Bending Fracture of Different Zirconia-Based Bioceramics for Dental Applications: A Comparative Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Morphology and Elemental Composition
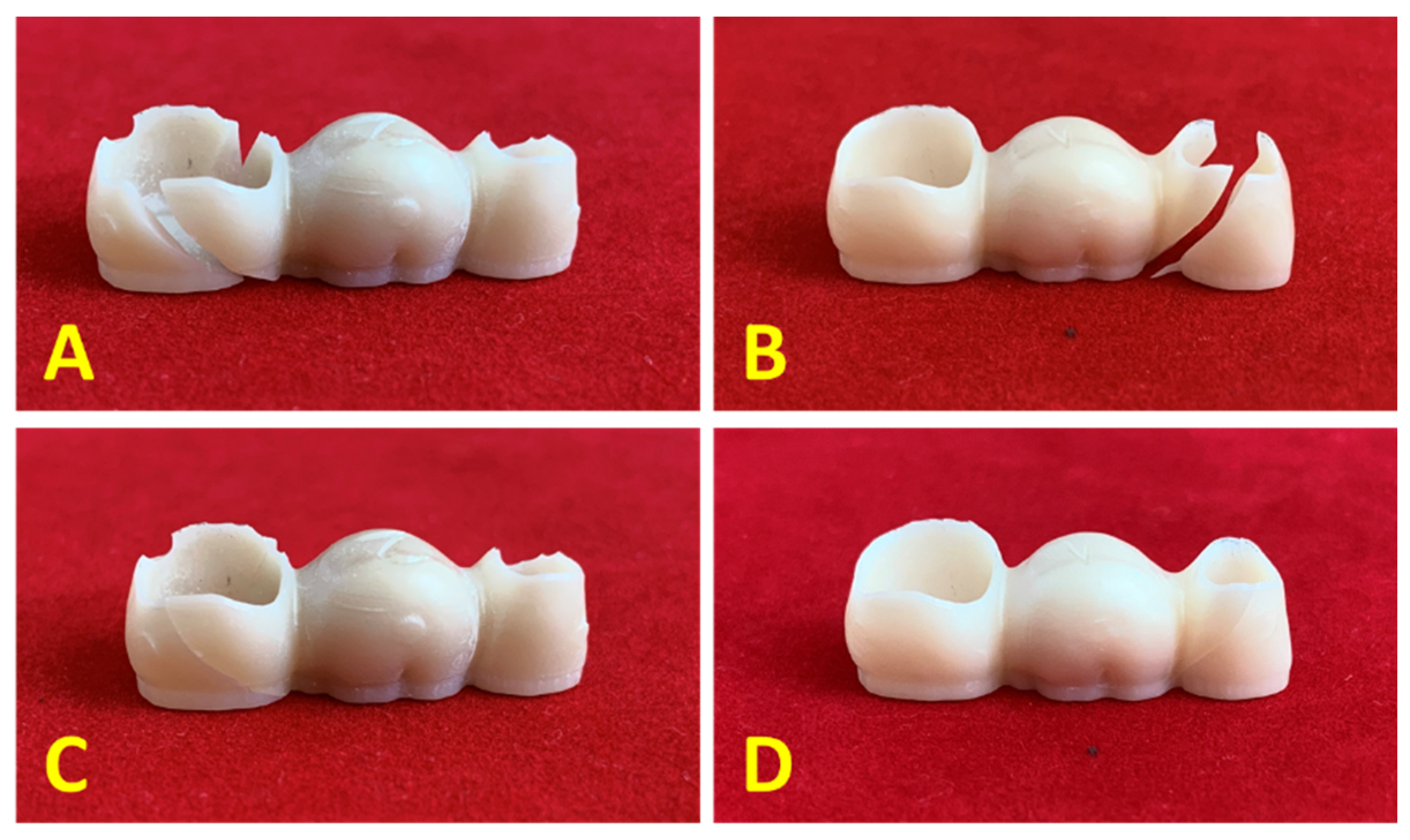
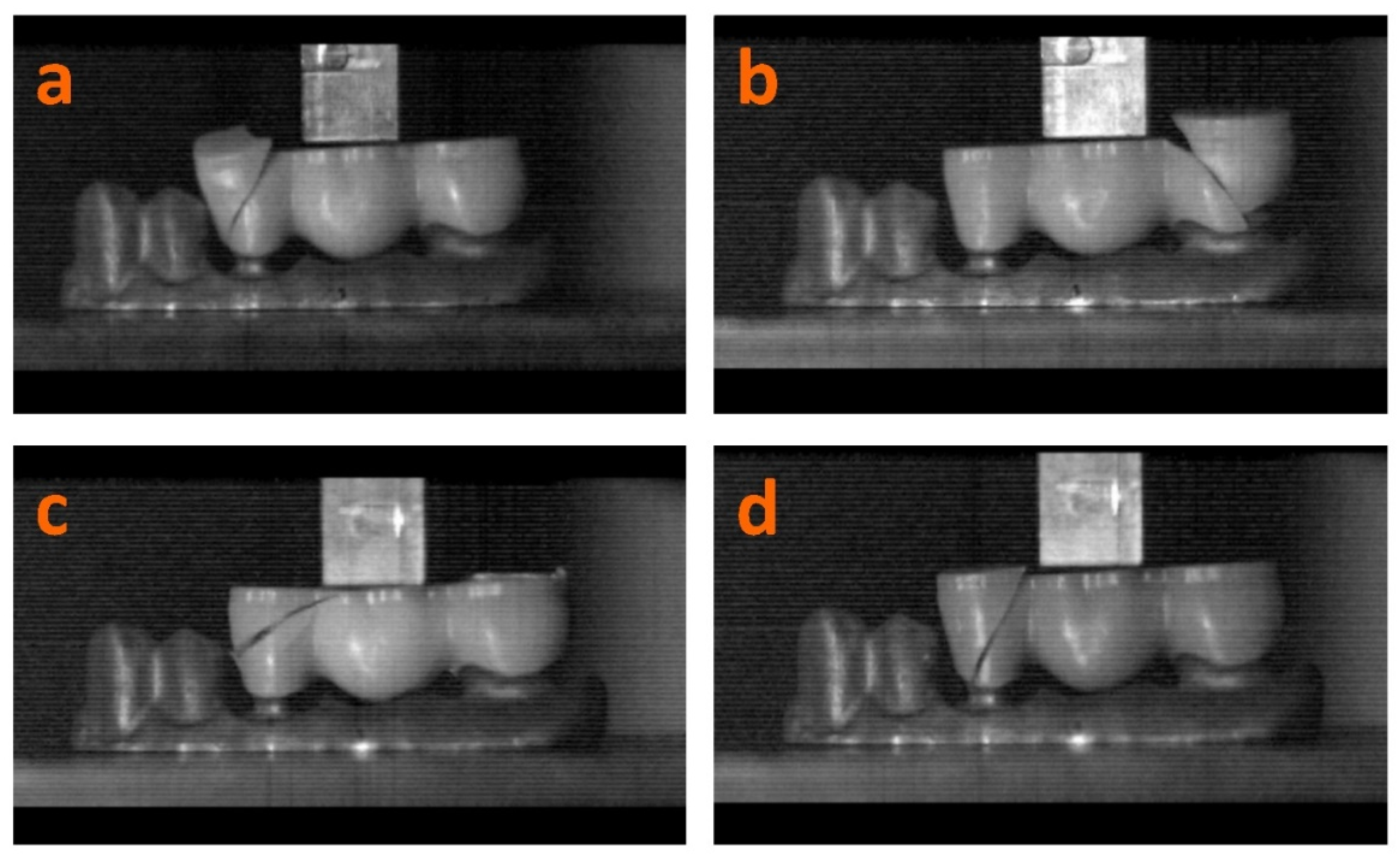
2.3. Bending Test
