Water Treatment from MB Using Zn-Ag MWCNT Synthesized by Double Arc Discharge
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Procured
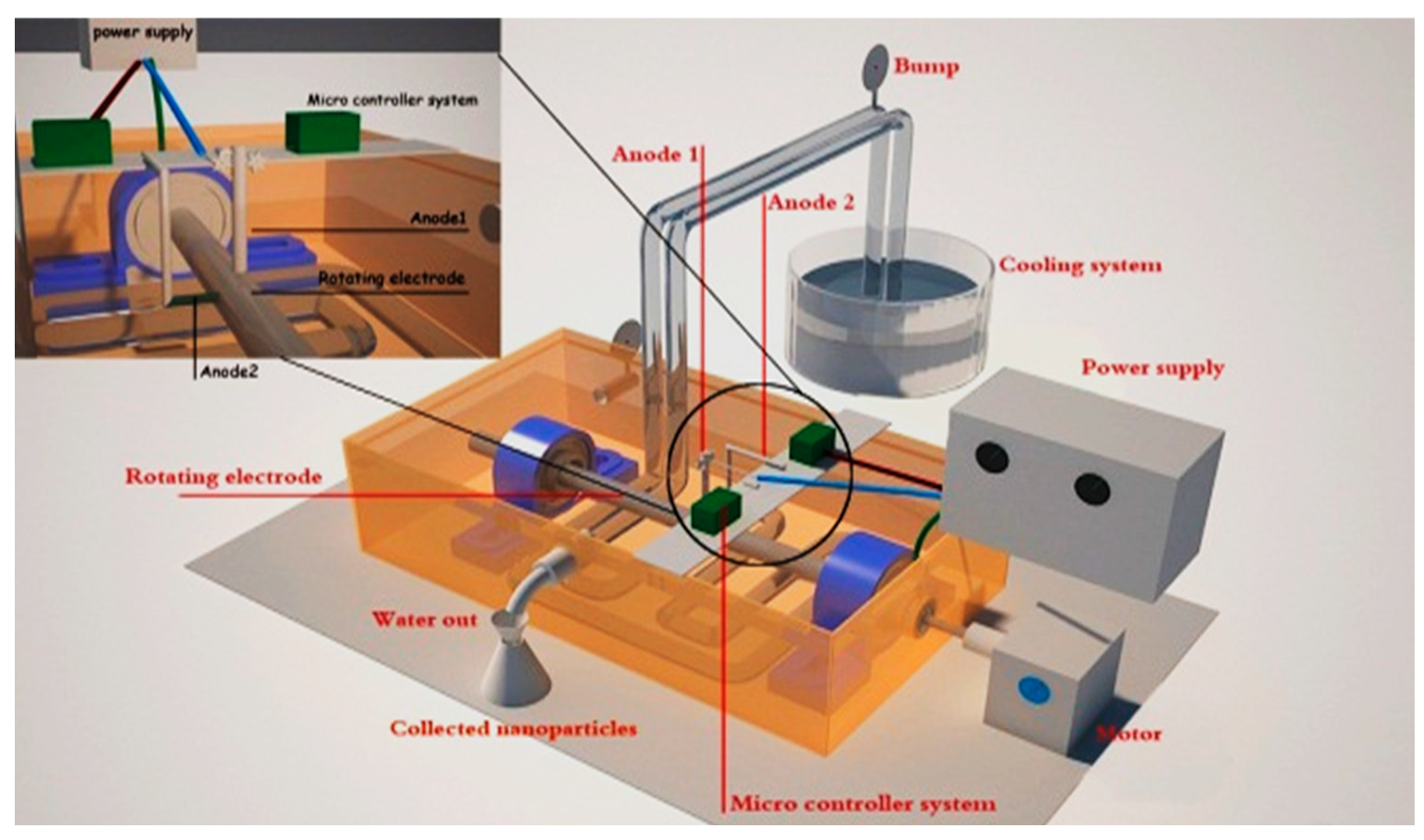
2.2. Experimental Setup of Methodology
2.3. Preparation of Zn-Ag MWCNT
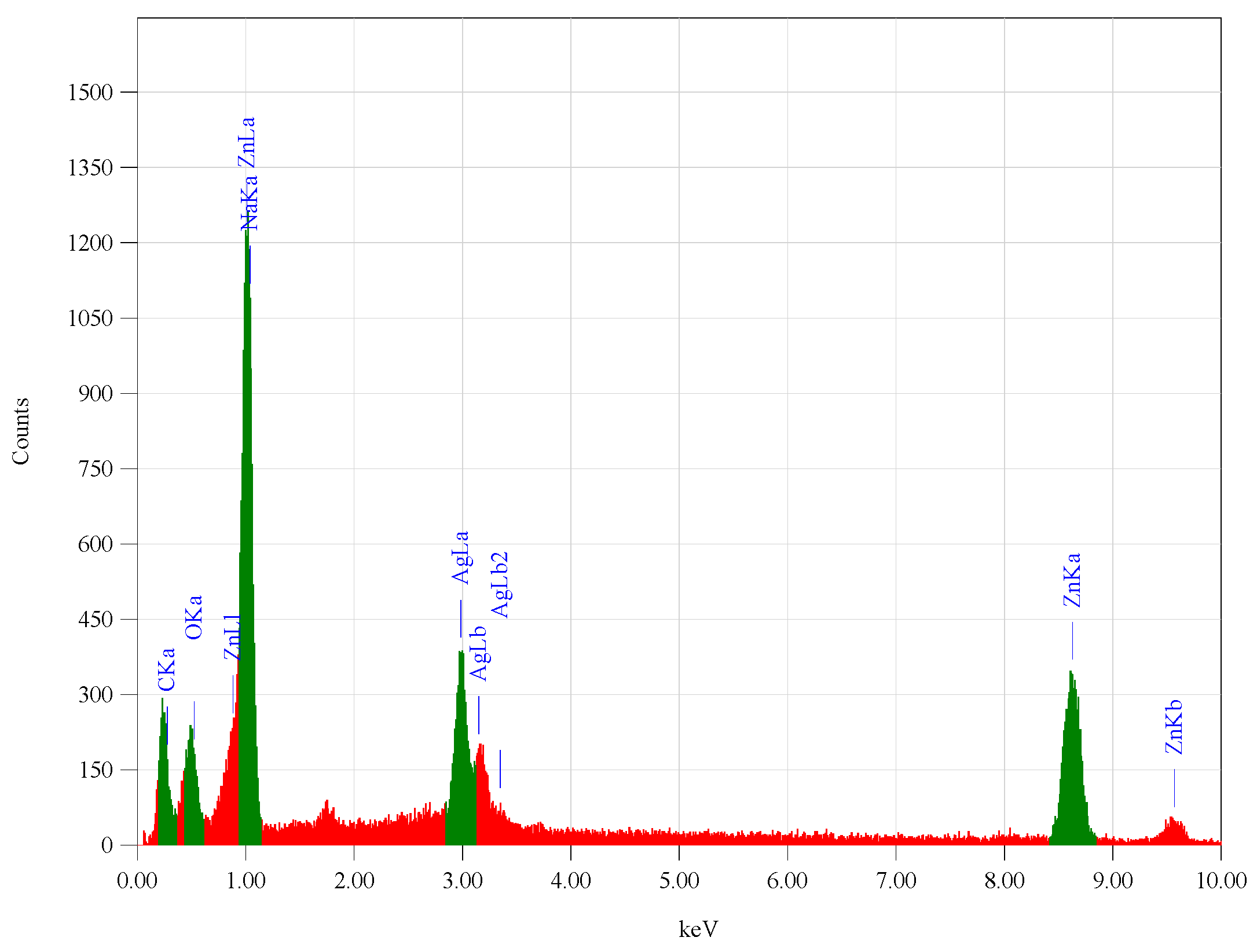
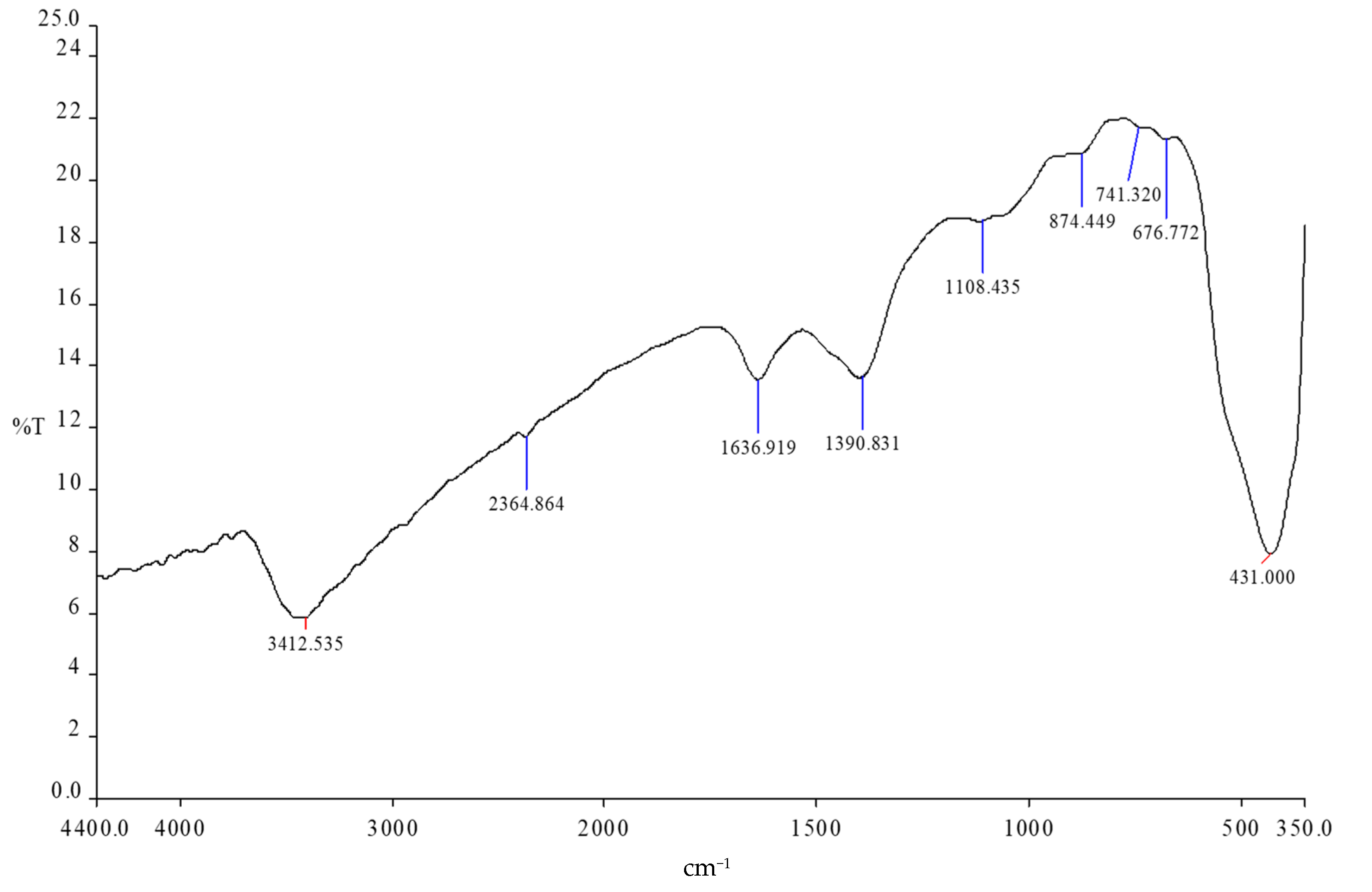
2.4. Characterization
2.5. Adsorption Experiments of MB Dye
- Contact time: to find the best for removal of MB from contaminated water, 100 mg of the prepared Zn-Ag MWCNT as adsorbent dose was added to 100 mL MB solution (40 mg/L) in a dark bottle at fixed pH = 5.8 then the bottle was placed on an electrical shaker for a time extended to 35 min.
