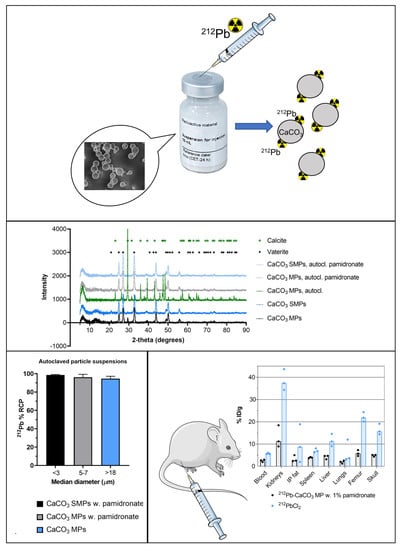
A Novel Single-Step-Labeled 212Pb-CaCO3 Microparticle for Internal Alpha Therapy: Preparation, Stability, and Preclinical Data from Mice
Abstract
:1. Introduction
2. Materials and Methods
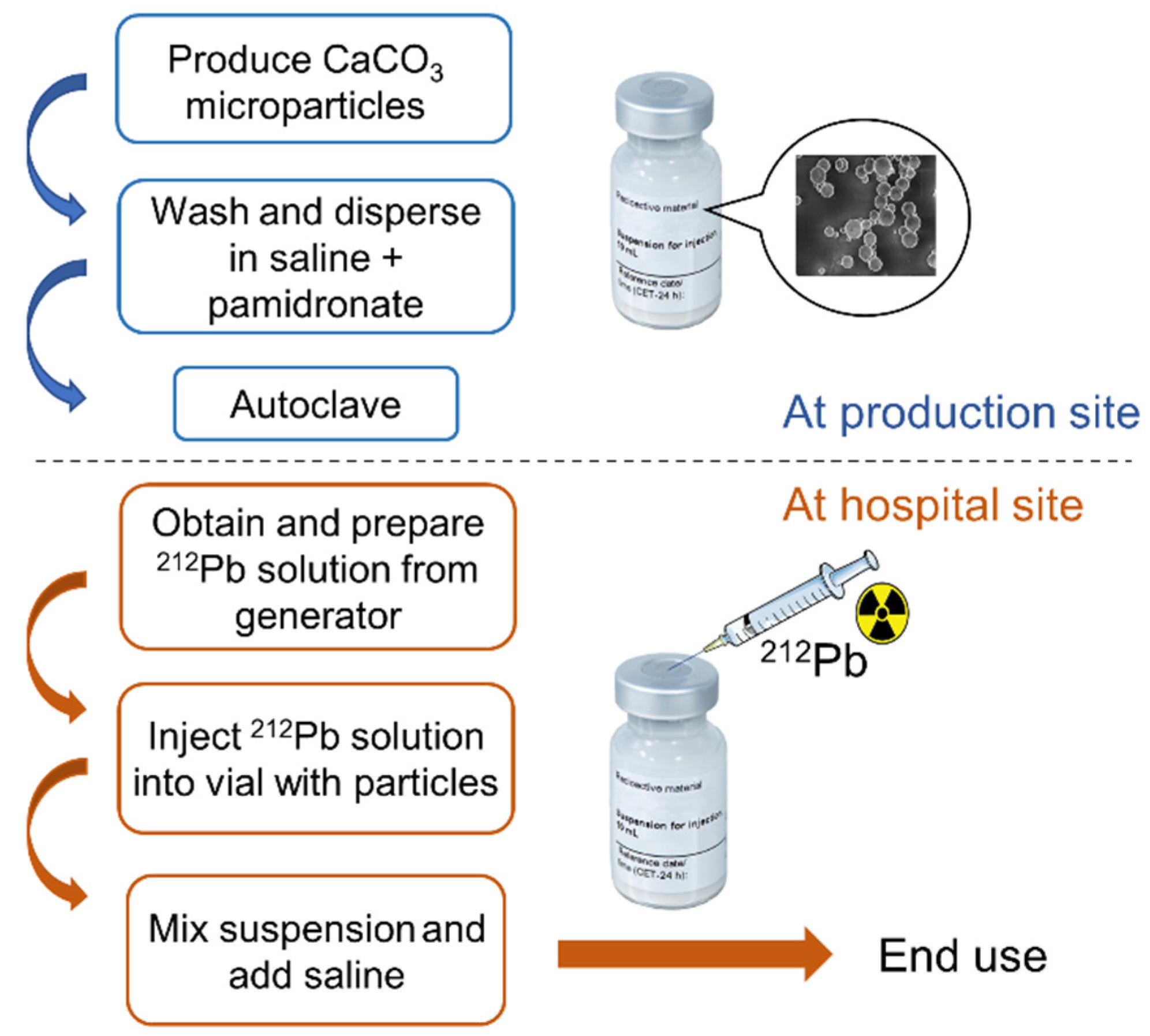
2.1. Preparation of CaCO3 Particles
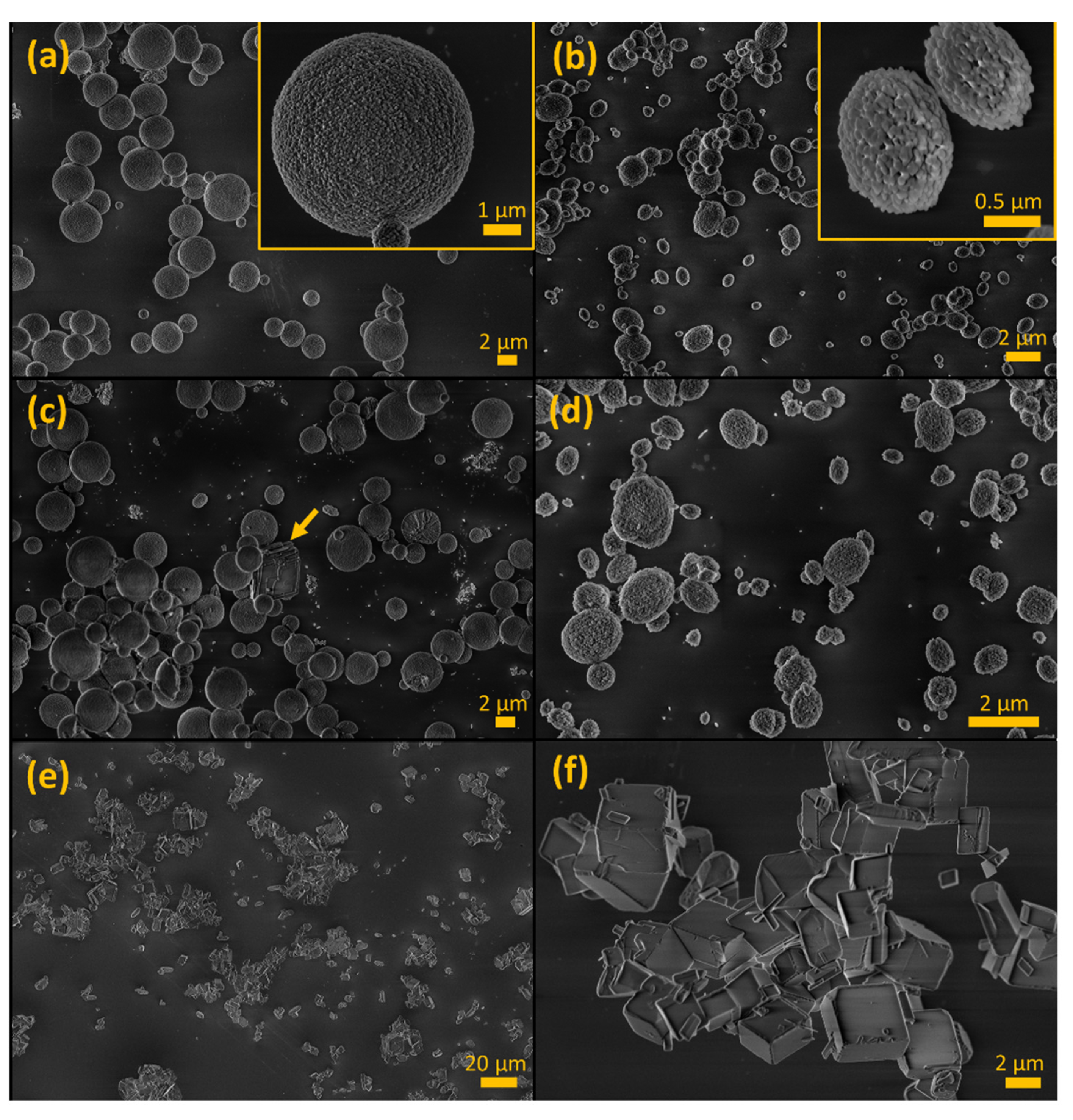
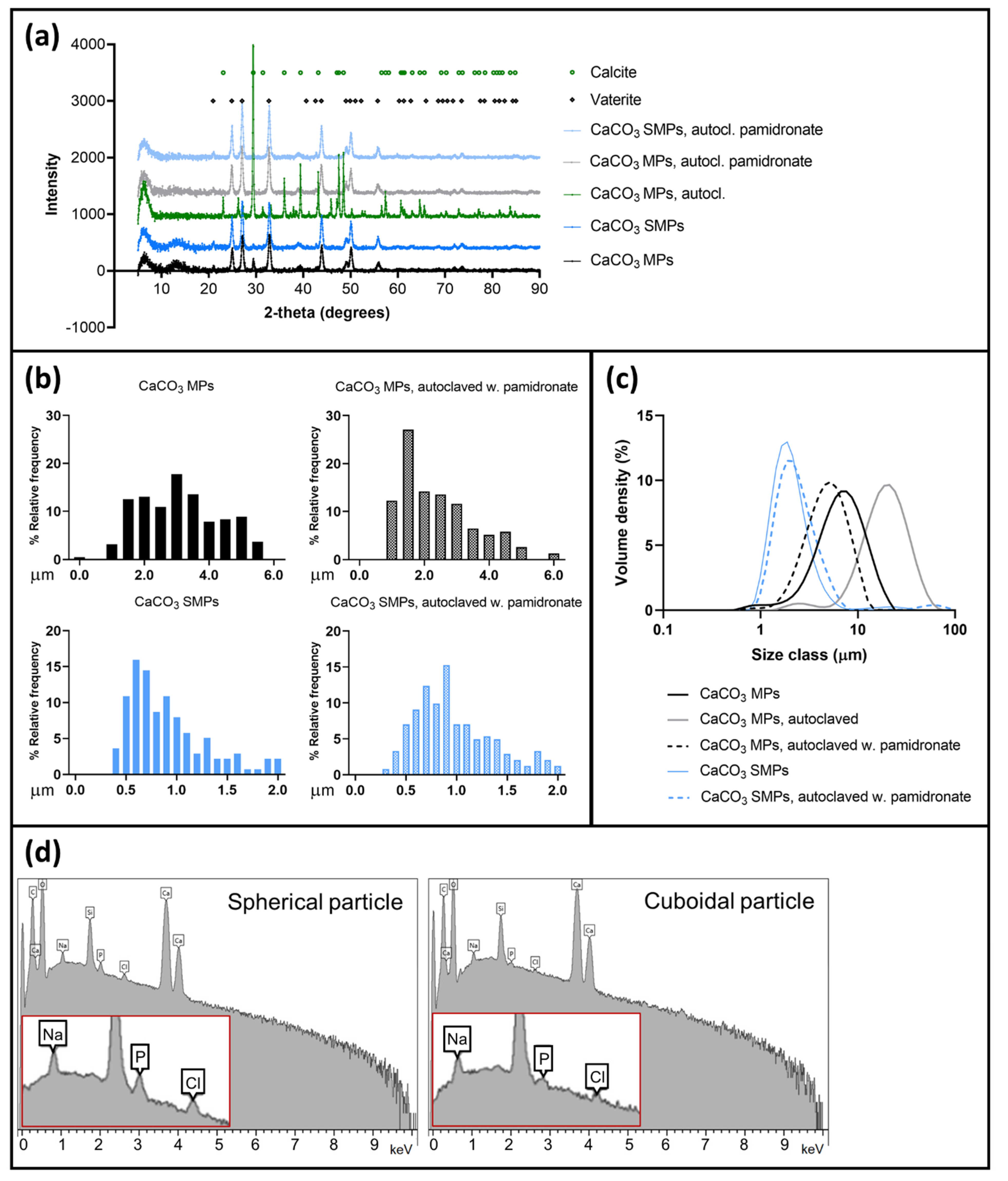
2.2. Characterization of CaCO3 Particles
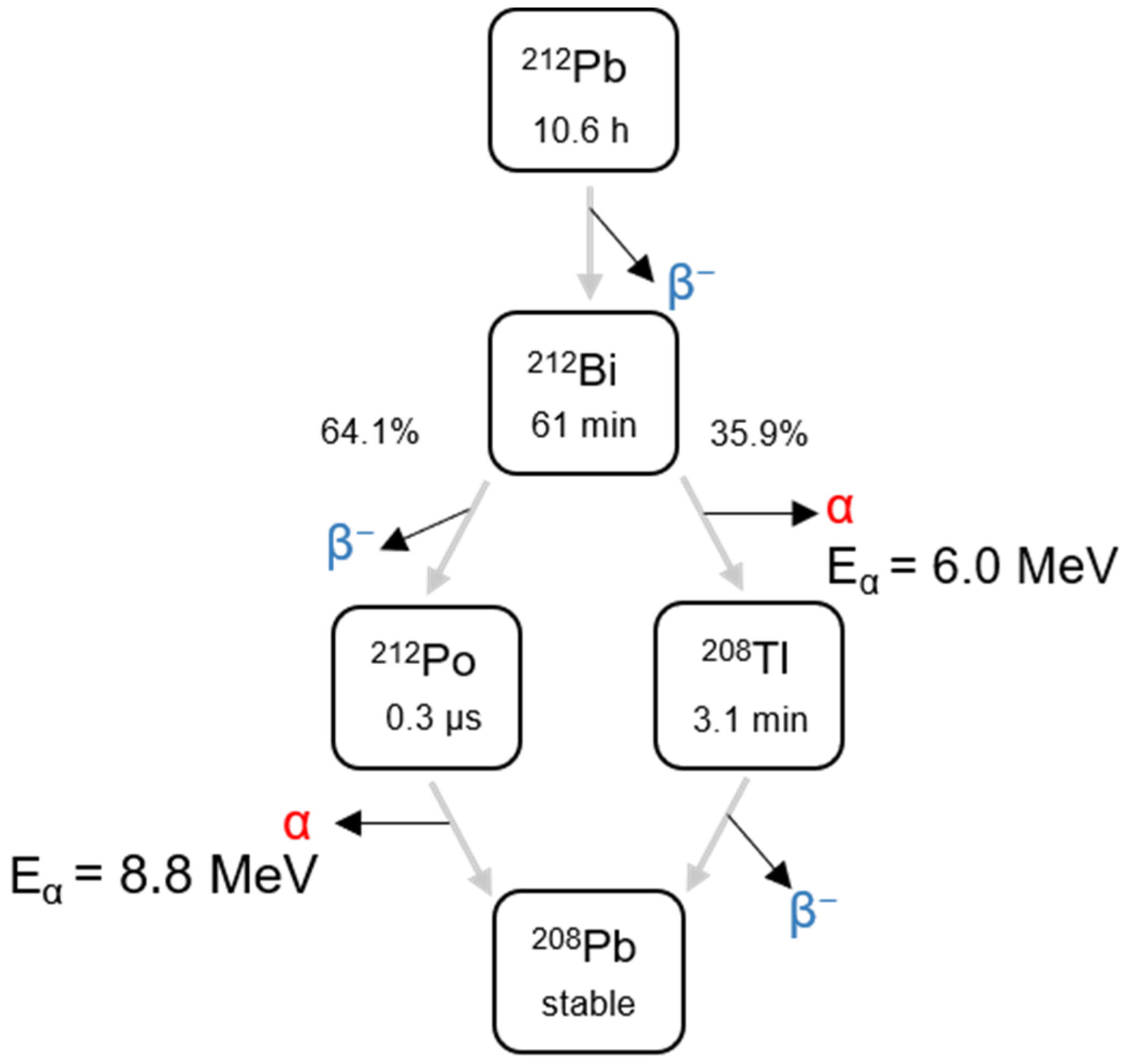
2.3. Radiolabeling CaCO3 Particles with 212Pb
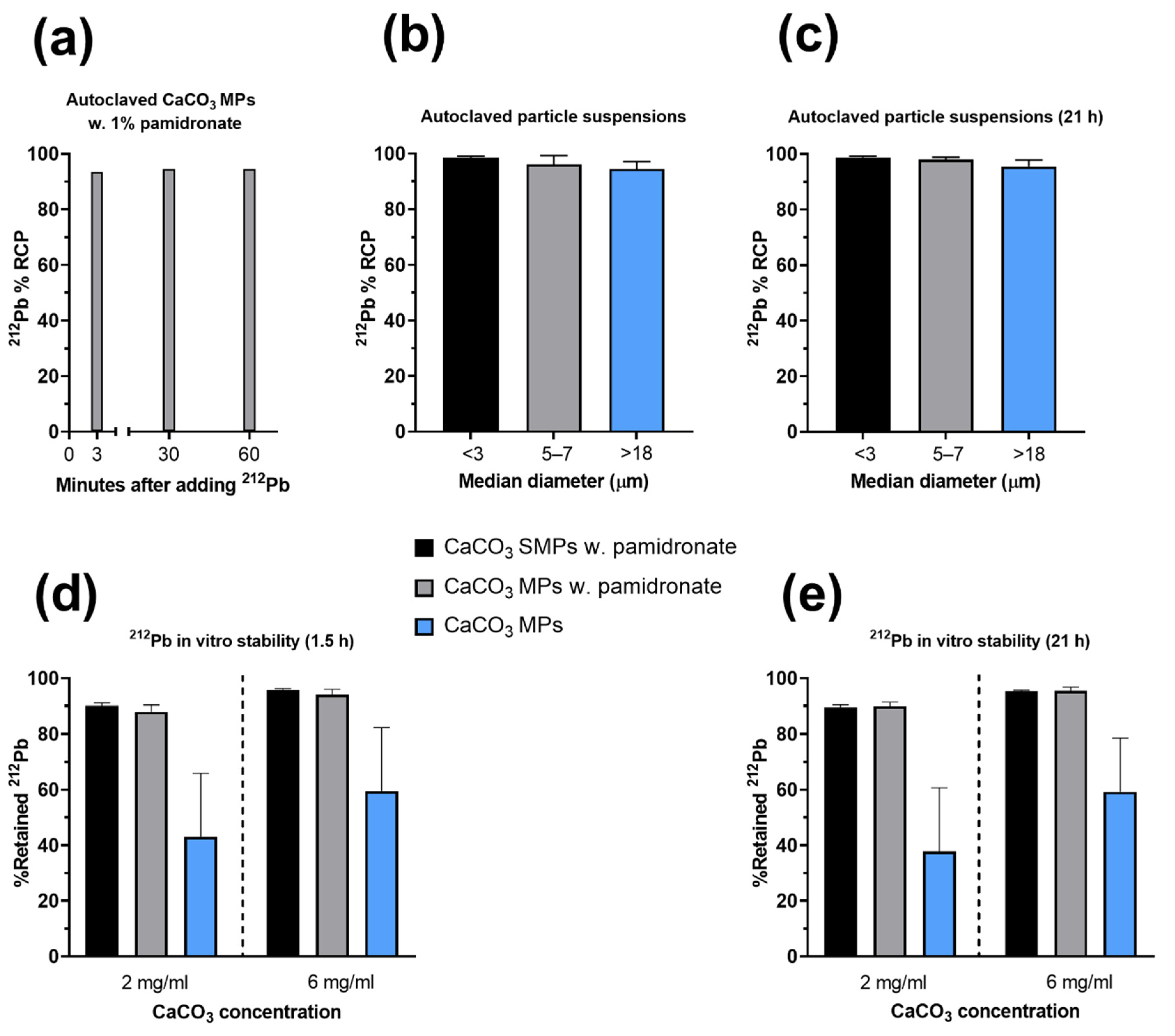
2.4. Evaluation of Radiolabeling
2.5. Animals
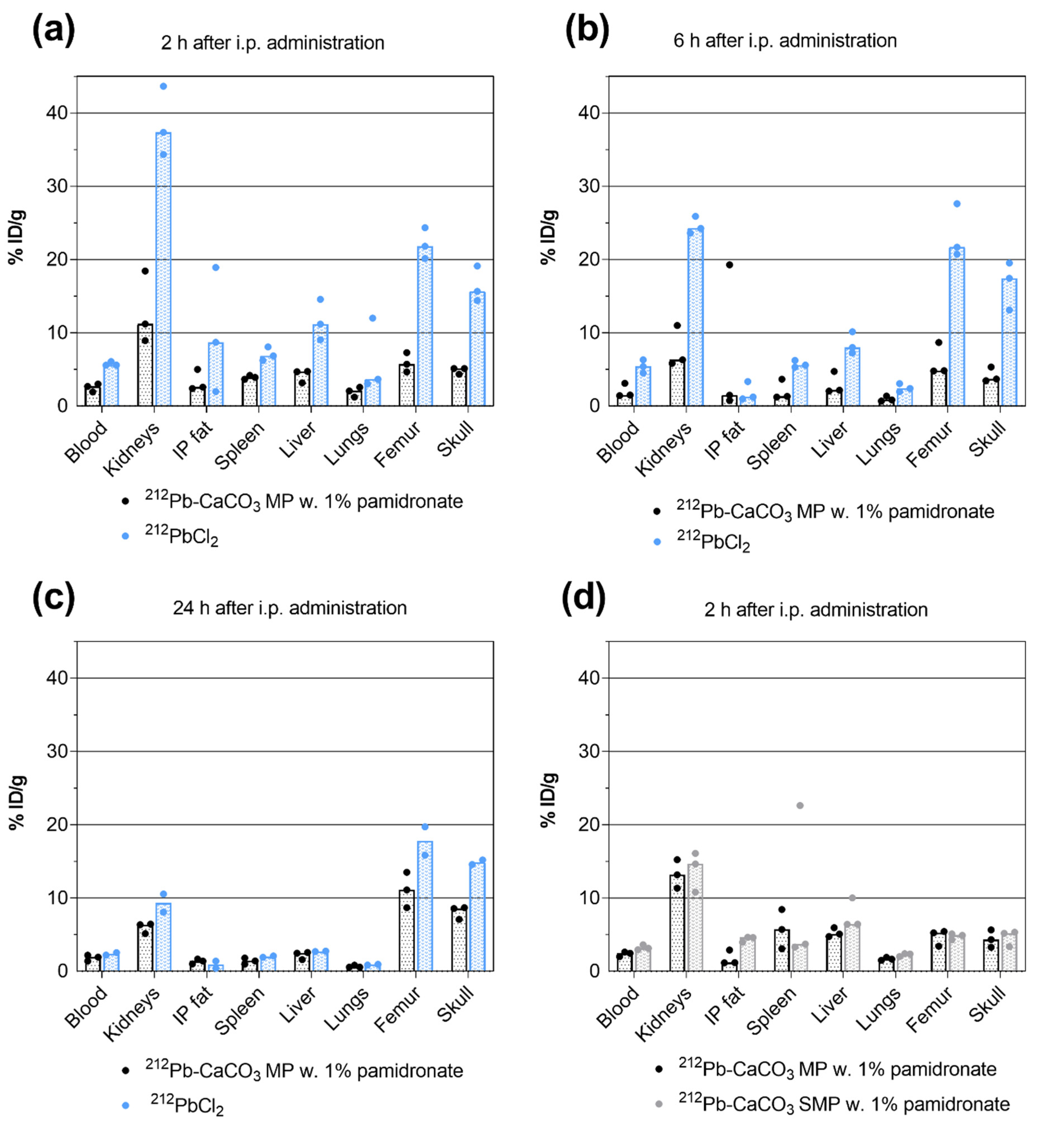
2.6. Biodistribution
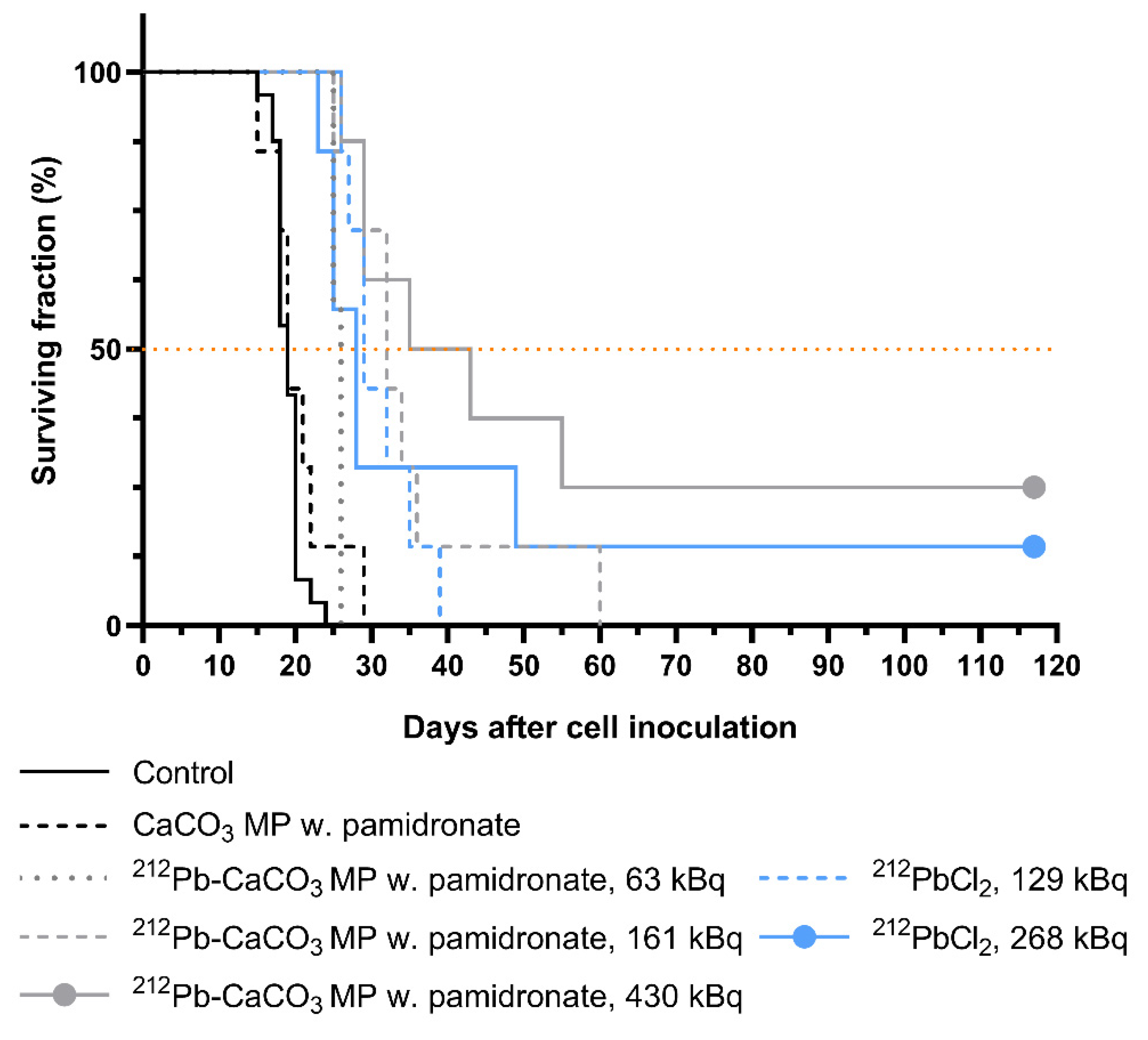
2.7. Therapeutic Efficacy
3. Results
3.1. Stabilization of CaCO3 MPs and SMPs by Pamidronate
3.2. Single-Step-Labeling of 212Pb-CaCO3 Particles: Yield and Stability
3.3. Biodistribution of 212Pb
3.4. Therapeutic Effect of 212Pb-CaCO3 MPs
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sgouros, G.; Bodei, L.; McDevitt, M.R.; Nedrow, J.R. Radiopharmaceutical Therapy in Cancer: Clinical Advances and Challenges. Nature 2020, 19, 589–608. [Google Scholar]
- Nelson, B.J.B.; Andersson, J.D.; Wuest, F. Targeted Alpha Therapy: Progress in Radionuclide Production, Radiochemistry, and Applications. Pharmaceutics 2021, 13, 49. [Google Scholar] [CrossRef] [PubMed]
- Makvandi, M.; Dupis, E.; Engle, J.W.; Nortier, F.M.; Fassbender, M.E.; Simon, S.; Birnbaum, E.R.; Atcher, R.W.; John, K.D.; Rixe, O.; et al. Alpha-Emitters and Targeted Alpha Therapy in Oncology: From Basic Science to Clinical Investigations. Target Oncol. 2018, 13, 189–203. [Google Scholar] [CrossRef] [PubMed]
