In Situ Transmission Electron Microscopy Investigation of Melting/Evaporation Kinetics in Anisotropic Gold Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Au Nanoparticles
2.2. Characterization Method
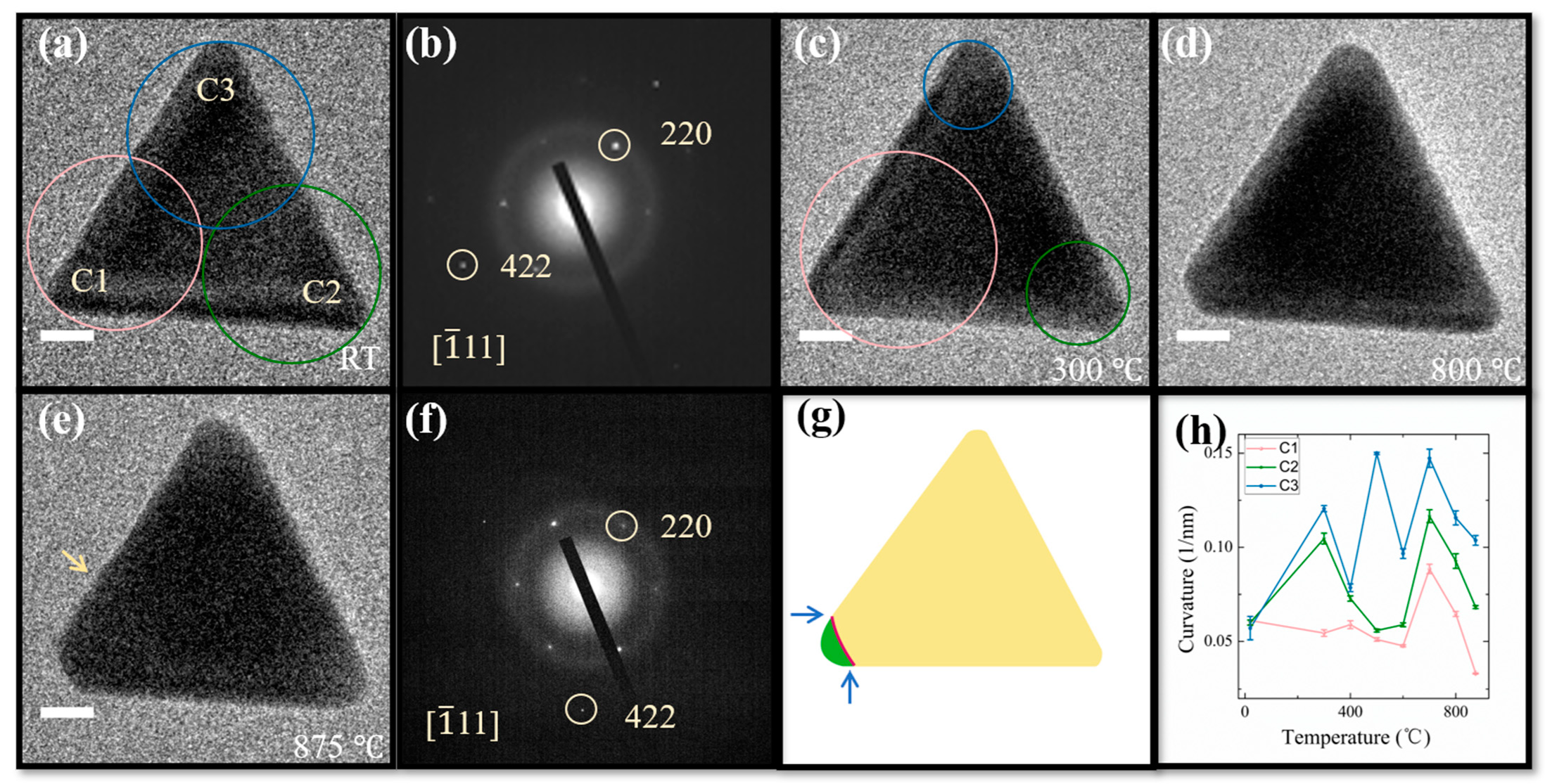
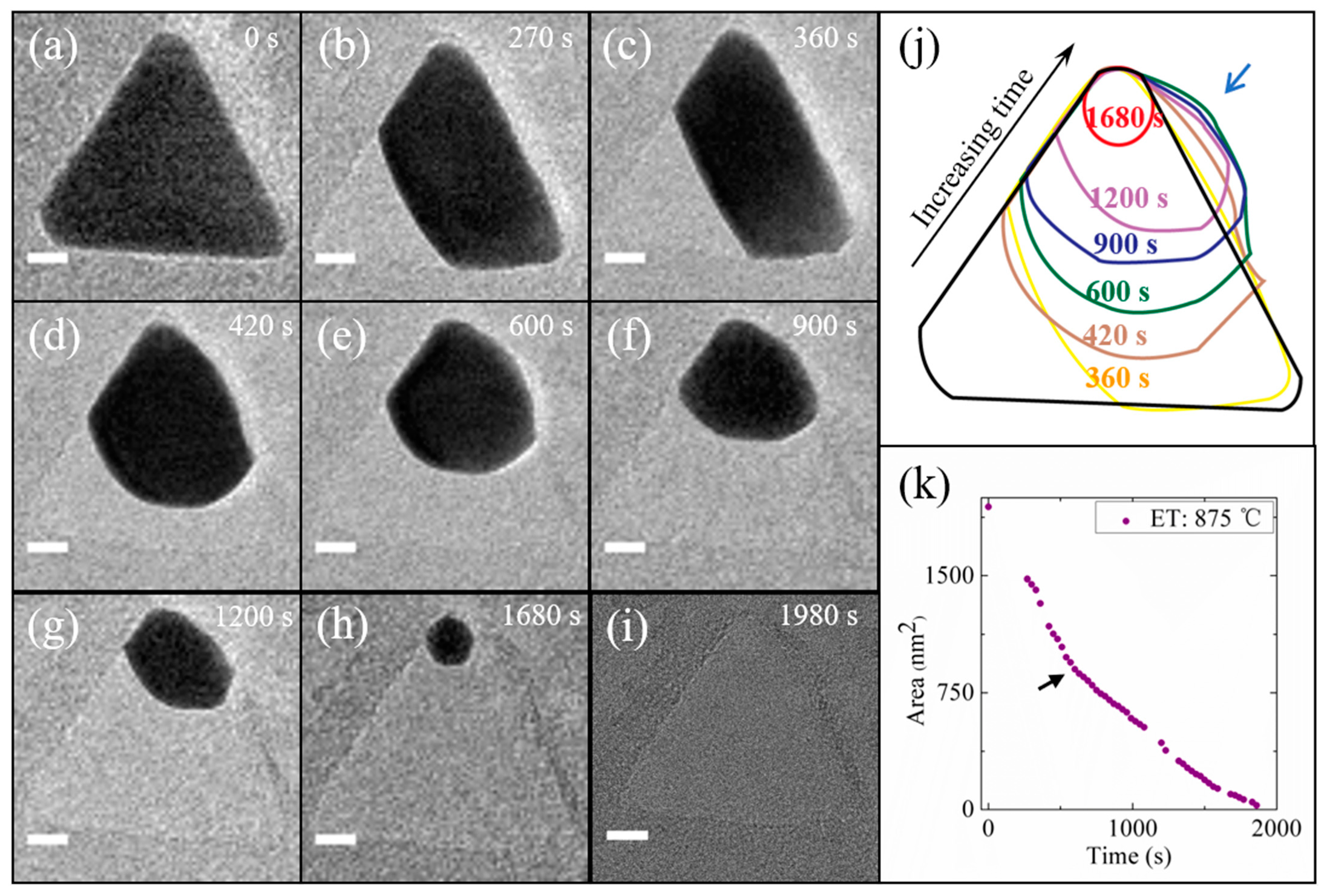
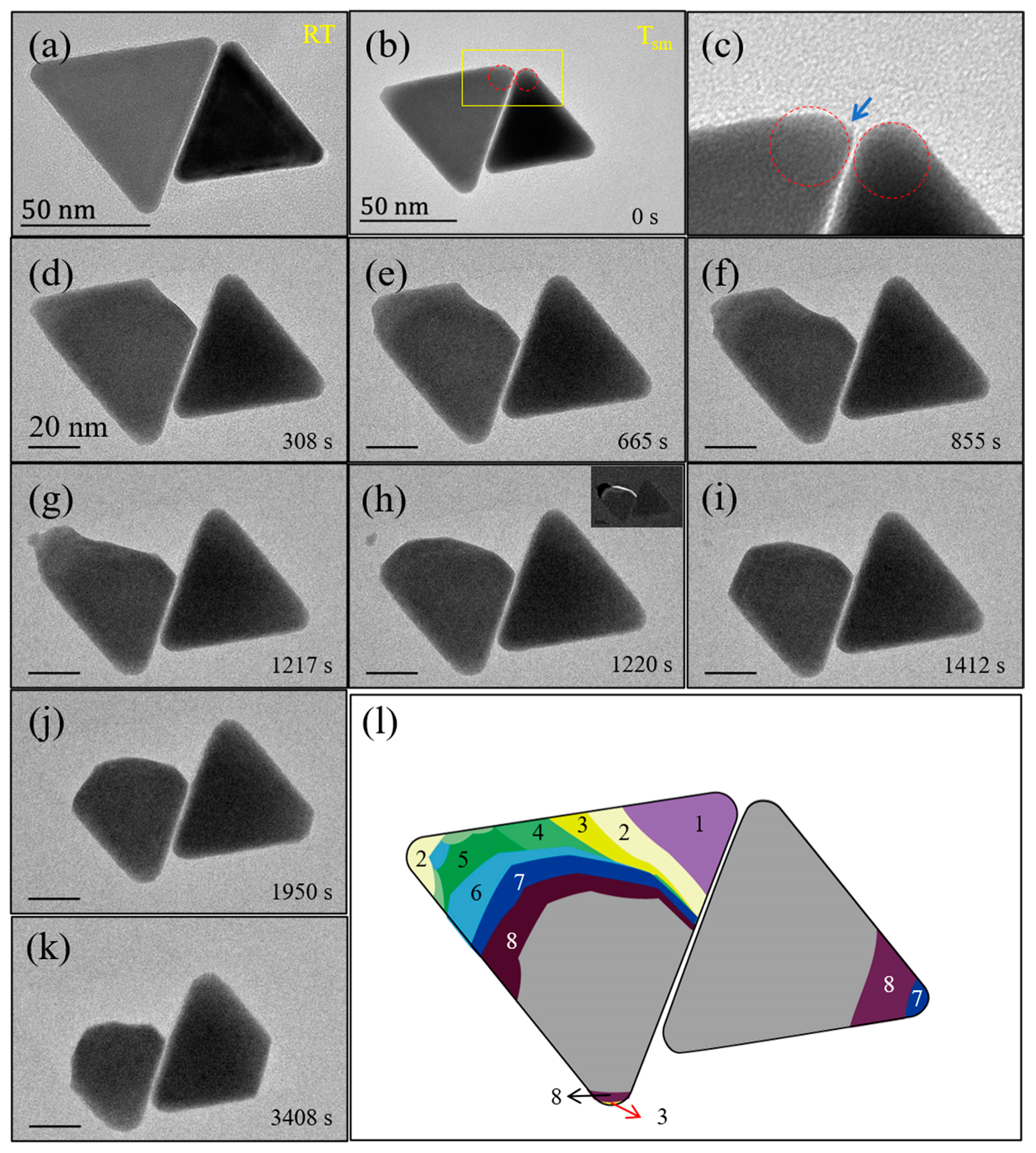
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, Y.; Lai, Z.; Zhang, X.; Fan, Z.; He, Q.; Tan, C.; Zhang, H. Phase engineering of nanomaterials. Nat. Rev. Chem. 2020, 4, 243–256. [Google Scholar] [CrossRef]
- Hoss, A.; Nold, M.; von Blanckenhagen, P.; Meyer, O. Order-disorder transition and melting of Au (110) surfaces. Nucl. Instrum. Methods B 1992, 64, 58–63. [Google Scholar] [CrossRef]
- Shah, N.; Nekrasov, K.A.; Gupta, S.K.; Gajjar, P.N.; Boyarchenkov, A.S. Atomistic investigations of melting characterization in metallic nanostructures. Surf. Sci. 2021, 707, 121803. [Google Scholar] [CrossRef]
- Millstone, J.E.; Hurst, S.J.; Metraux, G.S.; Cutler, J.I.; Mirkin, C.A. Colloidal gold and silver triangular nanoprisms. Small 2009, 5, 646–664. [Google Scholar] [CrossRef]
- Koth, A.; Appelhans, D.; Prietzel, C.; Koetz, J. Asymmetric gold nanoparticles synthesized in the presence of maltose-modified poly (Ethyleneimine). Colloids Surf. A 2012, 414, 50–56. [Google Scholar] [CrossRef]
- Simonsen, S.B.; Chorkendorff, I.; Dahl, S.; Skoglundh, M.; Sehested, J.; Helveg, S. Direct observations of oxygen-induced platinum nanoparticle ripening studied by in situ TEM. J. Am. Chem. Soc. 2010, 132, 7968–7975. [Google Scholar] [CrossRef] [Green Version]
