Preparation and Characterization of a Hybrid Complex of Cyclodextrin-Based Metal—Organic Frameworks-1 and Ascorbic Acid Derivatives
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
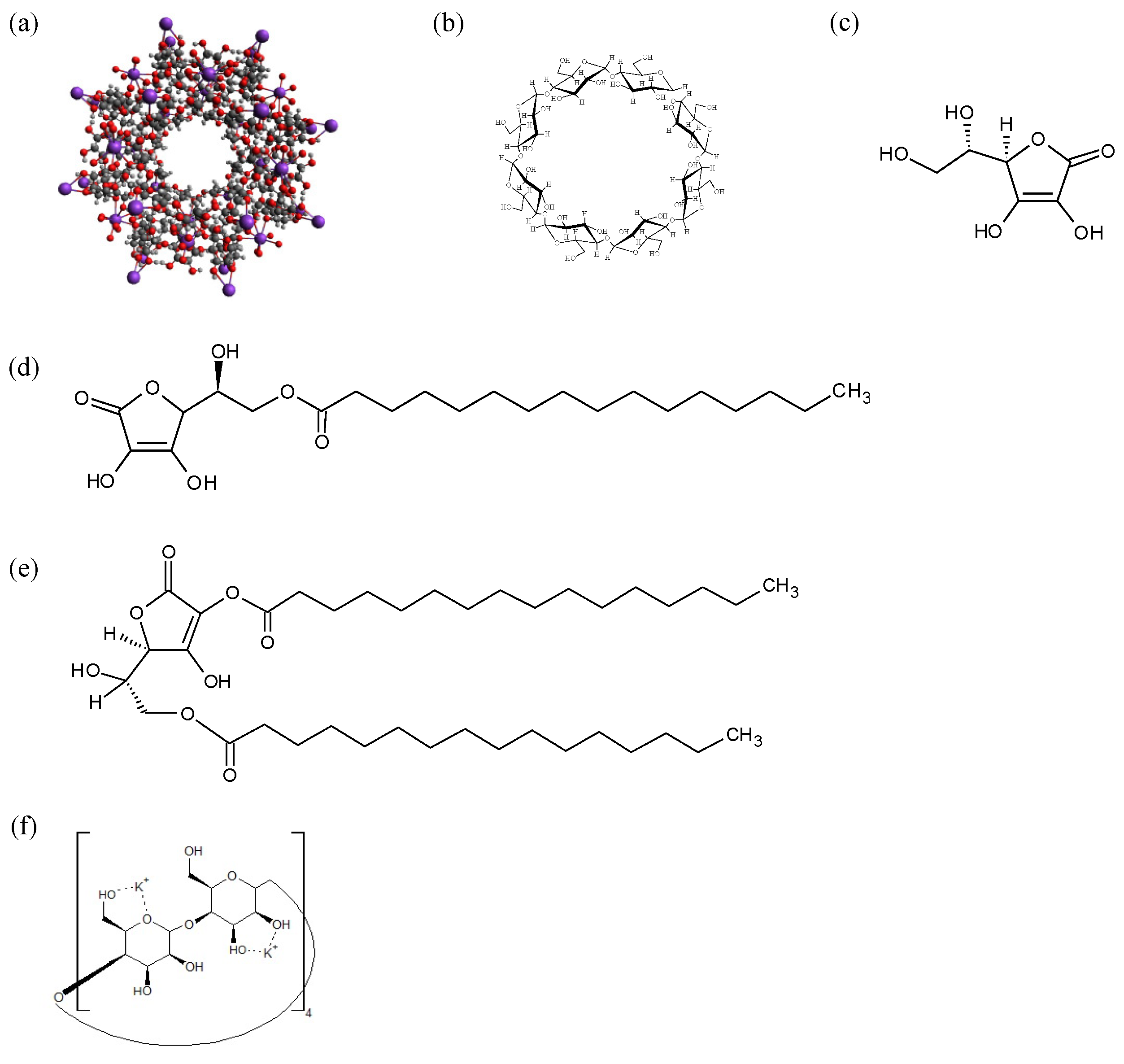
2.2. Preparation of CD-MOF-1
2.3. Preparation of ASCP or ASCDP Physical Mixture and Evaporated Samples
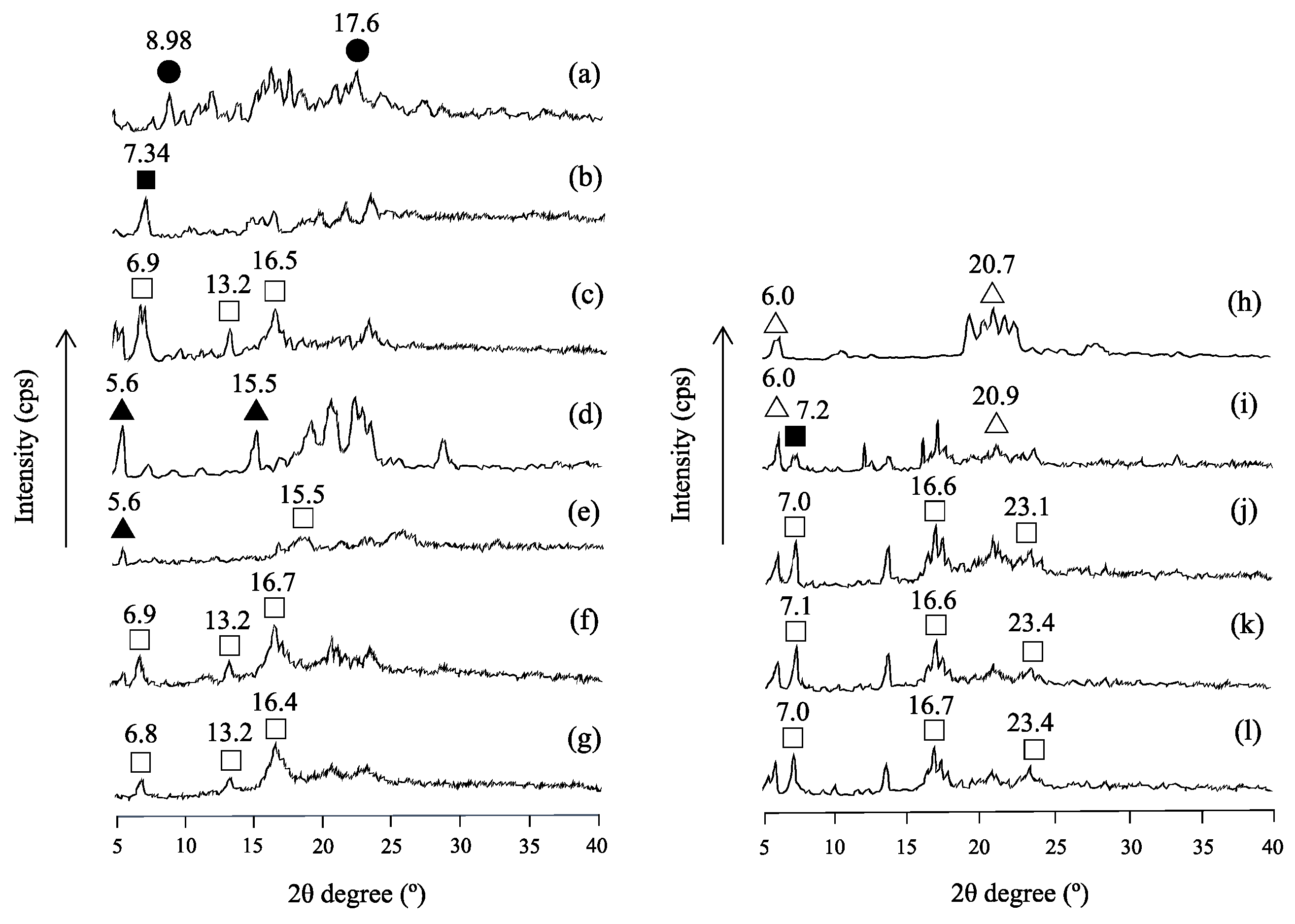
2.4. Powder X-ray Diffraction (PXRD) Measurement
2.5. Differential Scanning Calorimetry (DSC) Measurement
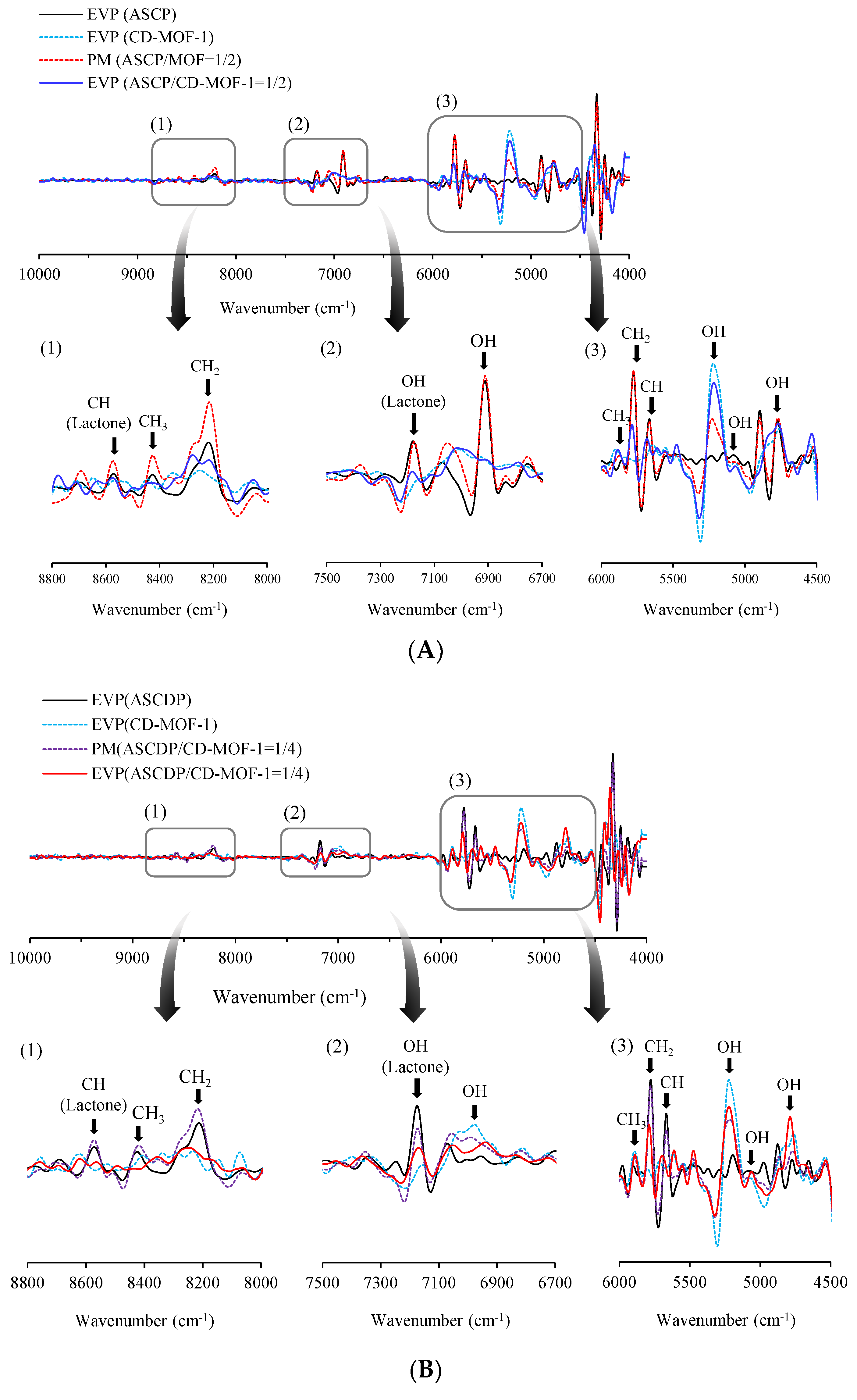
2.6. Near-Infrared Absorption Spectroscopy (NIR) Measurement
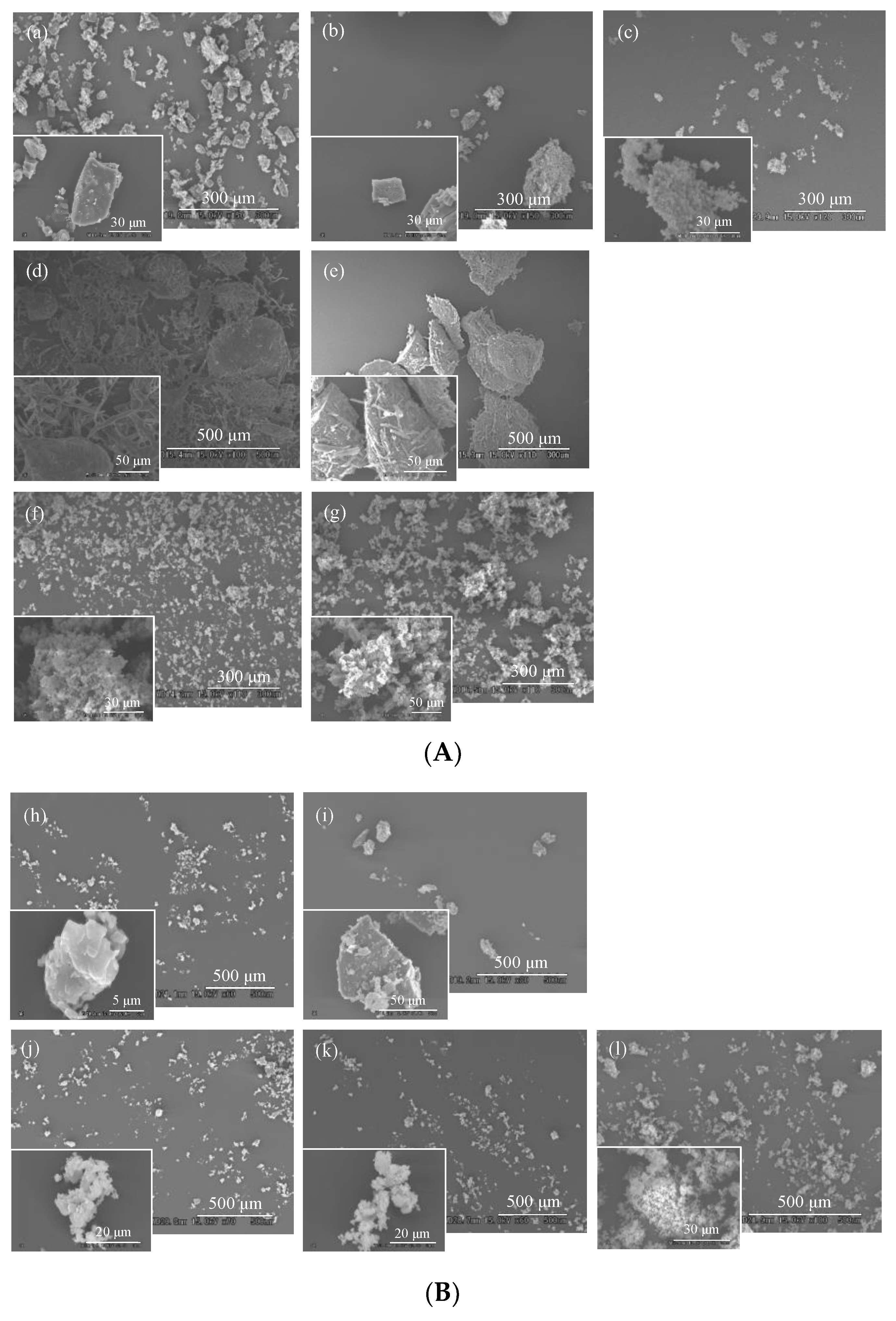
2.7. Scanning Electron Microscopy (SEM) Measurement
2.8. 1H-1H Nuclear Overhauser Effect Spectroscopy (NOESY) NMR Spectra Measurement
3. Results and Discussion
3.1. Evaluation of the Crystalline State
3.2. Evaluation of Thermal Behavior
3.3. Evaluation of Intermolecular Interaction
3.4. Evaluation of Morphological Properties
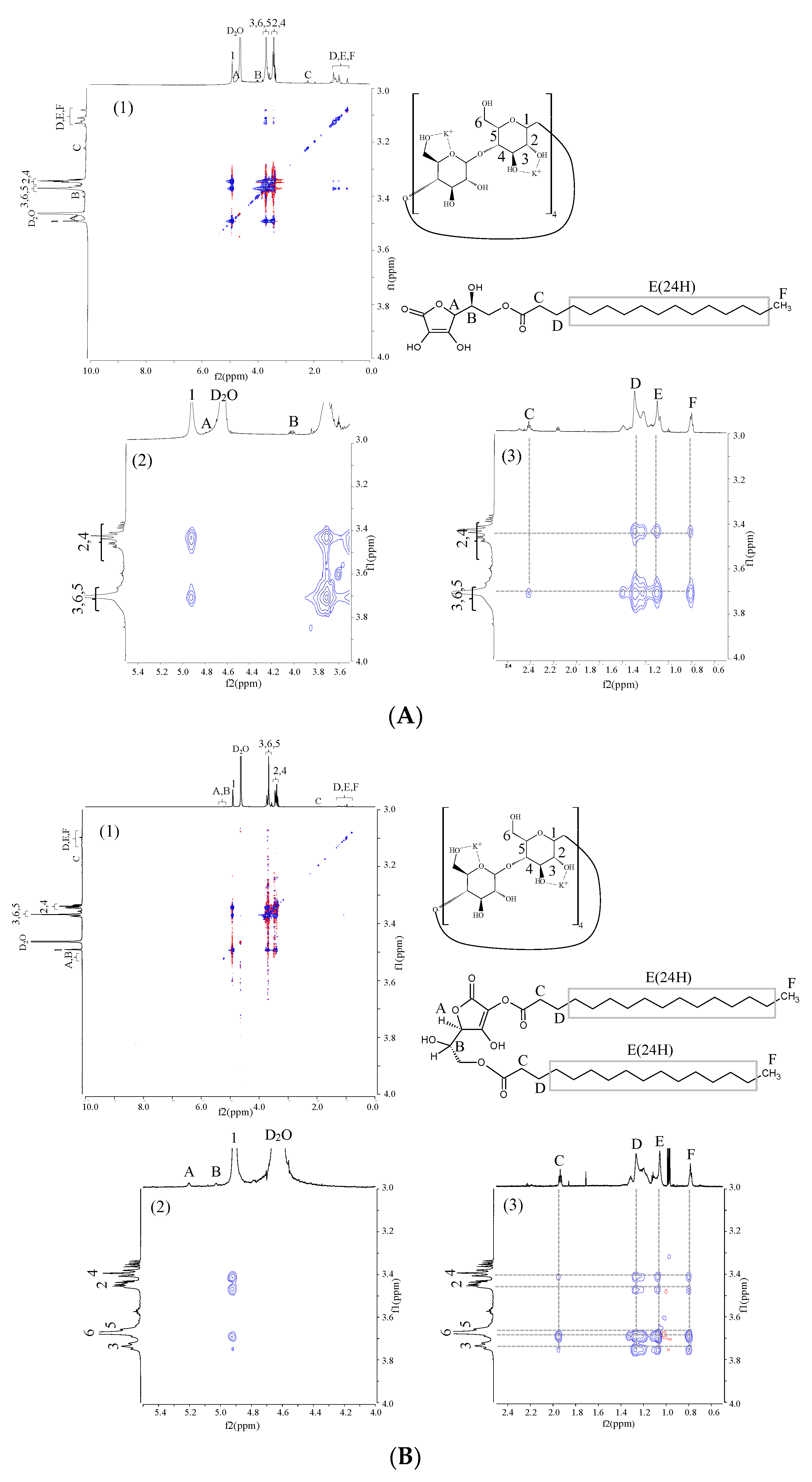
3.5. Evaluation of Relative Positional Relationship
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MOFs | Metal–organic frameworks |
