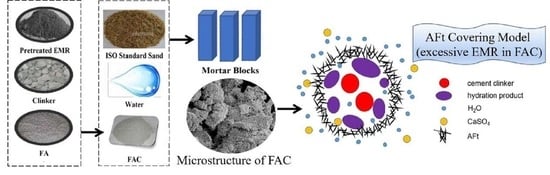
Effect of Electrolytic Manganese Residue in Fly Ash-Based Cementitious Material: Hydration Behavior and Microstructure
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
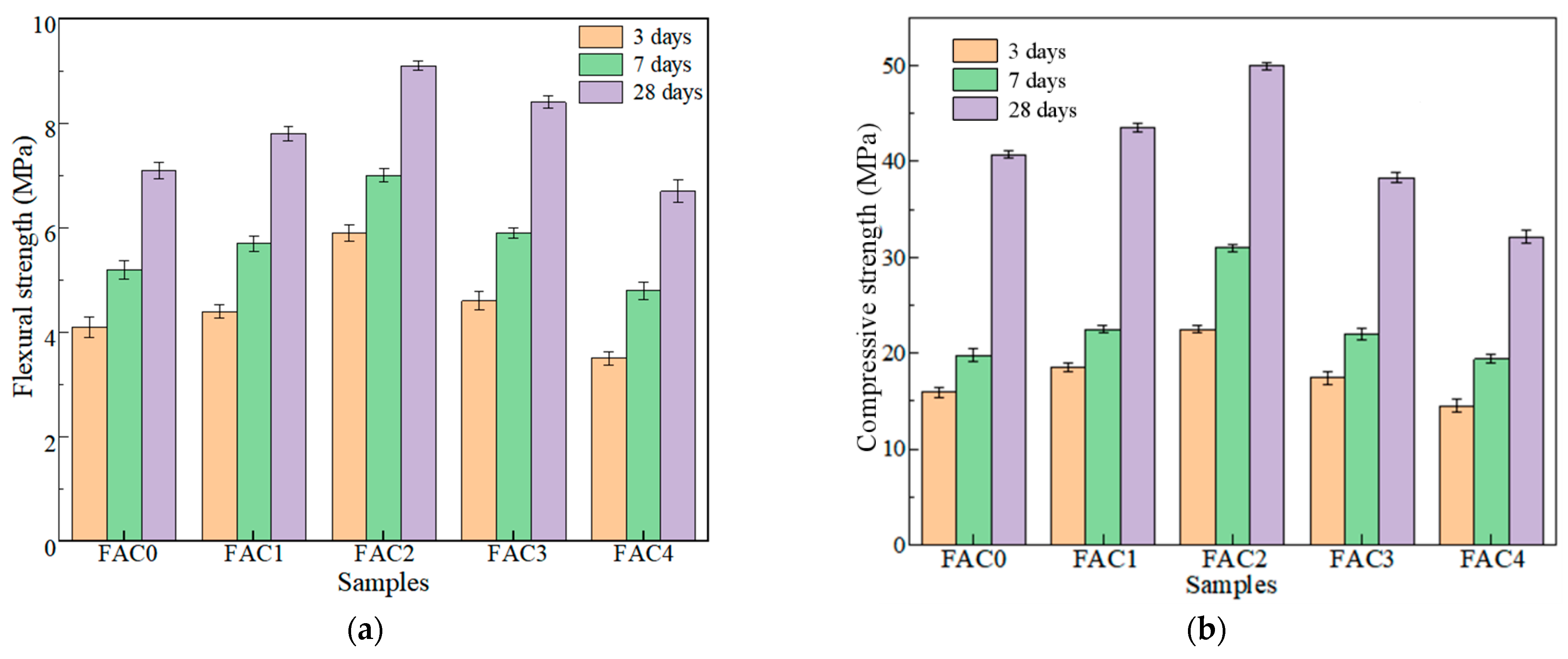
3.1. Effect of Electrolytic Manganese Residue on the Strength of FAC
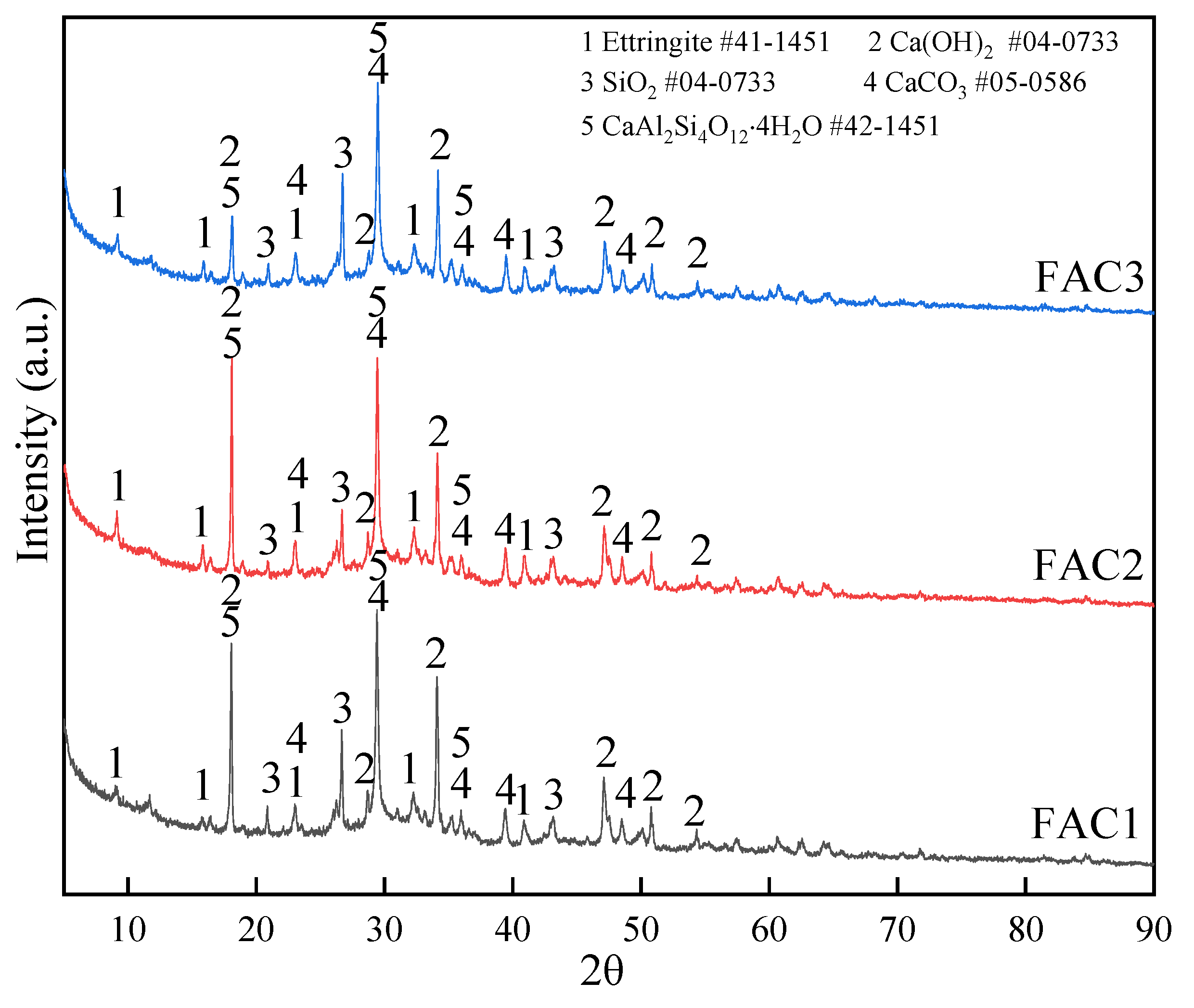
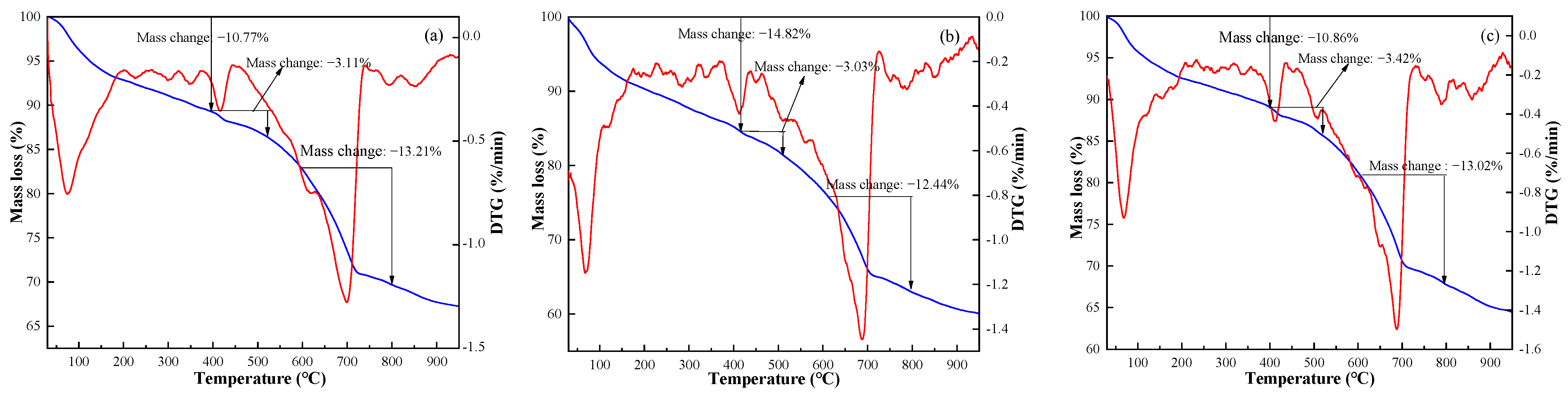
3.2. Effect of Electrolytic Manganese Residue on Hydration Products of FAC
3.3. Effect of Electrolytic Manganese Residue on Microstructure of FAC
3.4. Effect of Electrolytic Manganese Residue on Environmental Performance of FAC
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hagelstein, K. Globally sustainable manganese metal production and use. J. Environ. Manag. 2009, 90, 3736–3740. [Google Scholar] [CrossRef] [PubMed]
- Duan, N.; Wamg, F.; Zhou, C.; Zhu, C.; Yu, H. Analysis of pollution materials generated from electrolytic manganese industries in China. Resour. Conserv. Recycl. 2010, 54, 506–511. [Google Scholar] [CrossRef]
- Shu, J.; Liu, R.; Liu, Z.; Du, J.; Tao, C. Electrokinetic remediation of manganese and ammonia nitrogen from electrolytic manganese residue. Environ. Sci. Pollut. Res. 2015, 22, 16004–16013. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Liu, R.; Shu, J.; Li, W. Simultaneous stripping recovery of ammonia-nitrogen and precipitation of manganese from electrolytic manganese residue by air under calcium oxide assist. J. Environ. Sci. Health Part. A Toxic Hazard. Subst. Environ. Eng. 2015, 50, 1282–1290. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhong, H.; Wang, S.; Xue, J.; Zhang, Z. A novel conversion process for waste residue: Synthesis of zeolite from electrolytic manganese residue and its application to the removal of heavy metals. Colloids Surf. A 2015, 470, 258–267. [Google Scholar] [CrossRef]