- Kan, C.; Wang, G.; Zhu, X.; Li, C.; Cao, B. Structure and thermal stability of gold nanoplates. Appl. Phys. Lett. 2006, 88, 071904. [Google Scholar] [CrossRef]
- Li, D.; Wang, Z.L.; Wang, Z. Phase separation prior to alloying observed in vacuum heating of hybrid Au/Cu2O core–shell nanoparticles. J. Phys. Chem. C 2017, 121, 1387–1392. [Google Scholar] [CrossRef]
- Young, N.P.; van Huis, M.A.; Zandbergen, H.W.; Xu, H.; Kirkland, A.I. Transformations of gold nanoparticles investigated using variable temperature high-resolution transmission electron microscopy. Ultramicroscopy 2010, 110, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Shin, J.W.; Ryoo, R. Atomic scale mechanisms underlying thermal reshaping of anisotropic gold nanocrystals revealed by in situ electron microscopy. J. Phys. Chem. C 2020, 124, 12855–12863. [Google Scholar] [CrossRef]
- Asoro Michael, A.; Kovar, D.; Ferreira Paulo, J. In Situ transmission electron microscopy observations of sublimation in silver nanoparticles. ACS Nano 2013, 7, 7844. [Google Scholar] [CrossRef]
- Sambles, J.R. An electron microscope study of evaporating gold particles: The kelvin equation for liquid gold and the lowering of the melting point of solid gold particles. Proc. R. Soc. Lond. A 1971, 324, 339–351. [Google Scholar]
- Clancy, P.; Kuo, C. Melting and freezing characteristics and structural properties of supported and unsupported gold nanoclusters. J. Phys. Chem. B 2005, 109, 13743–13754. [Google Scholar]
- Lee, J.G.; Lee, J.; Tanaka, T.; Mori, H. In situ HREM observation of crystalline-to-gas transition in nanometer-sized Ag particles. Phys. Rev. Let. 2006, 96, 075504. [Google Scholar] [CrossRef] [Green Version]