| γ-CD | γ-Cyclodextrin |
| CD-MOF-1 | Cyclodextrin-based metal–organic frameworks-1 |
| ROS | Reactive oxygen species |
| ASC | L(+)-ascorbic Acid |
| ASCP | L-ascorbyl 6-palmitate |
| ASCDP | L-ascorbyl 2, 6-palmitate |
| CoQ10 | Coenzyme Q10 |
| FaSSIF | Fasted state simulated intestinal fluid |
| PM | Physical mixture |
| EVP | Evaporated sample |
| PXRD | Powder X-ray diffraction |
| DSC | Differential scanning calorimetry |
| NIR | Near-infrared absorption spectroscopy |
| SEM | Scanning electron microscopy |
| NOESY | Nuclear Overhauser effect spectroscopy |
| IR | Infrared absorption spectroscopy |
References
- Roy, I.; Stoddart, J.F. Cyclodextrin Metal-Organic Frameworks and Their Applications. Acc. Chem. Res. 2021, 16, 1440–1453. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Zhou, H.C. Gas storage in porous metal-organic frameworks for clean energy applications. Chem. Commun. 2010, 46, 44–53. [Google Scholar] [CrossRef]
- Li, B.; Wen, H.M.; Zhou, W.; Chen, B. Porous metal-organic frameworks for gas storage and separation: What, how, and why? J. Phys. Chem. Lett. 2014, 20, 3468–3479. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Xiang, S.; Qian, G. Metal-organic frameworks with functional pores for recognition of small molecules. Acc. Chem. Res. 2010, 43, 1115–1124. [Google Scholar] [CrossRef]
- Li, J.R.; Sculley, J.; Zhou, H.C. Metal-organic frameworks for separations. Chem. Rev. 2012, 112, 869–932. [Google Scholar] [CrossRef] [PubMed]
- Koo, W.T.; Jang, J.S.; Kim, I.D. Metal-organic frameworks for chemiresistive sensors. Chem 2019, 5, 1938–1963. [Google Scholar] [CrossRef]
- Horcajada, P.; Gref, R.; Baati, T.; Allan, P.K.; Maurin, G.; Couvreur, P.; Férey, G.; Morris, R.E.; Serre, C. Metal-organic frameworks in biomedicine. Chem. Rev. 2012, 112, 1232–1268. [Google Scholar] [CrossRef] [PubMed]
- Smaldone, R.A.; Forgan, R.S.; Furukawa, H.; Gassensmith, J.J.; Slawin, A.M.Z.; Yaghi, O.M.; Stoddart, J.F. Metalorganic frameworks from edible natural products. Angew. Chem. Int. Ed. 2010, 49, 8630–8634. [Google Scholar] [CrossRef]
- Hartlieb, K.J.; Holcroft, J.M.; Moghadam, P.Z.; Vermeulen, N.A.; Algaradah, M.M.; Nassar, M.S.; Botros, Y.Y.; Snurr, R.Q.; Stoddart, J.F. CD-MOF: A versatile separation medium. J. Am. Chem. Soc. 2016, 138, 2292–2301. [Google Scholar] [CrossRef] [Green Version]