3. Results
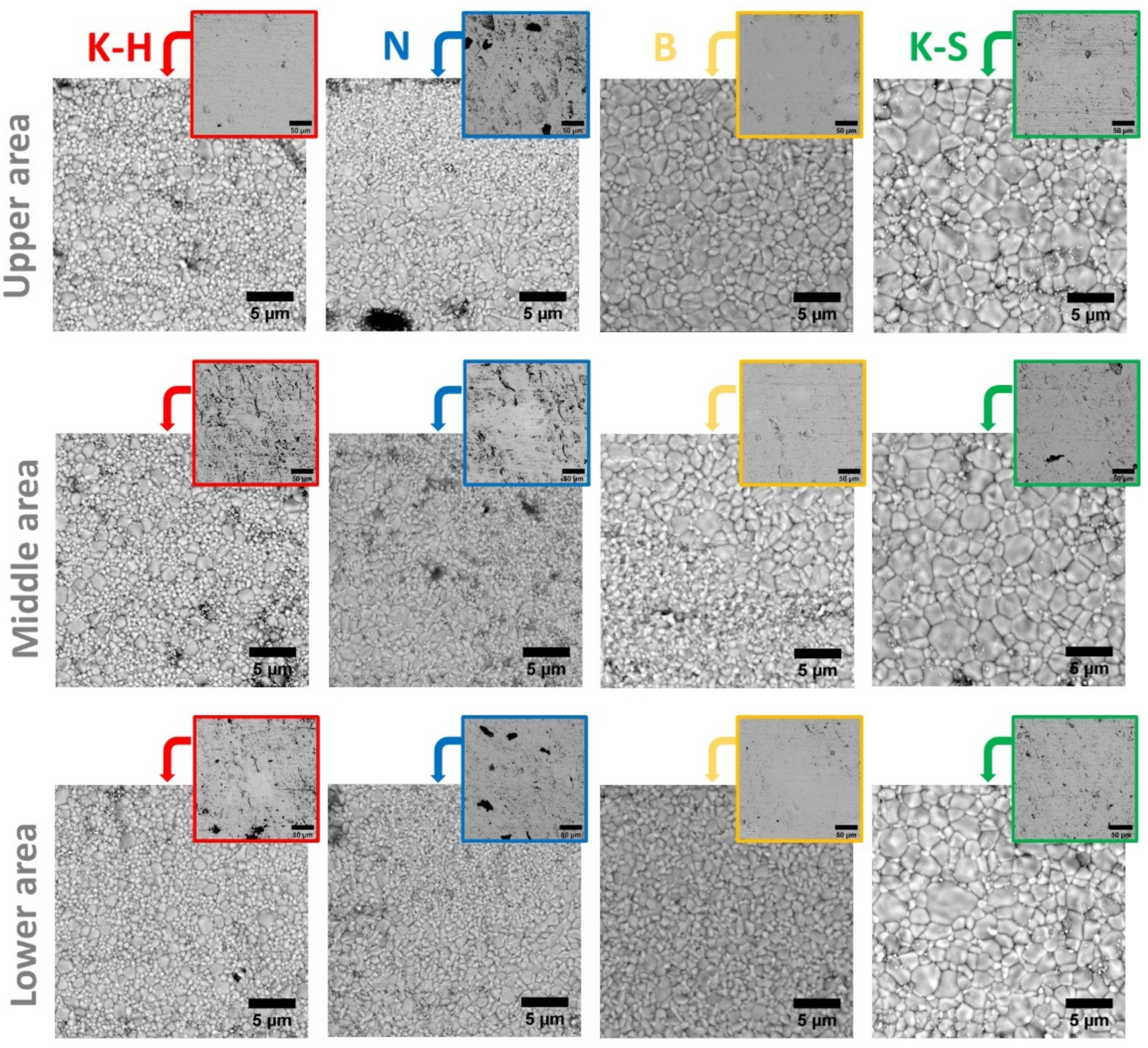
3.1. Morphology
- -
- The K-H specimens present both large and small grains, randomly distributed;
- -
- The B specimens present larger grains in the upper region, the grain size decreasing gradually to the lower region;
- -