- Dye concentration: the previous experiment was repeated several times under the same conditions except for the concentration of MB in deionized water was varied from 10 mg/L to 40 mg/L. Each time the dye’s removal percent was determined by the UV spectrophotometer measurements.
- Nanomaterial’s Zn-Ag MWCNT dosage: it was worthy to study the effect of the adsorbant dosage on the water treatment from MB. Here the same experiment was repeated using dosage, ranging from 100 mg up to 300 mg.
- pH: this experiment was carried out at various pH using drops of NaOH or HCl.
- pH of the solution was measured using a pH meter after it had been balanced with NaOH or HCl solutions.
- Point of zero charge (pzc): the point of zero charge was determined according to Albis et al. [20]. In brief, 50 mL of 0.01 M NaCl was adjusted to pH from 2 to 12 at 1 pH unit interval by using 0.01 M NaOH and HCl. 0.1 g of the sorbents was added, and the mixture was stirred for 48 h. The pH of each batch was measured (pH meter: Hach Sension 1, model 51700-23, Shanghai, China). Initial and final pH values were recorded and plotted. Moreover, after each experiment, the nano adsorbent content was eliminated from water by centrifuge with a speed of 4000 rpm. All the tests were performed in duplicate. The reduced amounts of MB were calculated by the following equation [12]:
3. Results and Discussions
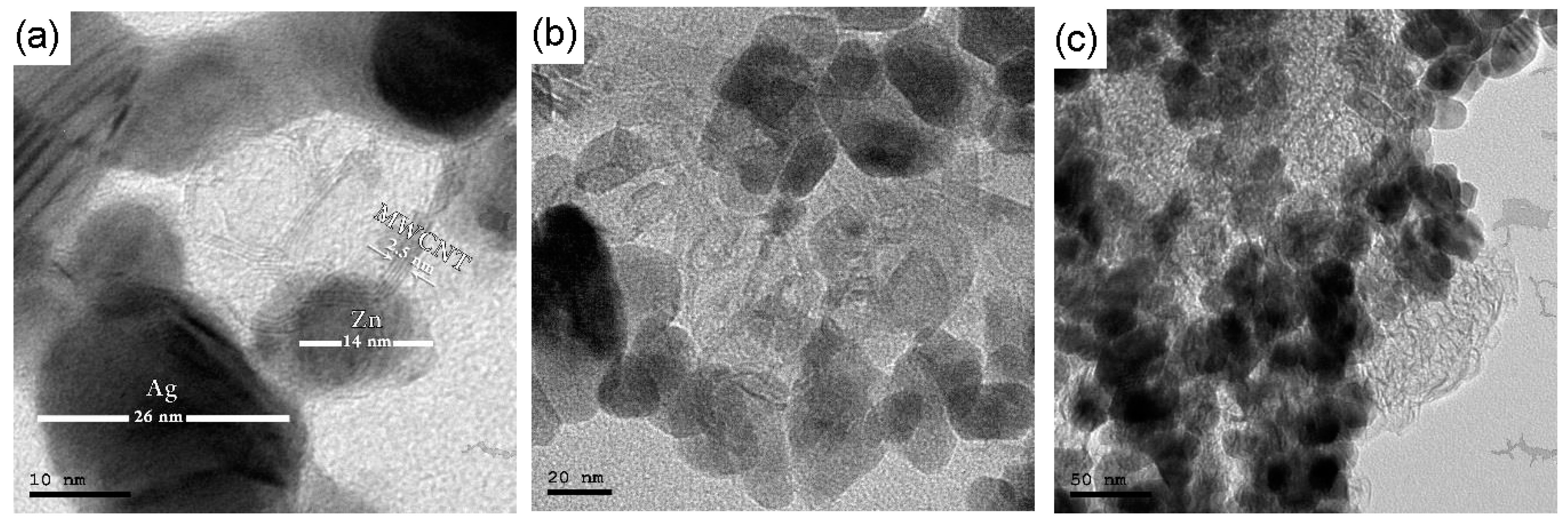
3.1. Characterization of Synthesized Zn-Ag MWCNT
3.2. Water Decontamination Results
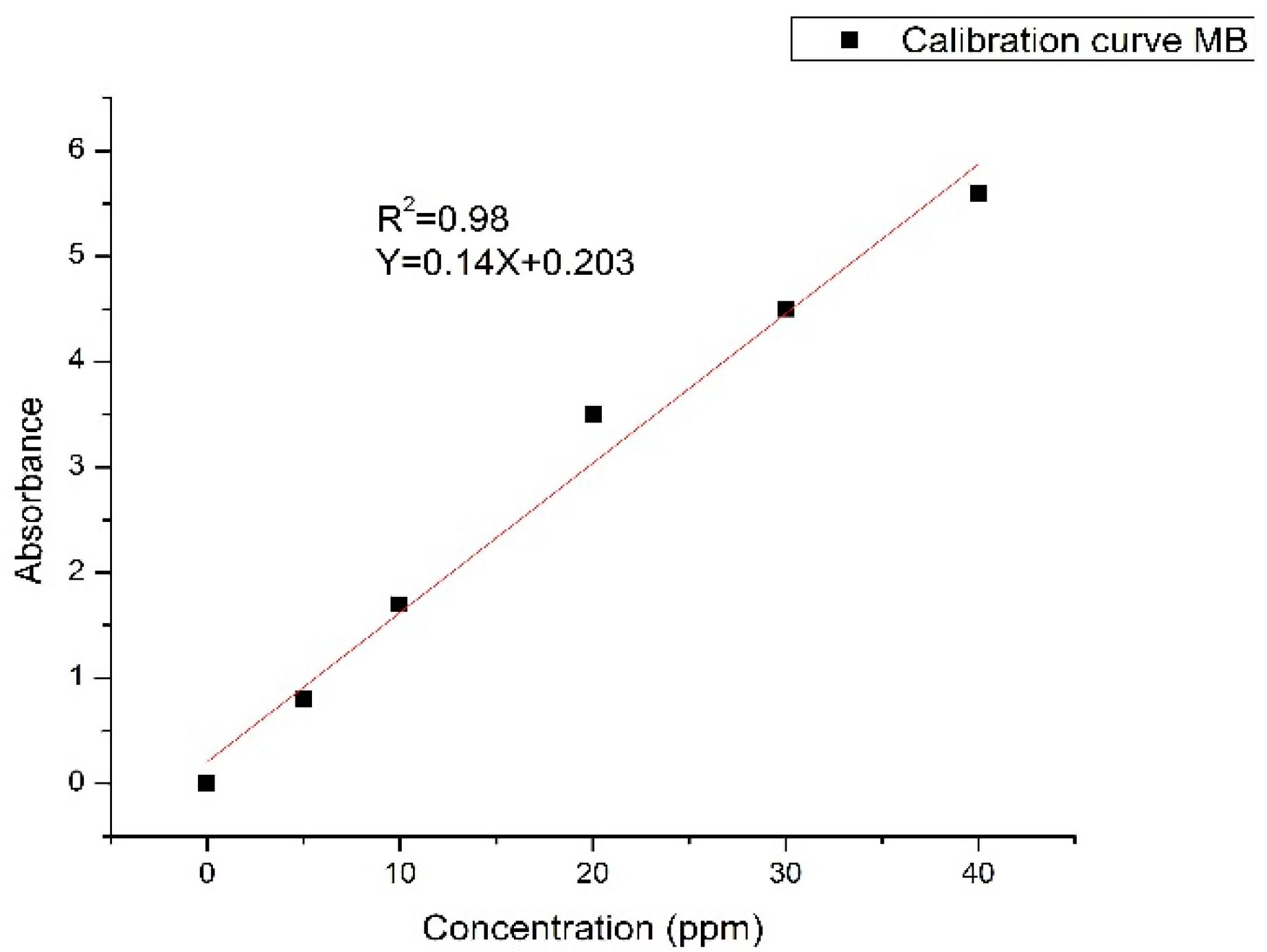
3.2.1. Calibration of the Spectrophotometer Results
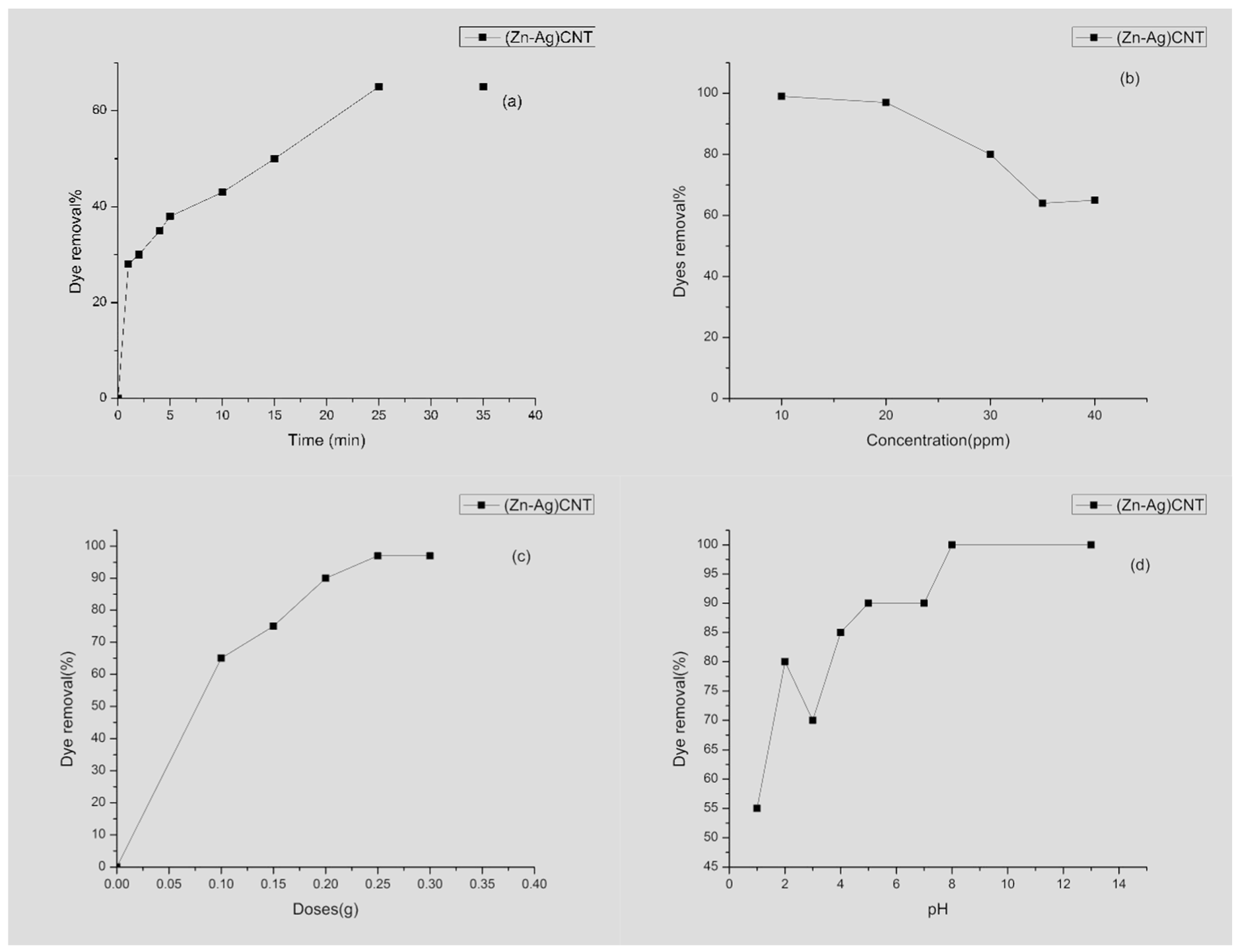
3.2.2. Impact of Contact Time
3.2.3. Impact of Initial Dye Concentration
3.2.4. The Effect of Adsorbent Dosage
3.2.5. Effect of pH
3.3. Kinetics Aspects
3.4. Isotherm Investigation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. WHO Drinking Water Guidelines for Drinking-Water Quality, 4th ed.; WHO: Geneva, Switzerland, 2017; CC BY-NC-SA 3.0 IGO; ISBN 978-92-4-154995-0. Available online: https://www.who.int/publications-detail-redirect/9789241549950 (accessed on 25 November 2021).
