3D Printed In Vitro Dentin Model to Investigate Occlusive Agents against Tooth Sensitivity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Nanocrystalline Hydroxyapatite Paste Preparation
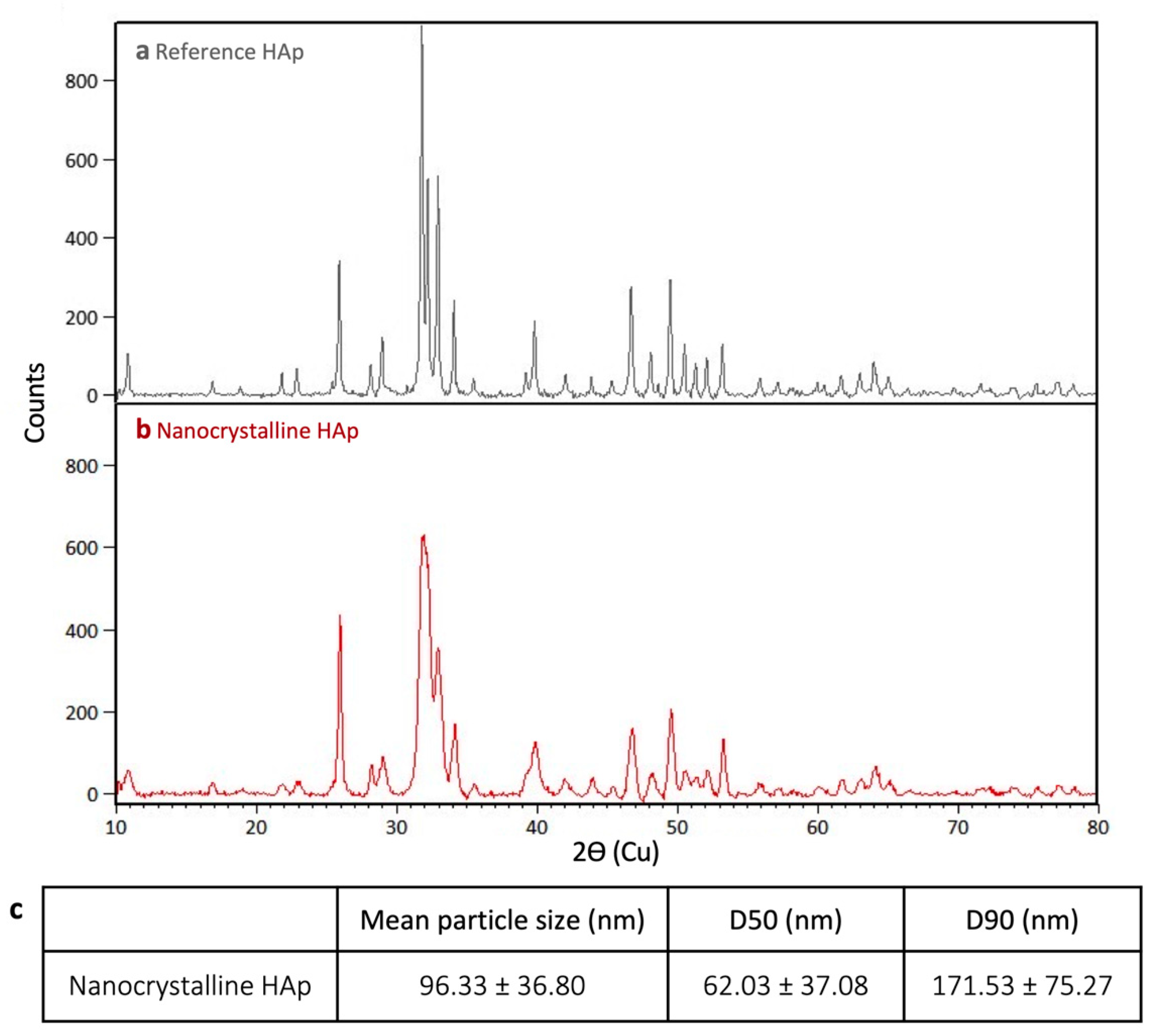
2.1.1. X-ray Diffraction (XRD)
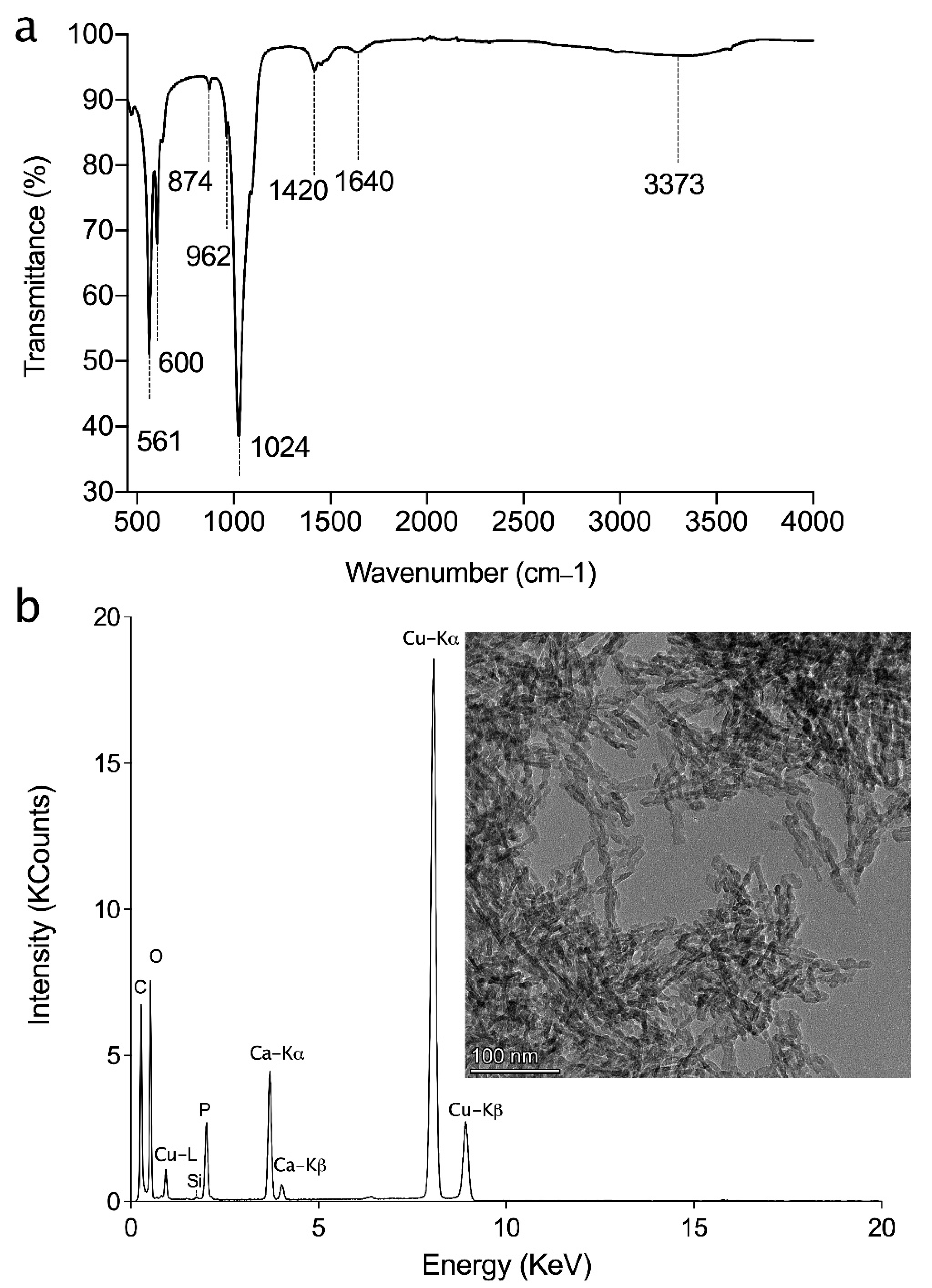
2.1.2. Attenuated Total Reflection-Fourier Transform Infrared (ATR-FTIR) Spectroscopy
2.1.3. Transition Electron Microscopy-Energy Dispersive X-ray (TEM-EDX)
2.1.4. Nanoparticle Tracking Analysis (NTA)
2.2. Ink Preparation
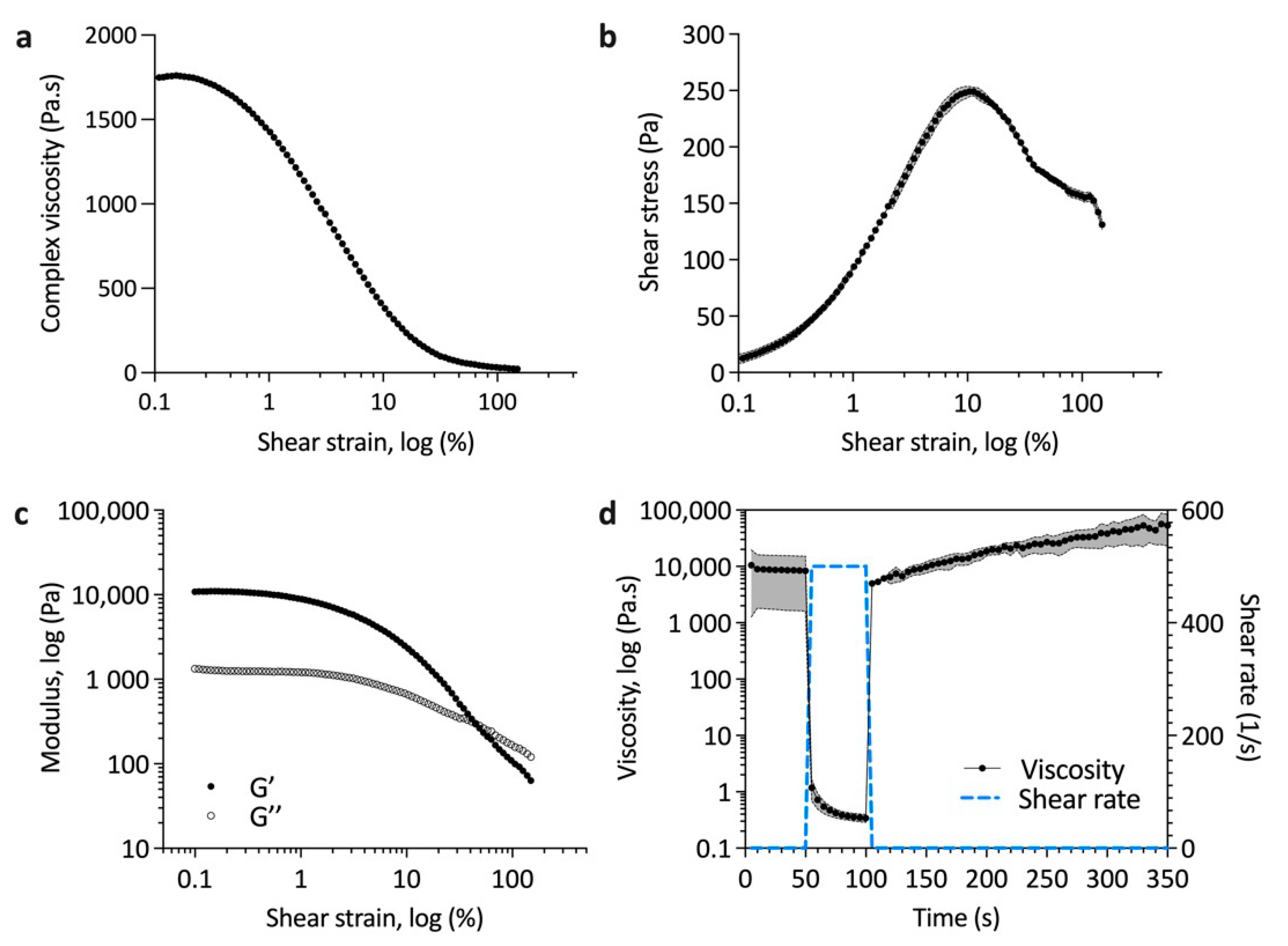
2.3. Ink Rheology
2.4. Scaffold Fabrication
2.5. Micro Computed Tomography
2.6. Natural Dentin Disk Preparation
2.7. Occlusion Test
2.8. Sputter Coating and Scanning Electron Microscopy (SEM)
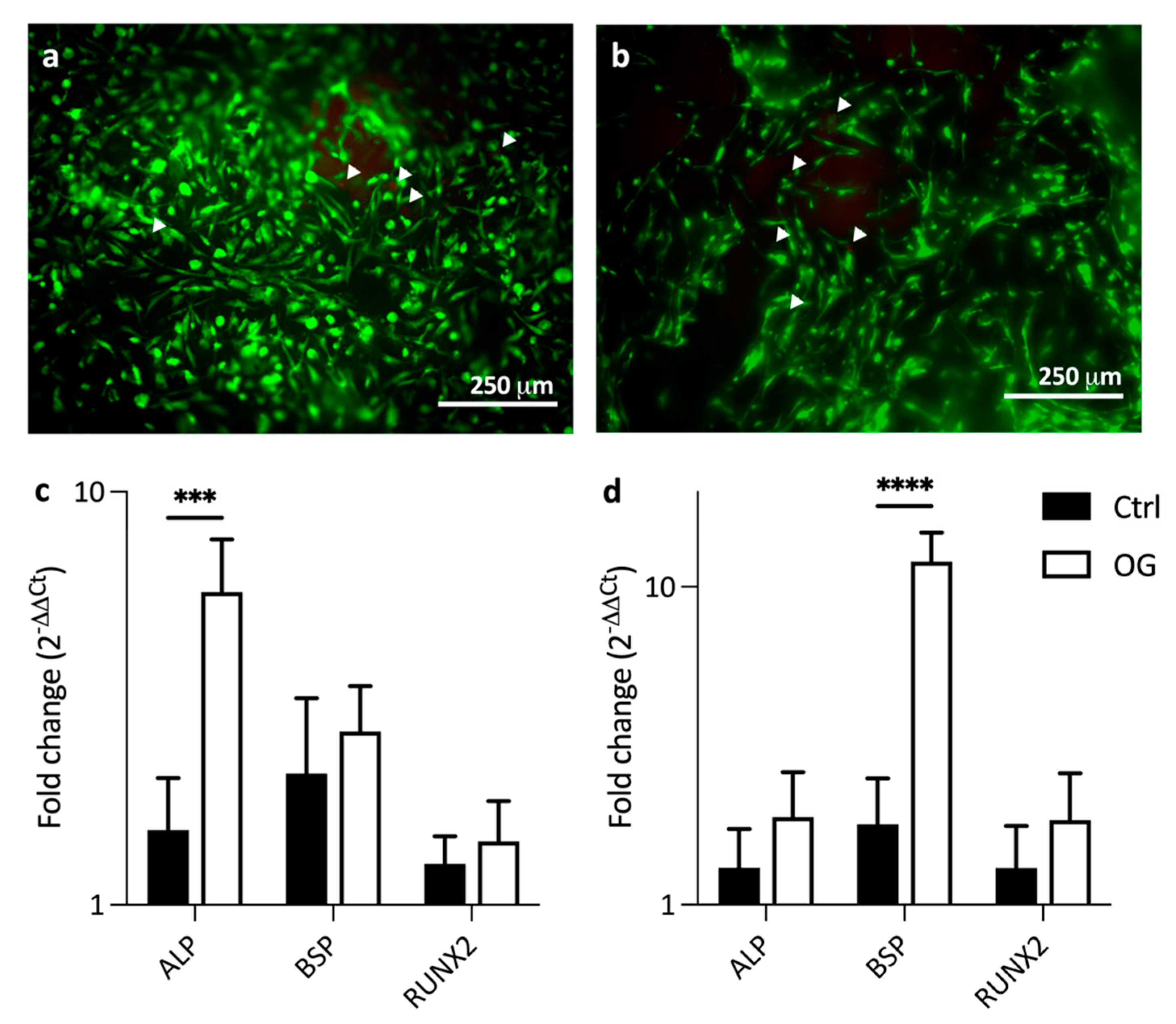
2.9. Cytocompatibility and Gene Expression
2.10. Statistical Analysis
3. Results and Discussion
3.1. HAp Characterization
3.2. Printability of Hap–Collagen Ink
3D Printed Scaffolds
3.3. Cytocompatibility of Dentin Mimics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pashley, D.H. Dentin-predentin complex and its permeability: Physiologic overview. J. Dent. Res. 1985, 64, 613–620. [Google Scholar] [CrossRef]
- Petersson, L.G. The role of fluoride in the preventive management of dentin hypersensitivity and root caries. Clin. Oral Investig. 2013, 17, 63–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brannstrom, M. Dentin sensitivity and aspiration of odontoblasts. J. Am. Dent. Assoc. 1963, 66, 366–370. [Google Scholar] [CrossRef] [PubMed]
