Phase-Changing Glauber Salt Solution for Medical Applications in the 28–32 °C Interval
Abstract
:1. Introduction
1.1. Clinical Background
1.2. PCM Background
1.3. Experimental Background
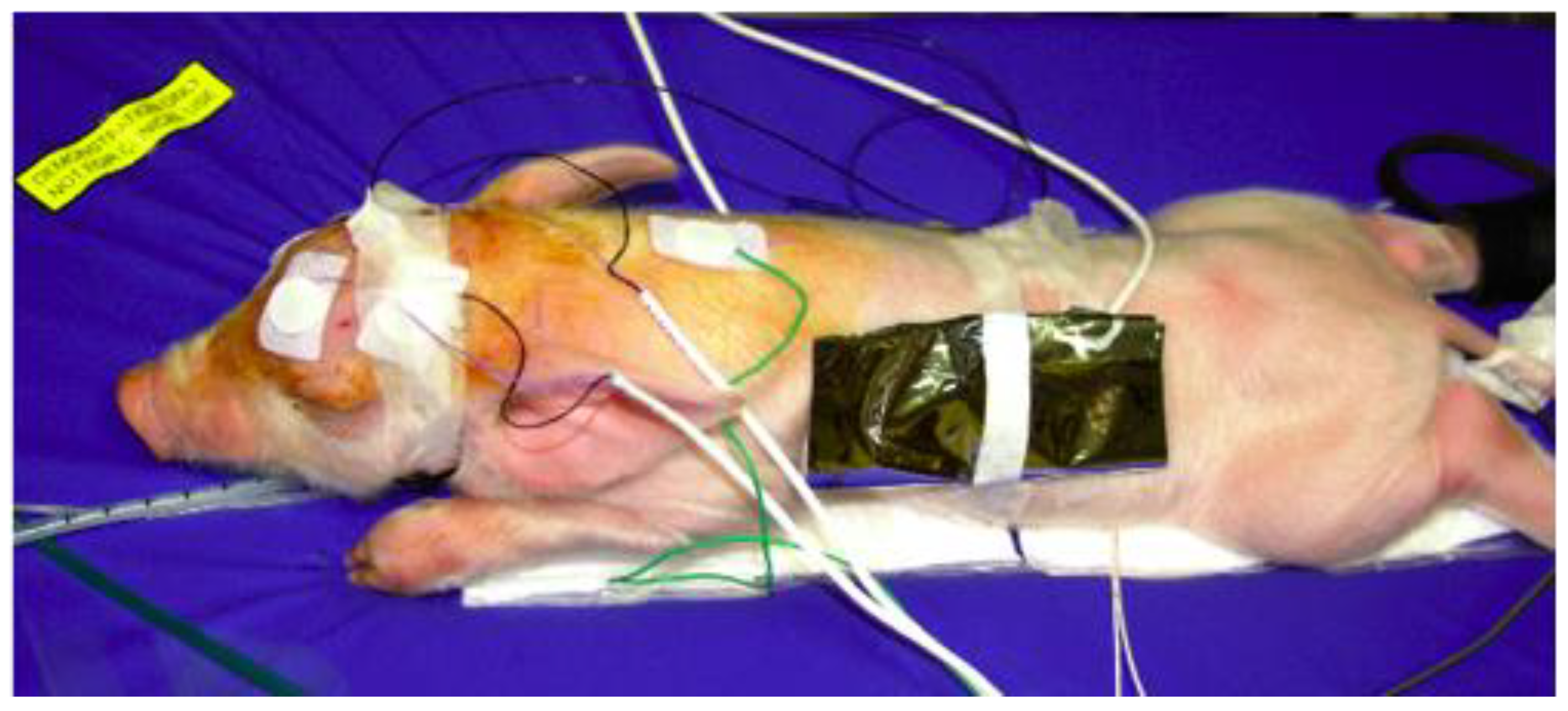
2. Materials and Methods
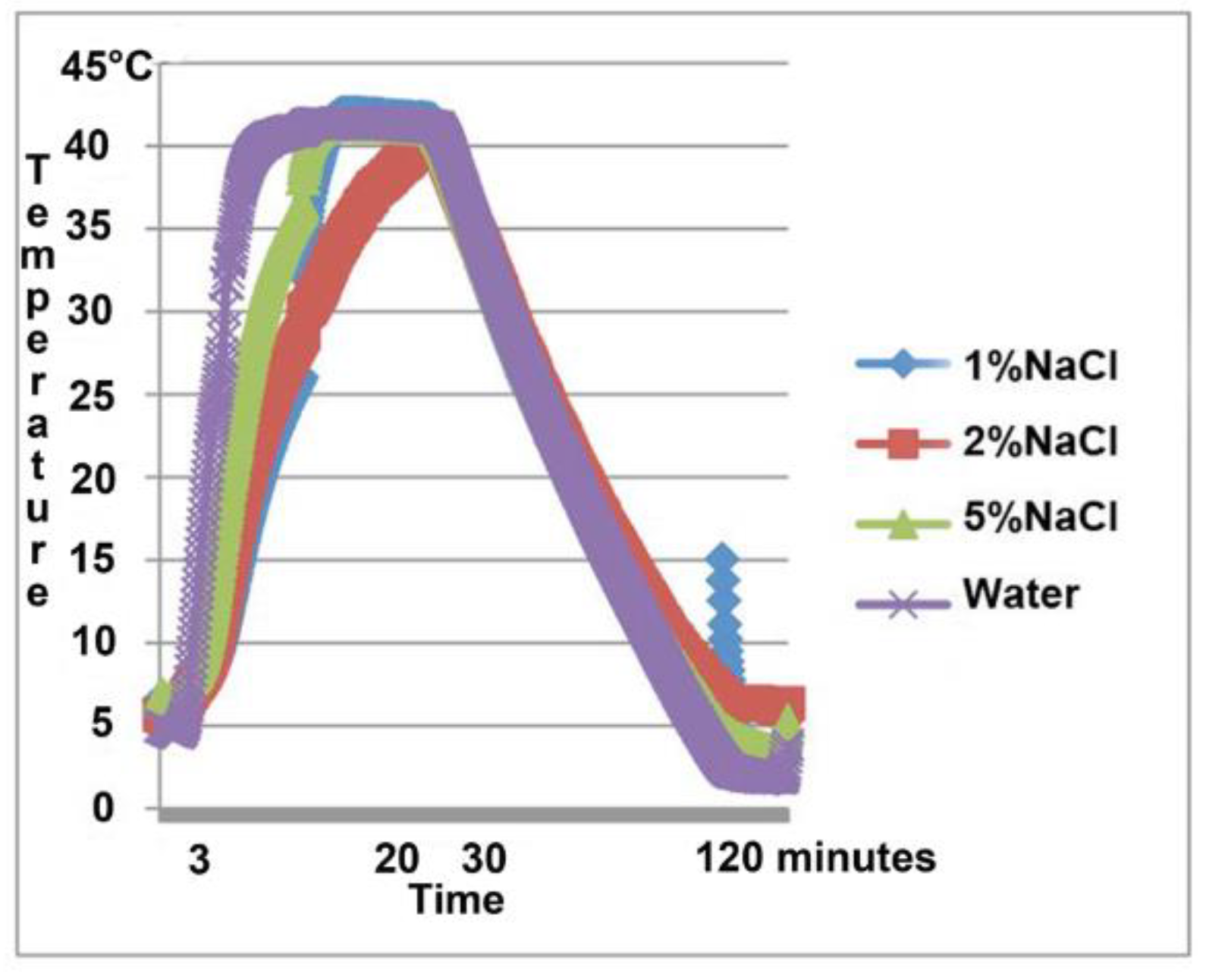
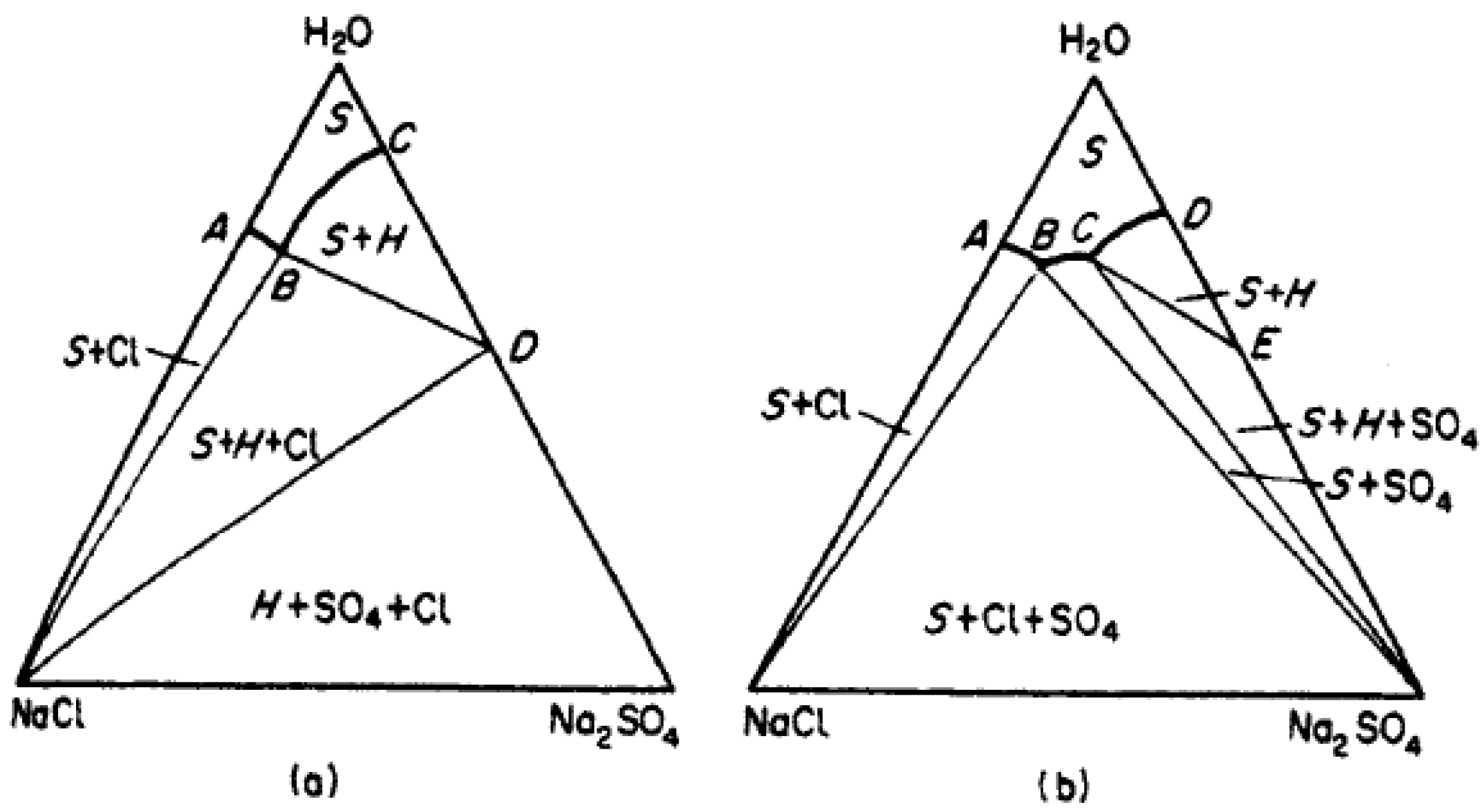
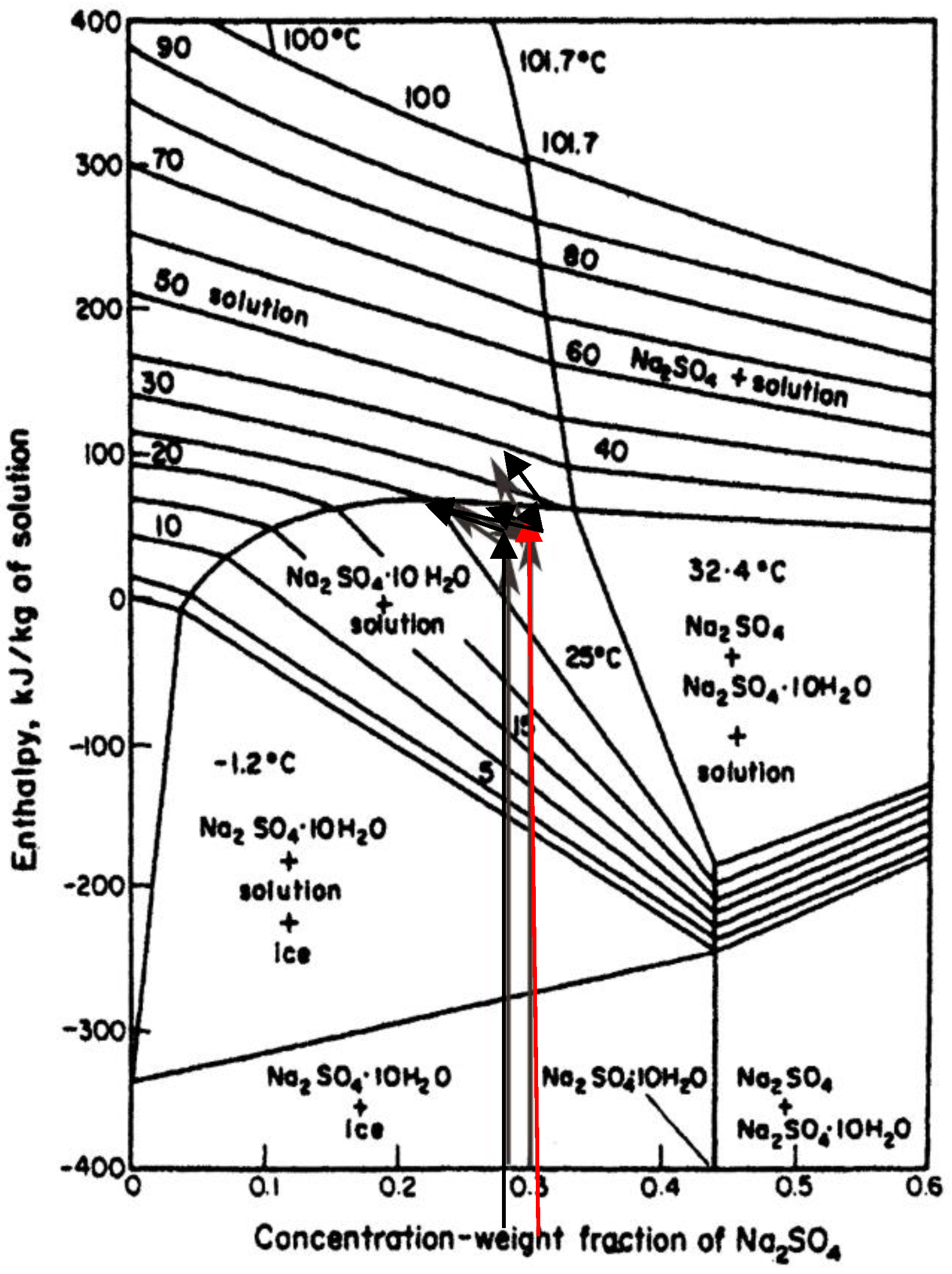
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Equipment Used
| Heated bath | Lauda RE106 with heater/cooler Lauda E100 |
| Computer | Fujitsu-Siemens Scaleo L with software: National Instrument Labview 8.2 |
| Dacpad | NI SC-2345 with termoprobes T100 elements K-Coupled |
| Scale | Presica BJ210C |
| Salt: NaCl | Falksalt, >99% without Ion-supplement |
| Na2SO4 | Merck, Sodium Sulfate Lot Ta518349843 |
| Water | Regular tap water. |
| Stirring rod | Bamboo rod |
| Mixing bowl | Plastic food cup |
References
- El-Sebaii, A.A.; Al-Ghamdi, A.A.; Al-Hazmi, F.S.; Faidah, A.S. Thermal performance of a single basin solar still with PCM as a storage medium. Appl. Energy 2009, 86, 1187–1195. [Google Scholar] [CrossRef]
- Joulin, A.; Younsi, Z.; Zalewski, L.; Lassue, S.; Rousse, D.R.; Cavrot, J.-P. Experimental and numerical investigation of a phase change material: Thermal-energy storage and release. Appl. Energy 2011, 88, 2454–2462. [Google Scholar] [CrossRef]
- Martin, V.; He, B.; Setterwall, F. Direct contact PCM-water cold storage. Appl. Energy 2010, 87, 2652–2659. [Google Scholar] [CrossRef]
- Wang, W.; Yang, X.; Fang, Y.; Ding, J.; Yan, J. Preparation and thermal properties of polyethylene glycol/expanded graphite blends for energy storage. Appl. Energy 2009, 86, 1479–1483. [Google Scholar] [CrossRef]
- Medrano, M.; Yilmaz, M.O.; Nogues, M.; Martorell, I.; Roca, J.; Cabeza, L.F. Experimental evaluation of commercial heat exchangers for use as PCM thermal storage systems. Appl. Energy 2009, 86, 2047–2055. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Xiao, W.; Zeng, R.; Zhang, Q.; Di, H. Review on thermal performance of phase change energy storage building envelope. Chin. Sci. Bull. 2009, 54, 920–928. [Google Scholar] [CrossRef] [Green Version]