- WHO. Ending Preventable Child Deaths from Pneumonia and Diarrhoea by 2025; WHO: Geneva, Switzerland, 2013; ISBN 978 92 4 150523 9. Available online: https://www.who.int/publications/i/item/9789241505239 (accessed on 25 November 2021).
- Yagub, M.T.; Sen, T.K.; Afroze, S.; Ang, H. Dye and its Removal from Aqueous Solution by Adsorption: A Review. Adv. Colloid Interface Sci. 2014, 209, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Nidheesh, P.V.; Gandhimathi, R.; Ramesh, S.T. Degradation of Dyes from Aqueous Solution by Fenton Processes: A Review. Environ. Sci. Pollut. Res. 2013, 20, 2099–2132. [Google Scholar] [CrossRef] [PubMed]
- Katheresan, V.; Kansedo, J.; Lau, S.Y. Efficiency of Various Recent Wastewater Dye Removal Methods: A Review. J. Environ. Chem. Eng. 2018, 6, 4676–4697. [Google Scholar] [CrossRef]
- Koh, H.K.; Geller, A.C.; VanderWeele, T.J. Deaths from COVID-19. JAMA 2021, 325, 133–134. [Google Scholar] [CrossRef] [PubMed]
- Mallick, K.; Witcomb, M.; Scurrell, M. Silver Nanoparticle Catalysed Redox Reaction: An Electron Relay Effect. Mater. Chem. Phys. 2006, 97, 283–287. [Google Scholar] [CrossRef]
- Gupta, N.; Singh, H.P.; Sharma, R.K. Metal Nanoparticles with High Catalytic Activity in Degradation of Methyl Orange: An Electron Relay Effect. J. Mol. Catal. A Chem. 2011, 335, 248–252. [Google Scholar] [CrossRef]
- Ai, L.; Zhang, C.; Chen, Z. Removal of Methylene Blue from Aqueous Solution by a Solvothermal-Synthesized Graphene/Magnetite Composite. J. Hazard. Mater. 2011, 192, 1515–1524. [Google Scholar] [CrossRef]
- El-Khatib, A.M.; Yousef, N.; Ghatass, Z.; Badawi, M.S.; Mohamed, M.; Elkhatib, M. Synthesized Silver Carbon Nanotubes and Zinc Oxide Nanoparticles and their Ability to Remove Methylene Blue Dye. J. Nano Res. 2019, 56, 1–16. [Google Scholar] [CrossRef]
- Ramesha, G.; Kumara, A.V.; Muralidhara, H.; Sampath, S. Graphene and Graphene Oxide as Effective Adsorbents toward Anionic and Cationic Dyes. J. Colloid Interface Sci. 2011, 361, 270–277. [Google Scholar] [CrossRef]
- Sadegh, H.; Shahryari-Ghoshekandi, R.; Agarwal, S.; Tyagi, I.; Asif, M.; Gupta, V.K. Microwave-Assisted Removal of Malachite Green by Carboxylate Functionalized Multi-Walled Carbon Nanotubes: Kinetics and Equilibrium Study. J. Mol. Liq. 2015, 206, 151–158. [Google Scholar] [CrossRef]
- Ghaedi, M.; Kokhdan, S.N. Oxidized Multiwalled Carbon Nanotubes for the Removal of Methyl Red (MR): Kinetics and Equilibrium Study. Desalination Water Treat 2012, 49, 317–325. [Google Scholar] [CrossRef]
- Ghaedi, M.; Khajehsharifi, H.; Yadkuri, A.H.; Roosta, M.; Asghari, A. Oxidized Multiwalled Carbon Nanotubes as Efficient Adsorbent for Bromothymol Blue. Toxicol. Environ. Chem. 2012, 94, 873–883. [Google Scholar] [CrossRef]
- Shahryari, Z.; Goharrizi, A.S.; Azadi, M. Experimental Study of Methylene Blue Adsorption from Aqueous Solutions onto Carbon Nano Tubes. Int. J. Water Res. Environ. Eng. 2010, 2, 16–28. [Google Scholar]
- Dodd, A.C.; McKinley, A.J.; Saunders, M.; Tsuzuki, T. Effect of Particle Size on the Photocatalytic Activity of Nanoparticulate Zinc Oxide. J. Nanopart. Res. 2006, 8, 43–51. [Google Scholar] [CrossRef]
- Jang, Y.; Simer, C.; Ohm, T. Comparison of Zinc Oxide Nanoparticles and its Nano-Crystalline Particles on the Photocatalytic Degradation of Methylene Blue. Mater. Res. Bull. 2006, 41, 67–77. [Google Scholar] [CrossRef]
- Ando, Y.; Zhao, X.; Hirahara, K.; Suenaga, K.; Bandow, S.; Iijima, S. Arc Plasma Jet Method Producing Single-Wall Carbon Nanotubes. Diam. Relat. Mater. 2001, 10, 1185–1189. [Google Scholar] [CrossRef]
