Evolution of Chemical Composition and Modeling of Growth Nonmetallic Inclusions in Steel Containing Yttrium
Abstract
:1. Introduction
2. Materials and Methods
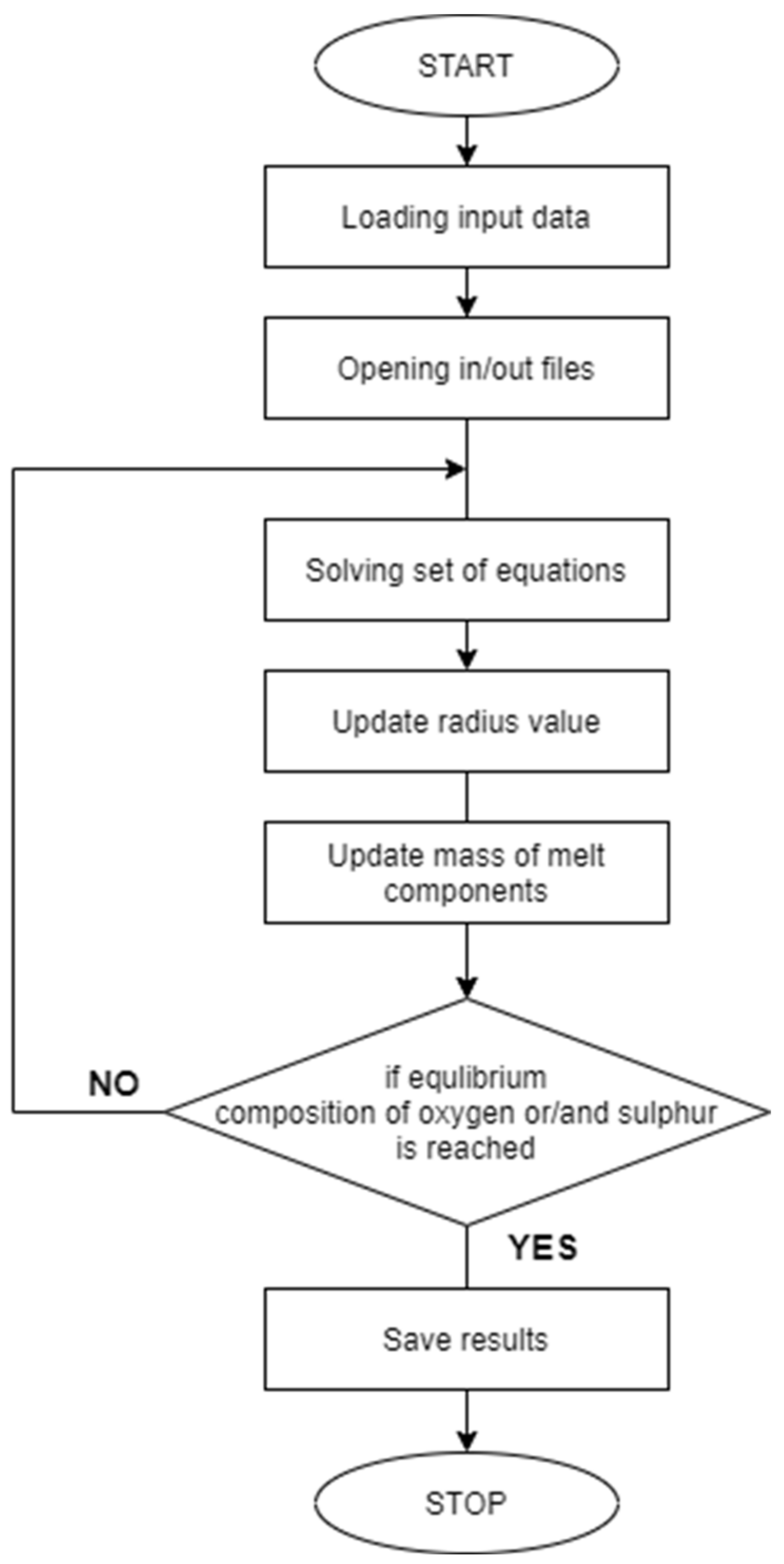
2.1. Calculation of Non-Metallic Phase Formation with Liquid Steel in Thermodynamic Equilibrium Conditions (WYK_STAL)
2.2. Bi-Component Nucleus Growth Model (Bi-Growth)
3. Simulation Results
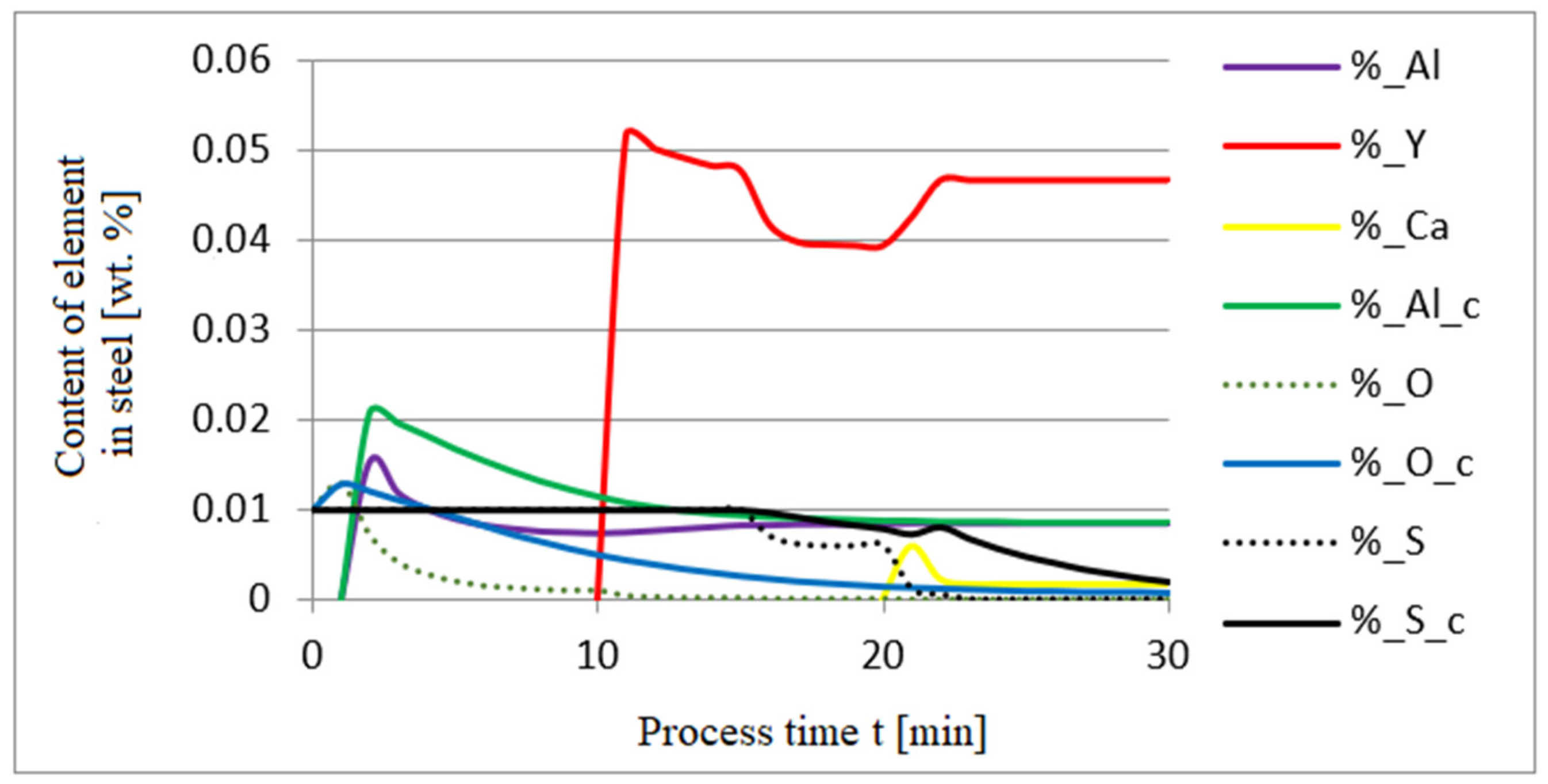
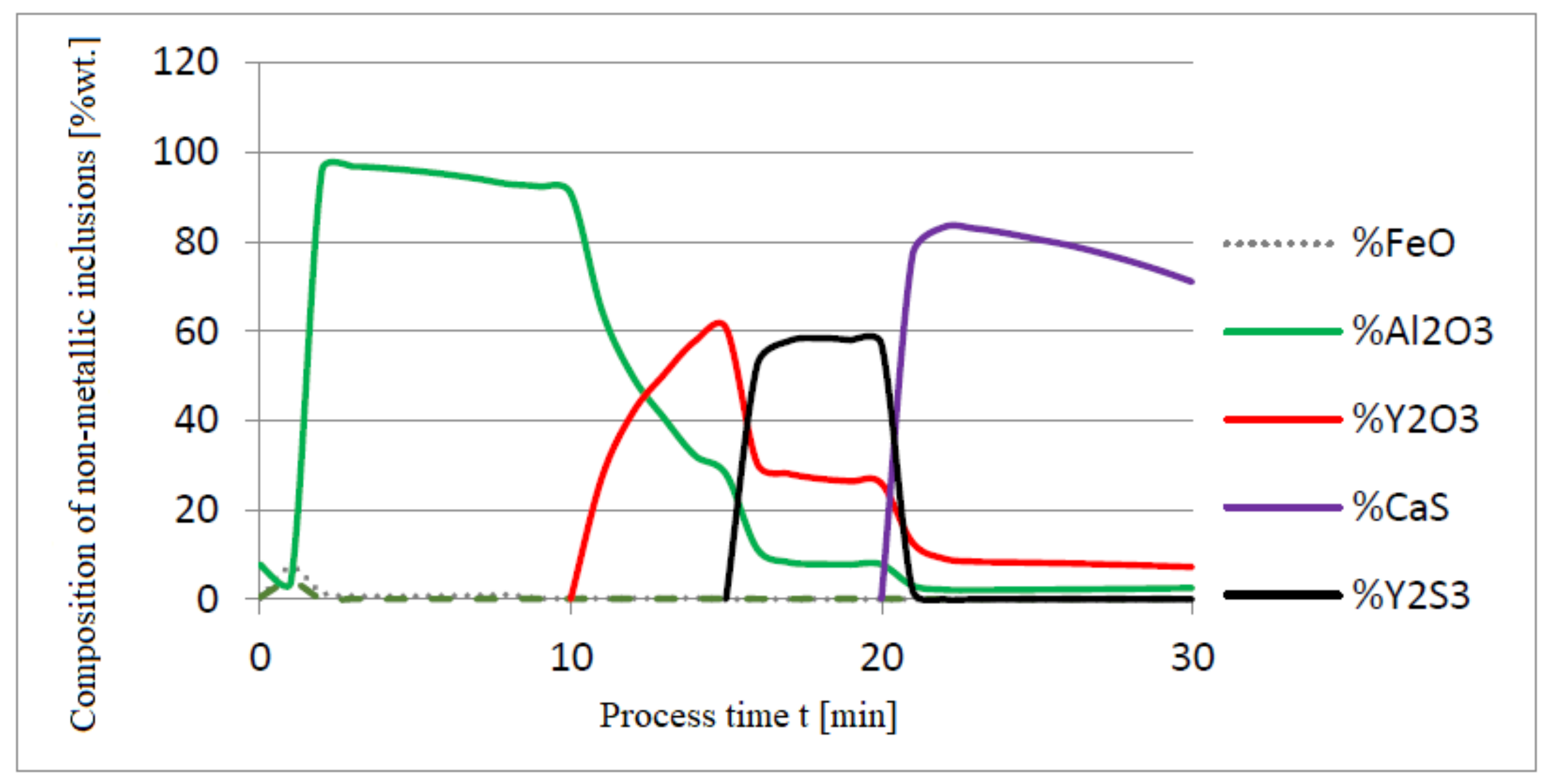
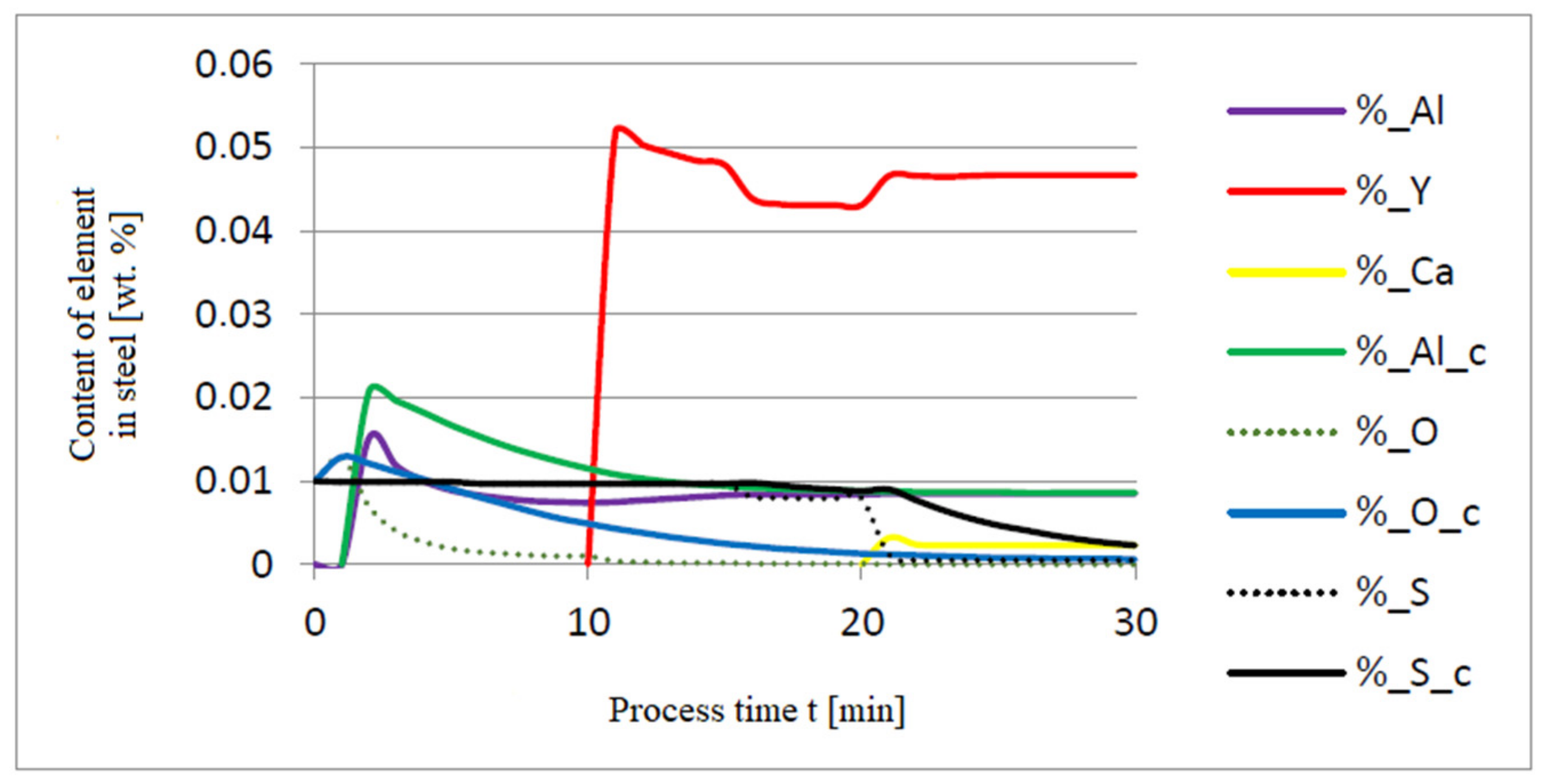
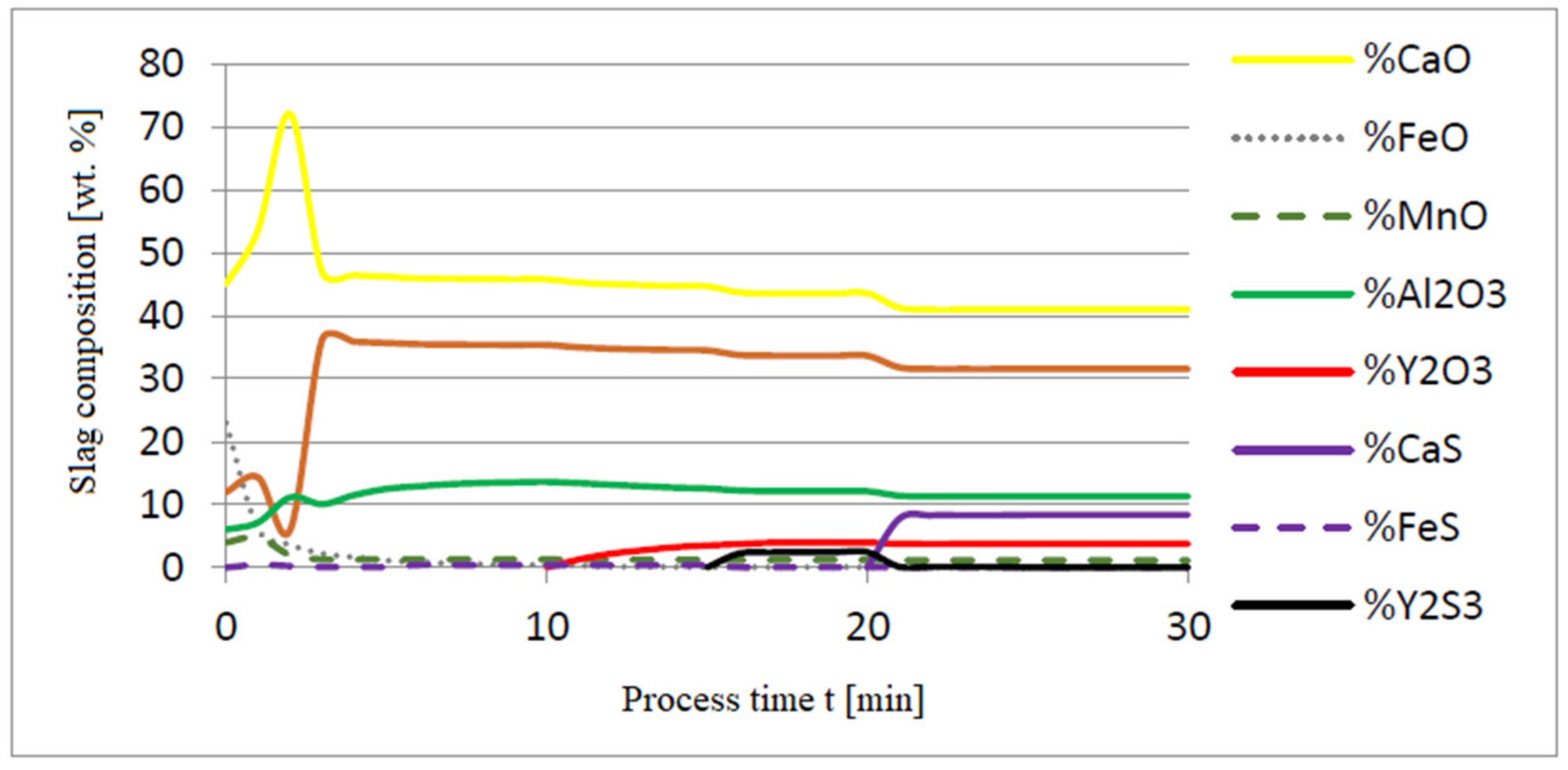
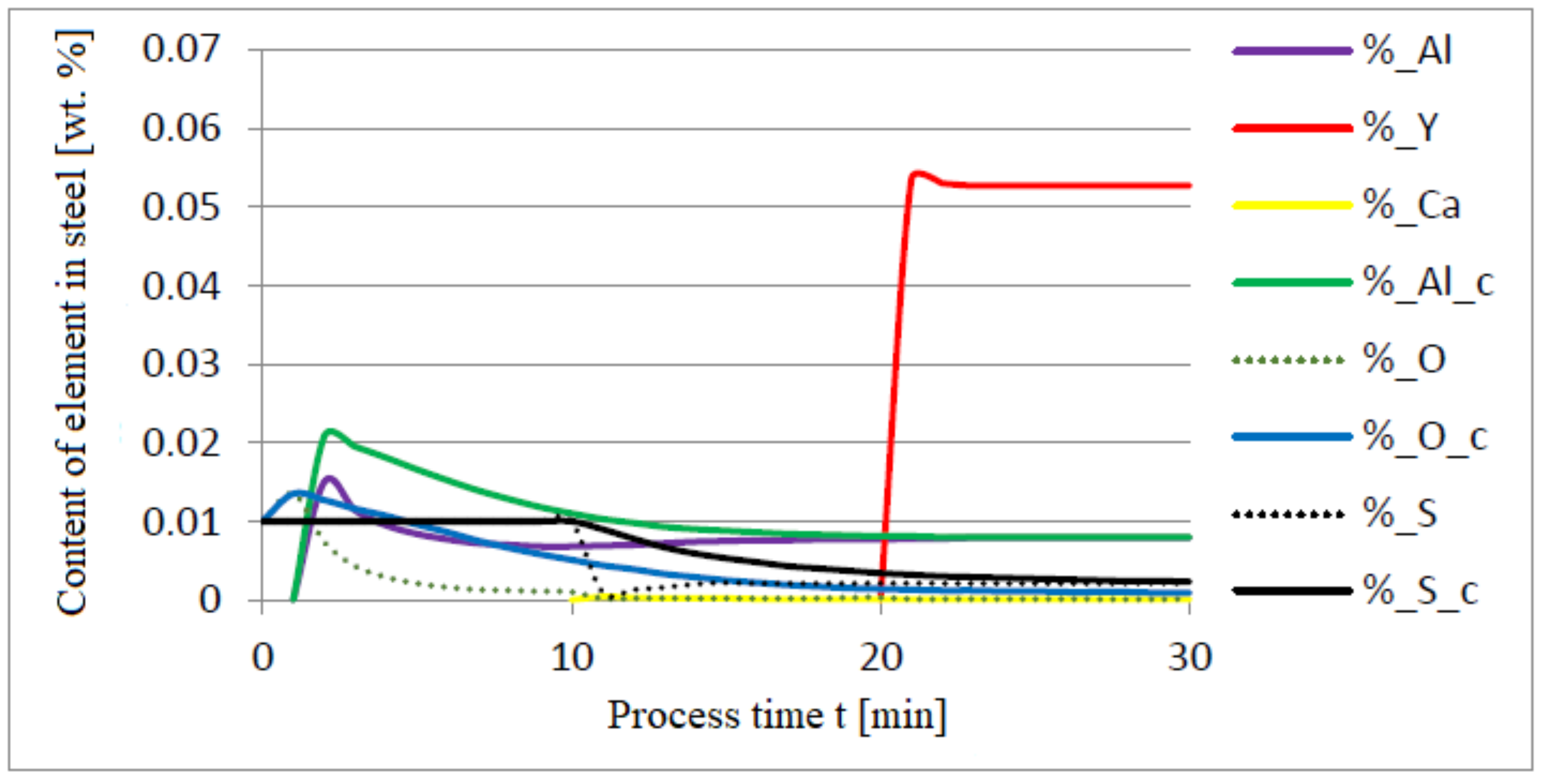
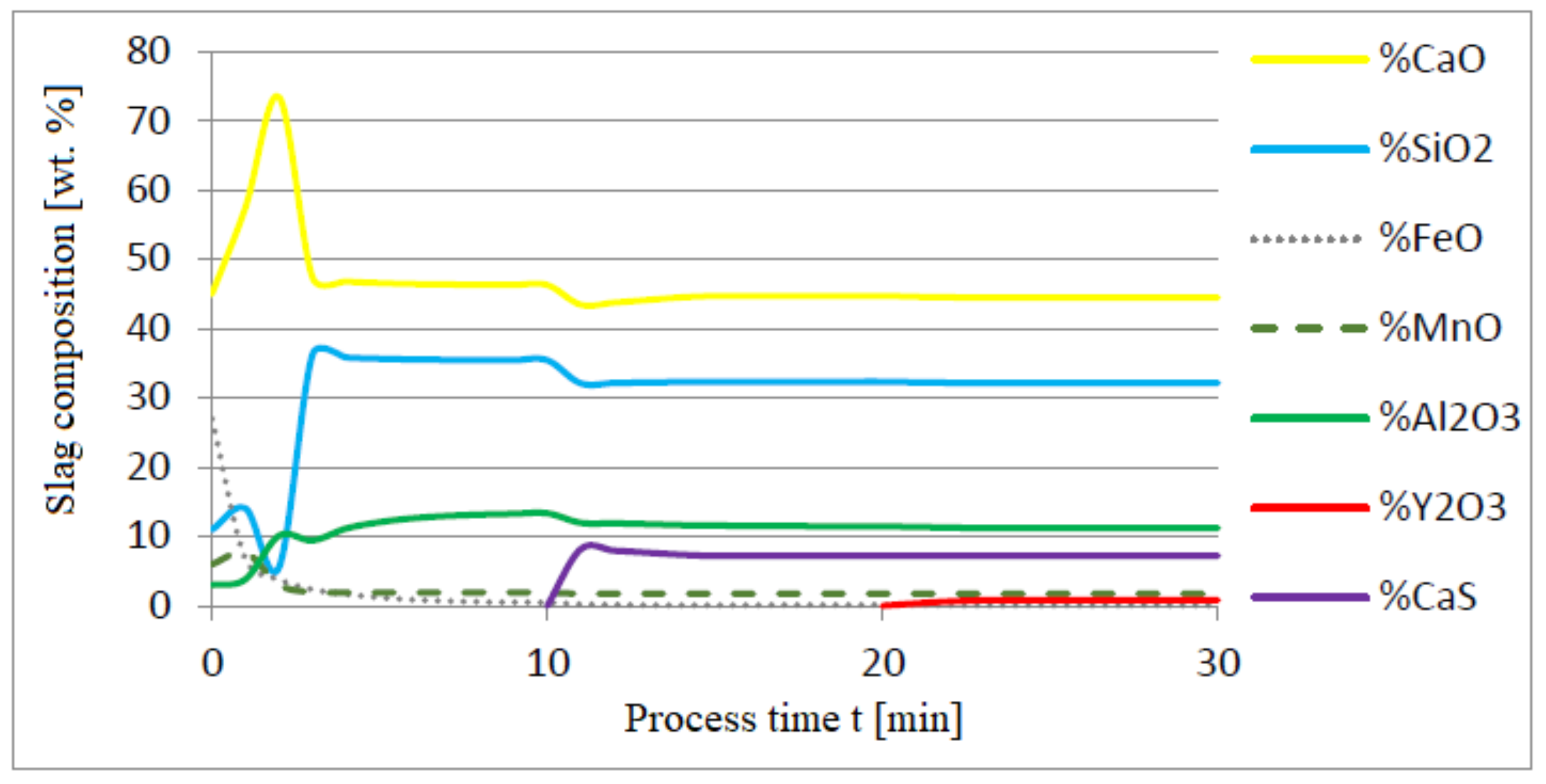
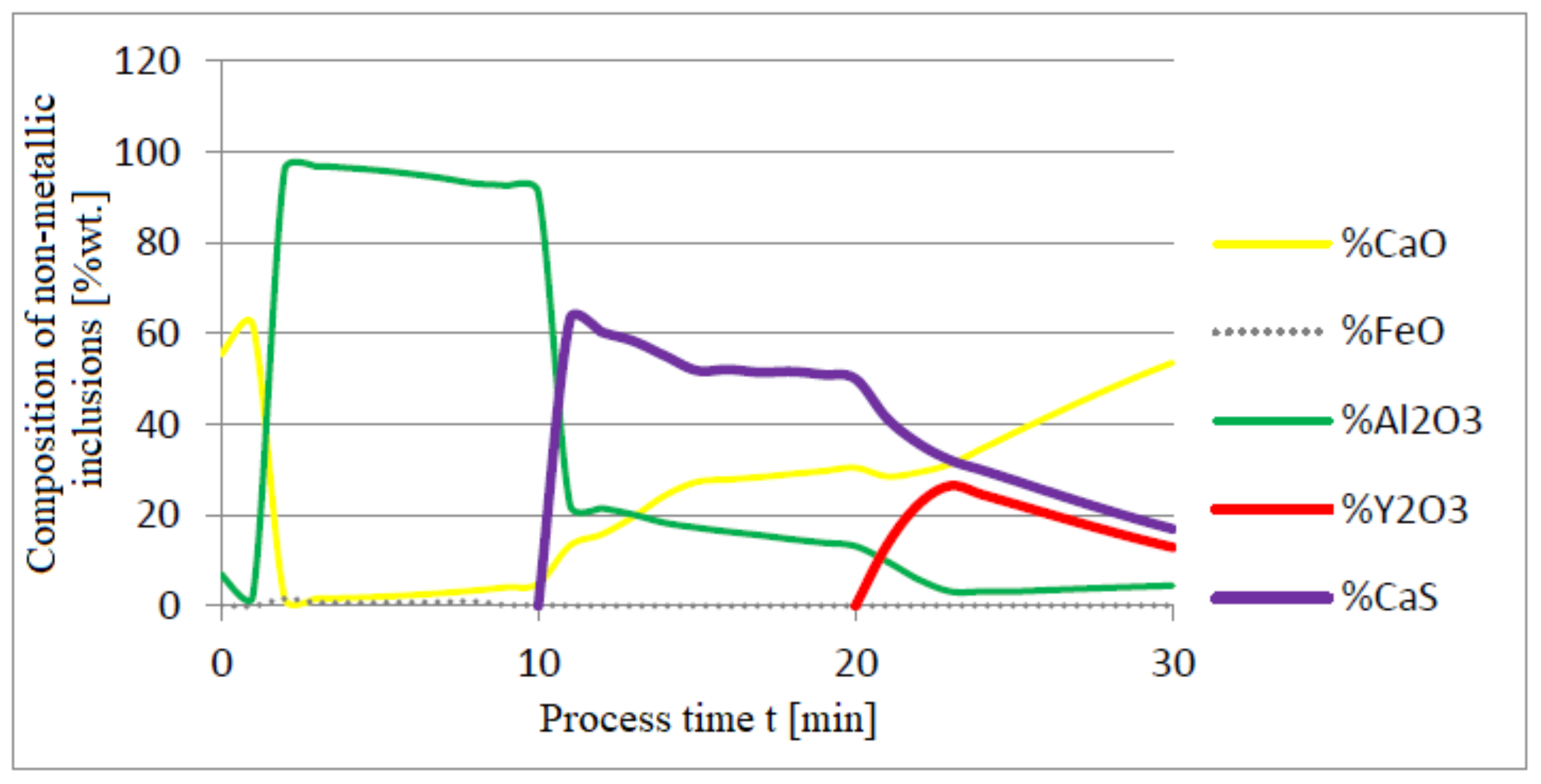
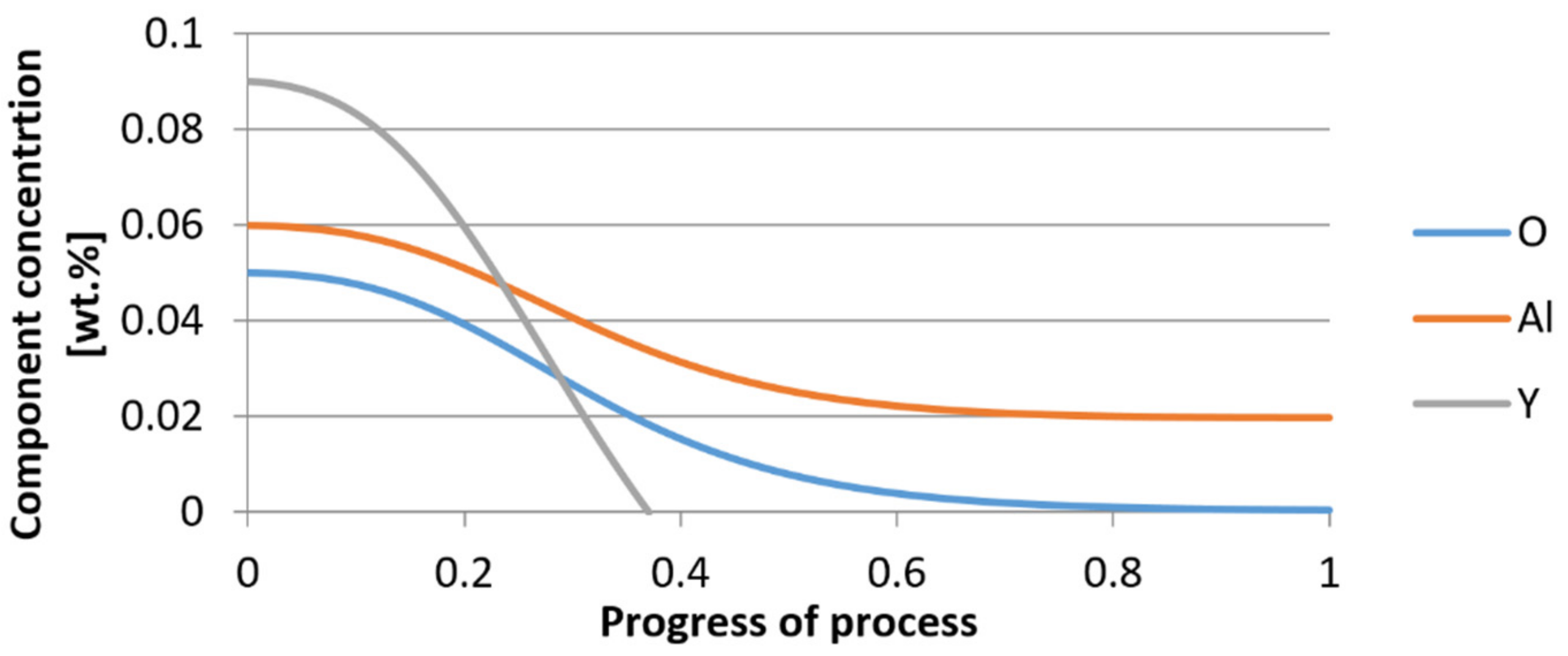
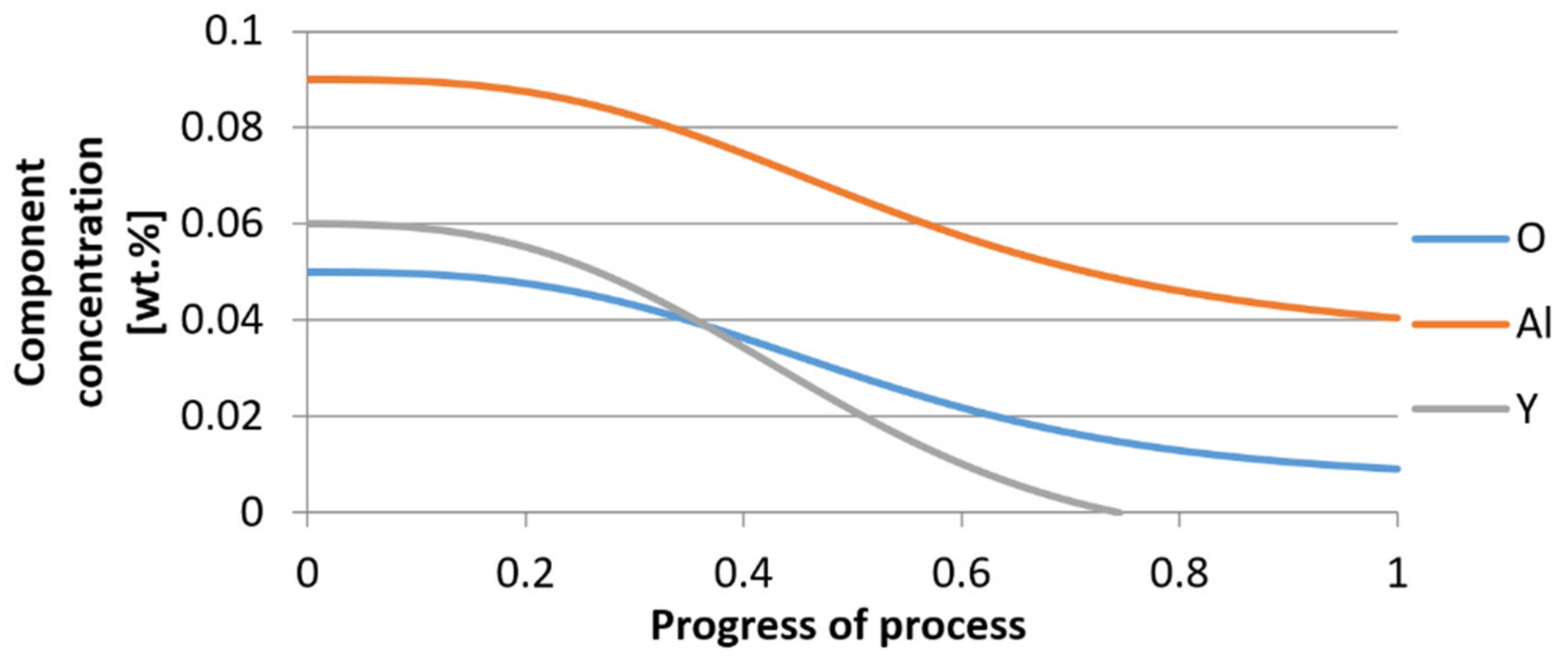
3.1. Simulation Results Obtained with the Computer Program WYK_STAL
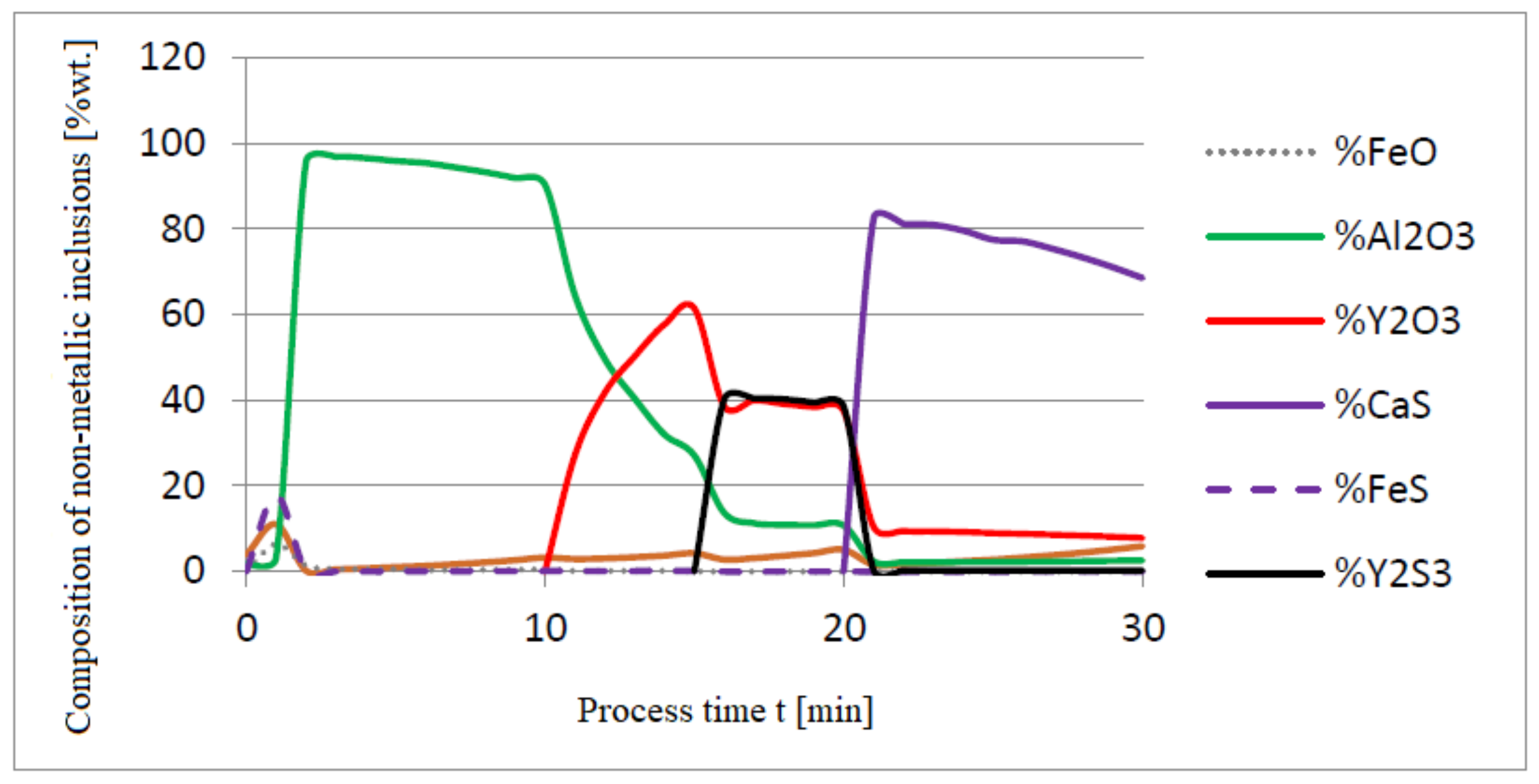
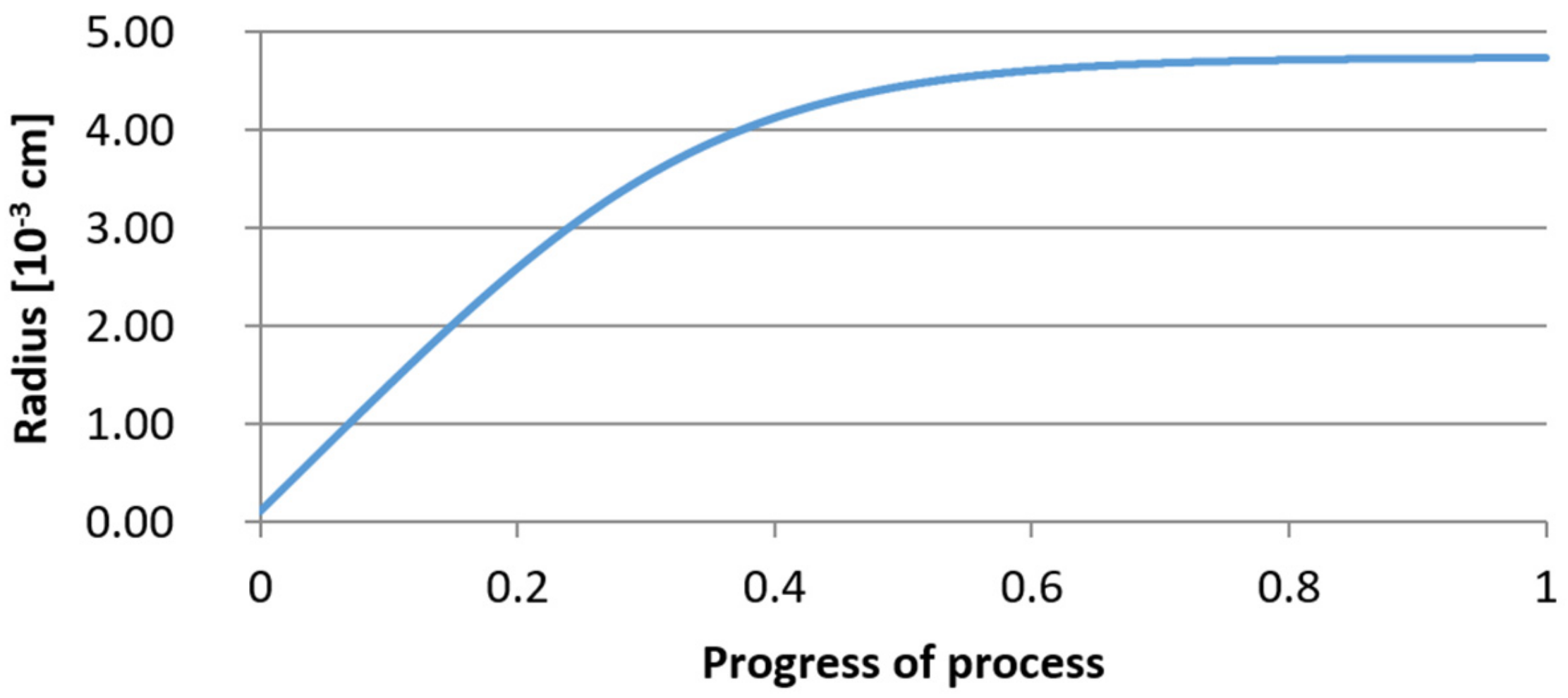
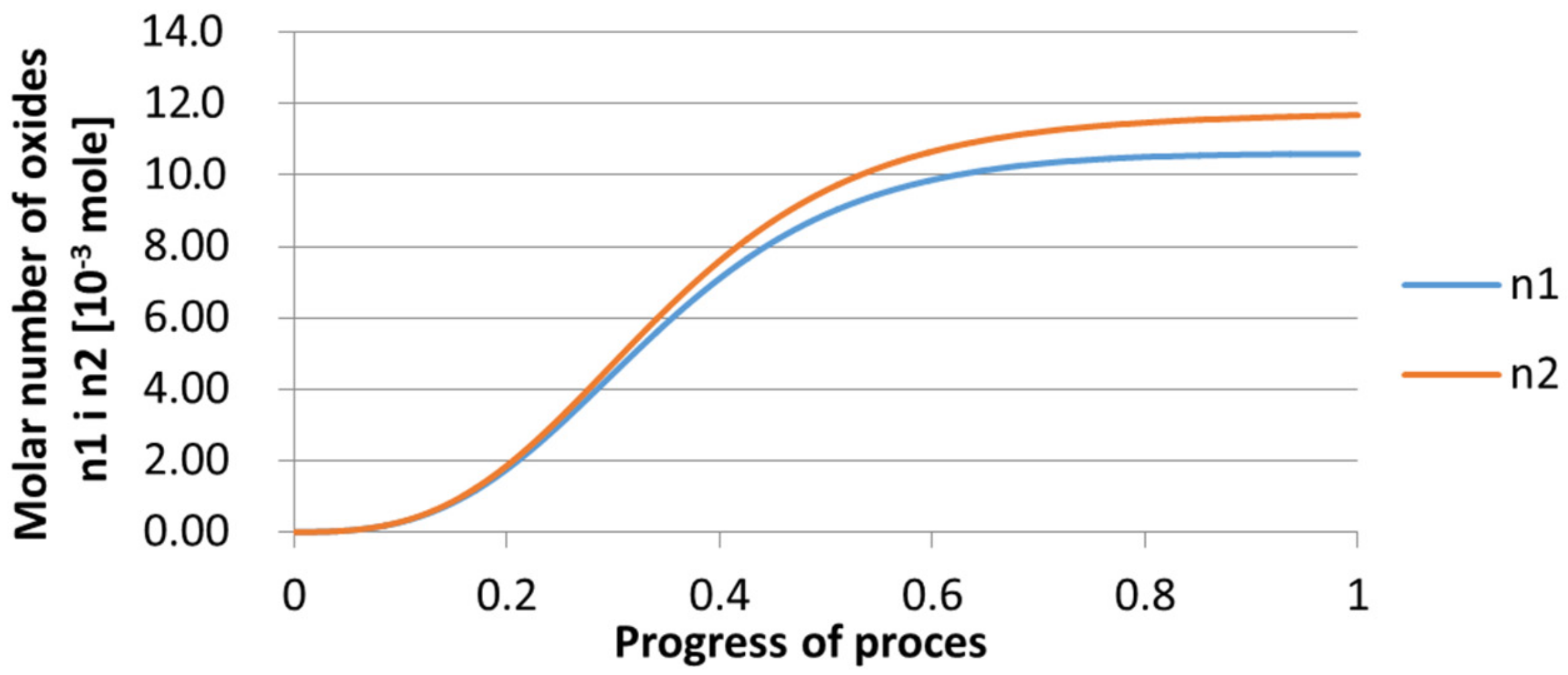
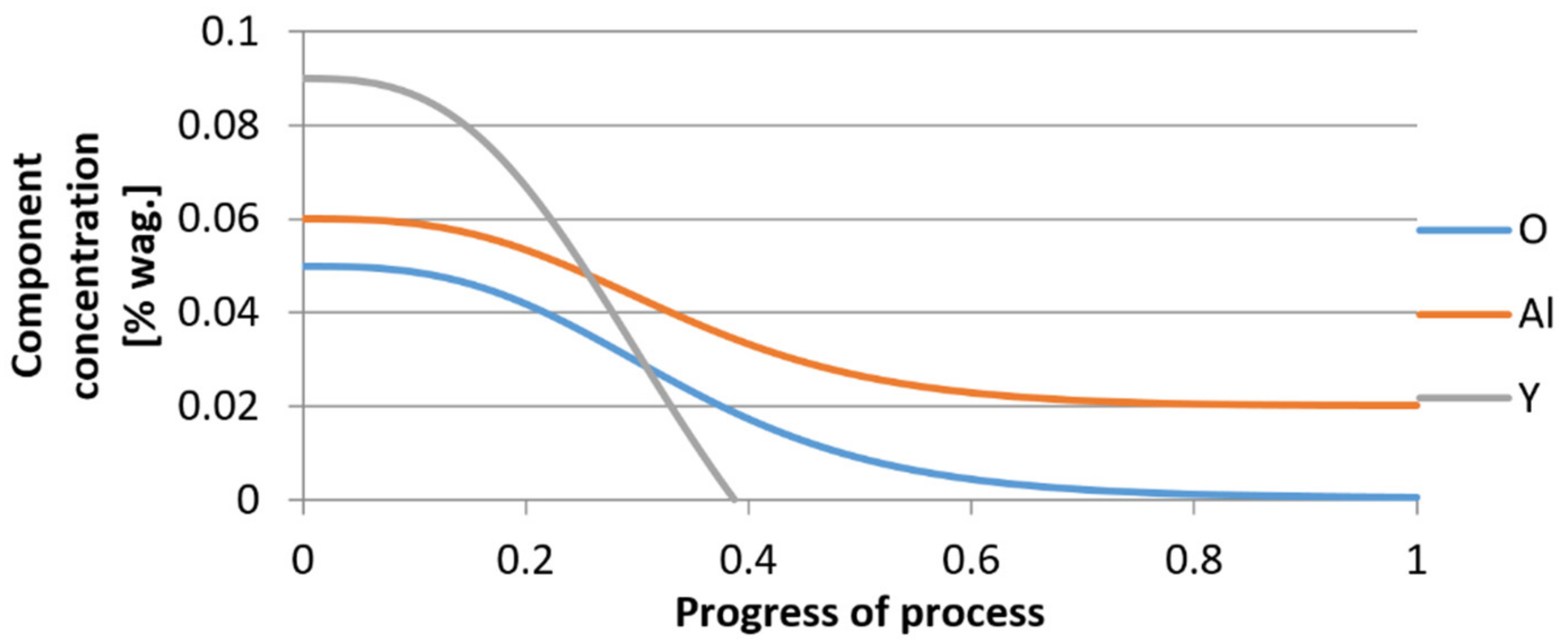
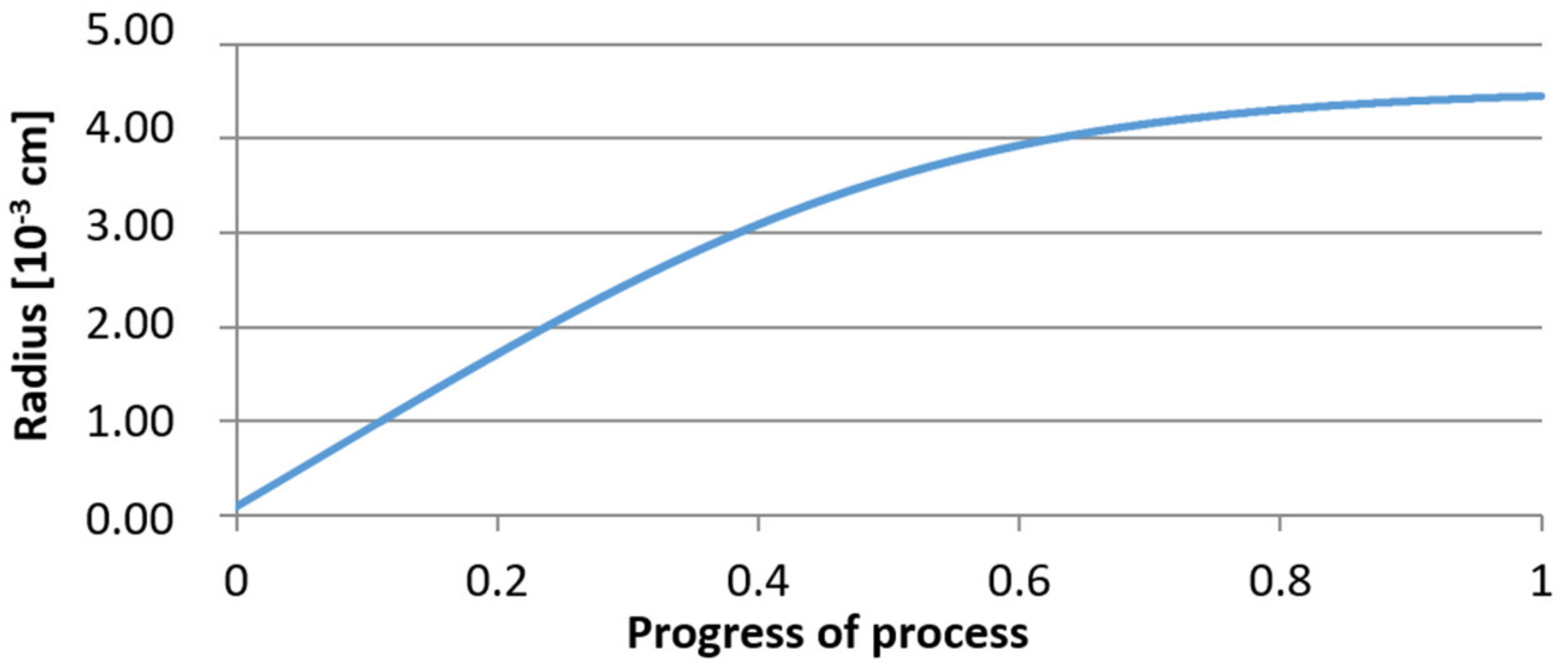
