The Role of Sintering Temperature and Dual Metal Substitutions (Al3+, Ti4+) in the Development of NASICON-Structured Electrolyte
Abstract
1. Introduction
2. Experimental Procedures
2.1. Materials
2.2. Li1+xAlxTixSn2−2xP3O12 Preparation
2.3. Li1+xAlxTixSn2−2xP3O12 Characterization
3. Result and Discussion
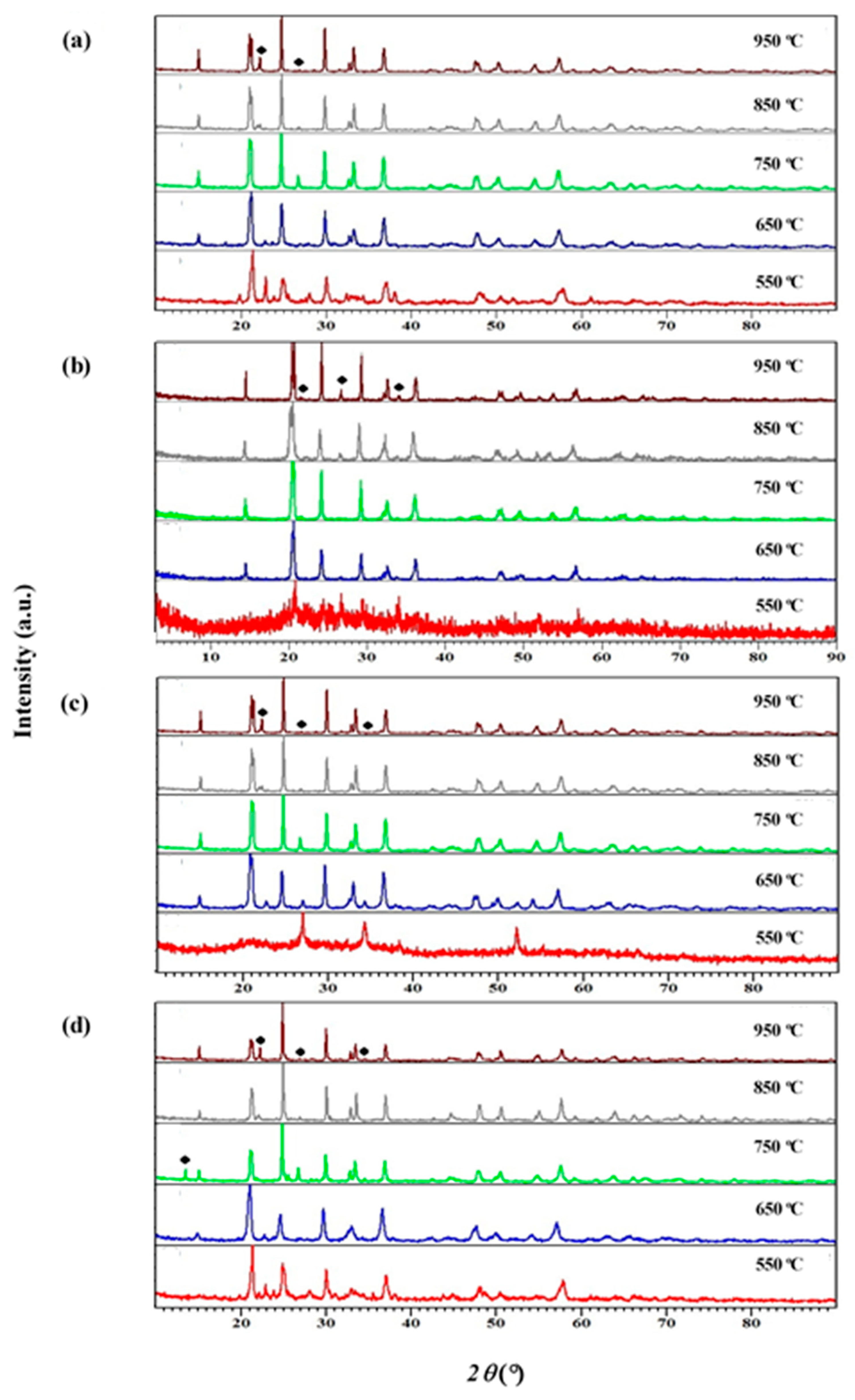
3.1. X-ray Diffraction Study
3.2. Conductivity and ImpedanceStudy
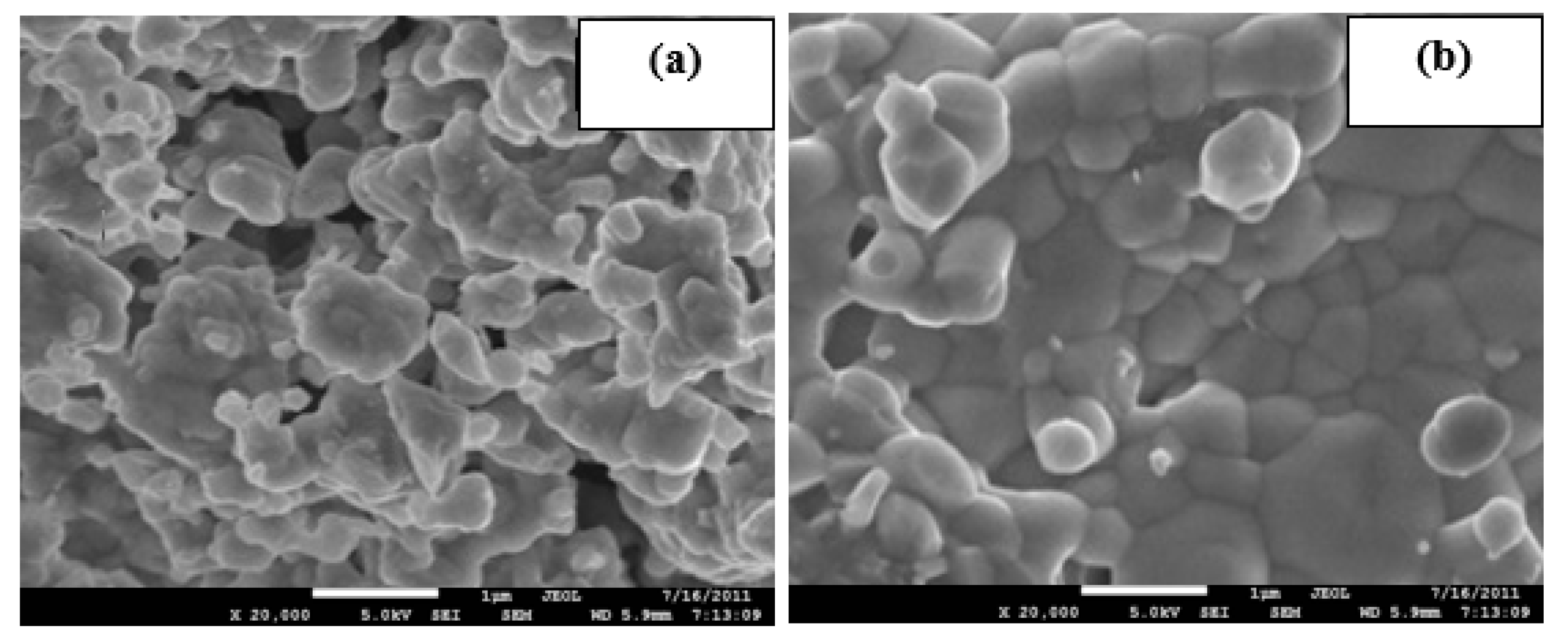
3.3. FESEM Analysis
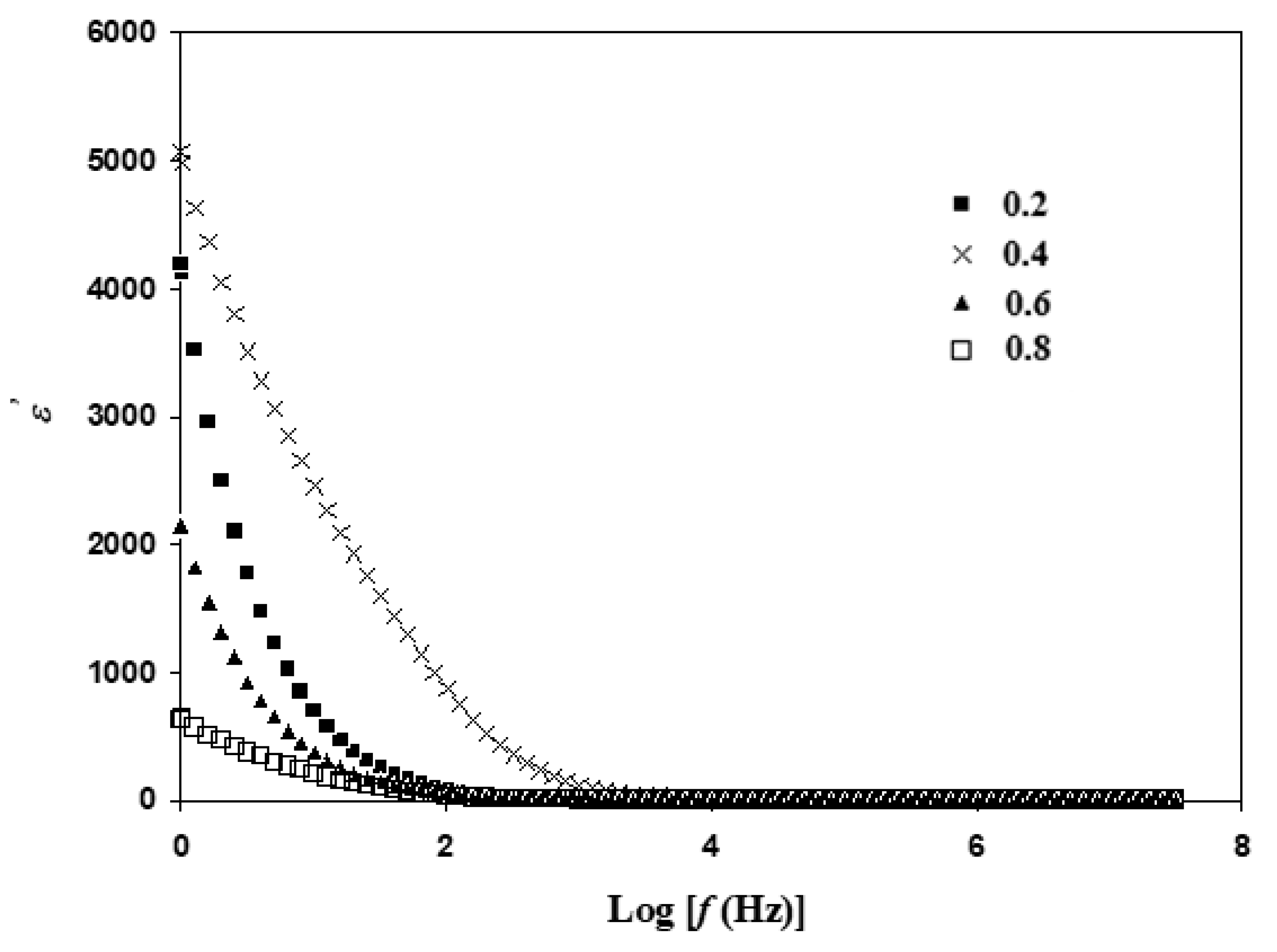
3.4. Dielectric Analysis
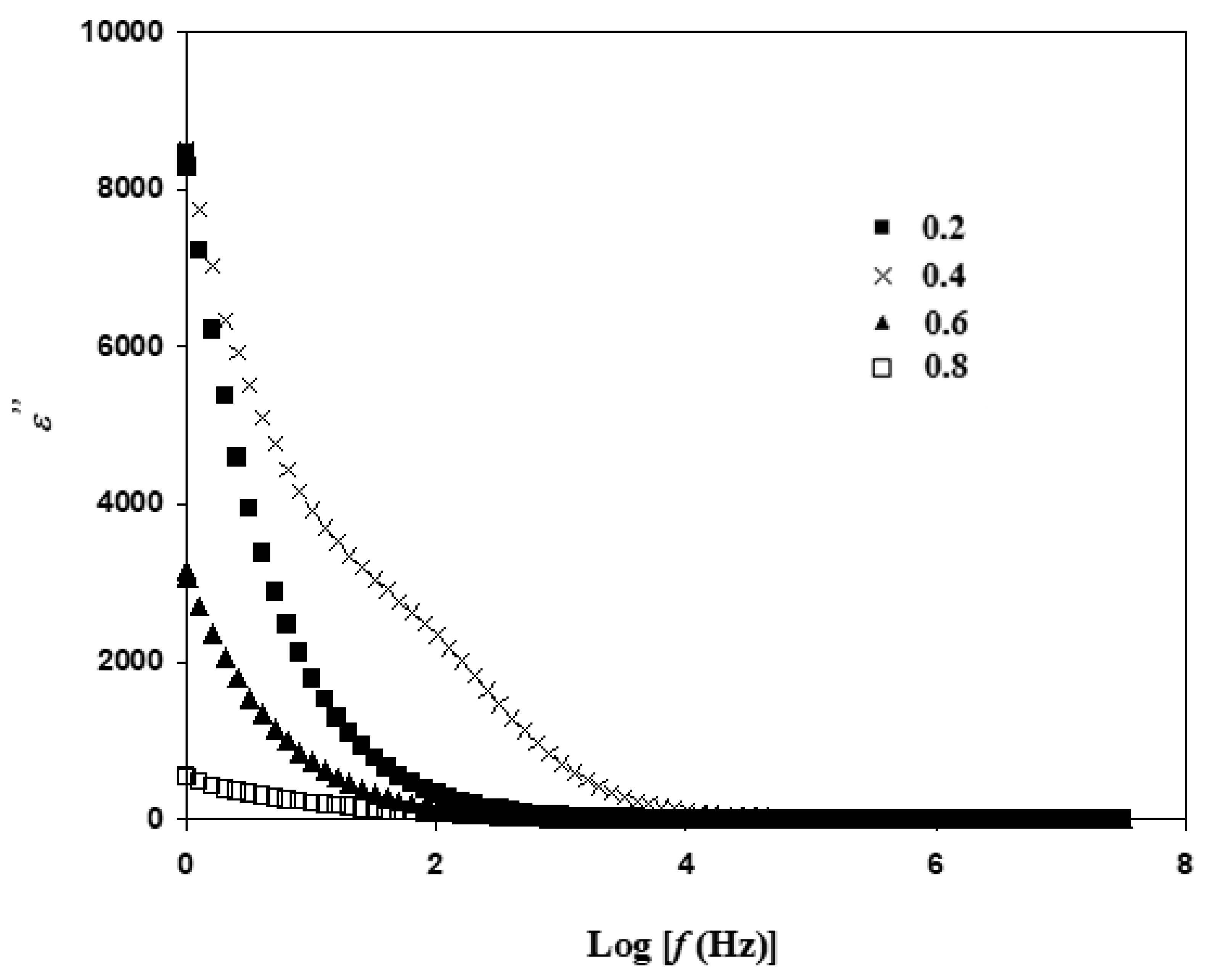
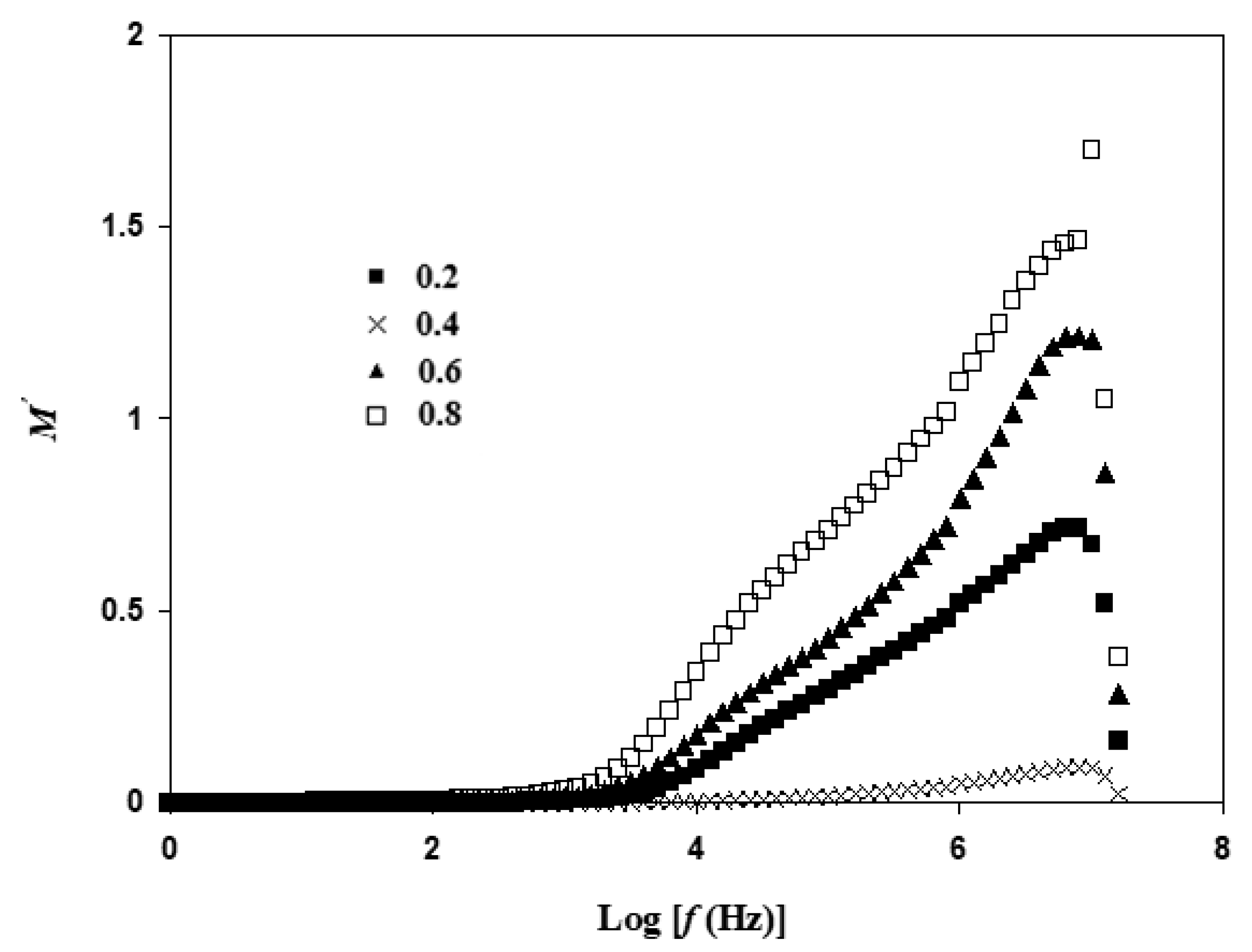
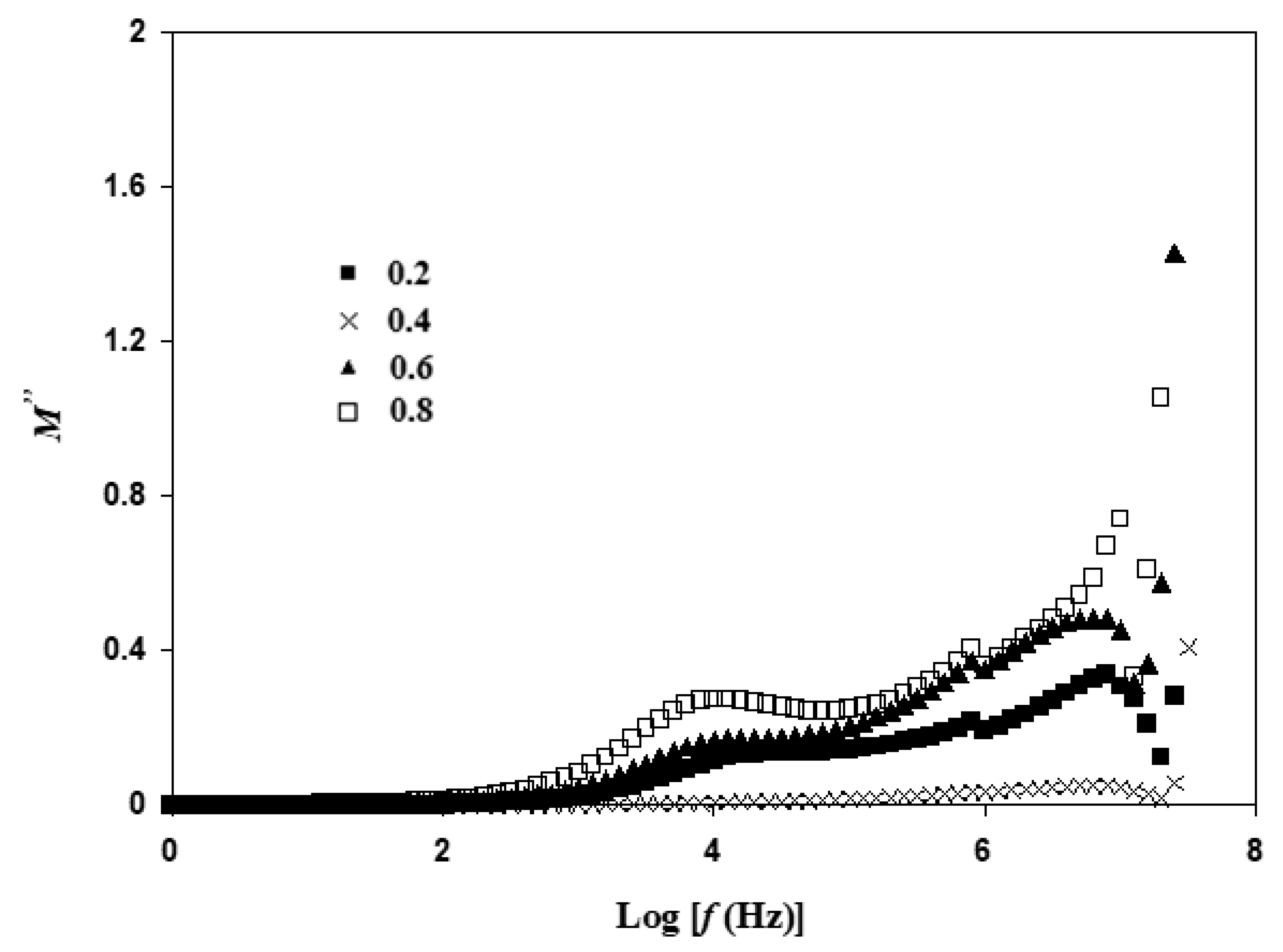
3.5. Electric Modulus Atable Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, F.; Wang, Y.; Zhang, X.; Liang, X.; Zhang, F.; Wang, L.; Li, Y.; Feng, Y.; Feng, W. Few-layer methyl-terminated germanene–graphene nanocomposite with high capacity for stable lithium storage. Carbon 2020, 161, 287–298. [Google Scholar] [CrossRef]
- Chen, S.; Li, Y.; Wang, Y.; Li, Z.; Peng, C.; Feng, Y.; Feng, W. Cross-linked Single-Ion Solid Polymer Electrolytes with Alternately Distributed Lithium Sources and Ion-Conducting Segments for Lithium Metal Batteries. Macromolecules 2021, 54, 9135–9144. [Google Scholar] [CrossRef]
- Ortiz, G.F.; López, M.C.; Lavela, P.; Vidal-Abarca, C.; Tirado, J.L. Improved lithium-ion transport in NASICON-type lithium titanium phosphate by calcium and iron doping. Solid State Ionics 2014, 262, 573–577. [Google Scholar] [CrossRef]
- Botros, M.; Scherer, T.; Popescu, R.; Kilmametov, A.; Clemens, O.; Hahn, H. Microstrain and electrochemical performance of garnet solid electrolyte integrated in a hybrid battery cell. RSC Adv. 2019, 9, 31102–31114. [Google Scholar] [CrossRef]
