interfaces 2015, 7, 4778–4783. [Google Scholar] [CrossRef]
- Scholz, S.; Kondakov, D.; Lussem, B.; Leo, K. Degradation Mechanisms and Reactions in Organic Light-Emitting Devices. Chem. Rev. 2015, 115, 8449–8503. [Google Scholar] [CrossRef]
- Khorasani, M.T.; Mirzadeh, H. Effect of oxygen plasma treatment on surface charge and wettability of PVC blood bag—In vitro assay. Radiat. Phys. Chem. 2007, 76, 1011–1016. [Google Scholar] [CrossRef]
- Cumpson, P.J. Angle-resolved XPS and AES: Depth-resolution limits and a general comparison of properties of depth-profile reconstruction methods. J. Electron Spectrosc. Relat. Phenom. 1995, 73, 25–52. [Google Scholar] [CrossRef]
- Yong, S.H.; Kim, S.J.; Cho, S.M.; Chae, H. Spatially-Resolved Remote Plasma Atomic Layer Deposition Process for Moisture Barrier Al2O3 Films. J. Korean Phys. Soc. 2018, 73, 45–52. [Google Scholar] [CrossRef]
- Graf, N.; Yegen, E.; Gross, T.; Lippitz, A.; Weigel, W.; Krakert, S.; Terfort, A.; Unger, W.E.S. XPS and NEXAFS studies of aliphatic and aromatic amine species on functionalized surfaces. Surf. Sci. 2009, 603, 2849–2860. [Google Scholar] [CrossRef]
- Lee, J.; Kim, T.; Ryu, S.U.; Choi, K.; Ahn, G.H.; Paik, J.G.; Ryu, B.; Park, T.; Won, Y.S. Study on the Aging Mechanism of Boron Potassium Nitrate (BKNO3) for Sustainable Efficiency in Pyrotechnic Mechanical Devices. Sci. Rep. 2018, 8, 11745. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.W.; Hsu, J.-C.; Lin, Y.-H.; Chen, H.-L. Structural investigation of high-transmittance aluminum oxynitride films deposited by ion beam sputtering. Surf. Interface Anal 2011, 43, 1089–1094. [Google Scholar] [CrossRef]
- Osadchii, D.Y.; Olivos-Suarez, A.I.; Bavykina, A.V.; Gascon, J. Revisiting Nitrogen Species in Covalent Triazine Frameworks. Langmuir 2017, 33, 14278–14285. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Tang, W.J.; Yang, H.D.; Dong, Z.P.; Huang, J.W.; Li, S.W.; Wang, J.; Jin, J.; Ma, J.T. Enhanced-electrocatalytic activity of Ni1-xFex alloy supported on polyethyleneimine functionalized MoS2 nanosheets for hydrazine oxidation. RSC Adv. 2014, 4, 1988–1995. [Google Scholar] [CrossRef]
- Liu, Q.; Qin, H.; Boscoboinik, J.A.; Zhou, G. Comparative Study of the Oxidation of NiAl(100) by Molecular Oxygen and Water Vapor Using Ambient-Pressure X-ray Photoelectron Spectroscopy. Langmuir 2016, 32, 11414–11421. [Google Scholar] [CrossRef]
- Klumbies, H.; Schmidt, P.; Hähnel, M.; Singh, A.; Schroeder, U.; Richter, C.; Mikolajick, T.; Hoßbach, C.; Albert, M.; Bartha, J.W.; et al. Thickness dependent barrier performance of permeation barriers made from atomic layer deposited alumina for organic devices. Org. Electron. 2015, 17, 138–143. [Google Scholar] [CrossRef] [Green Version]
- Park, S.H.K.; Oh, J.; Hwang, C.S.; Lee, J.I.; Yang, Y.S.; Chu, H.Y. Ultrathin film encapsulation of an OLED by ALD. ECS Solid State Lett. 2005, 8, H21–H23. [Google Scholar] [CrossRef]
- Langereis, E.; Creatore, M.; Heil, S.B.S.; Van de Sanden, M.C.M.; Kessels, W.M.M. Plasma-assisted atomic layer deposition of Al2O3 moisture permeation barriers on polymers. Appl. Phys. Lett. 2006, 89, 081915. [Google Scholar] [CrossRef]