- Brechbiel, M.W. Bifunctional Chelates for Metal Nuclides. Q. J. Nucl. Med. Mol. Imaging 2008, 52, 166. [Google Scholar] [PubMed]
- Meredith, R.F.; Torgue, J.; Azure, M.T.; Shen, S.; Saddekni, S.; Banaga, E.; Carlise, R.; Bunch, P.; Yoder, D.; Alvarez, R. Pharmacokinetics and Imaging of 212Pb-TCMC-Trastuzumab after Intraperitoneal Administration in Ovarian Cancer Patients. Cancer Biother. Radiopharm. 2014, 29, 12–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baidoo, K.E.; Milenic, D.E.; Brechbiel, M.W. Methodology for Labeling Proteins and Peptides with Lead-212 (212Pb). Nucl. Med. Biol. 2013, 40, 592–599. [Google Scholar] [CrossRef] [Green Version]
- Radchenko, V.; Morgenstern, A.; Jalilian, A.; Ramogida, C.; Cutler, C.S.; Duchemin, C.; Hoehr, C.; Haddad, F.; Bruchertseifer, F.; Gausemel, H.; et al. Production and Supply of Alpha Particles Emitting Radionuclides for Targeted Alpha Therapy (TAT). J. Nucl. Med. 2021, 62, 1495–1503. [Google Scholar] [CrossRef]
- Milenic, D.E.; Baidoo, K.E.; Kim, Y.S.; Brechbiel, M.W. Evaluation of Cetuximab as a Candidate for Targeted α-Particle Radiation Therapy of HER1-Positive Disseminated Intraperitoneal Disease. MAbs 2015, 7, 255–264. [Google Scholar] [CrossRef] [Green Version]
- Milenic, D.E.; Baidoo, K.E.; Kim, Y.S.; Barkley, R.; Brechbiel, M.W. Targeted α-Particle Radiation Therapy of HER1-Positive Disseminated Intraperitoneal Disease: An Investigation of the Human Anti-EGFR Monoclonal Antibody, Panitumumab. Trans. Oncol. 2017, 10, 535–545. [Google Scholar] [CrossRef]
