Selected Derivatives of Erythromycin B-In Silico and Anti-Malarial Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Instrumentation
2.1.1. NMR (Nuclear Magnetic Resonance) Spectrometers
Bruker Avance 300 Spectrometer
Varian Unity 500 Spectrometer
Bruker AMX 500 Spectrometer
2.1.2. Mass Spectrometer
2.1.3. pH Meter
2.1.4. Melting Point Apparatus
2.1.5. Thin Layer Chromatography (TLC)
2.2. Two-Dimensional NMR Spectroscopy
2.2.1. TOCSY and DQF-COSY
2.2.2. HMQC and HMBC
2.2.3. DOSY
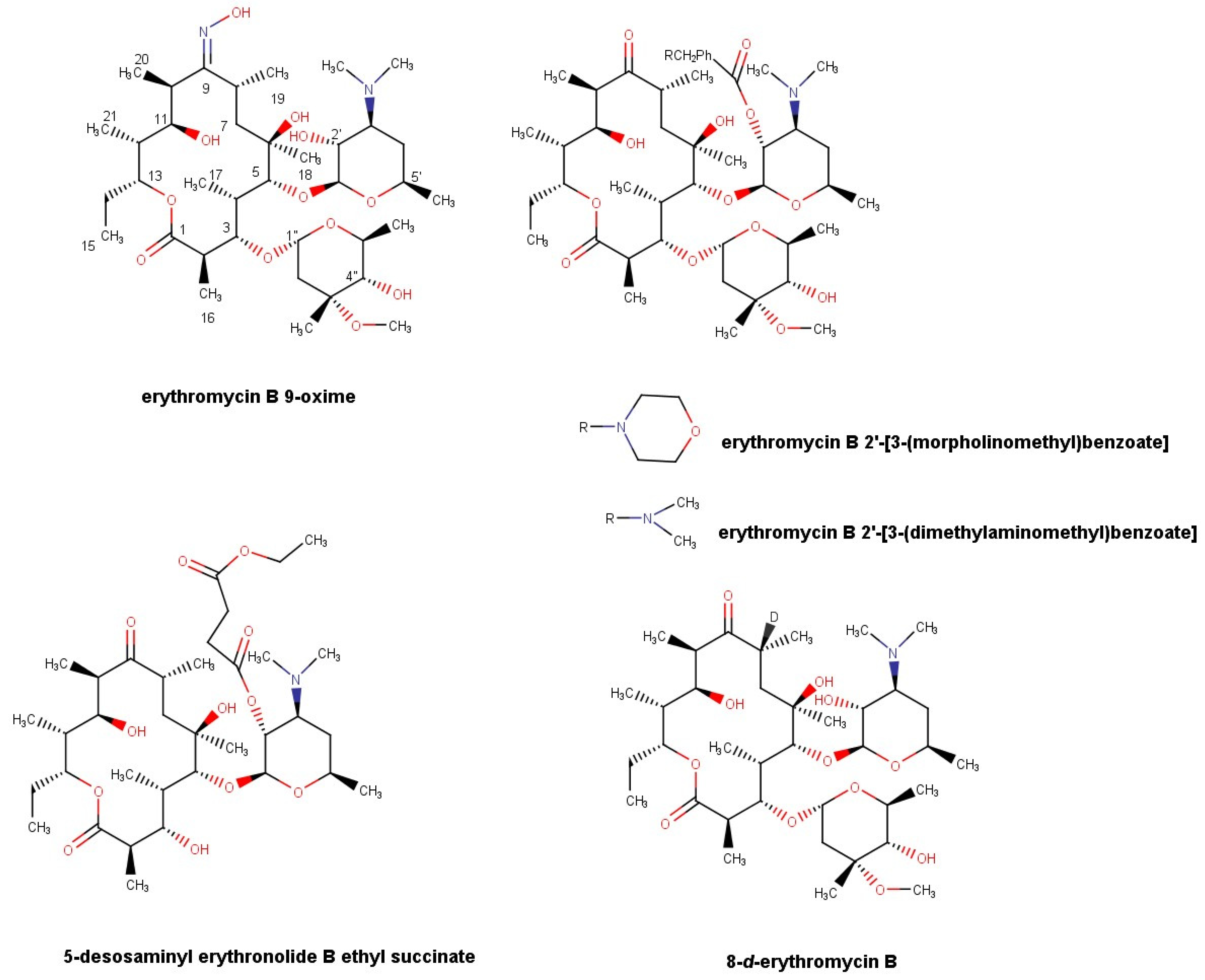
2.3. Synthesis of Erythromycin B Derivatives
2.3.1. Synthesis of Erythromycin B Oxime
The First Method
The Second Method
2.3.2. Synthesis of Erythromycin B 2′-(3-chloromethylbenzoate) (EBCMB)
2.3.3. Synthesis of Erythromycin B 2′-[3-(morpholinomethyl)benzoate] (EBMMB)
2.3.4. Synthesis of Erythromycin B 2′-[3-(dimethylaminomethyl)benzoate] (EBDMAMB)
2.3.5. Synthesis of 5-Desosaminyl Erythronolide B Ethyl Succinate
2.3.6. Synthesis of Erythromycin B Enol Ether
2.3.7. Synthesis of 8-d-erythromycin B (8D-EB)
2.4. In Vitro Determination of the Anti-Malarial Activity of Selected Erythromycin B Derivatives
2.4.1. Cultivation of P. falciparum Parasite
2.4.2. Synchronisation of K1 P. falciparum
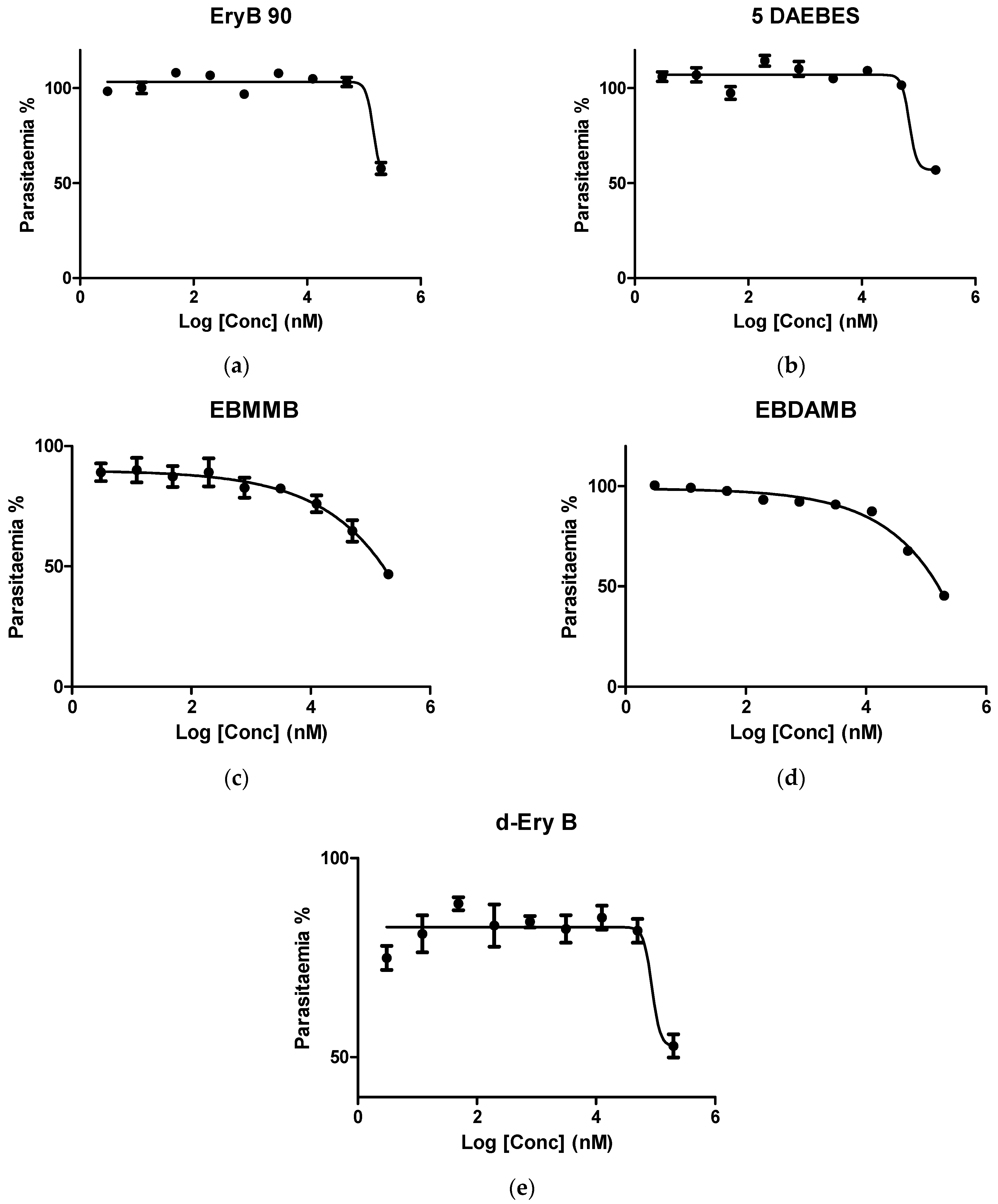
2.4.3. Determination of IC50 in K1 P. falciparum
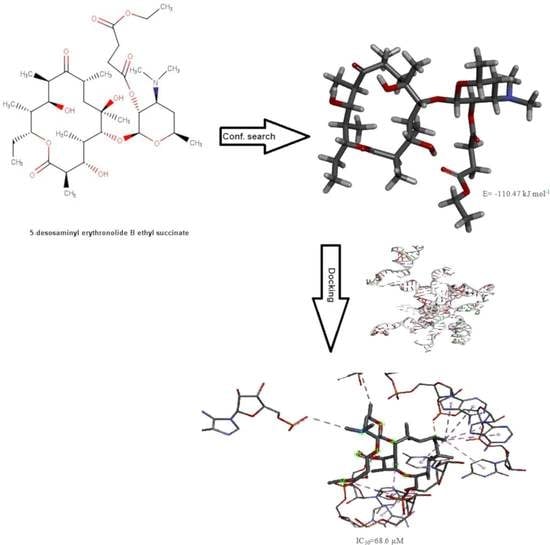
2.5. Conformational Search Studies
2.5.1. Unconstrained Conformational Search
2.5.2. Constrained Conformational Search
2.6. Molecular Docking Studies
3. Results and Discussion
3.1. Synthesis of N-Substituted 2′-(3-aminomethylbenzoate) Esters of Erythromycin B
3.2. In Vitro Investigation of Synthesised Erythromycin B Derivatives against P. falciparum K1 Strain
3.3. Conformational Search Studies of the Investigated Derivatives of Erythromycin B in Chloroform
3.3.1. Unconstrained Conformational Search of the Investigated Derivatives of Erythromycin B in Chloroform
3.3.2. Constrained Conformational Search of Erythromycin B 2′-[3-(dimethylaminomethyl)benzoate]
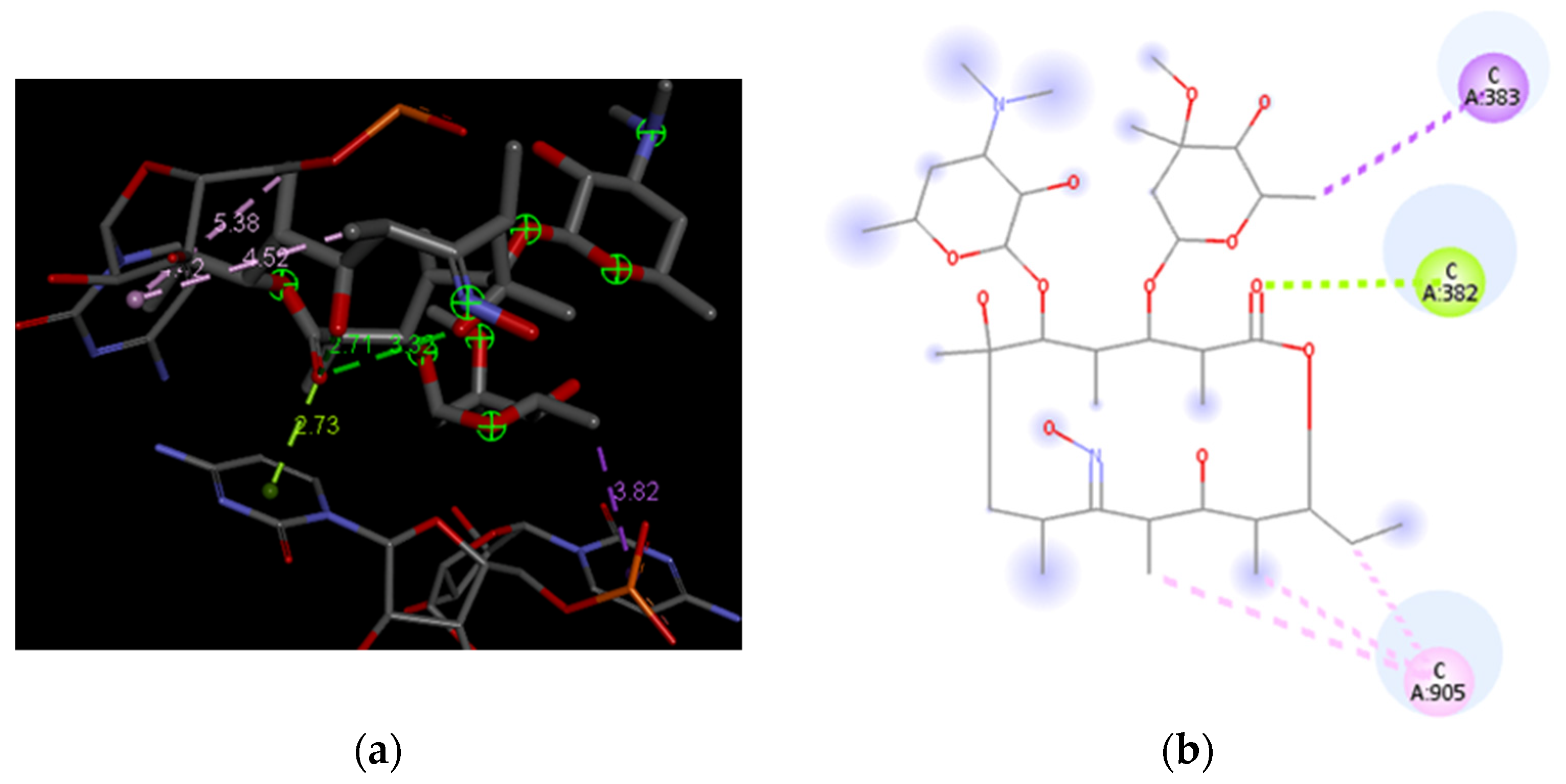
3.4. Docking Analysis of the Investigated Derivatives of Erythromycin B into In Silico Constructed Segment of the Exit Tunnel from the Apicoplast Ribosome of P. falciparum