- Borreguero, A.M.; Sanchez, M.L.; Valverde, J.L.; Carmona, M.; Rodriguez, J.F. Thermal testing and numerical simulation of gypsum wallboards incorporated with different PCMs content. Appl. Energy 2011, 88, 930–937. [Google Scholar] [CrossRef]
- Eicker, U. Cooling strategies, summer comfort and energy performance of a rehabilitated passive standard office building. Appl. Energy 2010, 87, 2031–2039. [Google Scholar] [CrossRef]
- Tzivanidis, C.; Antonopoulos, K.A.; Gioti, F. Numerical simulation of cooling energy consumption in connection with thermostat operation mode and comfort requirements for the Athens buildings. Appl. Energy 2011, 88, 2871–2884. [Google Scholar] [CrossRef]
- Xiao, W.; Wang, X.; Zhang, Y.P. Analytical optimization of interior PCM for energy storage in a lightweight passive solar room. Appl. Energy 2009, 86, 2013–2018. [Google Scholar] [CrossRef]
- Weng, Y.-C.; Cho, H.-P.; Chang, C.-C.; Chen, S.-L. Heat pipe with PCM for electronic cooling. Appl. Energy 2011, 88, 1825–1833. [Google Scholar] [CrossRef]
- Chou, C.; Tochihara, Y.; Kim, T. Physiological and subjective responses to cooling devices on firefighting protective clothing. Eur. J. Appl. Physiol. 2008, 104, 369–374. [Google Scholar] [CrossRef]
- Mondieig, D.; Rajabalee, F.; Laprie, A.; Oonk, H.A.J.; Calvet, T.; Angel Cuevas-Diarte, M. Protection of temperature sensitive biomedical products using molecular alloys as phase change material. Transfus. Apher. Sci. 2003, 28, 143–148. [Google Scholar] [CrossRef] [Green Version]
- Hauer, A.; Mehling, H.; Schossig, P.; Yamaha, M.; Cabeza, L.; Martin, V.; Setterwall, F.; Expertgroup IEA. Implementing Agreement on Energy Conservation through Energy Storage; Annex 17; Eces, I., Ed.; International Energy Agency: Arvika, Sweden, 2008; pp. 1–132. [Google Scholar]
- Zalba, B.; Marin, J.M.; Cabeza, L.F.; Mehling, H. Review on thermal energy storage with phase change: Materials, heat transfer analysis and applications. Appl. Therm. Eng. 2003, 23, 251–283. [Google Scholar] [CrossRef]