- Milenic, D.E.; Kim, Y.S.; Baidoo, K.E.; Wong, K.J.; Barkley, R.; Delgado, J.; Brechbiel, M.W. Exploration of a F(ab’)2 Fragment as the Targeting Agent of α-Radiation Therapy: A Comparison of the Therapeutic Benefit of Intraperitoneal and Intravenous Administered Radioimmunotherapy. Cancer Biother. Radiopharm. 2018, 33, 182–193. [Google Scholar] [CrossRef]
- Kasten, B.B.; Katre, A.; Kim, H.; Arend, R.; Fan, J.; Ferrone, S.; Zinn, K.R.; Buchsbaum, D. Targeted Radioimmunotherapy with B7-H3-Specific 212Pb-mAb 376.96 in Models of Human Ovarian Cancer. J. Nucl. Med. 2016, 57, 55. [Google Scholar]
- Kasten, B.B.; Arend, R.C.; Katre, A.A.; Kim, H.; Fan, J.; Ferrone, S.; Zinn, K.R.; Buchsbaum, D. B7-H3-Targeted 212Pb Radioimmunotherapy of Ovarian Cancer in Preclinical Models. Nucl. Med. Biol. 2017, 47, 23–30. [Google Scholar] [CrossRef] [Green Version]
- Kasten, B.B.; Gangrade, A.; Kim, H.; Fan, J.; Ferrone, S.; Ferrone, C.R.; Zinn, K.R.; Buchsbaum, D. 212Pb-Labeled B7-H3-Targeting Antibody for Pancreatic Cancer Therapy in Mouse Models. Nucl. Med. Biol. 2018, 58, 67–73. [Google Scholar] [CrossRef]
- Kasten, B.B.; Oliver, P.G.; Kim, H.; Fan, J.; Ferrone, S.; Zinn, K.R.; Buchsbaum, D. 212Pb-Labeled Antibody 225.28 Targeted to Chondroitin Sulfate Proteoglycan 4 for Triple-Negative Breast Cancer Therapy in Mouse Models. Int. J. Mol. Sci. 2018, 19, 925. [Google Scholar] [CrossRef] [Green Version]