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Malaria. Available online: https://www.who.int/news-room/fact-sheets/detail/malaria (accessed on 13 November 2021).
- Ford, J.M.; Hait, W.N. Pharmacology of drugs that alter multidrug resistance in cancer. Pharmacol. Rev. 1990, 42, 155–199. [Google Scholar] [PubMed]
- Menezes, M.S.; Kirchgatter, K.; Di Santi, S.M.F.; Savalli, C.; Monteiro, F.G.; Paula, G.A.; Ferreira, E.I. In vitro evaluation of erythromycin in chloroquine resistant Brazilian P. falciparum freshly isolates: Modulation effect and antimalarial activity evidence. Rev. Inst. Med. Trop. São Paulo 1999, 41, 249–253. [Google Scholar] [CrossRef]
- Hofsli, E.; Nissen-Meyer, J. Effect of erythromycin and tumour necrosis factor on the drug resistance of multidrug-resistant cells: Reversal of drug resistance by erythromycin. Int. J. Cancer 1989, 43, 520–525. [Google Scholar] [CrossRef]
- Hofsli, E.; Nissen-Meyer, J. Reversal of multidrug resistance by lipophilic drugs. Cancer Res. 1990, 50, 3997–4002. [Google Scholar] [PubMed]
- Robinson, B.L.; Warhurst, D.C. Antimalarial activity of erythromycin. Trans. R. Soc. Trop. Med. Hyg. 1972, 66, 525. [Google Scholar]
- Gaillard, T.; Madamet, M.; Tsombeng, F.F.; Dormoi, J. Antibiotics in malaria therapy: Which antibiotics except tetracyclines and macrolides may be used against malaria? Malar. J. 2016, 15, 556. [Google Scholar] [CrossRef]
- Sowunmi, A.; Gbotosho, G.O.; Fateye, B.A.; Adedeji, A.A. Predictors of the failure of treatment with trimethoprim-sulfamethoxazole in children with uncomplicated, Plasmodium falciparum malaria. Ann. Trop. Med. Parasitol. 2006, 100, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Mermin, J.; Ekwaru, J.P.; Liechty, C.A.; Were, W.; Downing, R.; Ransom, R.; Weidle, P.; Lule, J.; Coutinho, A.; Solberg, P. Effect of co-trimoxazole prophylaxis, antiretroviral therapy, and insecticide-treated bednets on the frequency of malaria in HIV-1-infected adults in Uganda: A prospective cohort study. Lancet 2006, 367, 1256–1261. [Google Scholar] [CrossRef]
- Manyando, C.; Njunju, E.M.; Mwakazanga, D.; Chongwe, G.; Mkandawire, R.; Champo, D.; Mulenga, M.; De Crop, M.; Claeys, Y.; Ravinetto, R.M.; et al. Safety of daily co-trimoxazole in pregnancy in an area of changing malaria epidemiology: A phase 3b randomized controlled clinical trial. PLoS ONE 2014, 9, 96017. [Google Scholar]