- Chi, C.; Li, Y.; Li, D.; Huang, H.; Wang, Q.; Yang, Y.; Huang, B. Flexible solvent-free supercapacitors with high energy density enabled by electrical-ionic hybrid polymer nanocomposites. J. Mater. Chem. A 2019, 7, 16748–16760. [Google Scholar] [CrossRef]
- Li, Q.; Xu, C.; Huang, B.; Yin, X. Rhombohedral Li1+xYxZr2−x(PO4)3 Solid Electrolyte Prepared by Hot-Pressing for All-Solid-State Li-Metal Batteries. Materials 2020, 13, 1719. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Zhong, S.; Luo, J.; Huang, Z.; Wang, C.A. Highly dense perovskite electrolyte with a high Li+ conductivity for Li–ion batteries. J. Power Sources 2019, 429, 75–79. [Google Scholar] [CrossRef]
- Liansheng, L.; Yuanfu, D.; Guohua, C. Status and prospect of garnet/polymer solid composite electrolytes for all-solid-state lithium batteries. J. Energy Chem. 2020, 50, 154–177. [Google Scholar] [CrossRef]
- Otoyama, M.; Sakuda, A.; Tatsumisago, M.; Hayashi, A. Sulfide Electrolyte Suppressing Side Reactions in Composite Positive Electrodes for All-Solid-State Lithium Batteries. ACS Appl. Mater. Interfaces 2020, 26, 29228–29234. [Google Scholar] [CrossRef] [PubMed]
- Zhuoran, Z.; Jianxing, Z.; Huanhuan, J.; Linfeng, P. Enhancing ionic conductivity of solid electrolyte by lithium substitution in halogenated Li-Argyrodite. J. Power Sources 2020, 450, 227601. [Google Scholar] [CrossRef]
- Harada, M.; Takeda, H.; Suzuki, S.; Nakano, K.; Tanibata, N.; Nakayama, M.; Karasuyama, M.; Takeuchi, I. Bayesian-optimization-guided experimental search of NASICON-type solid electrolytes for all-solid-state Li-ion batteries. J. Mater. Chem. A 2020, 8, 15103–15109. [Google Scholar] [CrossRef]
- Rudyi, A.S.; Lebedev, M.E.; Mironenko, A.A.; Mazaletskii, L.A.; Naumov, V.V.; Novozhilova, A.V.; Fedorov, I.S.; Churilov, A.B. Study of the Relaxational Polarization Dynamics of the LiPON Solid Electrolyte. Russ. Microelectron. 2020, 49, 345–357. [Google Scholar] [CrossRef]