- The N specimens have a multilayered structure regardless of the examined area, which consists of one layer with smaller grains, followed by a layer with larger ones;
- -
- Compared to the other investigated materials, the K-S specimens present the largest grains.
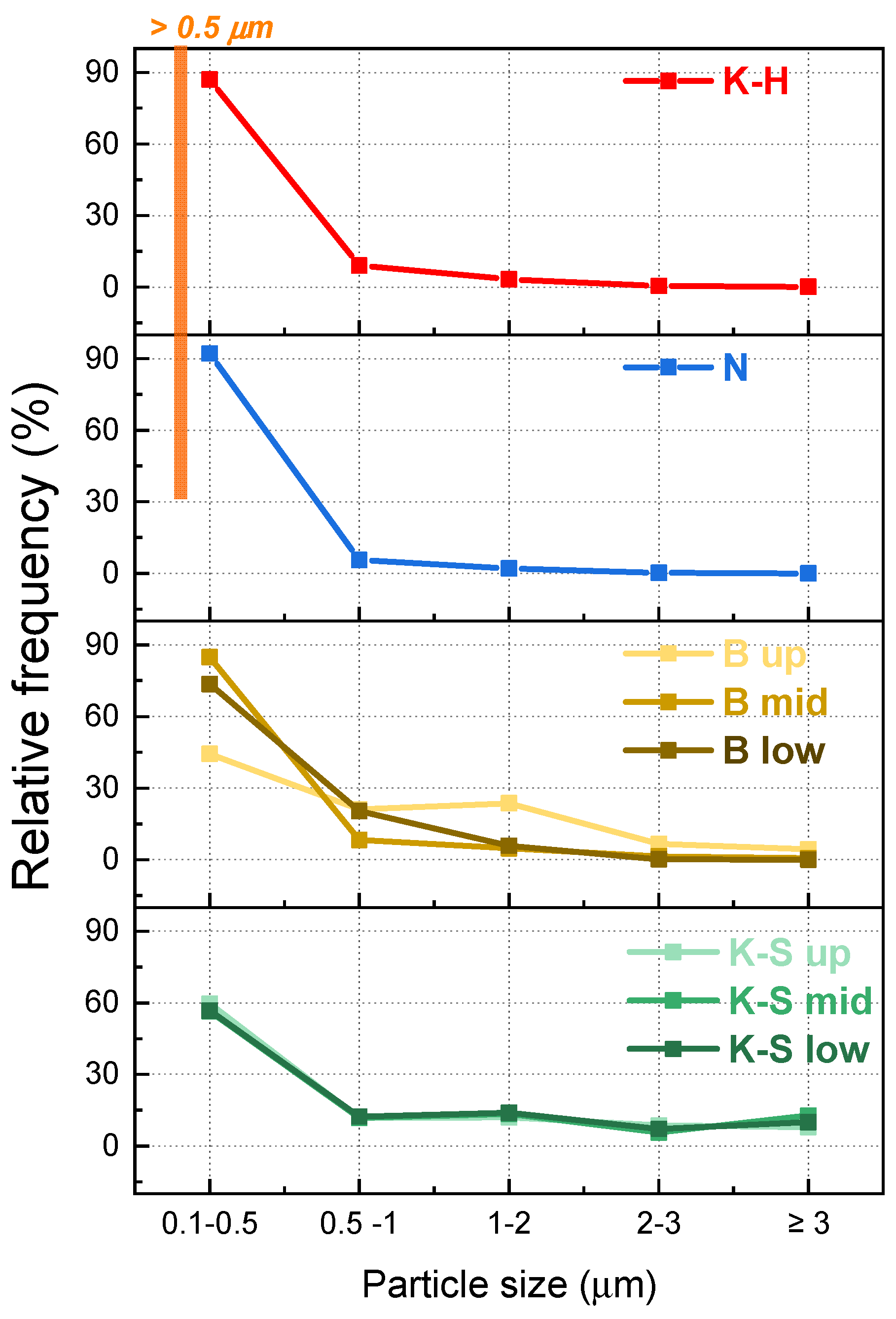
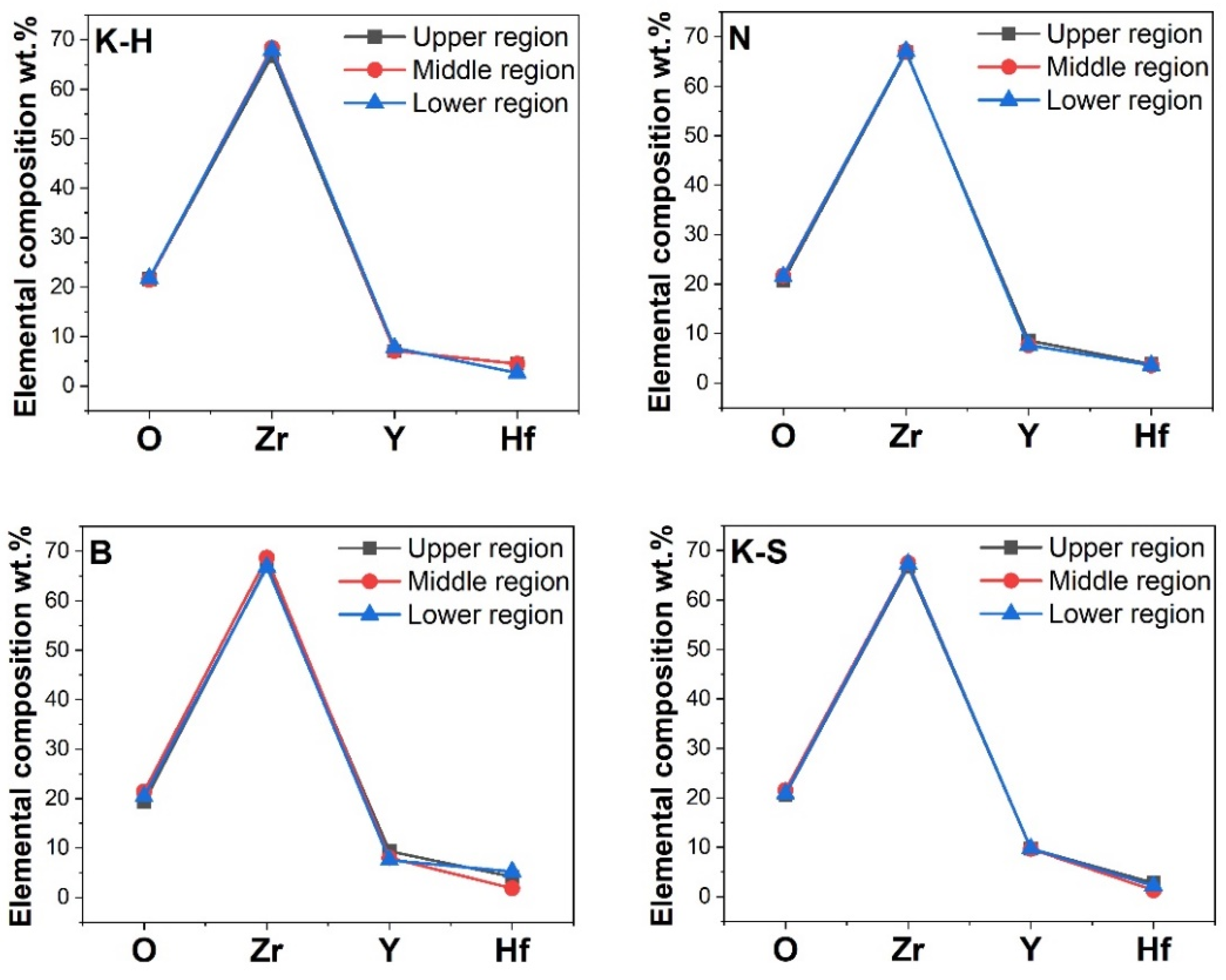
3.2. Chemical Composition
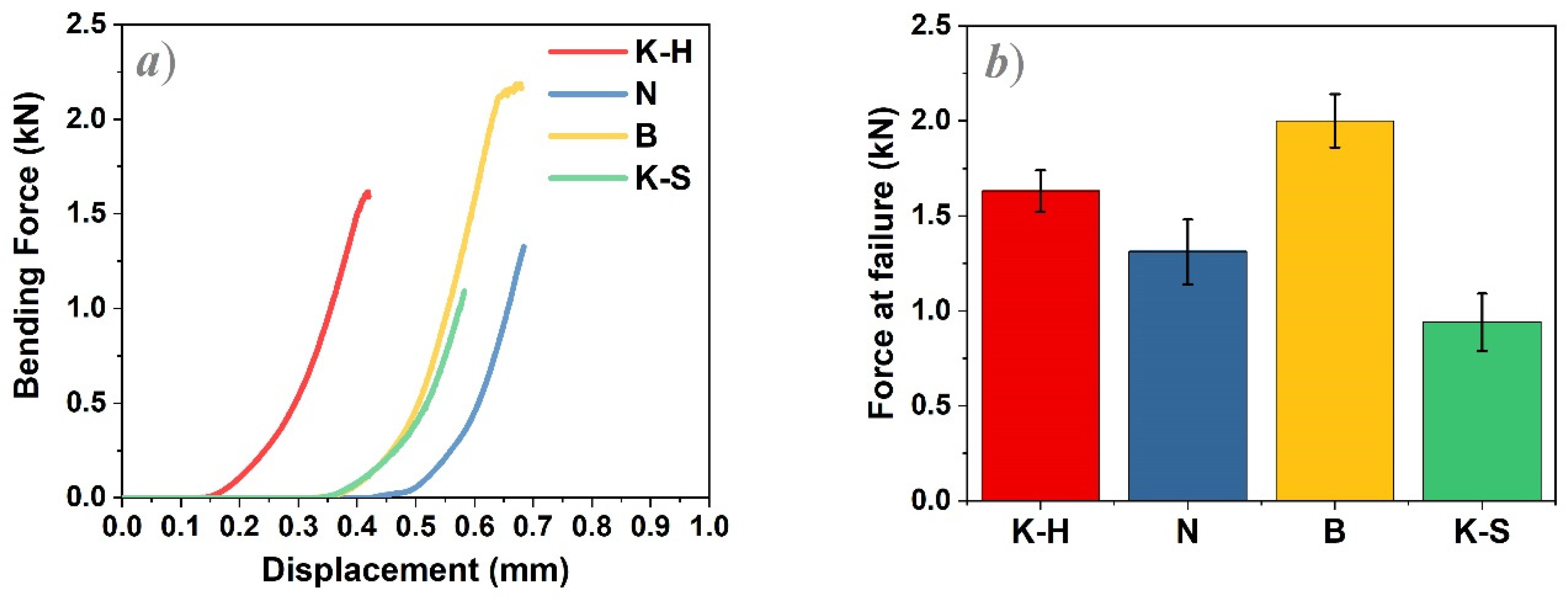
3.3. Bending Test
- -
- The best results were obtained for sample B as its investigated upper and middle areas consist of smaller (≤1 µm2) and larger particles (between 1 and 5 µm2), while the lower areas are comprised of a very large number of grains whose maximum size is 2 µm2;
- -
- K-S zirconia samples presented the largest grain size, irrespective of the investigated areas, concomitantly with the lowest force at failure values.
