Graphene and Other 2D Layered Nanomaterials and Hybrid Structures: Synthesis, Properties and Applications
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [Green Version]
- Glavin, N.R.; Rao, R.; Varshney, V.; Bianco, E.; Apte, A.; Roy, A.; Ringe, E.; Ajayan, P.M. Emerging Applications of Elemental 2D Materials. Adv. Mater. 2020, 32, 1904302. [Google Scholar] [CrossRef]
- Khan, K.; Tareen, A.K.; Aslam, M.; Wang, R.; Zhang, Y.; Mahmood, A.; Ouyang, Z.; Zhang, H.; Guo, Z. Recent developments in emerging two-dimensional materials and their applications. JPCC 2020, 8, 387–440. [Google Scholar] [CrossRef]
- Ares, P.; Novoselov, K.S. Recent advances in graphene and other 2D materials. Nano Mater. Sci. 2021. In Press. [Google Scholar] [CrossRef]
- Zeng, M.; Xiao, Y.; Liu, J.; Yang, K.; Fu, L. Exploring Two-Dimensional Materials toward the Next-Generation Circuits: From Monomer Design to Assembly Control. Chem. Rev. 2018, 118, 6236–6296. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Xue, F.; Hu, W.-J.; Li, L.-J. Two-dimensional materials with piezoelectric and ferroelectric functionalities. NPJ 2D Mater. Applic. 2018, 2, 18. [Google Scholar] [CrossRef]
- Mounet, N.; Gibertini, M.; Schwaller, P.; Campi, D.; Merkys, A.; Marrazzo, A.; Sohier, T.; Castelli, I.E.; Cepellotti, A.; Pizzi, G.; et al. Two-dimensional materials from high-throughput computational exfoliation of experimentally known compounds. Nat. Nanotechnol. 2018, 13, 246–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grazianetti, C.; Martella, C.; Molle, A. The Xenes Generations: A Taxonomy of Epitaxial Single-Element 2D Materials. Phys. Stat. Sol. Rap. Res. Lett. 2020, 14, 1900439. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Hu, P.; Long, Y.; Liu, Z.; He, X. Recent advances in ternary two-dimensional materials: Synthesis, properties and applications. J. Mater. Chem. A 2017, 5, 22855–22876. [Google Scholar] [CrossRef]