- Patyk-Kaźmierczak, E.; Warren, M.R.; Allan, D.R.; Katrusiak, A. Pressure inverse solubility and polymorphism of an edible γ-cyclodextrin-based metal–organic framework. Phys. Chem. Chem. Phys. 2017, 19, 9086–9091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Bao, T.Y.; Yang, X.Y.; Zhu, P.P.; Wu, L.H.; Sha, J.Q.; Zhang, L.; Dong, L.Z.; Cao, X.L.; Lan, Y.Q. Controllable porosity conversion of metal-organic frameworks composed of natural ingredients for drug delivery. Chem. Commun. 2017, 53, 7804–7807. [Google Scholar] [CrossRef]
- Masaki, H. Role of antioxidants in the skin: Anti-aging effects. J. Dermatol. Sci. 2010, 58, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Bickers, D.R.; Athar, M. Oxidative stress in the pathogenesis of skin disease. J. Investig. Dermatol. 2006, 126, 2565–2575. [Google Scholar] [CrossRef] [Green Version]
- Brenner, M.; Hearing, V.J. The Protective Role of Melanin Against UV Damage in Human Skin. Photochem. Photobiol. 2008, 84, 539–549. [Google Scholar] [CrossRef] [Green Version]
- Segall, A.I.; Moyano, M.A. Stability of vitamin C derivatives in topical formulations containing lipoic acid, vitamins A and E. Int. J. Cosmet. Sci. 2008, 30, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Telang, P. Vitamin C in dermatology. Indian Dermatol. Online J. 2013, 4, 143–146. [Google Scholar]
- Fujinami, Y.; Tai, A.; Yamamoto, I. Radical Scavenging Activity against 1, 1-Diphenyl-2-picrylhydrazyl of Ascorbic Acid 2-Glucoside (AA-2G) and 6-Acyl-AA-2G. Chem. Pharm. Bull. 2001, 49, 642–644. [Google Scholar] [CrossRef] [Green Version]
- Chang, T. An Updated Review of Tyrosinase Inhibitors. Int. J. Mol. Sci. 2009, 10, 2440–2475. [Google Scholar] [CrossRef] [Green Version]
- Yoshimura, M.; Watanabe, Y.; Kasaim, K.; Yamakoshi, J.; Koga, T. Inhibitory Effect of an Ellagic Acid-Rich Pomegranate Extract on Tyrosinase Activity and Ultraviolet-Induced Pigmentation. Biosci. Biotechnol. Biochem. 2005, 69, 2368–2373. [Google Scholar] [CrossRef]
- Ahmad, I.; Sheraz, M.A.; Ahmed, S.; Shaikh, R.H.; Vaid, F.H.M.; Khattak, S.R.; Ansari, S.A. Photostability and interaction of ascorbic acid in cream formulations. AAPS Pharm. Sci. Tech. 2011, 12, 917–923. [Google Scholar] [CrossRef] [Green Version]