- Strauss, F.; Teo, J.H.; Janek, J.; Brezesinski, T. Investigations into the superionic glass phase of Li4PS4I for improving the stability of high-loading all-solid-state batteries. Inorg. Chem. Front. 2020, 7, 3953–3960. [Google Scholar] [CrossRef]
- El-Shinawi, H.; Regoutz, A.; Payne, D.J.; Cussen, E.J.; Corr, S.A. NASICON LiM2(PO4)3 electrolyte (M = Zr) and electrode (M = Ti) materials for all solid-state Li-ion batteries with high total conductivity and low interfacial resistance. J. Mater. Chem. A 2018, 6, 5296–5303. [Google Scholar] [CrossRef]
- Guin, M.; Tietz, F.; Guillon, O. New promising NASICON material as solid electrolyte for sodium-ion batteries: Correlation between composition, crystal structure and ionic conductivity of Na3+xSc2SixP3−xO12. Solid State Ionics 2016, 293, 18–26. [Google Scholar] [CrossRef]
- Norhaniza, R.; Subban, R.H.Y.; Mohamed, N.S.; Ahmad, A. Chromium substituted LiSn2P3O12 solid electrolyte. Int. J. Electrochem. Sci. 2012, 7, 10254–10265. [Google Scholar]
- Norhaniza, R.; Subban, R.H.Y.; Mohamed, N.S. Ion conduction in vanadium-substituted LiSn2P3O12 electrolyte nanomaterials. J. Mat. Sci. 2011, 46, 7815–7821. [Google Scholar] [CrossRef]
- Lazarraga, M.G.; Ibañez, J.; Tabellout, M.; Rojo, J.M. On the aggregation process of ceramic LiSn2P3O12 particles embedded in Teflon matrix. Compos. Sci. Technol. 2004, 64, 759–765. [Google Scholar] [CrossRef]
- Lang, B.; Ziebarth, B.; Elsässer, C. Lithium Ion Conduction in LiTi2(PO4)3 and Related Compounds Based on the NASICON Structure: A First-Principles Study. Chem. Mater. 2015, 27, 5040–5048. [Google Scholar] [CrossRef]
- Arya, A.; Sadiq, M.; Sharma, A. Salt concentration and temperature dependent dielectric properties of blend solid polymer electrolyte complexed with NaPF6. Mater. Today Proc. 2019, 12, 554–564. [Google Scholar] [CrossRef]