- Feng, Y.; Zhang, S.; Li, H. Reductive leaching of manganese from low-grade pyrolusite ore in sulfuric acid using pyrolysis-pretreated sawdust as a reductant. Int. J. Miner. Metall. Mater. 2016, 23, 241–246. [Google Scholar] [CrossRef]
- Li, C.; Zhong, H.; Wang, S.; Xue, J. Leaching Behavior and risk assessment of heavy metals in a landfill of electrolytic manganese residue in western Hunan, China. Hum. Ecol. Risk Assess. 2014, 20, 1249–1263. [Google Scholar] [CrossRef]
- Duan, N.; Dan, Z.; Wang, F.; Pan, C.; Zhou, C.; Jiang, L. Electrolytic manganese metal industry experience based China’s new model for cleaner production promotion. J. Clean. Prod. 2011, 19, 2082–2087. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, J.; Wang, N. Treating electrolytic manganese residue with alkaline additives for stabilizing manganese and removing ammonia. Korean J. Chem. Eng. 2013, 30, 2037–2042. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, J.; Qian, J.; Hou, P.; Cao, J. Study on sulfate of electrolytic manganese residue. Mater. Rev. 2010, 24, 61–64. [Google Scholar]
- Li, T.; Xie, H.; He, X.; Zhou, X. Experimental study of calcined electrolysis manganese residue and fly ash complex admixture. Bull. Chin. Ceram. Soc. 2007, 26, 567–571. [Google Scholar]
- Hou, P.; Qian, J.; Wang, Z.; Deng, C. Production of quasi-sulphoaluminate cementitious materials with electrolytic manganese residue. Cem. Concr. Compos. 2011, 34, 248–254. [Google Scholar] [CrossRef]
- Yang, C.; Lv, X.; Tian, X.; Wang, Y.; Komarneni, S. An investigation on the use of electrolytic manganese residue as filler in sulfur concrete. Constr. Build. Mater. 2014, 73, 305–310. [Google Scholar] [CrossRef]
- Li, C.; Zhong, H.; Wang, S.; Xue, J.; Wu, F.; Zhang, Z. Preparation of MnO2 and calcium silicate hydrate from electrolytic manganese residue and evaluation of adsorption properties. J. Cent. South. Univ. 2015, 22, 2493–2502. [Google Scholar] [CrossRef]