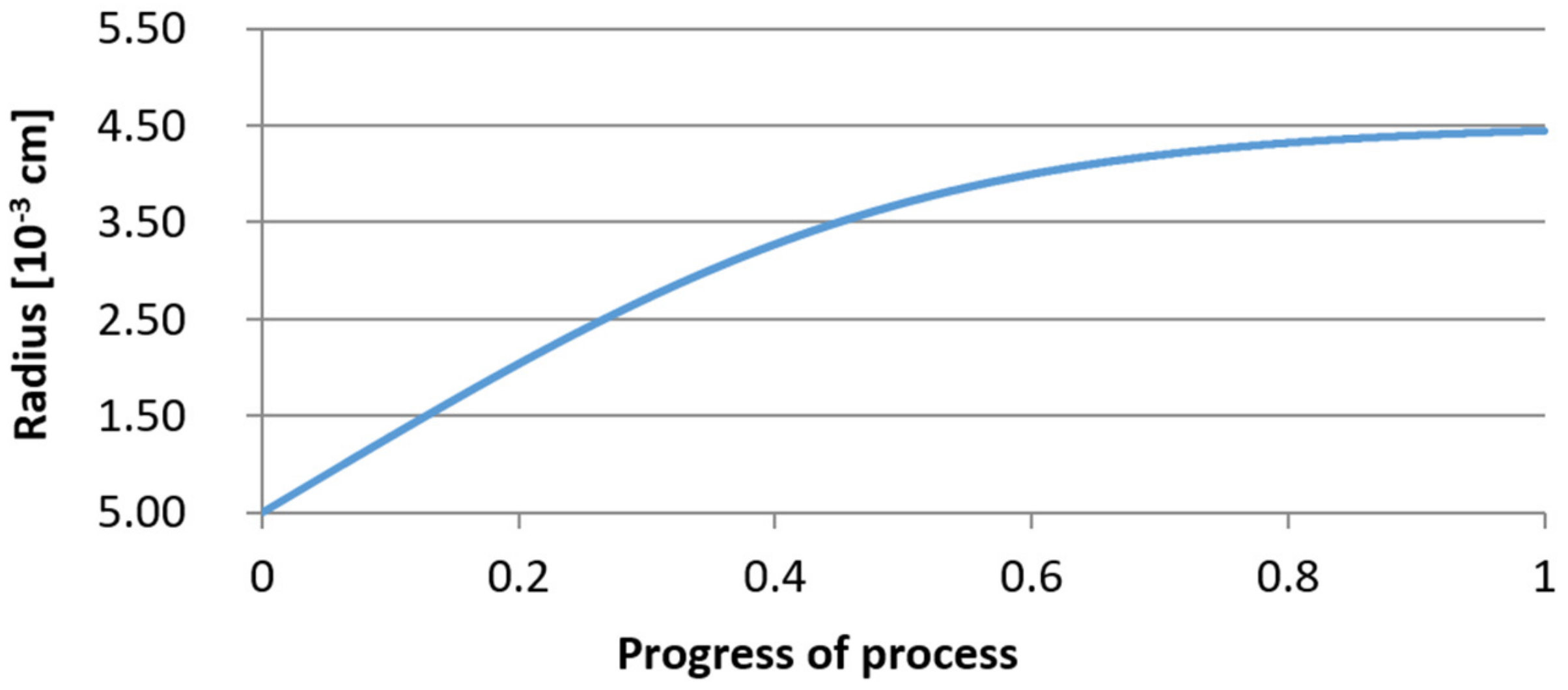
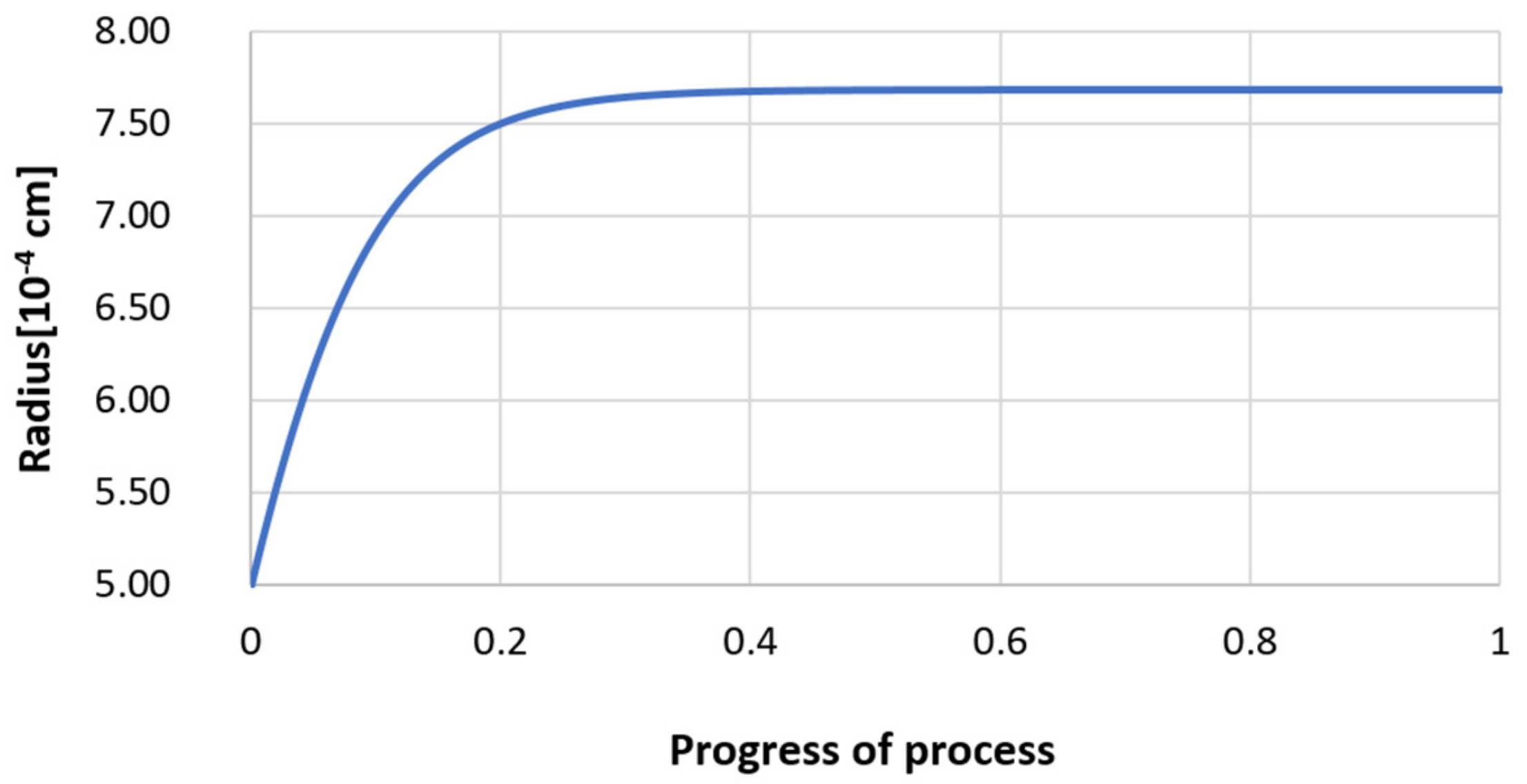
3.2. Results of Calculations of the Growth of Bi-Component Non-Metallic Inclusions in Liquid Steel
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ohta, H.; Suito, H. Activities in MnO−SiO2−Al2O3 slags and deoxidation equilibria of Mn and Si. Met. Mater. Trans. B 1996, 27, 263–270. [Google Scholar] [CrossRef]
- Tanahashi, M.; Furuta, N.; Yamauchi, C.; Fujisawa, T. Phase equilibria of the MnO−SiO2– CrOx system at 1873 K under controlled oxygen partial pressure. ISIJ Int. 2001, 41, 1309–1315. [Google Scholar] [CrossRef]
- Liu, C.D.; Bassim, M.N.; Stlawrence, S. Evaluation of fatigue crack growth initiation at inclusions in fully pearlitic steels. Mater. Sci. Eng. A. 1993, 167, 107–113. [Google Scholar] [CrossRef]
- Lis, T. Metalurgia stali o Wysokiej Czystości; Wyd. Politechnika Śląska: Gliwice, Poland, 2009. [Google Scholar]
- Lis, T.; Nowacki, K.; Kania, H. Poprawa czystości stali metodami metalurgii pozapiecowej. Hut. Wiad. Hut. 2001, 10, 356–361. [Google Scholar]