- Corbett, E.L.; Churchyard, G.J.; Charalambos, S.; Samb, B.; Moloi, V.; Clayton, T.C.; Grant, A.D.; Murray, J.; Hayes, R.J.; De Cock, K.M. Morbidity and mortality in South African gold miners: Impact of untreated disease due to human immunodeficiency virus. Clin. Infect. Dis. 2002, 34, 1251–1258. [Google Scholar] [CrossRef]
- Bukvić Krajačić, M.; Perić, M.; Smith, K.S.; Ivezić Schonfeld, Z.; Žiher, D.; Fajdetić, A.; Kujundžić, N.; Schonfeld, W.; Landek, G.; Padovan, J.; et al. Synthesis, structure-activity relationship, and antimalarial activity of ureas and thioureas of 15-membered azalides. J. Med. Chem. 2011, 54, 3595–3605. [Google Scholar] [CrossRef] [PubMed]
- Perić, M.; Fajdetić, A.; Rupčić, R.; Alihodžić, S.; Žiher, D.; Bukvić Krajačić, M.; Smith, K.S.; Ivezić-Schonfeld, Z.; Padovan, J.; Landek, G.; et al. Antimalarial activity of 9a-N substituted 15-membered azalides with improved in vitro and in vivo activity over azithromycin. J. Med. Chem. 2012, 55, 1389–1401. [Google Scholar] [CrossRef]
- Gaillard, T.; Dormoi, J.; Madamet, M.; Pradines, B. Macrolides and associated antibiotics based on similar mechanism of action like lincosamides in malaria. Malar. J. 2016, 15, 85. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, A.B.; Sun, Q.; Nkrumah, L.J.; Dunne, M.W.; Sacchettini, J.C.; Fidock, D.A. In vitro efficacy, resistance selection, and structural modeling studies implicate the malarial parasite apicoplast as the target of azithromycin. J. Biol. Chem. 2007, 282, 2494–2504. [Google Scholar] [CrossRef]
- Ohrt, C.; Willingmyre, G.D.; Lee, P.; Knirsch, C.; Milhous, W. Assessment of azithromycin in combination with other antimalarial drugs against Plasmodium falciparum in vitro. Antimicrob. Agents Chemother. 2002, 46, 2518–2524. [Google Scholar] [CrossRef] [PubMed]
- Mordi, M.N.; Pelta, M.D.; Boote, V.; Morris, G.A.; Barber, J. Acid-catalysed degradation of clarithromycin and erythromycin B: A comparative study using NMR spectroscopy. J. Med. Chem. 2000, 43, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Cachet, T.; Roets, E.; Hoogmartens, J.; Vanderhaeghe, H. Separation of novel derivatives from commercial erythromycin samples by thin layer chromatography. J. Chrom. 1987, 403, 343–349. [Google Scholar] [CrossRef]