- Du, B.; Zhou, C.; Dan, Z.; Luan, Z.; Duan, N. Preparation and characteristics of steam-autoclaved bricks produced from electrolytic manganese solid waste. Constr. Build. Mater. 2014, 50, 291–299. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, Z.; Hou, P.; Qian, J.; Cao, J. Experimental Study on Preparation of Non-burnt Brick from Electrolytic Manganese Residue. Non-Met. Mines. 2010, 33, 2008–2011. [Google Scholar]
- Phoo-ngernkham, T.; Chindaprasirt, P.; Sata, V.; Pangdaeng, S.; Sinsiri, T. Properties of high calcium fly ash geopolymer pastes with Portland cement as an additive. Int. J. Miner. Metall. Mater. 2013, 20, 214–220. [Google Scholar] [CrossRef]
- Zhu, J.; Yan, H. Microstructure and properties of mullite-based porous ceramics produced from coal fly ash with added Al2O3. Int. J. Miner. Metall. Mater. 2017, 24, 309–315. [Google Scholar] [CrossRef]
- Ahmaruzzaman, M. A review on the utilization of fly ash. Prog. Energy Combust. Sci. 2010, 36, 327–363. [Google Scholar] [CrossRef]
- Xu, Y.; Yang, B.; Liu, X.; Gao, S.; Li, D.; Mukiza, E.; Li, H. Investigation of the medium calcium based non-burnt brick made by red mud and fly ash: Durability and hydration characteristics. Int. J. Miner. Metall. Mater. 2019, 26, 983–991. [Google Scholar] [CrossRef]
- Gu, K.; Chen, B. Research on the incorporation of untreated flue gas desulfurization gypsum into magnesium oxysulfate cement. J. Clean. Prod. 2020, 271, 122497. [Google Scholar] [CrossRef]
- Demir, İ.; Güzelkücük, S.; Sevim, Ö. Effects of sulfate on cement mortar with hybrid pozzolan substitution. Eng. Sci. Technol. 2018, 21, 275–283. [Google Scholar] [CrossRef]