- Kasten, B.B.; Fan, J.; Ferrone, S.; Zinn, K.; Buchsbaum, D. Targeted Radioimmunotherapy of Triple Negative Breast Cancer with CSPG4-Specific 212Pb-Labeled Monoclonal Antibody. J. Nucl. Med. 2016, 57, 114. [Google Scholar]
- Maaland, A.F.; Saidi, A.; Torgue, J.; Heyerdahl, H.; Stallons, T.A.R.; Kolstad, A.; Dahle, J. Targeted Alpha Therapy for Chronic Lymphocytic Leukaemia and Non-Hodgkin’s Lymphoma with the Anti-CD37 Radioimmunoconjugate 212Pb-NNV003. PLoS ONE 2020, 15, e0230526. [Google Scholar] [CrossRef] [Green Version]
- Saidi, A.; Maaland, A.; Torgue, J.; Heyerdahl, H.; Dahle, J. Targeted Alpha Therapy with 212Pb-NNV003 for the Treatment of CD37 Positive B-Cell Chronic Lymphocytic Leukemia (CLL) and Non-Hodgkin Lymphoma (NHL). J. Nucl. Med. 2019, 60, 354. [Google Scholar] [CrossRef]
- Quelven, I.; Monteil, J.; Sage, M.; Saidi, A.; Mounier, J.; Bayout, A.; Garrier, J.; Cogne, M.; Durand-Panteix, S. 212Pb α-Radioimmunotherapy Targeting CD38 in Multiple Myeloma: A Preclinical Study. J. Nucl. Med. 2020, 61, 1058–1065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banerjee, S.R.; Minn, I.; Kumar, V.; Josefsson, A.; Lisok, A.; Brummet, M.; Chen, J.; Kiess, A.P.; Baidoo, K.; Brayton, C.; et al. Preclinical Evaluation of 203/212Pb-Labeled Low-Molecular-Weight Compounds for Targeted Radiopharmaceutical Therapy of Prostate Cancer. J. Nucl. Med. 2020, 61, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Stenberg, V.Y.; Juzeniene, A.; Chen, Q.; Yang, X.; Bruland, Ø.S.; Larsen, R.H. Preparation of the Alpha-Emitting Prostate-Specific Membrane Antigen Targeted Radioligand [212Pb] Pb-NG001 for Prostate Cancer. J. Labelled Compd. Radiopharm. 