- Rusdi, H.; Mohamed, N.S.; Subban, R.H.Y. Effects of temperatures to the electrical properties of Li1.6Al0.6Sn1.4P3O12 NASICON type solid electrolytes. AIP Conf. Proc. 2017, 1877, 050005. [Google Scholar] [CrossRef]
- Paolella, A.; Zhu, W.; Xu, G.L.; Monaca, A.L.; Savoie, S.; Girard, G.; Vijh, A.; Demex, H.; Perea, A.; Delaporte, N.; et al. Lithium Anodes: Understanding the reactivity of a thin Li1.5Al0.5Ge1.5(PO4)3solid-State electrolyte towards metallic lithium anode. Adv. Energy Mat. 2020, 10, 2070136. [Google Scholar] [CrossRef]
- Zhao, W.; Yi, J.; He, P.; Zhou, H. Solid-State Electrolytes for Lithium-Ion Batteries: Fundamentals, Challenges and Perspectives. Electrochem. Energy Rev. 2019, 2, 574–605. [Google Scholar] [CrossRef]
- Zhou, D.; Usher, B.F. Deviation of the AlGaAs lattice constant from Vegard’s law. J. Phys. D Appl. Phys. 2001, 34, 1461. [Google Scholar] [CrossRef]
- Kahlaoui, R.; Arbi, K.; Jimenez, R.; Sobrados, M.I.; Mehnaoui, M.; Sanz, J.; Ternane, R. Synthesis, structural characterization and ionic conductivity of NASICON-type Bax/2Li1−xTi2(PO4)3 (0.4 ≤ x ≤ 1) materials. Ionics 2016, 23, 837–846. [Google Scholar] [CrossRef]
- Narayanan, S.; Reid, S.; Butler, S.; Thangadurai, V. Sintering temperature, excess sodium, and phosphorous dependencies on morphology and ionic conductivity of NASICON Na3Zr2Si2PO12. Solid State Ionics 2019, 331, 22–29. [Google Scholar] [CrossRef]
- Hamsan, M.H.; Aziz, S.B.; Nofal, M.M.; Brza, M.A.; Abdulwahid, R.T.; Hadi, J.M.; Karim, W.O.; Kadir, M.F.Z. Characteristics of EDLC device fabricated from plasticized chitosan:MgCl2 based polymer electrolyte. J. Mater. Res. Technol. 2020, 9, 10635–10646. [Google Scholar] [CrossRef]
- Rao, M.K.; Babu, K.V.; Veeraiah, V.; Samatha, K. Effect of Nb substitution on structural, electrical and electrochemical properties of LiTi2(PO4)3 as electrolyte materials for lithium ion batteries. Asian Ceram. Soc. 2018, 6, 109–120. [Google Scholar] [CrossRef]