- El-Khatib, A.M.; Elsafi, M.; Sayyed, M.; Abbas, M.; El-Khatib, M. Impact of Micro and Nano Aluminium on the Efficiency of Photon Detectors. Results Phys. 2021, 30, 104908. [Google Scholar] [CrossRef]
- Albis, A.; Llanos, H.; Galeano, J.; García, D. Adsorción de Azul de Metileno Utilizando Cáscara de Yuca (Manihot Esculenta) Modificada Químicamente con Ácido Oxálico. Rev. Ion 2018, 31, 99–110. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, L.; Liao, C.; Yan, C. A Simple Route towards Tubular ZnO. Chem. Commun. 2002, 262–263. [Google Scholar] [CrossRef] [PubMed]
- Lanje, A.S.; Sharma, S.J.; Pode, R.B. Synthesis of silver nanoparticles: A safer alternative to conventional antimicrobial and antibacterial agents. J. Chem. Pharm. Res. 2010, 2, 478–483. [Google Scholar]
- Oh, W.C.; Zhang, F.J.; Chen, M.L. Characterization and Photodegradation Characteristics of Organic Dye for Pt-Titania Combined Multi-Walled Carbon Nanotube Composite Catalysts. J. Ind. Eng. Chem. 2010, 16, 321–326. [Google Scholar] [CrossRef]
- Yedurkar, S.; Maurya, C.; Mahanwar, P. Biosynthesis of Zinc Oxide Nanoparticles Using Ixora Coccinea Leaf Extract—A Green Approach. Open J. Synth. Theory Appl. 2016, 5, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Rao, C.N.R. Chemical Applications of Infrared Spectroscopy; Academic Press: New York, NY, USA; London, UK, 1963; pp. 377–379. [Google Scholar]
- Hwa, K.-Y.; Subramani, B. Synthesis of Zinc Oxide Nanoparticles on Graphene–Carbon Nanotube Hybrid for Glucose Biosensor Applications. Biosens. Bioelectron. 2014, 62, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Dinh, N.X.; Van Quy, N.; Huy, T.Q.; Le, A.-T. Decoration of Silver Nanoparticles on Multiwalled Carbon Nanotubes: Antibacterial Mechanism and Ultrastructural Analysis. J. Nanomater. 2015, 2015, 814379. [Google Scholar] [CrossRef] [Green Version]
- Hamadanian, M.; Jabbari, V.; Shamshiri, M.; Asad, M.; Mutlay, I. Preparation of Novel Hetero-Nanostructures and High Efficient Visible Light-Active Photocatalyst Using Incorporation of CNT as an Electron-Transfer Channel into the Support TiO2 and PbS. J. Taiwan Inst. Chem. Eng. 2013, 44, 748–757. [Google Scholar] [CrossRef]
- Yadav, S.; Asthana, A.; Singh, A.K.; Chakraborty, R.; Vidya, S.S.; Susan, A.B.H.; Carabineiro, S.A. Adsorption of Cationic Dyes, Drugs and Metal from Aqueous Solutions Using a Polymer Composite of Magnetic/Β-cyclodextrin/Activated Charcoal/Na Alginate: Isotherm, Kinetics and Regeneration Studies. J. Hazard. Mater. 2020, 409, 124840. [Google Scholar] [CrossRef]
- Ho, Y.S.; Mckay, G. The Kinetics of Sorption of Basic Dyes from Aqueous Solution by Sphagnum Moss Peat. Can. J. Chem. Eng. 1998, 76, 822–827. [Google Scholar] [CrossRef]
- Low, M.J.D. Kinetics of Chemisorption of Gases on Solids. Chem. Rev. 1960, 60, 267–312. [Google Scholar] [CrossRef]
- Kalavathy, M.H.; Karthikeyan, T.; Rajgopal, S.; Miranda, L.R. Kinetic and Isotherm Studies of Cu(II) Adsorption onto H3PO4-Activated Rubber Wood Sawdust. J. Colloid Interface Sci. 2005, 292, 354–362. [Google Scholar] [CrossRef]
- Weber, W.J., Jr.; Morriss, J.C. Kinetics of Adsorption on Carbon from Solution. J. Sanit. Eng. 1963, 89, 31–60. [Google Scholar]
- Boyd, G.E.; Adamson, A.W.; Myers, L.S. The Exchange Adsorption of Ions from Aqueous Solutions by Organic Zeolites, II, Kinetics. J. Amer. Chem. Soc. 1947, 69, 2836–2848. [Google Scholar] [CrossRef]