- Lis, T. Zmniejszenie zanieczyszczenia stali wtrąceniami niemetalicznymi obróbką pozapiecową. Hut. Wiad. Hut. 1999, 2, 54–61. [Google Scholar]
- Kalicka, Z. Rola Układów Dyspersyjnych w Opisie Rafinacji Ciekłej Stali; Uczelniane Wydawnictwo Naukowo−Dydaktyczne: Kraków, Poland, 1998. [Google Scholar]
- Lamut, J.; Falkus, J.; Jurjevec, B.; Knap, M. Influence of inclusions modification on nozzle clogging. Arch. Met. Mat. 2012, 57, 319–324. [Google Scholar] [CrossRef]
- Lu, D.Z.; Irons, G.A.; Lu, W.K. Kinetics and Mechanisms of calcium absorption and inclusion modification of steel. In Proceedings of the Scaninject VI, Lulea, Sweden, 2−4 June 1992. [Google Scholar]
- Holappa, L.; Ylonene, H. Effect of sulphur on non−metallic inclusion in Ca−treated, Al−killed steel. Steelmak. Proc. 1986, 69, 277–283. [Google Scholar]
- Tahtinen, K.; Vainola, R.; Sandholm, R. Ladle injection—A way to continuously cast aluminium killed steels fo billets at Ovako. In Proceedings of the Scaninject II, International Conference on Injection Metallurgy, Lulea, Sweden, 12–13 June 1980. [Google Scholar]