- Liu, S.; Liu, W.; Jiao, F.; Qin, W.; Yang, C. Production and resource utilization of flue gas desulfurized gypsum in China—A review. Environ. Pollut. 2021, 288, 117799. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, X.; Zhang, Y.; Tang, B.; Mukiza, E. Investigation on sulfate activation of electrolytic manganese residue on early activity of blast furnace slag in cement-based cementitious material. Constr. Build. Mater. 2019, 229, 116831. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, J.; Li, S.; Gao, Y.; Liu, C.; Qi, Y. Effect of different gypsums on the workability and mechanical properties of red mud-slag based grouting materials. J. Clean. Prod. 2020, 245, 118759. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, Q.; Jiang, X.; Hou, P.; Qian, J. Sulphate Activating of Electrolytic Manganese Residue to Fly Ash. Non-Met. Mines. 2011, 34, 5–8. [Google Scholar]
- Wang, Q.; Yang, J.; Chen, H. Long-term properties of concrete containing limestone powder. Mater. Struct. 2017, 50, 168. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.; Xu, Y.; Tang, B.; Wang, Y.; Mukiza, E. Preparation and characterization of cement treated road base material utilizing electrolytic manganese residue. J. Clean. Prod. 2019, 232, 980–992. [Google Scholar] [CrossRef]
- Maciel, M.; Soares, G.; Romano, R.; Cincotto, M. Monitoring of Portland cement chemical reaction and quantification of the hydrated products by XRD and TG in function of the stoppage hydration technique. J. Therm. Anal. Calorim. 2019, 136, 1269–1284. [Google Scholar] [CrossRef]
- He, T.; Gao, Z.; Li, S.; Zhao, S.; Bao, C. Influence of Gypsum Dosage on Performances of Silicate Mixed by Slag. Bull. Chin. Ceram. Soc. 2012, 31, 521–525. [Google Scholar]
- Ylmén, R.; Jäglid, U.; Steenari, B.; Panas, I. Early hydration and setting of Portland cement monitored by IR, SEM and Vicat techniques. Cem. Concr. Res. 2009, 39, 433–439. [Google Scholar] [CrossRef]