- Rengaraj, S.; Yeon, J.-W.; Kim, Y.; Jung, Y.; Ha, Y.-K.; Kim, W.-H. Adsorption characteristics of Cu(II) onto Ion Exchange Resins 252H and 1500H: Kinetics, Isotherms and Error Analysis. J. Hazard. Mater. 2007, 143, 469–477. [Google Scholar] [CrossRef]
- Freundlich, H.M. Over the Adsorption in Solution. Z. Phys Chem. 1906, 57, 385–470. [Google Scholar]
- Temkin, M.I.; Pyzhev, V. Kinetics of Ammonia Synthesis on Promoted Iron Catalyst. Acta Physicochem. URSS 1940, 12, 217–222. [Google Scholar]
- Hou, Y.; Abrams, B.L.; Vesborg, P.C.K.; Björketun, M.E.; Herbst, K.; Bech, L.; Setti, A.M.; Damsgaard, C.D.; Pedersen, T.; Hansen, O.; et al. Bioinspired Molecular Co-Catalysts Bonded to a Silicon Photocathode for Solar Hydrogen Evolution. Nat. Mater. 2011, 10, 434–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Q.; Wu, Q. Preparation of Carbon Microspheres Decorated with Silver Nanoparticles and their Ability to Remove Dyes from Aqueous Solution. J. Hazard. Mater. 2015, 283, 193–201. [Google Scholar] [CrossRef]
- Jiang, L.; Gao, L. Fabrication and Characterization of ZnO-Coated Multi-Walled Carbon Nanotubes with Enhanced Photocatalytic Activity. Mater. Chem. Phys. 2005, 91, 313–316. [Google Scholar] [CrossRef]
- Wang, R.; Xin, J.H.; Yang, Y.; Liu, H.; Xu, L.; Hu, J. The Characteristics and Photocatalytic Activities of Silver Doped ZnO Nanocrystallites. Appl. Surf. Sci. 2004, 227, 312–317. [Google Scholar] [CrossRef]
- Lv, T.; Pan, L.; Liu, X.; Sun, Z. Enhanced Photocatalytic Degradation of Methylene Blue by ZnO–Reduced Graphene Oxide–Carbon Nanotube Composites Synthesized Via Microwave-Assisted Reaction. Catal. Sci. Technol. 2012, 2, 2297–2301. [Google Scholar] [CrossRef]
- Selen, V.; Göler, Ö.; Özer, D.; Evin, E. Synthesized Multi-Walledcarbon Nanotubes as a Potential Adsorbent for the Removal of Methylene Blue Dye: Kinetics, Isotherms, and Thermodynamics. Desalination Water Treat 2016, 57, 8826–8838. [Google Scholar] [CrossRef]
- Shi, H.; Li, W.; Zhong, L.; Xu, C. Methylene Blue Adsorption from Aqueous Solution by Magnetic Cellulose/Graphene Oxide Composite: Equilibrium, Kinetics, and Thermodynamics. Ind. Eng. Chem. Res. 2014, 53, 1108–1118. [Google Scholar] [CrossRef]
- Abdel Ghafar, H.H.; Ali, G.A.M.; Fouad, O.A.; Makhlouf, S.A. Enhancement of Adsorption Efficiency of Methylene Blue on Co3O4/SiO2 Nanocomposite. Desalin. Water Treat. 2015, 53, 2980–2989. [Google Scholar] [CrossRef] [Green Version]
- Xie, G.; Xi, P.; Liu, H.; Chen, F.; Huang, L.; Shi, Y.; Hou, F.; Zeng, Z.; Shao, C.; Wang, J. A Facile Chemical Method to Produce Superparamagnetic Graphene Oxide–Fe3O4hybrid Composite and its Application in the Removal of Dyes from Aqueous Solution. J. Mater. Chem. 2011, 22, 1033–1039. [Google Scholar] [CrossRef]
- Lakkaboyana, S.; Soontarapa, K.; Asmel, N.; Kumar, V.; Marella, R.K.; Yuzir, A.; Yaacob, W.Z.W. Synthesis and Characterization of Cu(OH)2-Nws-PVA-AC Nano Composite and its Use as an Efficient Adsorbent for Removal of Methylene Blue. Sci. Rep. 2021, 11, 5686. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Xu, F.; Chen, M.; Xu, Z.; Zhu, Z. Adsorption Behavior of Methylene Blue on Carbon Nanotubes. Bioresour. Technol. 2010, 101, 3040–3046. [Google Scholar] [CrossRef] [PubMed]
- Ayad, M.M.; El-Nasr, A.A. Adsorption of Cationic Dye (Methylene Blue) from Water Using Polyaniline Nanotubes Base. J. Phys. Chem. C 2010, 114, 14377–14383. [Google Scholar] [CrossRef]
- Xiong, L.; Yang, Y.; Mai, J.; Sun, W.; Zhang, C.; Wei, D.; Chen, Q.; Ni, J. Adsorption Behavior of Methylene Blue onto Titanate Nanotubes. Chem. Eng. J. 2010, 156, 313–320. [Google Scholar] [CrossRef]