- Turkdogan, E.T. Fundamentals of Steelmaking; The Institute of Materials, The University of Cambridge: Cambridge, UK, 1996. [Google Scholar]
- Holappa, L.E.K. Ladle injection metallurgy. Int. Met. Rev. 1982, 27, 53–76. [Google Scholar] [CrossRef]
- Chodhary, S.K.; Gosh, A. Thermodynamic evaluation of formation of oxide—Sulphide duplex inclusions in steel. ISIJ Int. 2008, 8, 1552–1559. [Google Scholar]
- Smirnov, L.A.; Rovnushkin, V.A.; Oryshchenko, A.S.; Kalinin, G.Y. Modification of steel and alloys with rare‐earth elements, Part 1. Metallurgy 2015, 11, 57–63. [Google Scholar]
- Wilson, W.G.; Heaslip, L.J.; Sommerville, I.D. Rare Earth additions in continuosly cast iron. J. Miner. Met. Mater. Soc. 1985, 37, 36–41. [Google Scholar] [CrossRef]
- Nunes, F.C.; Dille, J.; Delplancke, J.L.; de Almedia, L.H. Yttrium addition to heat‐resistant cast stainless steel. Scr. Mater. 2006, 54, 1553–1556. [Google Scholar] [CrossRef]
- Fujikawa, H.; Morimoto, T.; Nishiyama, Y.; Newcomb, S.B. The effects of small additions of yttrium on the high temperature oxidation resistance of a Si‐containing austenic stainless steel. Oxid. Met. 2003, 59, 23–40. [Google Scholar] [CrossRef]
- Mingya, W.; Songmei, H.; Feifei, S.; Yan, W. Influence of rare earth elements microstructure and mechanical properties of cast high‐speed steel rolls. J. Rare Earth 2007, 25, 490–494. [Google Scholar]
- Yamshinsky, M.M. New materials and technologies in mechanical engineering. In Proceedings of the 9th International Scientific Conference, Kiev, Ukraine; 2009. [Google Scholar]
- Longmei, W.; Ting, D.; Kexiang, Y. Study of thermodynamics and phase equilibria in order to predict the behavior of yttrium in iron and steel. Inorg. Chim. Acta 1987, 140, 189–191. [Google Scholar]
- Terehova, W.F.; Sawicki, E.M. Questions of the theory and application of rare earth metals. In Yttrium, Rare Earth Metals and Alloys; 1973. [Google Scholar]