2020, 63, 129–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stenberg, V.Y.; Larsen, R.H.; Ma, L.W.; Peng, Q.; Juzenas, P.; Bruland, Ø.S.; Juzeniene, A. Evaluation of the PSMA-Binding Ligand 212Pb-NG001 in Multicellular Tumour Spheroid and Mouse Models of Prostate Cancer. Int. J. Mol. Sci. 2021, 22, 4815. [Google Scholar] [CrossRef] [PubMed]
- Mirzadeh, S.; Kumar, K.; Gansow, O.A. The Chemical Fate of 212Bi-DOTA Formed by β− Decay of 212Pb(DOTA)2−. Radiochim. Acta 1993, 60, 1–10. [Google Scholar] [CrossRef]
- Westrøm, S.; Generalov, R.; Bønsdorff, T.B.; Larsen, R.H. Preparation of 212Pb-Labeled Monoclonal Antibody Using a Novel ²²⁴Ra-Based Generator Solution. Nucl. Med. Biol. 2017, 51, 1–9. [Google Scholar] [CrossRef]
- Majkowska-Pilip, A.; Gawęda, W.; Żelechowska-Matysiak, K.; Wawrowicz, K.; Bilewicz, A. Nanoparticles in Targeted Alpha Therapy. Nanomaterials 2020, 10, 1366. [Google Scholar] [CrossRef] [PubMed]
- de Kruijff, R.M.; Wolterbeek, H.T.; Denkova, A.G. A Critical Review of Alpha Radionuclide Therapy—How to Deal with Recoiling Daughters? Pharmaceuticals 2015, 8, 321–336. [Google Scholar] [CrossRef]
- Leggett, R.W. An Age-Specific Kinetic Model of Lead Metabolism in Humans. Environ. Health Perspect. 1993, 101, 598–616. [Google Scholar] [CrossRef]
- Yong, K.; Brechbiel, M.W. Towards Translation of ²¹²Pb as a Clinical Therapeutic; Getting the Lead In! Dalton Trans. 2011, 40, 6068–6076. [Google Scholar] [CrossRef]