- won Kim, J.; Park, J.C. Dentin hypersensitivity and emerging concepts for treatments. J. Oral Biosci. 2017, 59, 211–217. [Google Scholar] [CrossRef]
- Jacobsen, P.L.; Bruce, G. Clinical dentin hypersensitivity: Understanding the causes and prescribing a treatment. J. Contemp. Dent. Pract. 2001, 2, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gernhardt, C.R. How valid and applicable are current diagnostic criteria and assessment methods for dentin hypersensitivity? An overview. Clin. Oral Investig. 2013, 17, 31–40. [Google Scholar] [CrossRef] [Green Version]
- Cunha-Cruz, J.; Wataha, J.C.; Zhou, L.; Manning, W.; Trantow, M.; Bettendorf, M.M.; Heaton, L.J.; Berg, J. Treating dentin hypersensitivity: Therapeutic choices made by dentists of the northwest PRECEDENT network. J. Am. Dent. Assoc. 2010, 141, 1097–1105. [Google Scholar] [CrossRef]
- Bartold, P.M. Dentinal hypersensitivity: A review. Aust. Dent. J. 2006, 51, 212–218. [Google Scholar] [CrossRef] [Green Version]
- Longridge, N.N.; Youngson, C.C. Dental pain. Prim. Dent. J. 2019, 8, 44–51. [Google Scholar] [CrossRef]
- Jung, J.H.; Park, S.B.; Yoo, K.H.; Yoon, S.Y.; Bae, M.K.; Lee, D.J.; Ko, C.C.; Kwon, Y.H.; Kim, Y. Il Effect of different sizes of bioactive glass-coated mesoporous silica nanoparticles on dentinal tubule occlusion and mineralization. Clin. Oral Investig. 2019, 23, 2129–2141. [Google Scholar] [CrossRef]