- Lu, X.; Xiao, H. Increase in grain boundary conductivity of Li1+xAlxSn2−x(PO4)3 by mixing powders pretreated at different temperatures. Ceram-Silik. 2017, 61, 14–19. [Google Scholar] [CrossRef][Green Version]
- Narváez-Semanate, J.L.; Rodrigues, A.C.M. Microstructure and ionic conductivity of Li1+xAlxTi2−x(PO4)3 NASICON glass-ceramics. Solid State Ionics 2010, 181, 1197–1204. [Google Scholar] [CrossRef]
- Kumar, A.; Shahi, K. Particle size effect on ionic conductivity in NaCl-A12O3 composite solid electrolytes. Solid State Commun. 1995, 94, 813–816. [Google Scholar] [CrossRef]
- Boyano, I.; Mainar, A.R.; Blázquez, J.A.; Kvasha, A.; Bengoechea, M.; de Meatza, I.; García-Martín, S.; Varez, A.; Sanz, J.; García-Alvarado, F. Reduction of Grain Boundary Resistance of La0.5Li0.5TiO3 by the Addition of Organic Polymers. Nanomaterials 2021, 11, 61. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, R.O.; Figueiredo, F.M.; Marques, F.M.B.; Franco, J.I. Influence of microstructure on the electrical properties of NASICON materials. Solid State Ionics 2001, 140, 173. [Google Scholar] [CrossRef]
- Liu, J.; Liu, T.; Pu, Y.; Guan, M.; Tang, Z.; Ding, F.; Xu, Z.; Li, Y. Facile synthesis of NASICON-type Li1.3Al0.3Ti1.7(PO4)3 solid electrolyte and its application for enhanced cyclic performance in lithium ion batteries through the introduction of an artificial Li3PO4 SEI layer. RSC Adv. 2017, 7, 46545–46552. [Google Scholar] [CrossRef]
- Kimpa, M.I.; Mayzan, M.Z.H.; Yabagi, J.A.; Nmaya, M.M.; Isah, K.U.; Agam, M.A. Review on Material Synthesis and Characterization of Sodium (Na) Super-Ionic Conductor. IOP Conf. Ser. Earth Environ. Sci. 2018, 140, 012156. [Google Scholar] [CrossRef]