- Chen, J.; Sorelli, L.; Vandamme, M.; Ulm, F.; Chanvillard, G. A coupled nanoindentation/SEM-EDS study on low water/cement ratio portland cement paste: Evidence for C-S-H/Ca(OH)2 nanocomposites. J. Am. Ceram. Soc. 2010, 93, 1484–1493. [Google Scholar] [CrossRef] [Green Version]
- Feng, J.; Cheng, L.; Zhou, X.; Xu, H.; Weir, M.; Li, Q.; Hannig, M.; Rupf, S. Effects of water aging on the mechanical and anti-biofilm properties of glass-ionomer cement containing dimethylaminododecyl methacrylate. Dent. Mater. 2019, 35, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, D.; Zhuang, S. The soundness of steel slag with different free CaO and MgO contents. Constr. Build. Mater. 2017, 151, 138–146. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, N.; Li, C.; Zhang, Y. Strength development mechanism of a marine binding material with red mud and seawater. Constr. Build. Mater. 2021, 303, 124428. [Google Scholar] [CrossRef]
- Pavoine, A.; Brunetaud, X.; Divet, L. The impact of cement parameters on Delayed Ettringite Formation. Cem. Concr. Compos. 2012, 34, 521–528. [Google Scholar] [CrossRef]
- Gu, Y.; Martin, R.; Metalssi, O.; Fen-Chong, T.; Dangla, P. Pore size analyses of cement paste exposed to external sulfate attack and delayed ettringite formation. Cem. Concr. Res. 2019, 123, 105766. [Google Scholar] [CrossRef]
- Yu, J.; Qian, J.; Tang, J.; Ji, Z.; Fan, Y. Effect of ettringite seed crystals on the properties of calcium sulphoaluminate cement. Constr. Build. Mater. 2019, 207, 249–257. [Google Scholar] [CrossRef]
- Qoku, E.; Bier, T.; Westphal, T. Phase assemblage in ettringite-forming cement pastes: A X-ray diffraction and thermal analysis characterization. J. Build. Eng. 2017, 12, 37–50. [Google Scholar] [CrossRef]
- Leklou, N.; Nguyen, V.; Mounanga, P. The effect of the partial cement substitution with fly ash on Delayed Ettringite Formation in heat-cured mortars. KSCE J. Civ. Eng. 2017, 21, 1359–1366. [Google Scholar] [CrossRef]