- Blaug, S.M.; Hajratwala, B. Kinetics of Aerobic Oxidation of Ascorbic Acid. J. Pharm. Sci. 1972, 61, 556–562. [Google Scholar] [CrossRef]
- Ochiai, Y.; Kaburagi, S.; Obayashi, K.; Ujiie, N.; Hashimoto, S.; Okano, Y.; Masaki, H.; Ichihashi, M.; Sakurai, H. A new lipophilic pro-vitamin C, tetra-isopalmitoyl ascorbic acid (VC-IP), prevents UV-induced skin pigmentation through its anti-oxidative properties. J. Derma. Sci. 2006, 44, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.Y.; Lee, P.C.; Huang, L.K.; Lu, L.P.; Liao, W.C. Stability studies of ascorbic acid 2-glucoside in cosmetic lotion using surface response methodology. Bioorg. Med. Chem. Lett. 2013, 15, 1583–1587. [Google Scholar] [CrossRef]
- Palma, S.; Nostro, P.L.; Manzo, R.; Allemandi, D. Evaluation of the surfactant properties of ascorbyl palmitate sodium salt. Eur. J. Pharm. Sci. 2002, 16, 37–43. [Google Scholar] [CrossRef]
- Bilia, A.R.; Bergonzi, M.C.; Vincieri, F.F.; Nostro, P.L.; Morris, G.A. A diffusion-ordered NMR spectroscopy study of the solubilization of artemisinin by octanoyl-6-O-ascorbic acid micelles. J. Pharm. Sci. 2002, 91, 2265–2270. [Google Scholar] [CrossRef] [PubMed]