- Cravanzola, S.; Cesano, F.; Gaziano, F.; Scarano, D. Carbon domains on MoS2/TiO2 system via acetylene polymerization: Synthesis, structure and surface properties. Front. Chem. 2017, 5, 91. [Google Scholar] [CrossRef] [Green Version]
- Cesano, F.; Scarano, D. Graphene and Other 2D Layered Hybrid Nanomaterial-Based Films: Synthesis, Properties, and Applications. Coatings 2018, 8, 419. [Google Scholar] [CrossRef] [Green Version]
- Yu, T.; Breslin, C.B. Graphene-Modified Composites and Electrodes and Their Potential Applications in the Electro-Fenton Process. Materials 2020, 13, 2254. [Google Scholar] [CrossRef] [PubMed]
- Alves, D.C.D.S.; Healy, B.; Yu, T.; Breslin, C.B. Graphene-Based Materials Immobilized within Chitosan: Applications as Adsorbents for the Removal of Aquatic Pollutants. Materials 2021, 14, 3655. [Google Scholar] [CrossRef]
- Tan, H.; Wang, D.; Guo, Y. A Strategy to Synthesize Multilayer Graphene in Arc-Discharge Plasma in a Semi-Opened Environment. Materials 2019, 12, 2279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gudaitis, R.; Lazauskas, A.; Jankauskas, Š.; Meškinis, Š. Catalyst-Less and Transfer-Less Synthesis of Graphene on Si(100) Using Direct Microwave Plasma Enhanced Chemical Vapor Deposition and Protective Enclosures. Materials 2020, 13, 5630. [Google Scholar] [CrossRef]
- Farivar, F.; Yap, P.L.; Tung, T.T.; Losic, D. Highly Water Dispersible Functionalized Graphene by Thermal Thiol-Ene Click Chemistry. Materials 2021, 14, 2830. [Google Scholar] [CrossRef]
- Poyato, R.; Verdugo, R.; Muoz-Ferreiro, C.; Gallardo-Lpez, N. Electrochemically Exfoliated Graphene-Like Nanosheets for Use in Ceramic Nanocomposites. Materials 2020, 13, 2656. [Google Scholar] [CrossRef] [PubMed]