- Barjat, H.; Morris, G.A.; Smart, S.; Swanson, A.G.; William, S.C.R. Reference deconvolution using multiplet reference signal. J. Mag. Res. Ser. A 1995, 116, 206–214. [Google Scholar] [CrossRef]
- Barjat, H.; Morris, G.A.; Smart, S.; Swanson, A.G.; William, S.C.R. High-resolution diffusion-ordered 2D spectroscopy (HR-DOSY). J. Mag. Res. Ser. B 1995, 108, 171–172. [Google Scholar]
- Morris, G.A. Diffusion-Ordered Spectroscopy (DOSY). Encyclopedia of Nuclear Magnetic Resonance: Advances in NMR; John Wiley and Sons: Chichester, UK, 2002; pp. 35–44. [Google Scholar]
- Gasc, J.C.; D’Ambrieres, S.G.; Lutz, A.; Chantot, J.F. New ether oxime derivatives of erythromycin A: A structure activity relationship study. J. Antibiot. 1991, 44, 313–331. [Google Scholar] [CrossRef][Green Version]
- Watanabe, Y.; Morimoto, S.; Adachi, T.; Kashimura, M.; Asaka, T. Chemical modification of erythromycins IX. Selective methylation at the C-6 hydroxyl group of erythromycin A oxime derivatives and preparation of clarithromycin. J. Antibiot. 1993, 46, 647–660. [Google Scholar] [CrossRef] [PubMed]
- Jensen, E.; Bundgaard, H.; Falch, E. Design of a water-soluble, solution-stable and biolabile prodrug of metronidazole for parenteral administration: N-substituted aminomethylbenzoate esters. Int. J. Pharm. 1990, 58, 143–153. [Google Scholar] [CrossRef]
- Bhadra, P.K.; Morris, G.A.; Barber, J. Design, synthesis, and evaluation of stable and taste-free erythromycin proprodrugs. J. Med. Chem. 2005, 48, 3878–3884. [Google Scholar] [CrossRef] [PubMed]
- Hassanzadeh, A.; Gorry, P.A.; Morris, G.A.; Barber, J. Pediatric erythromycins: A comparison of the properties of erythromycins A and B 2′-ethyl succinates. J. Med. Chem. 2006, 49, 6334–6342. [Google Scholar] [CrossRef]
- Bhadra, P.K.; Hassanzadeh, A.; Arsic, B.; Allison, D.G.; Morris, G.A.; Barber, J. Enhancement of the properties of a drug by mono-deuteriation: Reduction of acid-catalysed formation of a gut-motilide enol ether from 8-deuterio-erythromycin B. Org. Biomol. Chem. 2016, 14, 6289–6296. [Google Scholar] [CrossRef] [PubMed]