- Inoue, Y.; Nanri, A.; Murata, I.; Kanamoto, I. Characterization of Inclusion Complex of Coenzyme Q10 with the New Carrier CD-MOF-1 Prepared by Solvent Evaporation. AAPS Pharm. Sci. Tech. 2018, 19, 3048–3056. [Google Scholar] [CrossRef]
- Li, X.; Guo, T.; Lachmanski, L.; Manoli, F.; Menendez-Miranda, M.; Manet, I.; Guo, Z.; Wu, L.; Zhang, J.; Gref, R. Cyclodextrin-based metal-organic frameworks particles as efficient carriers for lansoprazole: Study of morphology and chemical composition of individual particles. Int. J. Pharm. 2017, 531, 424–432. [Google Scholar] [CrossRef]
- Lv, N.; Guo, T.; Liu, B.; Wang, C.; Singh, V.; Xu, X.; Li, X.; Chen, D.; Gref, R.; Zhang, J. Improvement in Thermal Stability of Sucralose by γ-Cyclodextrin Metal-Organic Frameworks. Pharm. Res. 2017, 34, 269–278. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nanri, A.; Yoshida, M.; Ishida, Y.; Nakata, D.; Terao, K.; Arce, F.J.; See, G.L.; Tanikawa, T.; Inoue, Y. Preparation and Characterization of a Hybrid Complex of Cyclodextrin-Based Metal—Organic Frameworks-1 and Ascorbic Acid Derivatives. Materials 2021, 14, 7309. https://doi.org/10.3390/ma14237309