- Slepchenkov, M.M.; Nefedov, I.S.; Glukhova, O.E. Controlling the Electronic Properties of a Nanoporous Carbon Surface by Modifying the Pores with Alkali Metal Atoms. Materials 2020, 13, 610. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.; Lv, Y.; Huang, F.; Zhao, C.; Zhao, S.; Wei, G. ZnO-Controlled Growth of Monolayer WS2 through Chemical Vapor Deposition. Materials 2019, 12, 1883. [Google Scholar] [CrossRef] [Green Version]
- Jiménez-Arévalo, N.; Flores, E.; Giampietri, A.; Sbroscia, M.; Betti, M.G.; Mariani, C.; Ares, J.R.; Ferrer, I.J.; Leardini, F. Borocarbonitride Layers on Titanium Dioxide Nanoribbons for Efficient Photoelectrocatalytic Water Splitting. Materials 2021, 14, 5490. [Google Scholar] [CrossRef]
- Drici-Setti, N.; Lelli, P.; Jouini, N. LDH-Co-Fe-Acetate: A New Efficient Sorbent for Azoic Dye Removal and Elaboration by Hydrolysis in Polyol, Characterization, Adsorption, and Anionic Exchange of Direct Red 2 as a Model Anionic Dye. Materials 2020, 13, 3183. [Google Scholar] [CrossRef] [PubMed]
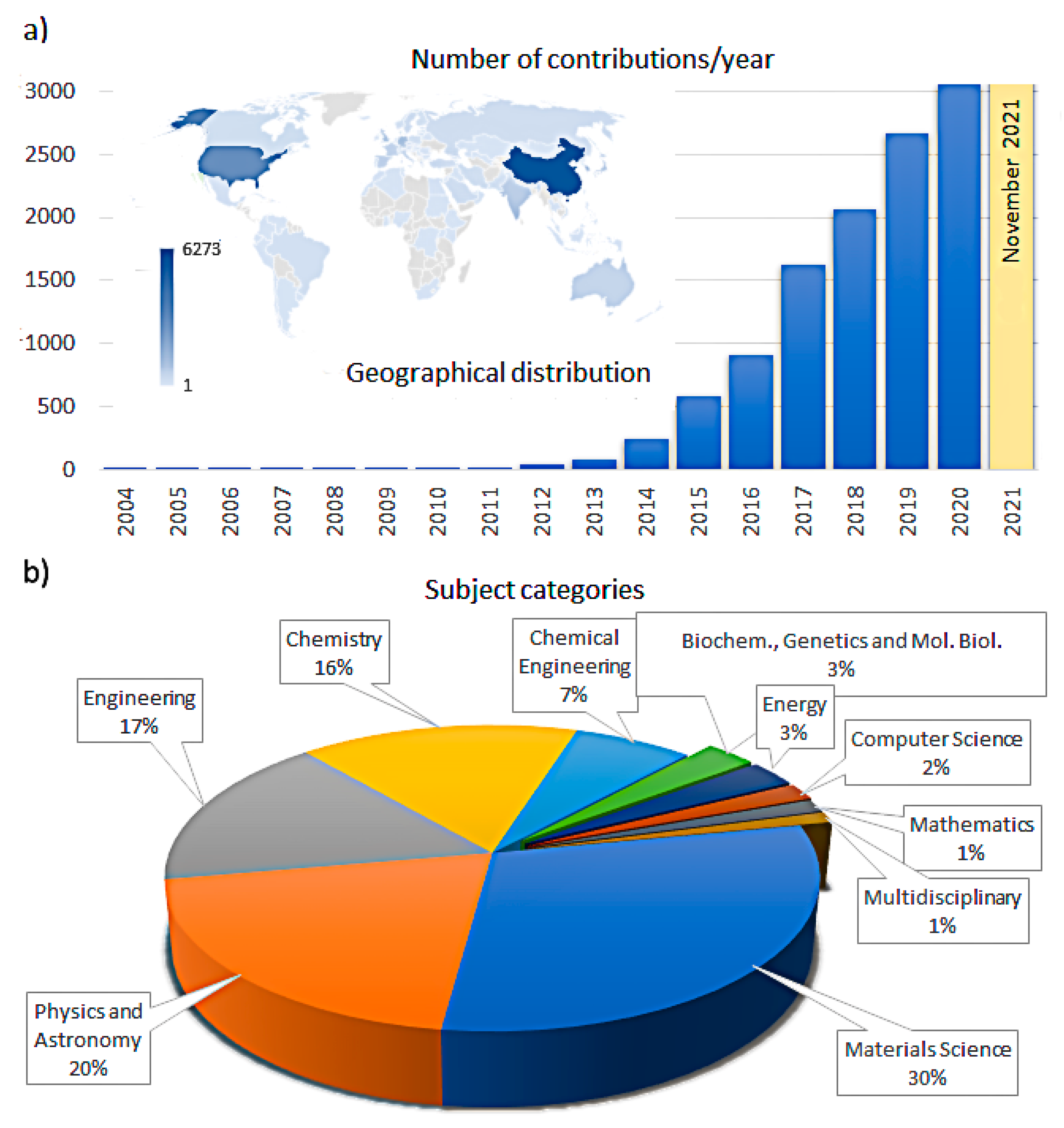
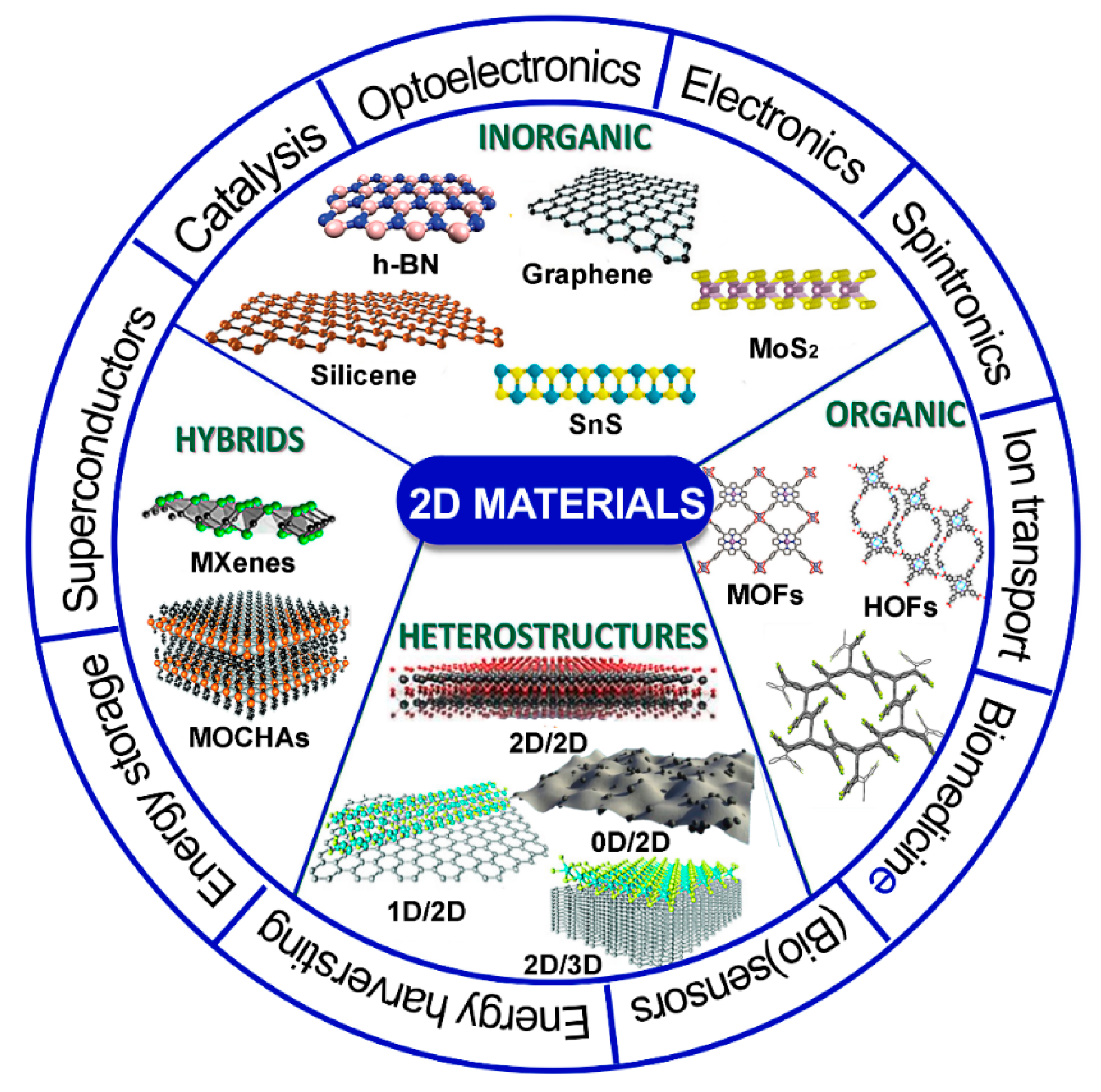
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scarano, D.; Cesano, F. Graphene and Other 2D Layered Nanomaterials and Hybrid Structures: Synthesis, Properties and Applications. Materials 2021, 14, 7108. https://doi.org/10.3390/ma14237108
Scarano D, Cesano F. Graphene and Other 2D Layered Nanomaterials and Hybrid Structures: Synthesis, Properties and Applications. Materials. 2021; 14(23):7108. https://doi.org/10.3390/ma14237108
Chicago/Turabian StyleScarano, Domenica, and Federico Cesano. 2021. "Graphene and Other 2D Layered Nanomaterials and Hybrid Structures: Synthesis, Properties and Applications" Materials 14, no. 23: 7108. https://doi.org/10.3390/ma14237108
APA StyleScarano, D., & Cesano, F. (2021). Graphene and Other 2D Layered Nanomaterials and Hybrid Structures: Synthesis, Properties and Applications. Materials, 14(23), 7108. https://doi.org/10.3390/ma14237108