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tin-Oo, M.M.; Saddki, N.; Hassan, N. Factors Influencing Patient Satisfaction with Dental Appearance and Treatments They Desire to Improve Aesthetics. BMC Oral Health 2011, 11, 6. [Google Scholar] [CrossRef] [Green Version]
- Rauch, A.; Hahnel, S.; Günther, E.; Bidmon, W.; Schierz, O. Tooth-Colored CAD/CAM Materials for Application in 3-Unit Fixed Dental Prostheses in the Molar Area: An Illustrated Clinical Comparison. Materials 2020, 13, 5588. [Google Scholar] [CrossRef]
- Turgut, S. Optical Properties of Currently Used Zirconia-based Esthetic Restorations Fabricated with Different Techniques. J. Esthet. Restor. Dent. 2020, 32, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Abhay, S.S.; Ganapathy, D.; Veeraiyan, D.N.; Ariga, P.; Heboyan, A.; Amornvit, P.; Rokaya, D.; Srimaneepong, V. Wear Resistance, Color Stability and Displacement Resistance of Milled PEEK Crowns Compared to Zirconia Crowns under Stimulated Chewing and High-Performance Aging. Polymers 2021, 13, 3761. [Google Scholar] [CrossRef]
- Syed, M.; Chopra, R.; Sachdev, V. Allergic Reactions to Dental Materials-A Systematic Review. J. Clin. Diagn. Res. JCDR 2015, 9, ZE04–ZE09. [Google Scholar] [CrossRef]
- Anusavice, K.J. Standardizing Failure, Success, and Survival Decisions in Clinical Studies of Ceramic and Metal–Ceramic Fixed Dental Prostheses. Dent. Mater. 2012, 28, 102–111. [Google Scholar] [CrossRef] [Green Version]
- Shenoy, A.; Shenoy, N. Dental Ceramics: An Update. J. Conserv. Dent. 2010, 13, 195. [Google Scholar] [CrossRef]
- Conrad, H.J.; Seong, W.-J.; Pesun, I.J. Current Ceramic Materials and Systems with Clinical Recommendations: A Systematic Review. J. Prosthet. Dent. 2007, 98, 389–404. [Google Scholar] [CrossRef]
- Chen, Y.; Yeung, A.W.K.; Pow, E.H.N.; Tsoi, J.K.H. Current Status and Research Trends of Lithium Disilicate in Dentistry: A Bibliometric Analysis. J. Prosthet. Dent. 2021, 126, 512–522. [Google Scholar] [CrossRef]
- Miyazaki, T.; Nakamura, T.; Matsumura, H.; Ban, S.; Kobayashi, T. Current Status of Zirconia Restoration. J. Prosthodont. Res. 2013, 57, 236–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischer, C.A.I.; Ghergic, D.L.; Vranceanu, D.M.; Ilas, S.A.; Comaneanu, R.M.; Baciu, F.; Cotrut, C.M. Assessment of Force Retention between Milled Metallic and Ceramic Telescopic Crowns with Different Taper Angles Used for Oral Rehabilitation. Materials 2020, 13, 4814. [Google Scholar] [CrossRef] [PubMed]
- Nistor, L.; Grădinaru, M.; Rîcă, R.; Mărășescu, P.; Stan, M.; Manolea, H.; Ionescu, A.; Moraru, I. Zirconia Use in Dentistry-Manufacturing and Properties. Curr. Health Sci. J. 2019, 45, 28–35. [Google Scholar] [CrossRef]
- Kelly, J.; Benetti, P. Ceramic Materials in Dentistry: Historical Evolution and Current Practice. Aust. Dent. J. 2011, 56, 84–96. [Google Scholar] [CrossRef]
- Silva, L.H.d.; Lima, E.d.; Miranda, R.B.d.P.; Favero, S.S.; Lohbauer, U.; Cesar, P.F. Dental Ceramics: A Review of New Materials and Processing Methods. Braz. Oral Res. 2017, 31, e58. [Google Scholar] [CrossRef] [PubMed]
- Tangsatchatham, S.; Juntavee, N. Flexural Strength of Various Types of Computerized Machinable Ceramic Veneered to Yttria Stabilized Tetragonal Zirconia Polycrystalline Ceramic upon Different Hybridized Techniques. Clin. Cosmet. Investig. Dent. 2019, 11, 61–71. [Google Scholar] [CrossRef] [Green Version]
- Brijawi, A.; Samran, A.; Samran, A.; Alqerban, A.; Murad, M. Effect of Different Core Design Made of Computer-Aided Design/Computer-Aided Manufacturing System and Veneering Technique on the Fracture Resistance of Zirconia Crowns: A Laboratory Study. J. Conserv. Dent. JCD 2019, 22, 59–63. [Google Scholar] [CrossRef]
- Malkondu, Ö.; Tinastepe, N.; Akan, E.; Kazazoğlu, E. An Overview of Monolithic Zirconia in Dentistry. Biotechnol. Biotechnol. Equip. 2016, 30, 644–652. [Google Scholar] [CrossRef] [Green Version]