- Kunam, D.; Manimaran, S.; Sampath, V.; Sekar, M. Evaluation of dentinal tubule occlusion and depth of penetration of nano-hydroxyapatite derived from chicken eggshell powder with and without addition of sodium fluoride: An in vitro study. J. Conserv. Dent. 2016, 19, 239–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, P.; Lu, W.; Xu, H.; Yang, J.; Liu, C.; Xu, P. In vitro dentin tubule occlusion by an arginine-containing dentifrice. Am. J. Dent. 2019, 32, 133–137. [Google Scholar] [PubMed]
- Kuntze, M.M.; Souza, B.D.M.; Schmidt, T.F.; De Almeida, J.; Bortoluzzi, E.A.; Felippe, W.T. Scanning electron microscopy evaluation of dentin ultrastructure after surface demineralization. J. Conserv. Dent. 2020, 23, 512–517. [Google Scholar] [CrossRef] [PubMed]
- Hadjichristou, C.; About, I.; Koidis, P.; Bakopoulou, A. Advanced in Vitro Experimental Models for Tissue Engineering-based Reconstruction of a 3D Dentin/pulp Complex: A Literature Review. Stem Cell Rev. Rep. 2021, 17, 785–802. [Google Scholar] [CrossRef]
- Goldberg, M.; Kulkarni, A.B.; Young, M.; Boskey, A. Dentin: Structure, Composition and Mineralization: The role of dentin ECM in dentin formation and mineralization. Front. Biosci. 2011, 3, 711. [Google Scholar] [CrossRef] [PubMed]
- Galler, K.M.; D’Souza, R.N.; Hartgerink, J.D.; Schmalz, G. Scaffolds for dental pulp tissue engineering. Adv. Dent. Res. 2011, 23, 333–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pashley, D.H. Clinical correlations of dentin structure and function. J. Prosthet. Dent. 1991, 66, 777–781. [Google Scholar] [CrossRef]