- Zak, P.L.; Kalisz, D.; Gerasin, S.; Kuglin, K. Growth model of Bi-component oxide nonmetallic inclusions in liquid steel. In Proceedings of the Metal 2017 26th International Conference on Metallurgy and Materials Abstracts, Brno, Czech Republic, 24–26 May 2017; pp. 273–278. [Google Scholar]
- Gerasin, S.; Kalisz, D.; Iwanciw, J. Thermodynamic and kinetic simulation of Y2O3 and Y2S3 nonmetallic phase formation in liquid steel. J. Min. Metall. Sect. B Metall. 2020, 56, 11–25. [Google Scholar] [CrossRef] [Green Version]
- Du, T.; Wang, L. Thermodynamics of Fe−Y−S, Fe−Y−O and Fe−Y−S−O metallic solutions. J. Less Common Met. 1985, 110, 179–185. [Google Scholar] [CrossRef]
- Iwanciw, J. Conditions and possibilities of mathematical models application to control of steelmaking. In Proceedings of the Conf. CIBEH on Informatics in Metallurgical Industry, Kozubnik, Poland; 1985. [Google Scholar]
- Iwanciw, J. Simulation of Steelmaking Processes for Work in Real Time. In Komitet Metalurgii PAN; Wyd. Nauk. Akapit: Kraków, Poland, 2002; Volume 1, pp. 186–192. [Google Scholar]
- Manual of WYK_STAL Simulation Software.