- Gluckman, P.D.; Wyatt, J.S.; Azzopardi, D.; Ballard, R.; Edwards, A.D.; Ferriero, D.M.; Polin, R.A.; Robertson, C.M.; Thoresen, M.; Whitelaw, A.; et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: Multicentre randomised trial. Lancet 2005, 365, 663–670. [Google Scholar] [CrossRef]
- Shankaran, S.; Laptook, A.R.; Ehrenkranz, R.A.; Tyson, J.E.; McDonald, S.A.; Donovan, E.F.; Fanaroff, A.A.; Poole, W.K.; Wright, L.L.; Higgins, R.D.; et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N. Engl. J. Med. 2005, 353, 1574–1584. [Google Scholar] [CrossRef] [PubMed]
- Madar, J.; Roehr, C.C.; Ainsworth, S.; Ersdal, H.; Morley, C.; Rüdiger, M.; Skåre, C.; Szczapa, T.; Te Pas, A.; Trevisanuto, D.; et al. European Resuscitation Council Guidelines 2021: Newborn resuscitation and support of transition of infants at birth. Resuscitation 2021, 161, 291–326. [Google Scholar] [CrossRef]
- Azzopardi, D.; Strohm, B.; Edwards, A.D.; Halliday, H.; Juszczak, E.; Levene, M.; Thoresen, M.; Whitelaw, A.; Brocklehurst, P. Treatment of asphyxiated newborns with moderate hypothermia in routine clinical practice: How cooling is managed in the UK outside a clinical trial. Arch. Dis. Child. Fetal Neonatal Ed. 2009, 94, F260–F264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colbourne, F.; Corbett, D. Delayed postischemic hypothermia: A six month survival study using behavioral and histological assessments of neuroprotection. J. Neurosci. 1995, 15, 7250–7260. [Google Scholar] [CrossRef] [Green Version]
- Taylor, D.L.; Mehmet, H.; Cady, E.B.; Edwards, A.D. Improved neuroprotection with hypothermia delayed by 6 h following cerebral hypoxia-ischemia in the 14-day-old rat. Pediatr. Res. 2002, 51, 13–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thoresen, M.; Penrice, J.; Lorek, A.; Cady, E.; Wylezinska, M.; Kirkbride, V. Mild Hypothermia following severe transient hypoxia-ischaemia ameliorates delayed cerebral energy failure in the newborn piglet. Pediatr. Res. 1995, 37, 667–670. [Google Scholar] [CrossRef] [Green Version]