- Shahbazi, M.; Jäger, H. Current Status in the Utilization of Biobased Polymers for 3D Printing Process: A Systematic Review of the Materials, Processes, and Challenges. ACS Appl. Bio Mater. 2021, 4, 325–369. [Google Scholar] [CrossRef]
- Cooke, M.E.; Rosenzweig, D.H. The rheology of direct and suspended extrusion bioprinting. APL Bioeng. 2021, 5. [Google Scholar] [CrossRef] [PubMed]
- Billiet, T.; Gevaert, E.; De Schryver, T.; Cornelissen, M.; Dubruel, P. The 3D printing of gelatin methacrylamide cell-laden tissue-engineered constructs with high cell viability. Biomaterials 2014, 35, 49–62. [Google Scholar] [CrossRef]
- Campodoni, E.; Dozio, S.M.; Panseri, S.; Montesi, M.; Tampieri, A.; Sandri, M. Mimicking Natural Microenvironments: Design of 3D-Aligned Hybrid Scaffold for Dentin Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 836. [Google Scholar] [CrossRef]
- Han, J.; Kim, D.S.; Jang, H.; Kim, H.R.; Kang, H.W. Bioprinting of three-dimensional dentin–pulp complex with local differentiation of human dental pulp stem cells. J. Tissue Eng. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Athirasala, A.; Tahayeri, A.; Thrivikraman, G.; Franca, C.M.; Monteiro, N.; Tran, V.; Ferracane, J.; Bertassoni, L.E. A Dentin-Derived Hydrogel Bioink for 3D Bioprinting of Cell- Laden Scaffolds in Regenerative Dentistry. Biofabrication 2019, 10, 024101. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Azmi, D.F.B.; Rosa, V.; Fawzy, A.S.; Fuh, J.Y.H.; Wong, Y.S.; Lu, W.F. Fabrication of dentin-like scaffolds through combined 3D printing and bio-mineralisation. Cogent Eng. 2016, 3, 1222777. [Google Scholar] [CrossRef]