- Ding, Y.; Fan, F.; Tian, Z.; Wang, Z.L. Sublimation-induced shape evolution of silver cubes. Small 2009, 5, 2812–2815. [Google Scholar] [CrossRef]
- He, L.B.; Zhang, L.; Tan, X.; Tang, L.; Xu, T.; Zhou, Y.; Ren, Z.; Wang, Y.; Teng, C.; Sun, L.; et al. Surface energy and surface stability of Ag nanocrystals at elevated temperatures and their dominance in sublimation-induced shape evolution. Small 2017, 13, 1700743. [Google Scholar] [CrossRef]
- Li, J.; Wang, Z.; Li, Y.; Deepak, F. In Situ atomic-scale observation of kinetic pathways of sublimation in silver nanoparticles. Adv. Sci. 2019, 6, 1802131. [Google Scholar] [CrossRef] [PubMed]
- Jones DR, H. The free energies of solid-liquid interfaces. Mater. Sci. 1974, 9, 1–17. [Google Scholar] [CrossRef]
- Wang, Z.; Palmer, R. Determination of the ground-state atomic structures of size-selected Au nanoclusters by electron-beam-induced transformation. Phys. Rev. Lett. 2012, 108, 245502. [Google Scholar] [CrossRef]
- Koga, K.; Ikeshoji, T.; Sugawara, K. Size- and temperature-dependent structural transitions in gold nanoparticles. Phys. Rev. Lett. 2004, 92, 115507. [Google Scholar] [CrossRef] [PubMed]
- Marks, L. Experimental studies of small particle structures. Rep. Prog. Phys. 1994, 57, 603–648. [Google Scholar] [CrossRef]
- Luo, W.; Su, K.; Li, K.; Li, Q. Connection between nanostructured materials’ size-dependent melting and thermodynamic properties of bulk materials. Solid State Commun. 2011, 151, 229–233. [Google Scholar] [CrossRef]