- Henriksen, G.; Schoultz, B.W.; Hoff, P.; Larsen, R.H. Potential In Vivo Generator for Alpha-Particle Therapy with 212Bi: Presentation of a System to Minimize Escape of Daughter Nuclide after Decay of 212Pb to 212Bi. Radiochim. Acta 2003, 91, 109–114. [Google Scholar] [CrossRef]
- Du, A.L.; Mougin-Degraef, M.; Botosoa, E.; Rauscher, A.; Chauvet, A.F.; Barbet, J.; Montavon, G. In Vivo 212Pb/212Bi Generator Using Indium-DTPA-Tagged Liposomes. Radiochim. Acta 2011, 99, 743–749. [Google Scholar]
- Diener, M.D.; Alford, J.M.; Kennel, S.J.; Mirzadeh, S. 212Pb@C60 and its Water-Soluble Derivatives: Synthesis, Stability, and Suitability for Radioimmunotherapy. J. Am. Chem. Soc. 2007, 129, 5131–5138. [Google Scholar] [CrossRef]
- Rosenow, M.K.; Zucchini, G.L.; Bridwell, P.M.; Stuart, F.P.; Friedman, A.M. Properties of Liposomes Containing 212Pb. Int. J. Nucl. Med. Biol. 1983, 10, 189–197. [Google Scholar] [CrossRef]
- Rotmensch, J.; Atcher, R.W.; Schlenker, R.; Hines, J.; Grdina, D.; Block, B.S.; Press, M.D.; Herbst, A.L.; Weichelsbaum, R.R. The Effect of the α-Emitting Radionuclide Lead-212 on Human Ovarian Carcinoma: A Potential New Form of Therapy. Gynecol. Oncol. 1989, 32, 236–239. [Google Scholar] [CrossRef]
- Rotmensch, J.; Atcher, R.W.; Hines, J.; Grdina, D.; Schwartz, J.S.; Toohill, M.; Herbst, A.L. The Development of α-Emitting Radionuclide Lead 212 for the Potential Treatment of Ovarian Carcinoma. Am. J. Obstet. 1989, 160, 789–797. [Google Scholar] [CrossRef]