- Tan, F.K.; Hassan, J.; Wahab, Z.A. Electrical conductivity and dielectric behavior of manganese and vanadium mixed oxide prepared by conventional solid state method. Int. J. Eng. Sci. Technol. 2016, 19, 2081. [Google Scholar] [CrossRef]
- Aziz, S.B.; Woo, T.J.; Kadir, M.; Ahmed, H.M. A conceptual review on polymer electrolytes and ion transport models. J. Sci. Adv. Mater. Devices 2018, 3, 1–17. [Google Scholar] [CrossRef]
- Zangina, T.; Hassan, J.; Matori, K.A.; Azis, R.S.; Ahmadu, U.; See, A. Sintering behavior, ac conductivity and dielectric relaxation of Li1.3Ti1.7Al0.3(PO4)3 NASICON compound. Results Phys. 2016, 6, 719–725. [Google Scholar] [CrossRef]
- Meena, P.L.; Kumar, R.; Prajapat, C.L.; Sreenivas, K.; Gupta, V. Dielectric studies of Co3−xMnxO4 (x = 0.1–1.0) cubic spinel multiferroic. J. Appl. Phys. 2009, 106, 024105. [Google Scholar] [CrossRef]
- Hyatt, N.; Reaney, I.; Knight, K. Ferroelectric-Paraelectric Phase Transition in the n = 2 Aurivillius Phase Bi3Ti1.5W0.5O9: A Neutron Powder Diffraction Study. Phys. Rev. B 2005, 71, 241191. [Google Scholar] [CrossRef]
- Luo, S.; Noguchi, Y.; Miyayama, M.; Kudo, T. Rietveld Analysis and Dielectric Properties of Bi2WO6-Bi4Ti3O12 Ferroelectric System. Mater. Res. Bull. 2001, 36, 531–540. [Google Scholar] [CrossRef]
- Fuzlin, A.F.; Rasali, N.M.J.; Samsudin, A.S. Effect on ammonium bromide in dielectric behavior based alginate solid biopolymer electrolytes. Mater. Sci. Eng. 2018, 342, 012080. [Google Scholar] [CrossRef]
- Tripathi, N.; Shukla, A.; Thakur, A.K.; Marx, D.T. Dielectric Modulus and Conductivity Scaling Approach to the Analysis of Ion Transport in Solid Polymer Electrolytes. Polym. Eng. Sci. 2019, 60, 297–305. [Google Scholar] [CrossRef]
- Nikam, P.N.; Deshpande, V.D. Dielectric Behavior of Plasticized PVC/Alumina Nanocomposites Influenced With DC Biasing Field. Mater. Today Proc. 2018, 5, 2254–2262. [Google Scholar] [CrossRef]