- Dick, K.; Dhanasekaran, T.; Zhang, Z.; Meisel, D. Size-dependent melting of silica-encapsulated gold nanoparticles. J. Am. Chem. Soc. 2002, 124, 2312–2317. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Teitel, S.; Dellago, C. Melting of icosahedral gold nanoclusters from molecular dynamics simulations. J. Chem. Phys. 2005, 122, 214722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niidom, Y.; Haine, A.T.; Niidome, T. Anisotropic gold-based nanoparticles: Preparation, properties, and applications. Chem. Lett. 2016, 45, 488–498. [Google Scholar] [CrossRef] [Green Version]
- Makarov, G.N. Laser applications in nanotechnology: Nanofabrication using laser ablation and laser nanolithography. Phys. Usp. 2013, 114, 673–718. [Google Scholar] [CrossRef]
- Nelli, D.; Rossi, G.; Wang, Z.W.; Palmer, R.; Ferrando, R. Structure and orientation effects in the coalescence of Au clusters. Nanoscale 2020, 12, 7688–7699. [Google Scholar] [CrossRef]
- Chen, L.; Ji, F.; Xu, Y.; He, L.; Mi, Y.; Bao, F.; Sun, B.; Zhang, X.; Zhang, Q. High-yield seedless synthesis of triangular gold nanoplates through oxidative etching. Nano Lett. 2014, 14, 7201–7206. [Google Scholar] [CrossRef]
- Luty-błocho, M.; Pacławski, K.; Wojnicki, M.; Fitzner, K. The kinetics of redox reaction of gold(III) chloride complex ions with l-ascorbic acid. Inorg. Chim. Acta. 2013, 395, 189–196. [Google Scholar] [CrossRef]
- Su, T.; Wang, Z.L.; Wang, Z.W. In situ observations of shell growth and oxidative etching behaviors of Pd nanoparticles in solutions by liquid cell transmission electron microscopy. Small 2019, 15, e1900050. [Google Scholar] [CrossRef]