- Ahangar, P.; Cooke, M.E.; Weber, M.H.; Rosenzweig, D.H. Current Biomedical Applications of 3D Printing and Additive Manufacturing. Appl. Sci. 2019, 9, 1713. [Google Scholar] [CrossRef] [Green Version]
- Mobasherpour, I.; Heshajin, M.S.; Kazemzadeh, A.; Zakeri, M. Synthesis of nanocrystalline hydroxyapatite by using precipitation method. J. Alloys Compd. 2007, 430, 330–333. [Google Scholar] [CrossRef]
- Drouet, C. Apatite formation: Why it may not work as planned, and how to conclusively identify apatite compounds. Biomed Res. Int. 2013, 2013. [Google Scholar] [CrossRef] [Green Version]
- McIntosh, I.M.; Nichols, A.R.L.; Tani, K.; Llewellin, E.W. Accounting for the species-dependence of the 3500 cm-1 H2Ot infrared molar absorptivity coefficient: Implications for hydrated volcanic glasses. Am. Mineral. 2017, 102, 1677–1689. [Google Scholar] [CrossRef] [Green Version]
- Kourkoumelis, N.; Tzaphlidou, M. Spectroscopic assessment of normal cortical bone: Differences in relation to bone site and sex. Sci. World J. 2010, 10, 402–412. [Google Scholar] [CrossRef] [Green Version]
- Gheisari, H.; Karamian, E.; Abdellahi, M. A novel hydroxyapatite -Hardystonite nanocomposite ceramic. Ceram. Int. 2015, 41, 5967–5975. [Google Scholar] [CrossRef]
- Sroka-Bartnicka, A.; Borkowski, L.; Ginalska, G.; Ślósarczyk, A.; Kazarian, S.G. Structural transformation of synthetic hydroxyapatite under simulated in vivo conditions studied with ATR-FTIR spectroscopic imaging. Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 2017, 171, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Wypych, F.; Carbajal Arízaga, G.G.; Da Costa Gardolinski, J.E.F. Intercalation and functionalization of zinc hydroxide nitrate with mono- and dicarboxylic acids. J. Colloid Interface Sci. 2005, 283, 130–138. [Google Scholar] [CrossRef]
- Petit, S.; Righi, D.; Madejová, J.; Decarreau, A. Interpretation of the infrared spectrum of the -clays: Application to the evaluation of the layer charge. Clay Miner. 1999, 34, 543–549. [Google Scholar] [CrossRef]
- Abifarin, J.K.; Obada, D.O.; Dauda, E.T.; Dodoo-Arhin, D. Experimental data on the characterization of hydroxyapatite synthesized from biowastes. Data Br. 2019, 26, 104485. [Google Scholar] [CrossRef]
- Chandrasekar, A.; Sagadevan, S.; Dakshnamoorthy, A. Synthesis and characterization of nano-hydroxyapatite (n-HAP) using the wet chemical technique. Int. J. Phys. Sci. 2013, 8, 1639–1645. [Google Scholar] [CrossRef]
- Paxton, N.; Smolan, W.; Bock, T.; Melchels, F.P.; Groll, J.; Jungst, T. Proposal to assess printability of bioinks for extrusion-based bioprinting and evaluation of rheological properties governing bioprintability. Biofabrication 2017, 9, 044107. [Google Scholar] [CrossRef] [PubMed]
- Bendtsen, S.T.; Quinnell, S.P.; Wei, M. Development of a novel alginate-polyvinyl alcohol-hydroxyapatite hydrogel for 3D bioprinting bone tissue engineered scaffolds. J. Biomed Mater. Res.-Part A 2017, 105, 1457–1468. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Liu, W.; Du, M.; Yang, C.; Li, X.; Yang, P. The differential effect of basic fibroblast growth factor and Stromal cell-derived factor-1 pretreatment on bone morrow mesenchymal stem cells osteogenic differentiation potency. Mol. Med. Rep. 2018, 17, 3715–3721. [Google Scholar] [CrossRef]