- Ramos, N.d.C.; Ramos, G.F.; Penteado, M.M.; de Melo, R.M.; Borges, A.L.S.; Bottino, M.A.; Tribst, J.P.M. Comparative Stress Evaluation between Bilayer, Monolithic and Cutback All-Ceramic Crown Designs: 3D Finite Element Study. Prosthesis 2021, 3, 173–180. [Google Scholar] [CrossRef]
- Kumchai, H.; Juntavee, P.; Sun, A.F.; Nathanson, D. Effects of Veneering Ceramic and Methods on Failure Load of Veneered Zirconia. Appl. Sci. 2021, 11, 2129. [Google Scholar] [CrossRef]
- Tang, Z.; Zhao, X.; Wang, H.; Liu, B. Clinical Evaluation of Monolithic Zirconia Crowns for Posterior Teeth Restorations. Medicine 2019, 98, e17385. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, T.A.; Abdulmajeed, A.A.; Delgado, A.; Donovan, T.E. Fracture Rate of 188695 Lithium Disilicate and Zirconia Ceramic Restorations after up to 7.5 Years of Clinical Service: A Dental Laboratory Survey. J. Prosthet. Dent. 2020, 123, 807–810. [Google Scholar] [CrossRef]
- Abdulmajeed, A.A.; Lim, K.G.; Närhi, T.O.; Cooper, L.F. Complete-Arch Implant-Supported Monolithic Zirconia Fixed Dental Prostheses: A Systematic Review. J. Prosthet. Dent. 2016, 115, 672–677.e1. [Google Scholar] [CrossRef]
- Zhang, Y.; Lawn, B.R. Novel Zirconia Materials in Dentistry. J. Dent. Res. 2018, 97, 140–147. [Google Scholar] [CrossRef]
- Kongkiatkamon, S.; Booranasophone, K.; Tongtaksin, A.; Kiatthanakorn, V.; Rokaya, D. Comparison of Fracture Load of the Four Translucent Zirconia Crowns. Molecules 2021, 26, 5308. [Google Scholar] [CrossRef]
- Kang, C.-M.; Peng, T.-Y.; Huang, H.-H. Effects of Thickness of Different Types of High-Translucency Monolithic Multilayer Precolored Zirconia on Color Accuracy: An in Vitro Study. J. Prosthet. Dent. 2021, 126, 587.e1–587.e8. [Google Scholar] [CrossRef] [PubMed]
- Pereira, G.K.R.; Guilardi, L.F.; Dapieve, K.S.; Kleverlaan, C.J.; Rippe, M.P.; Valandro, L.F. Mechanical Reliability, Fatigue Strength and Survival Analysis of New Polycrystalline Translucent Zirconia Ceramics for Monolithic Restorations. J. Mech. Behav. Biomed. Mater. 2018, 85, 57–65. [Google Scholar] [CrossRef]
- Manziuc, M.-M.; Gasparik, C.; Negucioiu, M.; Constantiniuc, M.; Burde, A.; Vlas, I.; Dudea, D. Optical Properties of Translucent Zirconia: A Review of the Literature. EuroBiotech J. 2019, 3, 45–51. [Google Scholar] [CrossRef] [Green Version]
- Fathy, S.M.; Al-Zordk, W.; E Grawish, M.; v Swain, M. Flexural Strength and Translucency Characterization of Aesthetic Monolithic Zirconia and Relevance to Clinical Indications: A Systematic Review. Dent. Mater. 2021, 37, 711–730. [Google Scholar] [CrossRef]
- Osman, R.; Swain, M. A Critical Review of Dental Implant Materials with an Emphasis on Titanium versus Zirconia. Materials 2015, 8, 932–958. [Google Scholar] [CrossRef] [Green Version]
- López-Suárez, C.; Castillo-Oyagüe, R.; Rodríguez-Alonso, V.; Lynch, C.D.; Suárez-García, M.-J. Fracture Load of Metal-Ceramic, Monolithic, and Bi-Layered Zirconia-Based Posterior Fixed Dental Prostheses after Thermo-Mechanical Cycling. J. Dent. 2018, 73, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-C.; Ding, S.-J.; Lin, T.-H.; Yan, M. Mechanical and Optical Properties Evaluation of Rapid Sintered Dental Zirconia. Ceram. Int. 2020, 46, 26668–26674. [Google Scholar] [CrossRef]
- Shah, M.K.; Bansal, S.; Pathak, V.; Bharadwaj, S.; Chauhan, A.; Nirwan, A.S. A Comparative Evaluation of Fracture Load of Monolithic and Bilayered Zirconia Crowns with and without a Cervical Collar: An In Vitro Study. Med. Pharm. Rep. 2019, 92, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; van Meerbeek, B.; Vleugels, J. Importance of Tetragonal Phase in High-Translucent Partially Stabilized Zirconia for Dental Restorations. Dent. Mater. 2020, 36, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Haraguchi, K.; Sugano, N.; Nishii, T.; Miki, H.; Oka, K.; Yoshikawa, H. Phase Transformation of a Zirconia Ceramic Head after Total Hip Arthroplasty. J. Bone Jt. Surg. 2001, 83, 996–1000. [Google Scholar] [CrossRef]