- Zheng, H.; Smith, R.K.; Jun, Y.; Kisielowski, C.; Dahmen, U.; Alivisatos, A. Observation of single colloidal platinum nanocrystal growth trajectories. Science 2009, 324, 1309–1312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Wang, Z.L.; Wang, Z.W. In situ tuning of crystallization pathways by electron beam irradiation and heating in amorphous bismuth ferrite films. RSC Adv. 2018, 8, 23522–23528. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Palmer, R.E.; Wilcoxon, J.P. Sintering of passivated gold nanoparticles under the electron beam. Langmuir 2006, 22, 2851–2855. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Yuan, H.; Wang, H.; Wang, Z. In Situ Transmission Electron Microscopy Investigation of Melting/Evaporation Kinetics in Anisotropic Gold Nanoparticles. Materials 2021, 14, 7332. https://doi.org/10.3390/ma14237332
Liu Y, Yuan H, Wang H, Wang Z. In Situ Transmission Electron Microscopy Investigation of Melting/Evaporation Kinetics in Anisotropic Gold Nanoparticles. Materials. 2021; 14(23):7332. https://doi.org/10.3390/ma14237332
Chicago/Turabian StyleLiu, Yunjie, Huanhuan Yuan, Hui Wang, and Zhiwei Wang. 2021. "In Situ Transmission Electron Microscopy Investigation of Melting/Evaporation Kinetics in Anisotropic Gold Nanoparticles" Materials 14, no. 23: 7332. https://doi.org/10.3390/ma14237332
APA StyleLiu, Y., Yuan, H., Wang, H., & Wang, Z. (2021). In Situ Transmission Electron Microscopy Investigation of Melting/Evaporation Kinetics in Anisotropic Gold Nanoparticles. Materials, 14(23), 7332. https://doi.org/10.3390/ma14237332