- Westrøm, S.; Malenge, M.; Jorstad, I.S.; Napoli, E.; Bruland, Ø.S.; Bønsdorff, T.B.; Larsen, R.H. Ra-224 Labeling of Calcium Carbonate Microparticles for Internal α-Therapy: Preparation, Stability, and Biodistribution in Mice. J. Label. Comp. Radiopharm. 2018, 61, 472–486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Westrøm, S.; Bønsdorff, T.B.; Bruland, Ø.S.; Larsen, R.H. Therapeutic Effect of α-Emitting 224Ra-Labeled Calcium Carbonate Microparticles in Mice with Intraperitoneal Ovarian Cancer. Trans. Oncol. 2018, 11, 259–267. [Google Scholar] [CrossRef]
- Napoli, E.; Bønsdorff, T.B.; Jorstad, I.S.; Bruland, Ø.S.; Larsen, R.H.; Westrøm, S. Radon-220 Diffusion from 224Ra-Labeled Calcium Carbonate Microparticles: Some Implications for Radiotherapeutic Use. PLoS ONE 2021, 16, e0248133. [Google Scholar] [CrossRef]
- Li, R.G.; Napoli, E.; Jorstad, I.S.; Bønsdorff, T.B.; Juzeniene, A.; Bruland, Ø.S.; Larsen, R.H.; Westrøm, S. Calcium Carbonate Microparticles as Carriers of 224Ra: Impact of Specific Activity in Mice with Intraperitoneal Ovarian Cancer. Curr. Radiopharm. 2021, 14, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Li, R.G.; Lindland, K.; Tonstad, S.K.; Bønsdorff, T.B.; Juzeniene, A.; Westrøm, S.; Larsen, R.H. Improved Formulation of 224Ra-Labeled Calcium Carbonate Microparticles by Surface Layer Encapsulation and Addition of EDTMP. Pharmaceutics 2021, 13, 634. [Google Scholar] [CrossRef]
- US National Library of Medicine ClinicalTrials.gov. Study of Radspherin® in Recurrent Ovarian Cancer Subjects with Peritoneal Carcinomatosis. Identifier NCT03732768. Available online: https://clinicaltrials.gov/ct2/show/NCT03732768 (accessed on 13 October 2021).