- Sinsiri, T.; Chindaprasirt, P.; Jaturapitakkul, C. Influence of fly ash fineness and shape on the porosity and permeability of blended cement pastes. Int. J. Miner. Metall. Mater. 2010, 17, 683–690. [Google Scholar] [CrossRef]
- Hanif, A.; Parthasarathy, P.; Ma, H.; Fan, T.; Li, Z. Properties improvement of fly ash cenosphere modified cement pastes using nano silica. Cem. Concr. Compos. 2017, 81, 35–48. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, D.; Chen, H. The role of fly ash microsphere in the microstructure and macroscopic properties of high-strength concrete. Cem. Concr. Compos. 2017, 83, 125–137. [Google Scholar] [CrossRef]
- Ma, H. Mercury intrusion porosimetry in concrete technology: Tips in measurement, pore structure parameter acquisition and application. J. Porous Mater. 2014, 21, 207–215. [Google Scholar] [CrossRef]
- Diamond, S. Mercury porosimetry: An inappropriate method for the measurement of pore size distributions in cement-based materials. Cem. Concr. Res. 2000, 30, 1517–1525. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, N.; Yao, Y.; Sun, H.; Feng, H. Micro-structural characterization of the hydration products of bauxite-calcination-method red mud-coal gangue based cementitious materials. J. Hazard. Mater. 2013, 262, 428–438. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Liu, X.; Sun, H. Hydration Characteristics of Intermediate-Calcium Based Cementitious Materials from Red Mud and Coal Gangue. Chin. J. Mater. Res. 2014, 28, 325–332. [Google Scholar]
- Zhang, J.; Sun, H.; Wang, J.; Zhang, N. 29Si polymerization degree of hydrates in coal gangue added cement. J. Cent. South. Univ. (Sci. Technol.) 2011, 42, 329–335. [Google Scholar]