- Choi, D.W.; Kim, S.J.; Lee, J.H.; Chung, K.B.; Park, J.S. A study of thin film encapsulation on polymer substrate using low temperature hybrid ZnO/Al2O3 layers atomic layer deposition. Curr. Appl. Phys. 2012, 12, S19–S23. [Google Scholar] [CrossRef]
- Kim, L.H.; Kim, K.; Park, S.; Jeong, Y.J.; Kim, H.; Chung, D.S.; Kim, S.H.; Park, C.E. Al2O3/TiO2 nanolaminate thin film encapsulation for organic thin film transistors via plasma-enhanced atomic layer deposition. ACS Appl. Mater. Interfaces 2014, 6, 6731–6738. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Choi, H.; Shin, S.; Park, J.; Ham, G.; Jung, H.; Jeon, H. Permeation barrier properties of an Al2O3/ZrO2 multilayer deposited by remote plasma atomic layer deposition. Curr. Appl. Phys. 2014, 14, 552–557. [Google Scholar] [CrossRef]
- Hoffmann, L.; Theirich, D.; Pack, S.; Kocak, F.; Schlamm, D.; Hasselmann, T.; Fahl, H.; Raupke, A.; Gargouri, H.; Riedl, T. Gas Diffusion Barriers Prepared by Spatial Atmospheric Pressure Plasma Enhanced ALD. ACS Appl. Mater. Inter. 2017, 9, 4171–4176. [Google Scholar] [CrossRef]
- Wu, J.; Fei, F.; Wei, C.T.; Chen, X.L.; Nie, S.H.; Zhang, D.Y.; Su, W.M.; Cui, Z. Efficient multi-barrier thin film encapsulation of OLED using alternating Al2O3 and polymer layers. RSC. Adv. 2018, 8, 5721–5727. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.X.; Weng, Y.L.; Sun, F.; Zhou, X.T.; Wu, C.X.; Yan, Q.; Guo, T.L.; Zhang, Y.G. Low-temperature atomic layer deposition of Al2O3/alucone nanolaminates for OLED encapsulation. RSC Adv. 2019, 9, 20884–20891. [Google Scholar] [CrossRef] [Green Version]
- Nayak, P.K. Pulsed-grown graphene for flexible transparent conductors. Nanoscale Adv. 2019, 1, 1215–1223. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Yang, Y.; Wu, R.; Fan, W.; Dai, Q.; He, J.; Bai, C. Boron nitride and hyperbranched polyamide assembled recyclable polyisoprene vitrimer with robust mechanical properties, high thermal conductivity and remoldability. Polymer 2020, 208, 122964. [Google Scholar] [CrossRef]
- Sadooghi, A.; Payganeh, G.; Tajdari, M.; Roohi, A.H. Experimental and Numerical Analysis of High-Cycle Fatigue Behavior of Steel Matrix Nanocomposites Reinforced by TiC/hBN Nanoparticles. Met. Mater. Int. 2019, 27, 802–814. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, W.; Han, S.; Gu, T.; Chae, H.; Whang, D. hBN Flake Embedded Al2O3 Thin Film for Flexible Moisture Barrier. Materials 2021, 14, 7373. https://doi.org/10.3390/ma14237373
Jang W, Han S, Gu T, Chae H, Whang D. hBN Flake Embedded Al2O3 Thin Film for Flexible Moisture Barrier. Materials. 2021; 14(23):7373. https://doi.org/10.3390/ma14237373
Chicago/Turabian StyleJang, Wonseok, Seunghun Han, Taejun Gu, Heeyeop Chae, and Dongmok Whang. 2021. "hBN Flake Embedded Al2O3 Thin Film for Flexible Moisture Barrier" Materials 14, no. 23: 7373. https://doi.org/10.3390/ma14237373
APA StyleJang, W., Han, S., Gu, T., Chae, H., & Whang, D. (2021). hBN Flake Embedded Al2O3 Thin Film for Flexible Moisture Barrier. Materials, 14(23), 7373. https://doi.org/10.3390/ma14237373