- Duhovny, D.; Nussinov, R.; Wolfson, H.J. Efficient Unbound Docking of Rigid Molecules. In Proceedings of the 2nd Workshop on Algorithms in Bioinformatics (WABI), Rome, Italy, 16–21 September 2002; Lecture Notes in Computer Science 2452. Guigo, G., Ed.; Springer: Berlin/Heidelberg, Germany, 2002; pp. 185–200. [Google Scholar]
- Schneidman-Duhovny, D.; Inbar, Y.; Polak, V.; Shatsky, M.; Halperin, I.; Benyamini, H.; Barzilai, A.; Dror, O.; Haspel, N.; Nussinov, R.; et al. Taking geometry to its edge: Fast unbound rigid (and hinge-bent) docking. Proteins 2003, 52, 107–112. [Google Scholar] [CrossRef]
- Schneidman-Duhovny, D.; Inbar, Y.; Nussinov, R.; Wolfson, H.J. PatchDock and SymmDock: Servers for rigid and symmetric docking. Nucleic Acids Res. 2005, 33, W363–W367. [Google Scholar] [CrossRef]
- Andrusier, N.; Nussinov, R.; Wolfson, H.J. FireDock: Fast Interaction Refinement in Molecular Docking. Proteins 2007, 69, 139–159. [Google Scholar] [CrossRef]
- Mashiach, E.; Schneidman-Duhovny, D.; Andrusier, N.; Nussinov, R.; Wolfson, H.J. FireDock: A web server for fast interaction refinement in molecular docking. Nucleic Acids Res. 2008, 36, W229–W232. [Google Scholar] [CrossRef]
- Pranab, B. Erythromycin B: An Old Drug for the New Millennium. Ph.D. Thesis, The University of Manchester, Manchester, UK, 2003. [Google Scholar]
- Vossen, M.G.; Pferschy, S.; Chiba, P.; Noedl, H. The SYBR green I malaria drug sensitivity assay: Performance in low parasitemia samples. Am. J. Trop. Med. Hyg. 2010, 82, 398–401. [Google Scholar] [CrossRef]
- Rason, M.A.; Randriantsoa, T.; Andrianantenaina, H.; Ratsimbasoa, A.; Menard, D. Performance and reliability of the SYBR Green I based assay for the routine monitoring of susceptibility of Plasmodium falciparum clinical isolates. Trans. R. Soc. Trop. Med. Hyg. 2008, 102, 346–351. [Google Scholar] [CrossRef]
- Li, L.; Li, C.; Zhang, Z.; Alexov, E. On the dielectric “constant” of proteins: Smooth dielectric function for macromolecular modeling and its implementation in DelPhi. J. Chem. Theory Comput. 2013, 9, 2126–2136. [Google Scholar] [CrossRef]
- Xi, K.; Wang, F.-H.; Xiong, G.; Zhang, Z.-L.; Tan, Z.-J. Competitive binding of Mg2+ and Na+ ions to nucleic acids: From helices to tertiary structures. Biophys. J. 2018, 114, 1776–1790. [Google Scholar] [CrossRef]