- Jacobs, S. Whole-body hypothermia for neonatal hypoxic-ischemic encephalopathy reduces mortality into childhood. J. Pediatr. 2012, 161, 968–969. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, S.; Hunt, R.; Tarnow-Mordi, W.; Inder, T.; Davis, P. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst. Rev. 2007, 4, CD003311. [Google Scholar]
- Nair, J.; Kumar, V.H.S. Current and Emerging Therapies in the Management of Hypoxic Ischemic Encephalopathy in Neonates. Children 2018, 5, 99. [Google Scholar] [CrossRef] [Green Version]
- Olson, L.; Faulkner, S.; Lundströmer, K.; Kerenyi, A.; Kelen, D.; Chandrasekaran, M.; Ådén, U.; Olson, L.; Golay, X.; Lagercrantz, H.; et al. Comparison of Three Hypothermic Target Temperatures for the Treatment of Hypoxic Ischemia: mRNA Level Responses of Eight Genes in the Piglet Brain. Transl. Stroke Res. 2012, 4, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Worner, J.; Oddo, M. Too cold may not be so cool: Spontaneous hypothermia as a marker of poor outcome after cardiac arrest. Crit. Care 2010, 14, 1002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rüegger, C.M.; Davis, P.G.; Cheong, J.L. Xenon as an adjuvant to therapeutic hypothermia in near-term and term newborns with hypoxic-ischaemic encephalopathy. Cochrane Database Syst. Rev. 2018, 8, Cd012753. [Google Scholar] [CrossRef]
- Thoresen, M.; Tooley, J.; Liu, X.; Jary, S.; Fleming, P.; Luyt, K.; Jain, A.; Cairns, P.; Harding, D.; Sabir, H. Time is brain: Starting therapeutic hypothermia within three hours after birth improves motor outcome in asphyxiated newborns. Neonatology 2013, 104, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Walloe, L.; Hjort, N.L.; Thoresen, M. Start cooling as soon as possible. Acta Paediatr. 2019, 108, 771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hauer, A.; Mehling, H.; Schossig, P.; Yamaha, M.; Cabeza, L.; Martin, V.; Setterwall, F. Advanced thermal energy storage through Phase Change Materials and Chemical Reactions-Feasibility studies and demostration projects, Final report. In Implementing Agreement on Energy Conservation through Energy Storage; Annex 17; Agency, I.E., Ed.; International Energy Agency: Arvika, Sweden, 2009; pp. 1–136. [Google Scholar]