- Arumugam, R.; Prasad, K.H.; Satyanarayana, N.; Srinadhu, E.S. Conductivity and electrical modulus studies of Li1.3Al0.3Ti1.7(PO4) electrolyte thin films grown by RF magnetron sputtering. TechConnect 2018, 2, 52–55. [Google Scholar]
- Zang, G.; Zhang, J.; Zheng, P.; Wang, J.; Wang, C. Grain boundary effect on the dielectric properties of CaCu3Ti4O12 ceramics. J. Phys. D Appl. Phys. 2005, 38, 1824–1827. [Google Scholar] [CrossRef]
- Supriya, S.; Kumar, S.; Kar, M. Grain size and grain boundary effect on dielectric behavior of nanocrystalline cobalt ferrite. In Proceedings of the 2017 IEEE 12th Nanotechnology Materials and Devices Conference (NMDC), Singapore, 2–4 October 2017; pp. 165–166. [Google Scholar]




| Sample | Impurity (%) | a(=b)/Å | c (Å) | V (Å3) | c/a | Rw | χ2 | s.o.f of Li in 6b | s.o.f of Sn in 12c | s.o.f of Al in 12c | s.o.f of P in 3b | s.o.f of O |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LSP (83831) | - | 8.63 | 21.53 | 1389.82 | 2.49 | - | - | 1.00 | 1.00 | 0.00 | 1.00 | 1.00 |
| x = 0 | 19.0 | 8.63 | 21.66 | 1396.48 | 2.51 | 9.60 | 3.27 | 1.00 | 1.00 | - | 1.00 | 1.00 |
| x = 0.2 | 2.0 | 8.55 | 21.29 | 1347.56 | 2.49 | 22.06 | 3.69 | 1.00 | 0.79 | 0.09 | 0.11 | 0.70 |
| x = 0.4 | 1.6 | 8.58 | 21.33 | 1360.55 | 2.49 | 40.72 | 1.24 | 1.00 | 0.45 | 0.50 | 0.05 | 0.70 |
| x = 0.6 | - | 8.61 | 21.50 | 1380.20 | 2.50 | 16.89 | 2.45 | 1.00 | 0.88 | 0.30 | 0.30 | 1.00 |
| x = 0.8 | 0.6 | 8.61 | 21.50 | 1380.20 | 2.50 | 20.68 | 2.45 | 1.00 | 0.39 | 0.35 | 0.13 | 1.00 |
| Sintering Temperature (°C) | Conductivity (S cm−1) | |||
|---|---|---|---|---|
| x = 0.2 | x = 0.4 | x = 0.6 | x = 0.8 | |
| 550 | 4.03 × 10−6 | 2.13 × 10−6 | 2.83 × 10−6 | 3.59 × 10−7 |
| 650 | 4.08 × 10−6 | 4.74 × 10−6 | 1.41 × 10−6 | 1.41 × 10−7 |
| 750 | 1.65 × 10−6 | 1.60 × 10−6 | 1.87 × 10−6 | 9.25 × 10−8 |
| 850 | 1.03 × 10−6 | 7.12 × 10−7 | 2.65 × 10−6 | 3.57 × 10−6 |