- Ai, L.; Jiang, J. Removal of Methylene Blue from Aqueous Solution with Self-Assembled Cylindrical Graphene–Carbon Nanotube Hybrid. Chem. Eng. J. 2012, 192, 156–163. [Google Scholar] [CrossRef]
- Kubra, K.T.; Salman, S.; Hasan, N. Enhanced Toxic Dye Removal from Wastewater Using Biodegradable Polymeric Natural Adsorbent. J. Mol. Liq. 2021, 328, 115468. [Google Scholar] [CrossRef]

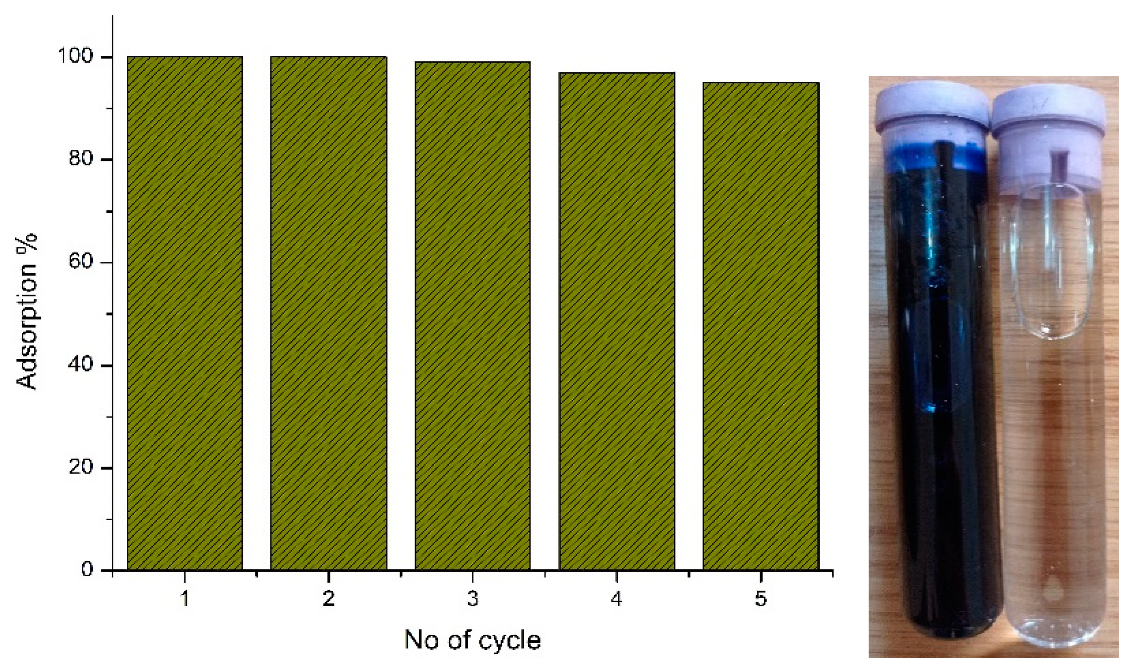
| Isotherms | Linear Expression | Plot | Parameters | R2 | Calculated Parameters | Ref. |
|---|---|---|---|---|---|---|
| 1st-order kinetic | ) vs. t | qt = exp(intercept) = −(slope × 2.303) | 0.88 | = 0.138 min−1 qe = 21.71 | [30] | |
| 2nd-order kinetic | vs. t | qe = (slope)−1 k2 = (slope)2 × (intercept)−1 | 0.98 | k2 = 5.8 × 103 (g/mg·min) qe = 27.91 | [31] | |
| Elovich | ln (t) | qt vs. ln (t) | β = slope, α = (slope)−1 exp(intercept/slope) | 0.92 | α =1.747 (mg/g·min) β = 4.430 (g/mg) | [32] |
| Intraparticle diffusion | qt = kint t1/2 + C | qt vs. t1/2 | kint = slope | 0.99 | kint =3.373 C = 7.359 | [33] |
| Film diffusion process | = −R’t | vs. t | R’ = −(slope) | 0.86 | R’ = 0.156 min−1 | [34] |
| Isotherms | Linear Expression | Plot | Parameters | R2 | Calculated Parameters | Ref. |
| Langmuir | = (intercept)−1 = intercept/slope | 0.995 | = 33.11 mg/g = 0.250 L/mg | [35] | ||
| Freundlich | = exp(intercept) nf = (slope)−1 | 0.910 | = 5.568 (mg/g)(L/mg)1/n = 2.358 | [36] | ||
| Temkin | = slope = exp (intercept/slope) | 0.959 | = 7.1031 mg/g = 1.22 L/g | [37] |
| Adsorbent | Prepared Method | Adsorption Capacity (mg g−1) | Reference |
|---|---|---|---|
| Ag-CNT | Physical Arc discharge | 45.87 | [10] |
| ZnO NPs | Physical Arc discharge | 25.12 | [10] |
| MWCNTs | Chemical Method | 95.30 | [43] |
| Magnetic cellulose/GO composite | Chemical Method | 70.03 | [44] |
| Nano-Co3O4/SiO2 | Chemical Method | 53.87 | [45] |
| Graphene oxide–Fe3O4 hybrid nano-composite | Chemical Method | 167.20 | [46] |
| Copper hydroxide nanowires decorated on activated carbon | Chemical Method | 139.9 | [47] |
| Carbon nanotubes | Chemical Method | 46.20 | [48] |
| Polyaniline nanotubes base | Chemical Method | 9.21 | [49] |
| Titanate nanotubes | Chemical Method | 133.33 | [50] |
| G–CNT hybrid | Chemical Method | 81.97 | [51] |
| Zn-Ag MWCNT | Physicsl Arc discharge | 33.11 | The present work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aljohani, F.S.; Elsafi, M.; Ghoneim, N.I.; Toderaş, M.; Sayyed, M.I.; Mohafez, H.; Islam, M.A.; Khandaker, M.U.; El-Khatib, M. Water Treatment from MB Using Zn-Ag MWCNT Synthesized by Double Arc Discharge. Materials 2021, 14, 7205. https://doi.org/10.3390/ma14237205
Aljohani FS, Elsafi M, Ghoneim NI, Toderaş M, Sayyed MI, Mohafez H, Islam MA, Khandaker MU, El-Khatib M. Water Treatment from MB Using Zn-Ag MWCNT Synthesized by Double Arc Discharge. Materials. 2021; 14(23):7205. https://doi.org/10.3390/ma14237205
Chicago/Turabian StyleAljohani, Faizah S., Mohamed Elsafi, Nourhan I. Ghoneim, M. Toderaş, M. I. Sayyed, Hamidreza Mohafez, Mohammad A. Islam, Mayeen Uddin Khandaker, and Mostafa El-Khatib. 2021. "Water Treatment from MB Using Zn-Ag MWCNT Synthesized by Double Arc Discharge" Materials 14, no. 23: 7205. https://doi.org/10.3390/ma14237205
APA StyleAljohani, F. S., Elsafi, M., Ghoneim, N. I., Toderaş, M., Sayyed, M. I., Mohafez, H., Islam, M. A., Khandaker, M. U., & El-Khatib, M. (2021). Water Treatment from MB Using Zn-Ag MWCNT Synthesized by Double Arc Discharge. Materials, 14(23), 7205. https://doi.org/10.3390/ma14237205