- Wang, W.L.; Yang, X.X.; Fang, Y.T.; Ding, J.; Yan, J.Y. Enhanced thermal conductivity and thermal performance of form-stable composite phase change materials by using beta-Aluminum nitride. Appl. Energy 2009, 86, 1196–1200. [Google Scholar] [CrossRef]
- Muir, I.H.; Bishop, P.A.; Ray, P. Effects of a novel ice-cooling technique on work in protective clothing at 28 degrees C, 23 degrees C, and 18 degrees C WBGTs. Am. Ind. Hyg. Assoc. J. 1999, 60, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Yim, Y.J.; Lee, J.W.; Heo, Y.J.; Park, S.J. Carbon-Filled Organic Phase-Change Materials for Thermal Energy Storage: A Review. Molecules 2019, 24, 2055. [Google Scholar] [CrossRef] [Green Version]
- Yinping, Z.; Yi, J. A simple method, the-history method, of determining the heat of fusion, specific heat and thermal conductivity of phase-change materials. Meas. Sci. Technol. 1999, 10, 201. [Google Scholar] [CrossRef]
- Chen, B.; Wang, X.; Zeng, R.; Zhang, Y.; Wang, X.; Niu, J.; Li, Y.; Di, H. An experimental study of convective heat transfer with microencapsulated phase change material suspension: Laminar flow in a circular tube under constant heat flux. Exp. Therm. Fluid Sci. 2008, 32, 1638–1646. [Google Scholar] [CrossRef]
- Günther, E.; Heibler, S.; Mehling, H.; Redlich, R. Enthalpy of Phase Change Materials as a Function of Temperature: Required Accuracy and Suitable Measurement Methods. Int. J. Thermophys. 2009, 30, 1257–1269. [Google Scholar] [CrossRef]
- Olson, L. Cooling of newborns with PCMs. In IEATA, PCM Future; IEATA: Arvika, Sweden, 2004. [Google Scholar]
- Olson, L. Cooling with phase changing material. In Neonatal Brain and Epigenetics Workshop; Karolinska Institutet: Stockholm, Sweden, 2011. [Google Scholar]