- Everett, J.R.; Tyler, J.W. The conformational analysis of erythromycin A. J. Chem. Soc. Perkin Trans. 2 1987, 11, 1659–1667. [Google Scholar] [CrossRef]
- Arsic, B.; Awan, A.; Brennan, R.J.; Aguilar, J.A.; Ledder, R.; McBain, A.J.; Regan, A.C.; Barber, J. Theoretical and experimental investigation on clarithromycin, erythromycin A and azithromycin and descladinosyl derivatives of clarithromycin and azithromycin with 3-O substitution as antibacterial agents. Med. Chem. Commun. 2014, 5, 1347–1354. [Google Scholar] [CrossRef]
- Arsic, B.; Barber, J. In silico study on the apicoplast L4 ribosomal protein and three domains from 23S rRNA from Plasmodium falciparum and comparison with the existing cocrystal structures. Chem. Naissensis 2019, 2, 50–61. [Google Scholar]

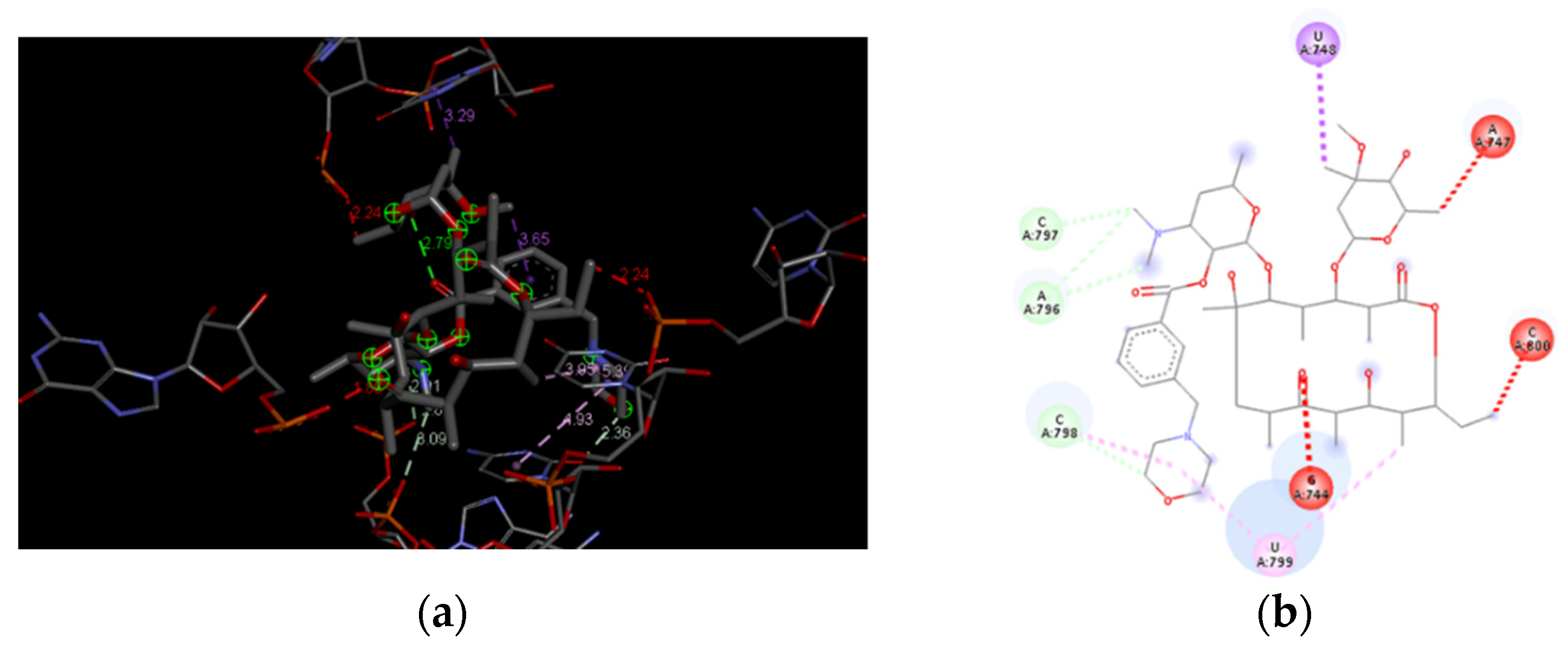
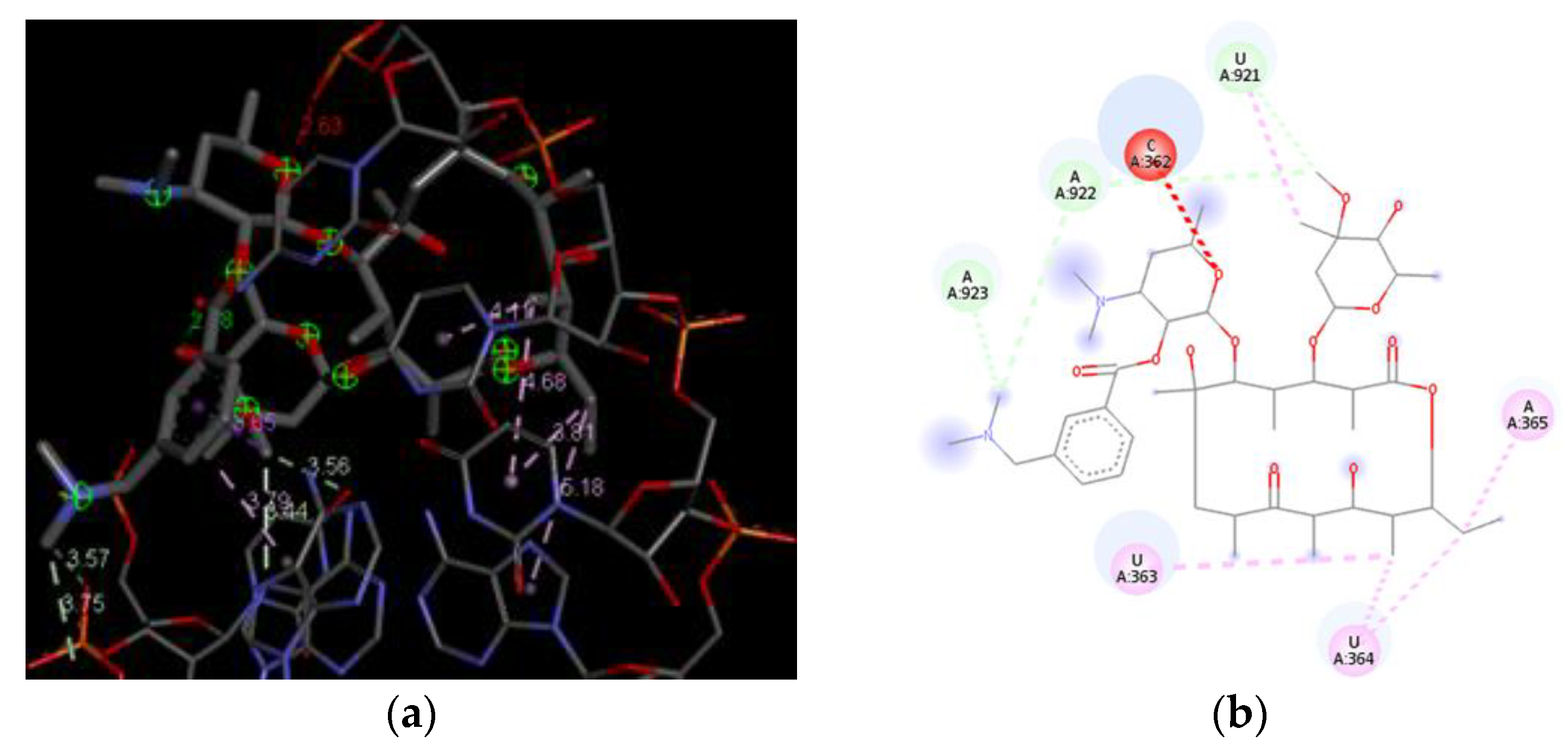

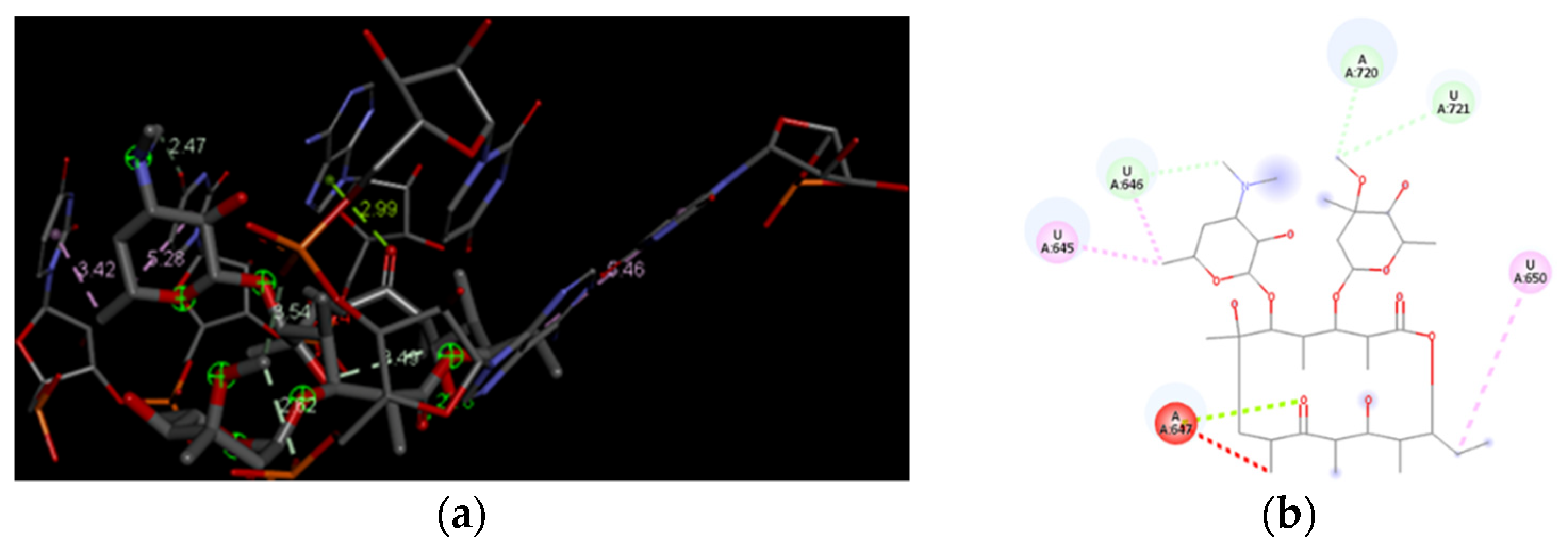
| Compounds | Global Minimum | Distances (Å) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Energy (kJ/mol) | Number of Repeats | H4-H11 | H15-H16 | H3-H8 | H3-H11 | H4-H18 | H5-H18 | H16-H17 | |
| Erythromycin B 9-oxime | 124.27 | 3 | 6.70 | 4.59 | 2.20 | 5.18 | 2.77 | 3.46 | 2.93 |
| Erythromycin B 2′-[3-(morpholinomethyl) benzoate] | 102.62 | 2 | 3.02 | 2.88 | 5.67 | 3.92 | 4.08 | 2.57 | 3.83 |
| Erythromycin B 2′-[3-(dimethylaminomethyl) benzoate] | 70.16 | 4 | 4.79 | 3.69 | 2.34 | 2.15 | 2.51 | 3.48 | 2.57 |
| 5-Desosaminyl erythronolide B ethyl succinate | −118.56 | 1 | 3.82 | 3.05 | 6.93 | 4.72 | 3.55 | 3.44 | 4.99 |
| 8-d-Erythromycin B | 111.13 | 1 | 3.97 | 3.76 | 2.35 * | 2.19 | 2.73 | 3.47 | 2.26 |
| Solutions | Solution Parameters | Derivatives | ||||
|---|---|---|---|---|---|---|
| EryB 90 | EBMMB | EBDAMB | 5DAEBES | d-Ery B | ||
| 1 | Score | 7332 | 7578 | 7362 | 6430 | 7132 |
| Area | 886.20 | 972.00 | 1012.70 | 740.40 | 800.00 | |
| ACE | −341.11 | −477.95 | −490.63 | −324.01 | −337.27 | |
| 2 | Score | 7032 | 7388 | 7030 | 6222 | 6416 |
| Area | 839.20 | 951.20 | 745.90 | 739.90 | 754.20 | |
| ACE | −339.59 | −419.55 | −275.08 | −467.41 | −362.32 | |
| 3 | Score | 6640 | 7376 | 6980 | 6162 | 6296 |
| Area | 845.50 | 896.30 | 785.80 | 865.40 | 900.70 | |