- Snoeck, D.; Pel, L.; De Belie, N. Superabsorbent polymers to mitigate plastic drying shrinkage in a cement paste as studied by NMR. Cem. Concr. Compos. 2018, 93, 54–62. [Google Scholar] [CrossRef]
- Peng, D.; Wang, Y.; Liu, X.; Tang, B.; Zhang, N. Preparation, characterization, and application of an eco-friendly sand-fixing material largely utilizing coal-based solid waste. J. Hazard. Mater. 2019, 373, 294–302. [Google Scholar] [CrossRef] [PubMed]
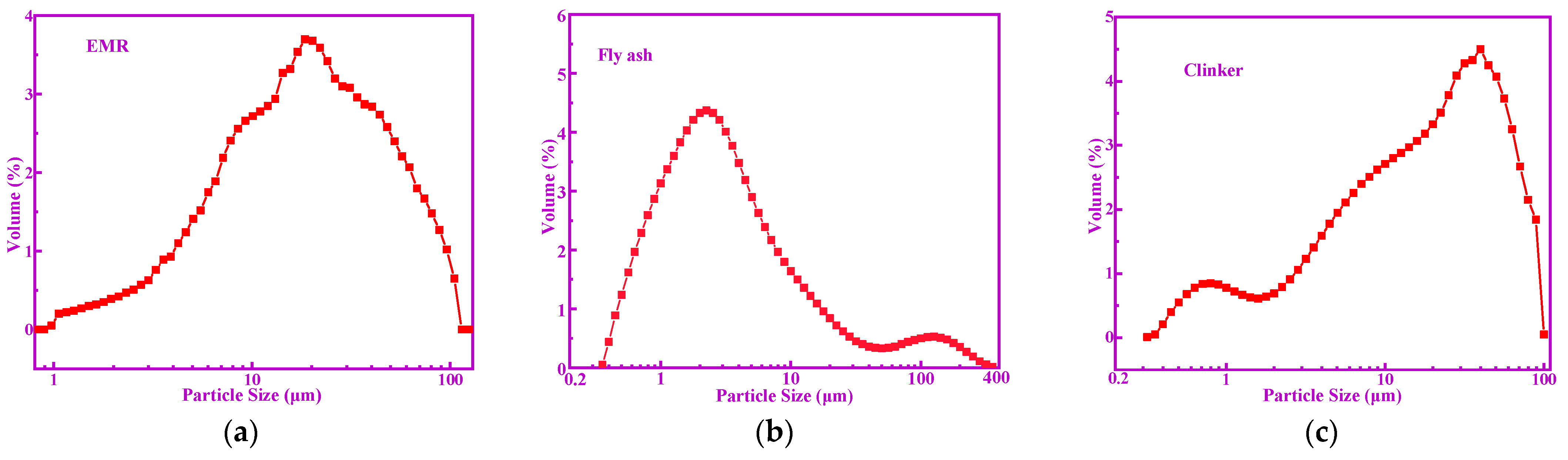
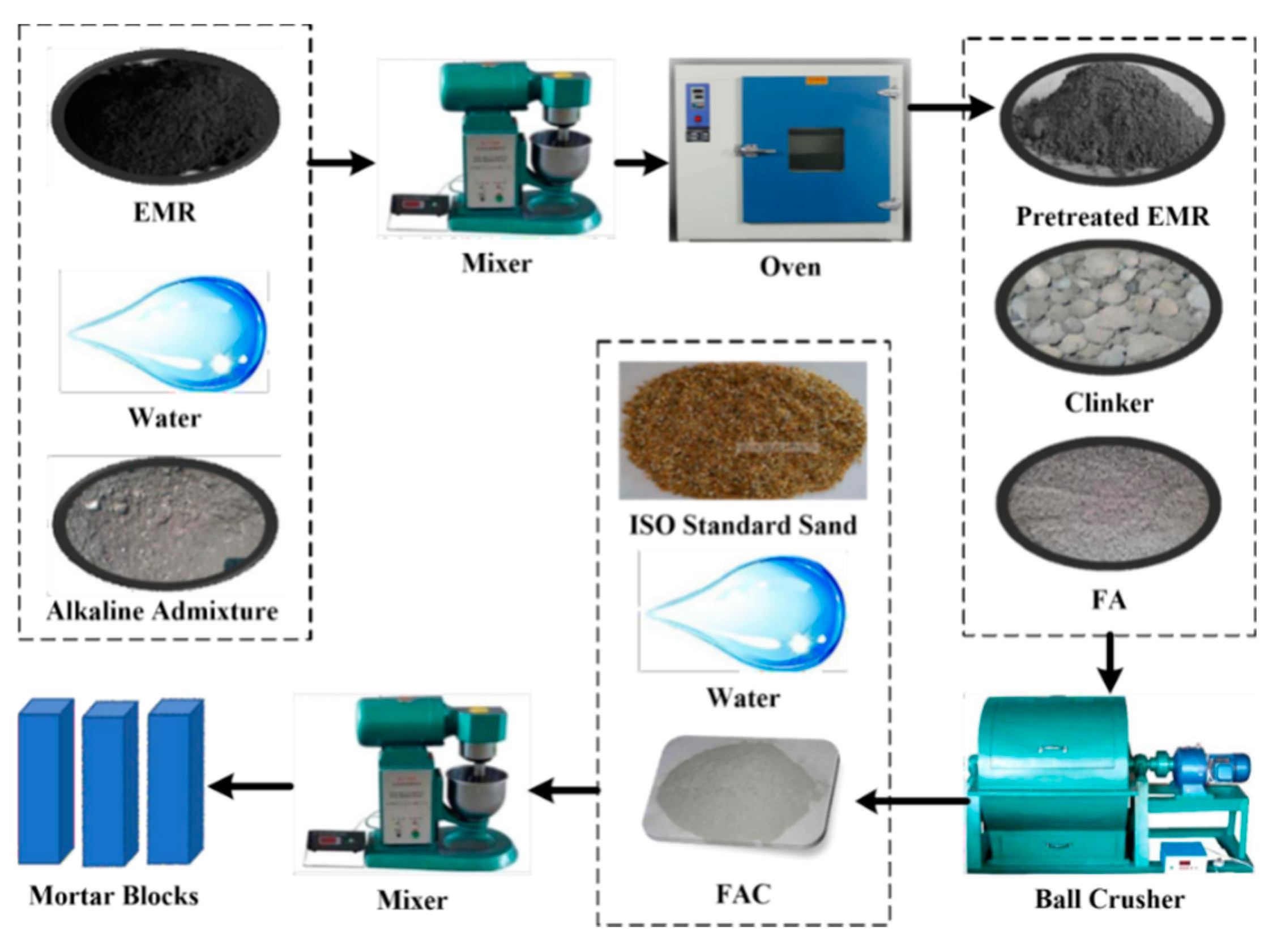



| Oxides | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | MnO | SO3 | LOI |
|---|---|---|---|---|---|---|---|---|
| EMR | 29.63 | 8.32 | 5.41 | 14.82 | 1.79 | 2.02 | 29.54 | 8.47 |
| FA | 47.38 | 37.09 | 8.02 | 3.12 | 0.34 | 0.05 | 1.35 | 2.63 |
| Clinker | 18.6 | 3.81 | 3.25 | 64.85 | 2.89 | 0.34 | 0.43 | 4.14 |
| Samples | Clinker | EMR | FA | SO3 | Fineness (m2/kg) |
|---|---|---|---|---|---|
| FAC0 | 55 | 0 | 45 | 0.8 | 377 |
| FAC1 | 55 | 5 | 40 | 2.3 | 380 |
| FAC2 | 55 | 10 | 35 | 3.1 | 385 |
| FAC3 | 55 | 15 | 30 | 4.2 | 376 |
| FAC4 | 55 | 20 | 25 | 5.4 | 373 |
| Samples | BET (m2/g) | Total Pore Volume (mg/L) | Average Pore Diameter (nm) | Porosity (%) | Bulk Density (g/cm3) | Apparent Density (g/cm3) |
|---|---|---|---|---|---|---|
| FAC1 | 231.00 | 0.31 | 17.6 | 40.32 | 1.30 | 2.22 |
| FAC2 | 230.00 | 0.29 | 15.8 | 39.44 | 1.34 | 2.24 |
| FAC3 | 232.00 | 0.32 | 18.4 | 41.76 | 1.30 | 2.19 |
| Samples | Porosity (%) | Pore Size Distribution (%) | ||
|---|---|---|---|---|
| <10 nm | 10–50 nm | >50 nm | ||
| FAC1 | 40.32 | 7.91 | 16.17 | 16.24 |
| FAC2 | 39.44 | 9.78 | 15.11 | 14.55 |
| FAC3 | 41.76 | 9.15 | 13.95 | 18.66 |
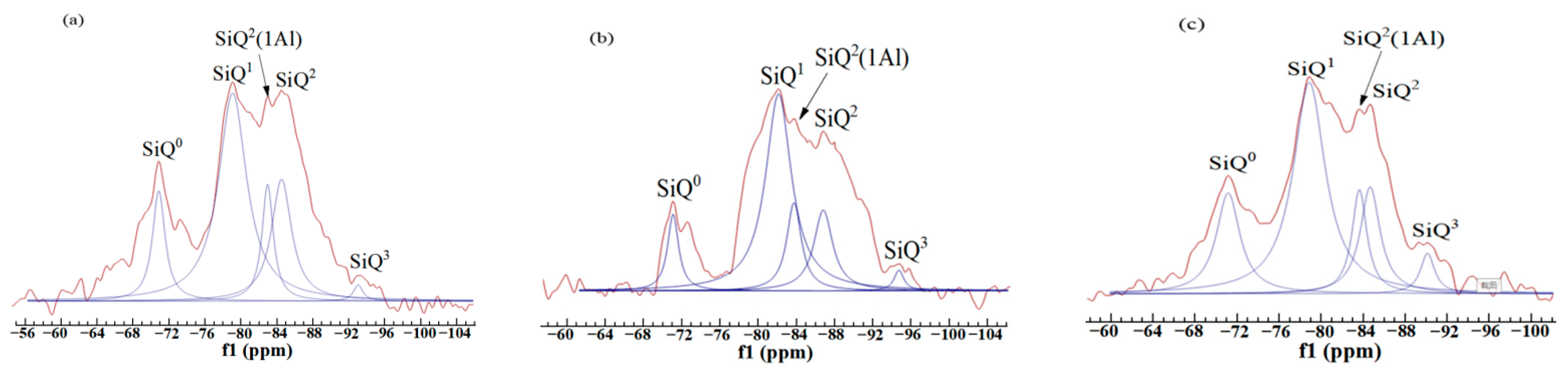
| Symbol | SiQ0 | SiQ1 | SiQ2 | SiQ3 | SiQ4 |
|---|---|---|---|---|---|
| Chemical shift (ppm) | −68–−76 | −76–−82 | −82–−88 | −88–−98 | −98–−129 |
| Samples | Peak Position (ppm) | Assign | Relative | RBO Value |