- Sharma, A.; Tyagi, V.V.; Chen, C.R.; Buddhi, D. Review on thermal energy storage with phase change materials and applications. Renew. Sustain. Energy Rev. 2009, 13, 318–345. [Google Scholar] [CrossRef]
- Olson, L. PCM for medical applications. In Annex 17; IEATA: Ankara, Turkey, 2005; Beijing, China, 2006. [Google Scholar]
- Hong, H.; Kim, S.K.; Kim, Y.-S. Accuracy improvement of T-history method for measuring heat of fusion of various materials. Int. J. Refrig. 2004, 27, 360–366. [Google Scholar] [CrossRef]
- Peck, J.H.; Kim, J.-J.; Kang, C.; Hong, H. A study of accurate latent heat measurement for a PCM with a low melting temperature using T-history method. Int. J. Refrig. 2006, 29, 1225–1232. [Google Scholar] [CrossRef]
- Mehling, H.; Cabeza, L.F. Heat and cold storage with PCM an up to date introduction into basics and applications. In Heat and Mass Transfer; Springer: Berlin/Heidelberg, Germany, 2008; Volume 308. [Google Scholar]
- Iwata, S.; Iwata, O.; Olson, L.; Kapetanakis, A.; Kato, T.; Evans, S.; Araki, Y.; Kakuma, T.; Matsuishi, T.; Setterwall, F.; et al. Therapeutic hypothermia can be induced and maintained using either commercial water bottles or a “phase changing material” mattress in a newborn piglet model. Arch. Dis. Child. 2009, 94, 387–391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hallberg, B.; Olson, L.; Bartocci, M.; Edqvist, I.; Blennow, M. Passive induction of hypothermia during transport of asphyxiated infants: A risk of excessive cooling. Acta Paediatr. 2009, 98, 942–946. [Google Scholar] [CrossRef]
- Tran, H.T.T.; Le, H.T.T.; Tran, H.T.P.; Khu, D.T.K.; Lagercrantz, H.; Tran, D.M.; Winbladh, B.; Hellström-Westas, L.; Alfvén, T.; Olson, L. Hypothermic treatment for neonatal asphyxia in low-resource settings using phase-changing material-An easy to use and low-cost method. Acta Paediatr. 2021, 110, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Hu, Y.; Hou, Y.; Shen, X.; Xu, G.; Dai, L.; Zhou, J.; Liu, Y.; Cai, K. Phase-change material filled hollow magnetic nanoparticles for cancer therapy and dual modal bioimaging. Nanoscale 2015, 7, 9004–9012. [Google Scholar] [CrossRef]