- Iwanciw, J.; Podorska, D.; Wypartowicz, J. Modeling of oxide precipitates chemical composition during steel deoxidization. Arch. Metall. Mater. 2011, 56, 999–1005. [Google Scholar] [CrossRef]
- Iwanciw, J.; Podorska, D.; Wypartowicz, J. Simulation of oxygen and nitrogen removal from steel by means of titanium and aluminum. Arch. Metall. Mater. 2011, 56, 635–644. [Google Scholar] [CrossRef]
- Mikhailov, G.G.; Makrovets, L.A.; Smirnov, L.A. Thermodynamic simulation of phase equlibria of oxide systems containing rare‐earth metals. Report 2. Phase Diagrams of Oxide Systems with Y2O3. Bull. S. Ural State Univ. Ser. Metall. 2015, 14, 5–10. [Google Scholar]
- Merder, T.; Pieprzyca, J.; Warzecha, M.; Warzecha, P.; Hutny, A. Evolution of the numerical model describing the distrbution of non-metallic inclusions in the tundish. Materials 2021, 14, 2229. [Google Scholar] [CrossRef] [PubMed]
- Tkadleckova, K.; Walek, M.; Michalek, J.; Huczala, K.T. Numerical analysis of rtd curves and inclusions removal in a multi-strand asymmetric tundish with different configuration of impact pad. Metals 2020, 10, 848. [Google Scholar] [CrossRef]
- Bulko, B.; Molnar, M.; Demeter, P.; Baricova, D.; Pribulova, A.; Futas, P. Study of the Influence of intermix conditions on steel cleanliness. Metals 2018, 8, 852. [Google Scholar] [CrossRef] [Green Version]
- Kalisz, D.; Migas, P.; Karbowniczek, M.; Moskal, M.; Hornk, A. Influence of selected deoxidizers on chemical composition of molten inclusions in liquid steel. J. Mater. Eng. Perform. 2020, 29, 1479–1487. [Google Scholar] [CrossRef]








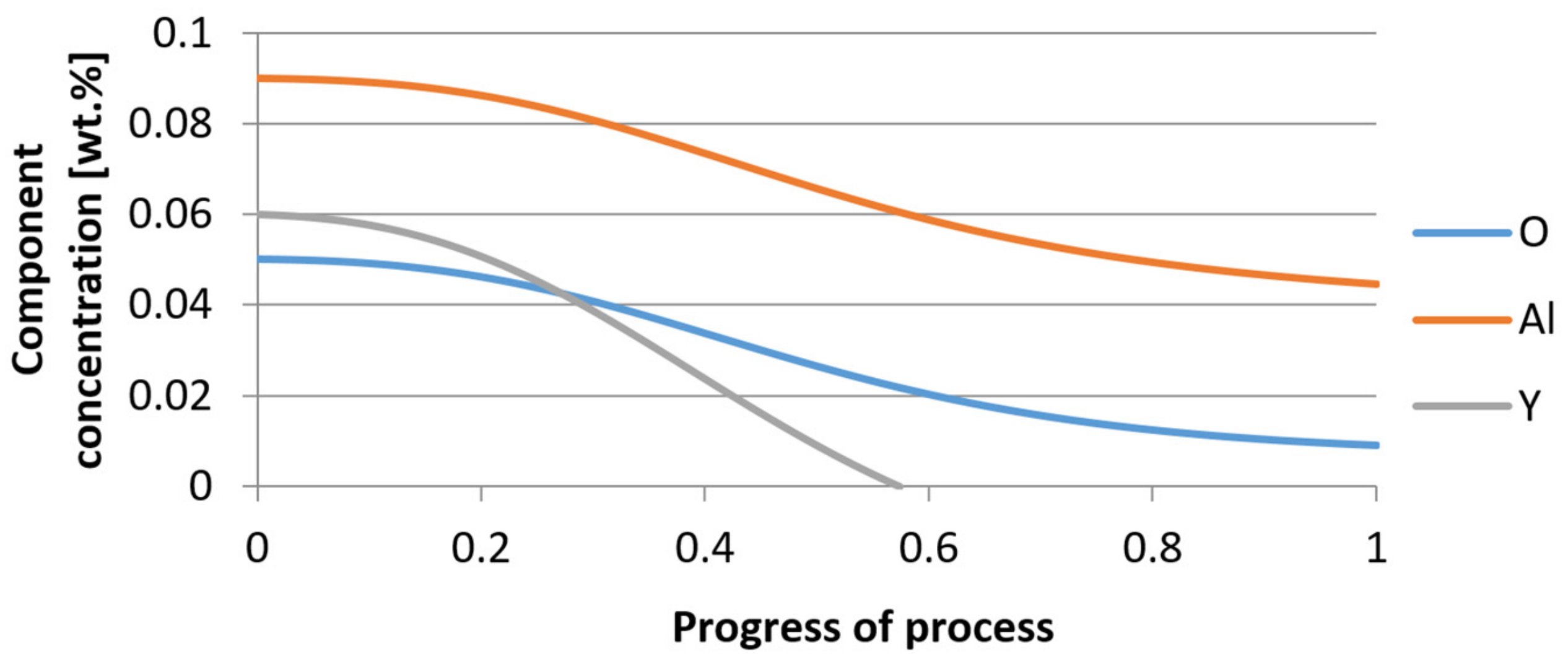
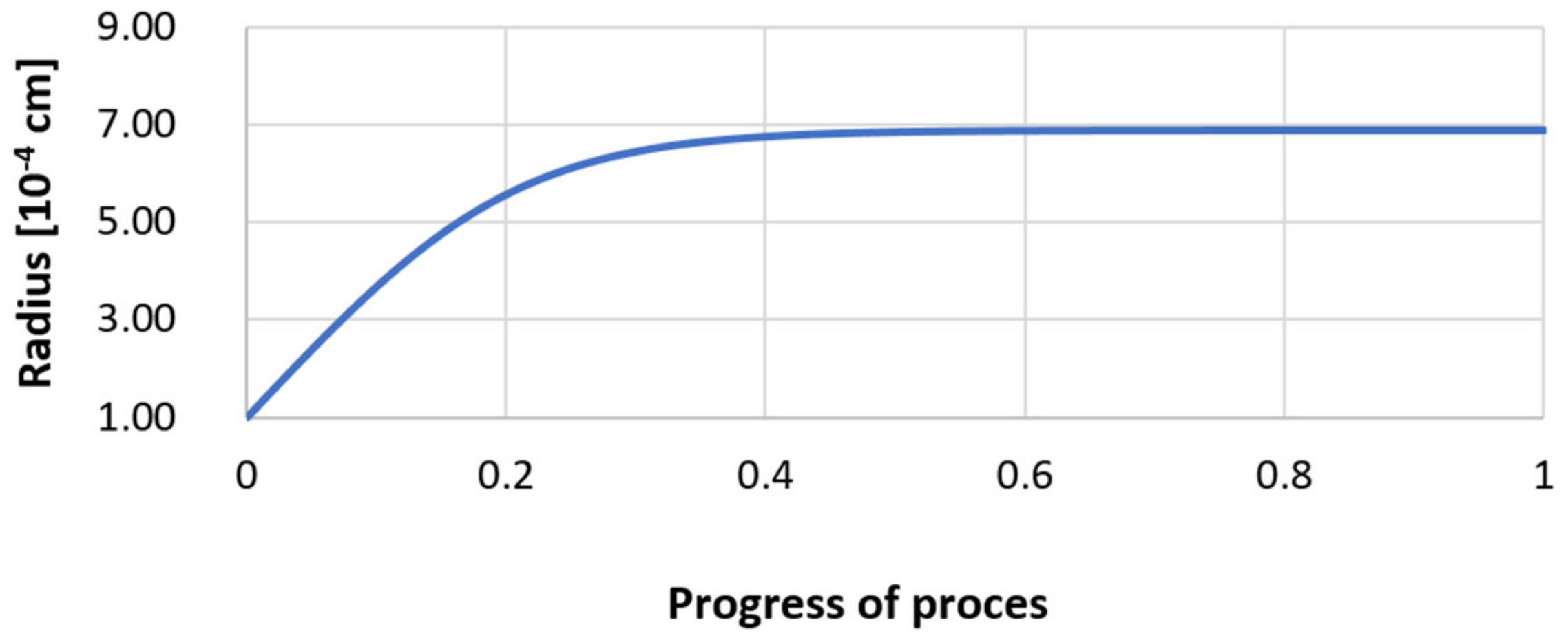
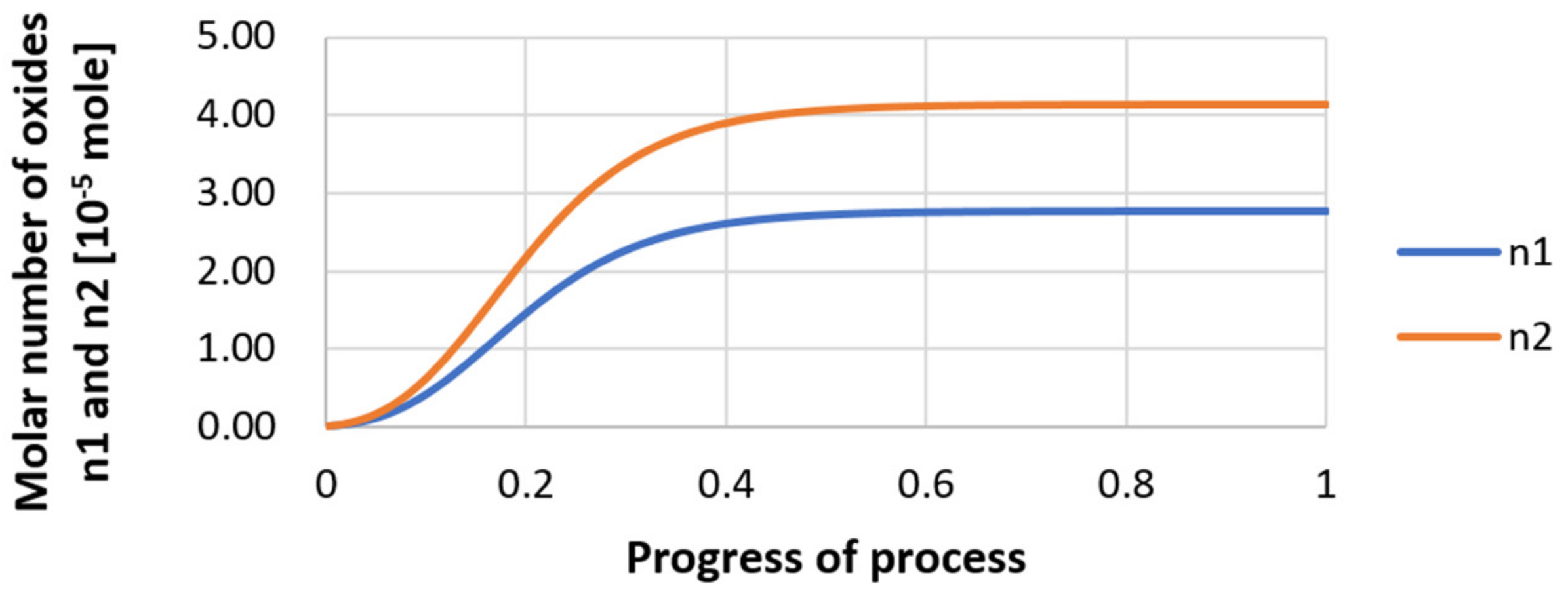
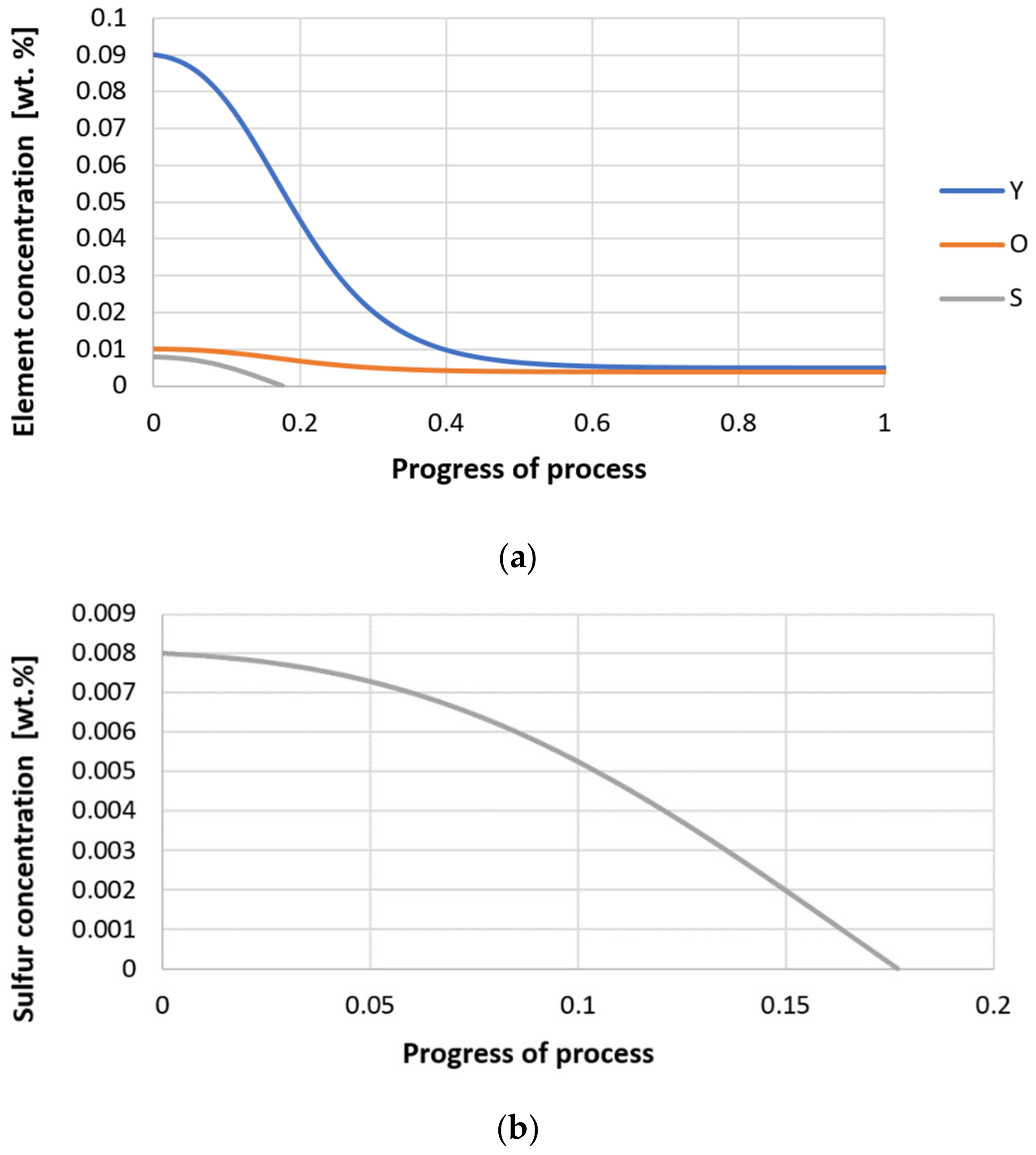


| Reaction | ∆G° = C – Dt [J·mol−1] | K | |||
|---|---|---|---|---|---|
| B | (1600 °C) | ||||
| Y2O3(s) = 2[Y] + 3[O] | 1.793 | 0.658 | 9.365 | 34.40 | 2.5 × 10−16 |
| Y2O2S(s) = 2[Y] + 2[O] + [S] | 1.521 | 0.536 | 7.949 | 28.03 | 4.1 × 10−15 |
| Y2S3(s) = 2[Y] + 3[S] | 1.171 | 0.441 | 6.119 | 23.10 | |