|---|---|---|---|---|
| FAC1 | −71.29 | SiQ0 | 23.83 | 30.99% |
| −79.63 | SiQ1 | 100 | ||
| −84.80 | SiQ2(1Al) | 22.27 | ||
| −85.15 | SiQ2 | 41.80 | ||
| −93.81 | SiQ3 | 2.73 | ||
| FAC2 | −71.45 | SiQ0 | 16.31 | 32.60% |
| −81.37 | SiQ1 | 100 | ||
| −83.97 | SiQ2(1Al) | 29.08 | ||
| −87.00 | SiQ2 | 34.75 | ||
| −94.84 | SiQ3 | 4.26 | ||
| FAC3 | −71.54 | SiQ0 | 35.02 | 29.58% |
| −79.42 | SiQ1 | 100 | ||
| −84.29 | SiQ2(1Al) | 22.96 | ||
| −85.34 | SiQ2 | 31.13 | ||
| −90.91 | SiQ3 | 8.56 |
| Samples | Mn | Cr | Cd | As | Pb | NH3-N |
|---|---|---|---|---|---|---|
| EMR | 1168 | 0.205 | 0.064 | 0.158 | 0.293 | 24.53 |
| FAC1 | <0.001 | <0.001 | <0.001 | <0.001 | 0.001 | 0.26 |
| FAC2 | <0.001 | 0.001 | <0.001 | <0.001 | 0.003 | 0.35 |
| FAC3 | <0.001 | 0.002 | <0.001 | <0.001 | 0.005 | 0.52 |
| GB/T 8978-1996 | 2.0 | 1.5 | 0.1 | 0.5 | 1.0 | 15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zhang, N.; Ren, Y.; Xu, Y.; Liu, X. Effect of Electrolytic Manganese Residue in Fly Ash-Based Cementitious Material: Hydration Behavior and Microstructure. Materials 2021, 14, 7047. https://doi.org/10.3390/ma14227047
Wang Y, Zhang N, Ren Y, Xu Y, Liu X. Effect of Electrolytic Manganese Residue in Fly Ash-Based Cementitious Material: Hydration Behavior and Microstructure. Materials. 2021; 14(22):7047. https://doi.org/10.3390/ma14227047
Chicago/Turabian StyleWang, Yaguang, Na Zhang, Yongyu Ren, Yingtang Xu, and Xiaoming Liu. 2021. "Effect of Electrolytic Manganese Residue in Fly Ash-Based Cementitious Material: Hydration Behavior and Microstructure" Materials 14, no. 22: 7047. https://doi.org/10.3390/ma14227047
APA StyleWang, Y., Zhang, N., Ren, Y., Xu, Y., & Liu, X. (2021). Effect of Electrolytic Manganese Residue in Fly Ash-Based Cementitious Material: Hydration Behavior and Microstructure. Materials, 14(22), 7047. https://doi.org/10.3390/ma14227047