- Wu, J.; Hu, R.; Zeng, S.; Xi, W.; Huang, S.; Deng, J.; Tao, G. Flexible and Robust Biomaterial Microstructured Colored Textiles for Personal Thermoregulation. ACS Appl. Mater. Interfaces 2020, 12, 19015–19022. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Li, W.; Zhu, R.; Lin, C.; Hufenus, R. Flexible Phase Change Material Fiber: A Simple Route to Thermal Energy Control Textiles. Materials 2021, 14, 401. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Long, J.; Du, Y.; Zhou, J.; Wang, Y.; Miao, Z.; Liu, Y.; Xu, S.; Cao, S. Recyclable low-temperature phase change microcapsules for cold storage. J. Colloid Interface Sci. 2020, 564, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Thayyil, S.; Costello, A.; Shankaran, S.; Robertson, N.J. Therapeutic hypothermia for neonatal encephalopathy implications for neonatal units in India. Indian Pediatr. 2009, 46, 283–289. [Google Scholar] [PubMed]
- Robertson, N.J.; Nakakeeto, M.; Hagmann, C.; Cowan, F.M.; Acolet, D.; Iwata, O.; Allen, E.; Elbourne, D.; Costello, A.; Jacobs, I. Therapeutic hypothermia for birth asphyxia in low-resource settings: A pilot randomised controlled trial. Lancet 2008, 372, 801–803. [Google Scholar] [CrossRef]
- Montaldo, P.; Pauliah, S.S.; Lally, P.J.; Olson, L.; Thayyil, S. Cooling in a low-resource environment: Lost in translation. Semin. Fetal Neonatal Med. 2015, 20, 72–79. [Google Scholar] [CrossRef] [Green Version]
- Thayyil, S.; Bhutta, Z.A.; Ramji, S.; Costello, A.M.; Robertson, N. J Global application of therapeutic hypothermia to treat perinatal asphyxial encephalopathy. Int. Health 2010, 2, 79–81. [Google Scholar] [CrossRef] [PubMed]
- Thomas, N.; Abiramalatha, T.; Bhat, V.; Varanattu, M.; Rao, S.; Wazir, S.; Lewis, L.; Balakrishnan, U.; Murki, S.; Mittal, J.; et al. Phase Changing Material for Therapeutic Hypothermia in Neonates with Hypoxic Ischemic Encephalopathy—Multi-centric Study. Indian Pediatr. 2018, 55, 201–205. [Google Scholar] [CrossRef]




| No. | Criterion | What Needs to Be Done |
|---|---|---|