| 950 | 2.46 × 10−6 | 3.85 × 10−6 | 2.82 × 10−6 | 2.62 × 10−6 |
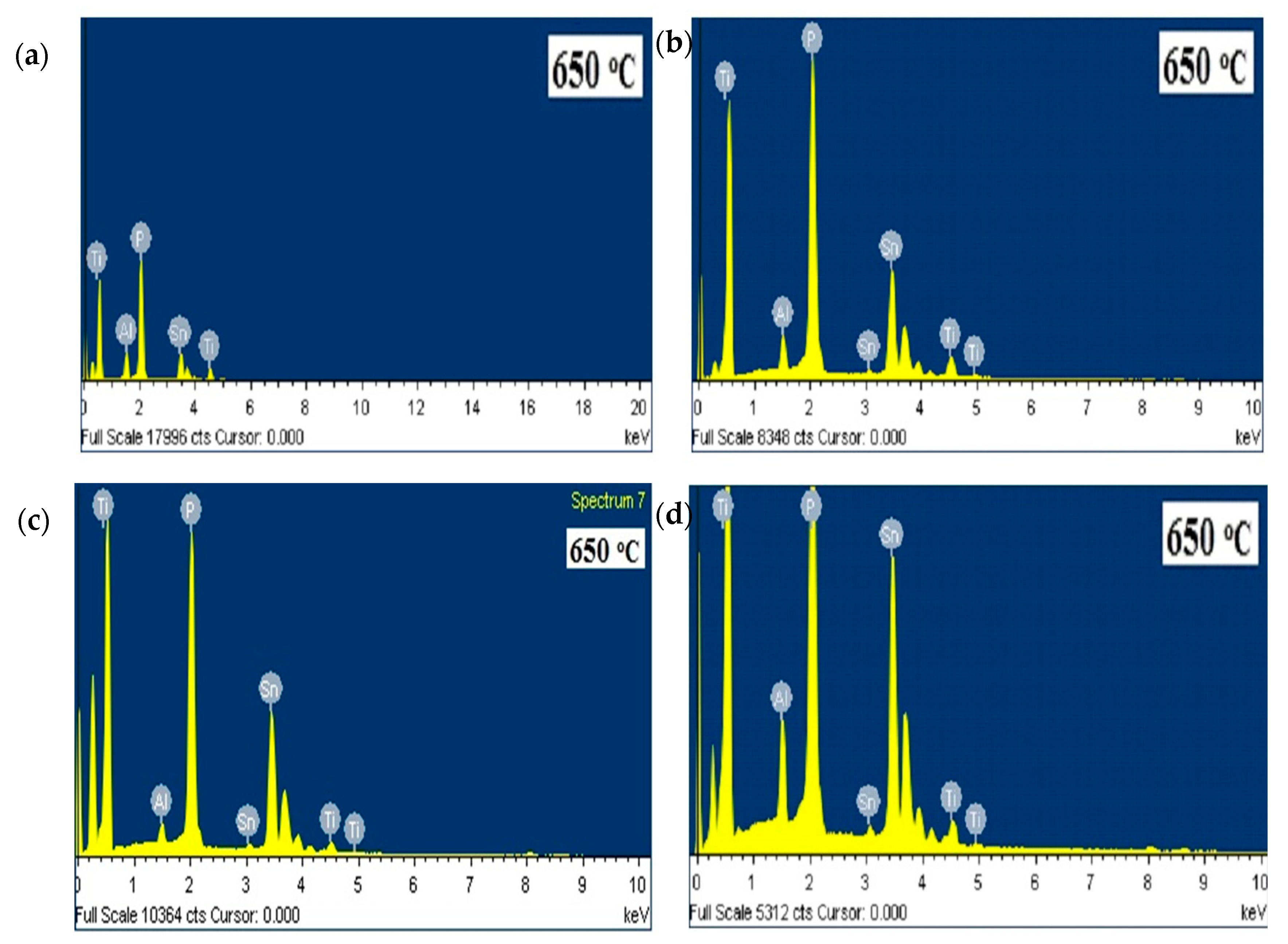
| x | Elements | Atomic Percentage (%) | |
|---|---|---|---|
| Theory | Calculated | ||
| 0.2 | Sn | 32 | 16.1 |
| P | 60 | 63.0 | |
| Al | 4 | 9.3 | |
| Ti | 4 | 11.6 | |
| 0.4 | Sn | 24 | 36.7 |
| P | 60 | 53.0 | |
| Al | 8 | 3.5 | |
| Ti | 8 | 6.8 | |
| 0.6 | Sn | 16 | 30.8 |
| P | 60 | 61.9 | |
| Al | 12 | 3.7 | |
| Ti | 12 | 3.6 | |
| 0.8 | Sn | 8 | 26.8 |
| P | 60 | 60.9 | |
| Al | 16 | 8.4 | |
| Ti | 16 | 3.9 | |
| x | trex (s) |
|---|---|
| 0.2 | 1.98 × 10−8 |
| 0.4 | 1.57 × 10−8 |
| 0.6 | 2.49 × 10−8 |
| 0.8 | 1.57 × 10−5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rusdi, H.; Rusdi, R.; Aziz, S.B.; Alsubaie, A.S.; Mahmoud, K.H.; Kadir, M.F.Z. The Role of Sintering Temperature and Dual Metal Substitutions (Al3+, Ti4+) in the Development of NASICON-Structured Electrolyte. Materials 2021, 14, 7342. https://doi.org/10.3390/ma14237342
Rusdi H, Rusdi R, Aziz SB, Alsubaie AS, Mahmoud KH, Kadir MFZ. The Role of Sintering Temperature and Dual Metal Substitutions (Al3+, Ti4+) in the Development of NASICON-Structured Electrolyte. Materials. 2021; 14(23):7342. https://doi.org/10.3390/ma14237342
Chicago/Turabian StyleRusdi, Hashlina, Roshidah Rusdi, Shujahadeen B. Aziz, Abdullah Saad Alsubaie, Khaled H. Mahmoud, and Mohd F. Z. Kadir. 2021. "The Role of Sintering Temperature and Dual Metal Substitutions (Al3+, Ti4+) in the Development of NASICON-Structured Electrolyte" Materials 14, no. 23: 7342. https://doi.org/10.3390/ma14237342
APA StyleRusdi, H., Rusdi, R., Aziz, S. B., Alsubaie, A. S., Mahmoud, K. H., & Kadir, M. F. Z. (2021). The Role of Sintering Temperature and Dual Metal Substitutions (Al3+, Ti4+) in the Development of NASICON-Structured Electrolyte. Materials, 14(23), 7342. https://doi.org/10.3390/ma14237342