| ACE | −277.37 | −424.72 | −376.62 | −404.81 | −342.87 | |
| 4 | Score | 6636 | 7276 | 6970 | 6110 | 6272 |
| Area | 700.10 | 966.70 | 827.10 | 840.20 | 749.90 | |
| ACE | −326.43 | −462.36 | −437.15 | −442.39 | −264.42 | |
| 5 | Score | 6598 | 6834 | 6954 | 6056 | 6260 |
| Area | 896.20 | 898.30 | 860.40 | 727.70 | 836.30 | |
| ACE | −408.91 | −461.77 | −418.69 | −365.69 | −280.40 | |
| 6 | Score | 6556 | 6830 | 6904 | 6000 | 6254 |
| Area | 704.80 | 814.00 | 858.30 | 759.10 | 701.70 | |
| ACE | −379.30 | −311.01 | −431.74 | −286.73 | −325.53 | |
| 7 | Score | 6554 | 6826 | 6896 | 5972 | 6250 |
| Area | 719.30 | 891.70 | 816.60 | 831.00 | 736.90 | |
| ACE | −336.11 | −334.69 | −362.42 | −319.28 | −386.86 | |
| 8 | Score | 6524 | 6824 | 6870 | 5952 | 6212 |
| Area | 802.30 | 885.40 | 812.00 | 716.10 | 722.80 | |
| ACE | −464.79 | −330.79 | −351.92 | −351.59 | −353.71 | |
| 9 | Score | 6500 | 6726 | 6868 | 5896 | 6190 |
| Area | 786.90 | 754.70 | 851.10 | 646.80 | 670.10 | |
| ACE | −389.76 | −291.68 | −349.78 | −267.87 | −306.69 | |
| 10 | Score | 6462 | 6722 | 6790 | 5892 | 6122 |
| Area | 714.60 | 1011.00 | 1014.50 | 693.60 | 804.90 | |
| ACE | −372.85 | −489.58 | −487.56 | −183.95 | −351.11 | |
| Rank | Derivatives | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| eryB9O | eryBMB | eryBDA | eryBD | eryB8D | |||||||||||||||||||||
| SN | GE | AVdW | RVdW | ACE | SN | GE | AVdW | RVdW | ACE | SN | GE | AVdW | RVdW | ACE | SN | GE | AVdW | RVdW | ACE | SN | GE | AVdW | RVdW | ACE | |
| 1 | 10 | −63.18 | −23.29 | 9.23 | −22.58 | 5 | −74.06 | −28.32 | 8.97 | −24.41 | 5 | −57.60 | −23.55 | 13.89 | −21.13 | 2 | −67.89 | −25.26 | 11.31 | −24.74 | 6 | −63.40 | −26.31 | 9.46 | −20.67 |
| 2 | 9 | −60.65 | −24.69 | 9.32 | −20.27 | 3 | −55.61 | −21.65 | 7.65 | −19.85 | 4 | −52.66 | −20.91 | 14.62 | −20.77 | 1 | −52.42 | −20.56 | 9.31 | −18.29 | 1 | −61.51 | −23.48 | 8.48 | −20.95 |
| 3 | 1 | −60.28 | −24.59 | 8.05 | −19.29 | 4 | −50.21 | −26.49 | 26.04 | −19.84 | 8 | −51.82 | −21.02 | 4.11 | −15.61 | 8 | −49.83 | −22.88 | 22.20 | −20.92 | 2 | −48.35 | −21.82 | 17.42 | −18.82 |
| 4 | 2 | −59.82 | −23.60 | 12.36 | −21.84 | 9 | −44.94 | −18.35 | 7.22 | −15.85 | 10 | −51.58 | −28.90 | 26.43 | −20.68 | 7 | −47.89 | −23.46 | 14.09 | −16.53 | 8 | −46.70 | −20.56 | 18.10 | −19.11 |