- US National Library of Medicine ClinicalTrials.gov. Study of Radspherin® in Colorectal Carcinoma Subjects with Peritoneal Carcinomatosis Treated with HIPEC. Identifier NCT03732781. Available online: https://clinicaltrials.gov/ct2/show/NCT03732781 (accessed on 13 October 2021).
- Trushina, D.B.; Sulyanov, S.N.; Bukreeva, T.V.; Kovalchuk, M.V. Size Control and Structure Features of Spherical Calcium Carbonate Particles. Crystallogr. Rep. 2015, 60, 570–577. [Google Scholar] [CrossRef]
- Trushina, D.B.; Bukreeva, T.V.; Antipina, M.N. Size-Controlled Synthesis of Vaterite Calcium Carbonate by the Mixing Method: Aiming for Nanosized Particles. Cryst. Growth Des. 2016, 16, 1311–1319. [Google Scholar] [CrossRef]
- Hassfjell, S.P.; Hoff, P.; Bruland, Ø.S.; Alstad, J. 212Pb/212Bi-EDTMP—Synthesis and Biodistribution of a Novel Bone Seeking Alpha-Emitting Radiopharmaceutical. J. Label. Comp. Radiopharm. 1994, 34, 717–734. [Google Scholar] [CrossRef]
- Juzeniene, A.; Bernoulli, J.; Suominen, M.; Halleen, J.; Larsen, R.H. Antitumor Activity of Novel Bone-Seeking, α-Emitting 224Ra-Solution in a Breast Cancer Skeletal Metastases Model. Anticancer Res. 2018, 38, 1947–1955. [Google Scholar]
- Napoli, E.; Stenberg, V.Y.; Juzeniene, A.; Hjellum, G.E.; Bruland, Ø.S.; Larsen, R.H. Calibration of Sodium Iodide Detectors and Reentrant Ionization Chambers for 212Pb Activity in Different Geometries by HPGe Activity Determined Samples. Appl. Radiat. Isot. 2020, 166, 109362. [Google Scholar] [CrossRef] [PubMed]
- Vikulina, A.; Voronin, D.; Fakhrullin, R.; Vinokurov, V.; Volodkin, D. Naturally Derived Nano-and Micro-Drug Delivery Vehicles: Halloysite, Vaterite and Nanocellulose. New J. Chem. 2020, 44, 5638–5655. [Google Scholar] [CrossRef] [Green Version]
- Boudousq, V.; Bobyk, L.; Busson, M.; Garambois, V.; Jarlier, M.; Charalambatou, P.; Pèlegrin, A.; Paillas, S.; Chouin, N.; Quenet, F.; et al. Comparison between Internalizing Anti-HER2 mAbs and Non-Internalizing Anti-CEA mAbs in Alpha-Radioimmunotherapy of Small Volume Peritoneal Carcinomatosis Using 212Pb. PLoS ONE 2013, 8, e69613. [Google Scholar] [CrossRef] [Green Version]
- Muslimov, A.R.; Antuganov, D.O.; Tarakanchikova, Y.V.; Zhukov, M.V.; Nadporojskii, M.A.; Zyuzin, M.V.; Timin, A.S. Calcium Carbonate Core–Shell Particles for Incorporation of 225Ac and Their Application in Local α-Radionuclide Therapy. ACS Appl. Mater. Interfaces 2021, 13, 25599–25610. [Google Scholar] [CrossRef] [PubMed]
- Muslimov, A.R.; Antuganov, D.; Tarakanchikova, Y.V.; Karpov, T.E.; Zhukov, M.V.; Zyuzin, M.V.; Timin, A.S. An Investigation of Calcium Carbonate Core-Shell Particles for Incorporation of 225Ac and Sequester of Daughter Radionuclides: In Vitro and In Vivo Studies. J. Control. Release 2021, 330, 726–737. [Google Scholar] [CrossRef]
- Zyuzin, M.V.; Antuganov, D.; Tarakanchikova, Y.V.; Karpov, T.E.; Mashel, T.V.; Gerasimova, E.N.; Peltek, O.O.; Alexandre, N.; Bruyere, S.; Kondratenko, Y.A.; et al. Radiolabeling Strategies of Micron- and Submicron-Sized Core–Shell Carriers for In Vivo Studies. ACS Appl. Mater. Interfaces 2020, 12, 31137–31147. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, R.G.; Lindland, K.; Bønsdorff, T.B.; Westrøm, S.; Larsen, R.H. A Novel Single-Step-Labeled 212Pb-CaCO3 Microparticle for Internal Alpha Therapy: Preparation, Stability, and Preclinical Data from Mice. Materials 2021, 14, 7130. https://doi.org/10.3390/ma14237130
Li RG, Lindland K, Bønsdorff TB, Westrøm S, Larsen RH. A Novel Single-Step-Labeled 212Pb-CaCO3 Microparticle for Internal Alpha Therapy: Preparation, Stability, and Preclinical Data from Mice. Materials. 2021; 14(23):7130. https://doi.org/10.3390/ma14237130
Chicago/Turabian StyleLi, Ruth Gong, Kim Lindland, Tina Bjørnlund Bønsdorff, Sara Westrøm, and Roy Hartvig Larsen. 2021. "A Novel Single-Step-Labeled 212Pb-CaCO3 Microparticle for Internal Alpha Therapy: Preparation, Stability, and Preclinical Data from Mice" Materials 14, no. 23: 7130. https://doi.org/10.3390/ma14237130
APA StyleLi, R. G., Lindland, K., Bønsdorff, T. B., Westrøm, S., & Larsen, R. H. (2021). A Novel Single-Step-Labeled 212Pb-CaCO3 Microparticle for Internal Alpha Therapy: Preparation, Stability, and Preclinical Data from Mice. Materials, 14(23), 7130. https://doi.org/10.3390/ma14237130