| YS(s) = [Y] + [S] | 0.321 | 0.091 | 1.677 | 4.74 | |
| YN(s) = [Y] + [N] | 0.391 | 0.150 | 2.044 | 7.86 | |
| YC2(s) = [Y]+2[C] | 1.704 | 0.124 | 0.809 | 6.49 | |
| Variant | Input Parameters | System Properties |
|---|---|---|
| 1-model a | 1min 30 kg Al 10 min 76 kg Y 20 min 20 kg Ca | activity of the formed compound a = 1 |
| 2-model c | 1min 30 kg Al 10 min 76 kg Y 20 min 20 kg Ca | metal-slag interfacial partition coefficient |
| 3-model a | 1 min 30 kg Al 10 min 30 kg Ca 20 min 76 kg Y | activity of the formed compound a = 1 |
| 4-model c | 1 min 30 kg Al 10 min 30 kg Ca 20 min 76 kg Y | metal-slag interfacial partition coefficient |
| Component | C | Mn | Si | P | N | S |
|---|---|---|---|---|---|---|
| wt.% | 0.054 | 0.05 | 0.23 | 0.007 | 0.005 | max 0.02 |
| Chemical Compound | Molar Volume v of Precipitate (cm3·mole−1) |
|---|---|
| Y2O3 | 45.07 |
| Y2S3 | 70.80 |
| Y2O2S | 49.39 |
| Al2O3 | 25.82 |
| Chemical Compound | Molar Volume v of Precipitate (cm3·mol−1) |
|---|---|
| Y2O3 | 45.07 |
| Y2S3 | 70.80 |
| Y2O2S | 49.39 |
| Al2O3 | 25.82 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalisz, D.; Żak, P.L.; Semiryagin, S.; Gerasin, S. Evolution of Chemical Composition and Modeling of Growth Nonmetallic Inclusions in Steel Containing Yttrium. Materials 2021, 14, 7113. https://doi.org/10.3390/ma14237113
Kalisz D, Żak PL, Semiryagin S, Gerasin S. Evolution of Chemical Composition and Modeling of Growth Nonmetallic Inclusions in Steel Containing Yttrium. Materials. 2021; 14(23):7113. https://doi.org/10.3390/ma14237113
Chicago/Turabian StyleKalisz, Dorota, Paweł L. Żak, Sergey Semiryagin, and Sergey Gerasin. 2021. "Evolution of Chemical Composition and Modeling of Growth Nonmetallic Inclusions in Steel Containing Yttrium" Materials 14, no. 23: 7113. https://doi.org/10.3390/ma14237113
APA StyleKalisz, D., Żak, P. L., Semiryagin, S., & Gerasin, S. (2021). Evolution of Chemical Composition and Modeling of Growth Nonmetallic Inclusions in Steel Containing Yttrium. Materials, 14(23), 7113. https://doi.org/10.3390/ma14237113