| 1. | Must deliver controlled set temperature that gives the cooled object a temperature of 33.5–34.5 °C. | New mixtures that can deliver the temperatures the medical market asks for. |
| 2. | Must be non-toxic. | The ingredients in the solution need to be tested for toxicity or the material needs to be securely encapsulated. |
| 3. | Must not expand or in any other way cause injuries to the intended target. | Needs to be tested for each solution in a helicopter test. |
| 4. | Must have a rapid cooling effect but with no cooling undershoot. | Needs to be tested. |
| 5. | Should be stable for at least 72 h. | If cooling time is shorter, a fast change should be possible without problems for staff or patient. |
| 6. | Be of low cost. | Enables use in developing countries |
| 7. | No interbatch variability. | Each mixture needs to be tested to fulfill criteria. |
| 8. | Able to cycle through cooling phase change at least 100 times. | Needs to be tested. |
| 9. | Effective without electricity or water supply is a major advantage. | Choose PCM as the cooling agent. |
| 10. | Reversed phase change no longer than 4 h. | Reversed cycle needed to be tested. |
| 11. | Magnetic resonance imaging compatible. | No metallic or magnetic compounds can be involved in end-product. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olson, L.; Lothian, C.; Ådén, U.; Lagercrantz, H.; Robertson, N.J.; Setterwall, F. Phase-Changing Glauber Salt Solution for Medical Applications in the 28–32 °C Interval. Materials 2021, 14, 7106. https://doi.org/10.3390/ma14237106
Olson L, Lothian C, Ådén U, Lagercrantz H, Robertson NJ, Setterwall F. Phase-Changing Glauber Salt Solution for Medical Applications in the 28–32 °C Interval. Materials. 2021; 14(23):7106. https://doi.org/10.3390/ma14237106
Chicago/Turabian StyleOlson, Linus, Carina Lothian, Ulrika Ådén, Hugo Lagercrantz, Nicola J. Robertson, and Fredrik Setterwall. 2021. "Phase-Changing Glauber Salt Solution for Medical Applications in the 28–32 °C Interval" Materials 14, no. 23: 7106. https://doi.org/10.3390/ma14237106