| 5 | 8 | −53.61 | −23.03 | 16.31 | −21.13 | 7 | −44.11 | −24.22 | 27.44 | −18.84 | 6 | −48.66 | −23.05 | 16.02 | −19.41 | 3 | −45.82 | −19.87 | 8.91 | −15.22 | 9 | −45.24 | −15.63 | 3.87 | −15.69 |
| 6 | 6 | −47.33 | −16.88 | 9.54 | −18.68 | 6 | −41.75 | −23.33 | 15.91 | −14.88 | 2 | −48.50 | −18.58 | 4.20 | −15.64 | 10 | −44.62 | −22.38 | 8.79 | −14.04 | 7 | −42.49 | −17.24 | 11.94 | −16.47 |
| 7 | 4 | −44.88 | −16.35 | 7.59 | −16.79 | 2 | −30.70 | −25.80 | 40.54 | −17.23 | 3 | −45.67 | −22.70 | 23.01 | −19.13 | 9 | −38.29 | −17.57 | 11.63 | −14.30 | 4 | −40.76 | −19.02 | 11.57 | −15.62 |
| 8 | 7 | −42.39 | −16.63 | 9.09 | −15.56 | 10 | −29.42 | −18.97 | 19.66 | −12.45 | 7 | −41.62 | −19.57 | 13.90 | −16.31 | 5 | −38.09 | −21.25 | 25.36 | −18.31 | 3 | −33.22 | −14.06 | 6.78 | −13.20 |
| 9 | 3 | −23.15 | −7.92 | 2.81 | −9.12 | 8 | −21.96 | −11.50 | 4.54 | −5.58 | 1 | −40.86 | −25.65 | 23.11 | −15.79 | 4 | −34.84 | −14.83 | 7.62 | −12.25 | 5 | −24.99 | −12.77 | 12.30 | −11.45 |
| 10 | 5 | −15.07 | −9.36 | 7.03 | −4.80 | 1 | −15.89 | −27.10 | 72.48 | −21.55 | 9 | −24.27 | −8.63 | 3.70 | −9.54 | 6 | −32.26 | −20.80 | 22.81 | −13.81 | 10 | −11.66 | −22.26 | 59.71 | −16.76 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhadra, P.K.; Magwaza, R.N.; Nirmalan, N.; Freeman, S.; Barber, J.; Arsic, B. Selected Derivatives of Erythromycin B-In Silico and Anti-Malarial Studies. Materials 2021, 14, 6980. https://doi.org/10.3390/ma14226980
Bhadra PK, Magwaza RN, Nirmalan N, Freeman S, Barber J, Arsic B. Selected Derivatives of Erythromycin B-In Silico and Anti-Malarial Studies. Materials. 2021; 14(22):6980. https://doi.org/10.3390/ma14226980
Chicago/Turabian StyleBhadra, Pranab K., Rachael N. Magwaza, Niroshini Nirmalan, Sally Freeman, Jill Barber, and Biljana Arsic. 2021. "Selected Derivatives of Erythromycin B-In Silico and Anti-Malarial Studies" Materials 14, no. 22: 6980. https://doi.org/10.3390/ma14226980
APA StyleBhadra, P. K., Magwaza, R. N., Nirmalan, N., Freeman, S., Barber, J., & Arsic, B. (2021). Selected Derivatives of Erythromycin B-In Silico and Anti-Malarial Studies. Materials, 14(22), 6980. https://doi.org/10.3390/ma14226980