| Gene | Forward | Reverse |
|---|---|---|
| Alkaline phosphatase (ALP) | AGAACCCCAAAGGCTTCTTC | CTTGGCTTTTCCTTCATGGT |
| Bone sialoprotein (BSP) | AAGCTCCAGCCTGGGATGA | TATTGCACCTTCCTGAGTTGAACT |
| Runt-related transcription factor 2 (RUNX2) | TCAGCCCAGAACTGAGAAACTC | TTATCACAGATGGTCCCTAATGGT |
| Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) | TCCCTGAGCTGAACGGGAAG | GGAGGAGTGGGTGTCGCTGT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naseri, S.; Cooke, M.E.; Rosenzweig, D.H.; Tabrizian, M. 3D Printed In Vitro Dentin Model to Investigate Occlusive Agents against Tooth Sensitivity. Materials 2021, 14, 7255. https://doi.org/10.3390/ma14237255
Naseri S, Cooke ME, Rosenzweig DH, Tabrizian M. 3D Printed In Vitro Dentin Model to Investigate Occlusive Agents against Tooth Sensitivity. Materials. 2021; 14(23):7255. https://doi.org/10.3390/ma14237255
Chicago/Turabian StyleNaseri, Shiva, Megan E. Cooke, Derek H. Rosenzweig, and Maryam Tabrizian. 2021. "3D Printed In Vitro Dentin Model to Investigate Occlusive Agents against Tooth Sensitivity" Materials 14, no. 23: 7255. https://doi.org/10.3390/ma14237255
APA StyleNaseri, S., Cooke, M. E., Rosenzweig, D. H., & Tabrizian, M. (2021). 3D Printed In Vitro Dentin Model to Investigate Occlusive Agents against Tooth Sensitivity. Materials, 14(23), 7255. https://doi.org/10.3390/ma14237255