Nanri A, Yoshida M, Ishida Y, Nakata D, Terao K, Arce FJ, See GL, Tanikawa T, Inoue Y. Preparation and Characterization of a Hybrid Complex of Cyclodextrin-Based Metal—Organic Frameworks-1 and Ascorbic Acid Derivatives. Materials. 2021; 14(23):7309. https://doi.org/10.3390/ma14237309
Chicago/Turabian StyleNanri, Ayumi, Masaaki Yoshida, Yoshiyuki Ishida, Daisuke Nakata, Keiji Terao, Florencio Jr Arce, Gerard Lee See, Takashi Tanikawa, and Yutaka Inoue. 2021. "Preparation and Characterization of a Hybrid Complex of Cyclodextrin-Based Metal—Organic Frameworks-1 and Ascorbic Acid Derivatives" Materials 14, no. 23: 7309. https://doi.org/10.3390/ma14237309
APA StyleNanri, A., Yoshida, M., Ishida, Y., Nakata, D., Terao, K., Arce, F. J., See, G. L., Tanikawa, T., & Inoue, Y. (2021). Preparation and Characterization of a Hybrid Complex of Cyclodextrin-Based Metal—Organic Frameworks-1 and Ascorbic Acid Derivatives. Materials, 14(23), 7309. https://doi.org/10.3390/ma14237309