- Ruiz, L.; Readey, M.J. Effect of Heat Treatment on Grain Size, Phase Assemblage, and Mechanical Properties of 3 Mol% Y-TZP. J. Am. Ceram. Soc. 1996, 79, 2331–2340. [Google Scholar] [CrossRef]
- Mihai, L.L.; Parlatescu, I.; Gheorghe, C.; Andreescu, C.; Bechir, A.; Pacurar, M.; Cumpata, C.N. In Vitro Study of the Effectiveness to Fractures of the Aesthetic Fixed Restorations Achieved from Zirconium and Alumina. Rev. Chim. 2014, 65, 725–729. [Google Scholar]
- Piconi, C.; Maccauro, G. Zirconia as a Ceramic Biomaterial. Biomaterials 1999, 20, 1–25. [Google Scholar] [CrossRef]
- Kelly, J.R.; Denry, I. Stabilized Zirconia as a Structural Ceramic: An Overview. Dent. Mater. 2008, 24, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Marrelli, M.; Maletta, C.; Inchingolo, F.; Alfano, M.; Tatullo, M. Three-Point Bending Tests of Zirconia Core/Veneer Ceramics for Dental Restorations. Int. J. Dent. 2013, 2013, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Zarone, F.; Di Mauro, M.I.; Ausiello, P.; Ruggiero, G.; Sorrentino, R. Current Status on Lithium Disilicate and Zirconia: A Narrative Review. BMC Oral Health 2019, 19, 134. [Google Scholar] [CrossRef] [Green Version]
- Exare, C.; Kiat, J.-M.; Guiblin, N.; Petricek, V. Mechanical Properties of ZTA: Correlation with Structural Properties and Influence of Ageing. J. Ceram. 2016, 2016, 4264062. [Google Scholar] [CrossRef] [Green Version]
- Kontonasaki, E.; Giasimakopoulos, P.; Rigos, A.E. Strength and Aging Resistance of Monolithic Zirconia: An Update to Current Knowledge. Jpn. Dent. Sci. Rev. 2020, 56, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Lucas, T.J.; Lawson, N.C.; Janowski, G.M.; Burgess, J.O. Effect of Grain Size on the Monoclinic Transformation, Hardness, Roughness, and Modulus of Aged Partially Stabilized Zirconia. Dent. Mater. 2015, 31, 1487–1492. [Google Scholar] [CrossRef] [PubMed]
- Pantea, M.; Antoniac, I.; Trante, O.; Ciocoiu, R.; Fischer, C.A.; Traistaru, T. Correlations between Connector Geometry and Strength of Zirconia-Based Fixed Partial Dentures. Mater. Chem. Phys. 2019, 222, 96–109. [Google Scholar] [CrossRef]
- Chougule, K.J. An In Vitro Comparative Evaluation of Flexural Strength of Monolithic Zirconia after Surface Alteration Utilising Two Different Techniques. J. Clin. Diagn. Res. 2017, 11, ZC20–ZC23. [Google Scholar] [CrossRef]
- Xu, Y.; Han, J.; Lin, H.; An, L. Comparative Study of Flexural Strength Test Methods on CAD/CAM Y-TZP Dental Ceramics. Regen. Biomater. 2015, 2, 239–244. [Google Scholar] [CrossRef] [Green Version]
- Luthardt, R.G.; Holzhüter, M.S.; Rudolph, H.; Herold, V.; Walter, M.H. CAD/CAM-Machining Effects on Y-TZP Zirconia. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2004, 20, 655–662. [Google Scholar] [CrossRef]
- Mecholsky, J.J. Fracture Mechanics Principles. Dent. Mater. 1995, 11, 111–112. [Google Scholar] [CrossRef]
- Piconi, C.; Condo, S.G.; Kosmač, T. Alumina- and Zirconia-based Ceramics for Load-bearing Applications. In Advanced Ceramics for Dentistry; Shen, J.Z., Kosmač, T., Eds.; Elsevier: Massachusetts, MA, USA, 2014; pp. 219–253. [Google Scholar]
- Bakke, M. Bite Force and Occlusion. Semin. Orthod. 2006, 12, 120–126. [Google Scholar] [CrossRef]
- Diaz Lantada, A.; González Bris, C.; Lafont Morgado, P.; Sanz Maudes, J. Novel System for Bite-Force Sensing and Monitoring Based on Magnetic Near Field Communication. Sensors 2012, 12, 11544–11558. [Google Scholar] [CrossRef] [Green Version]
- Takaki, P.; Vieira, M.; Bommarito, S. Maximum Bite Force Analysis in Different Age Groups. Int. Arch. Otorhinolaryngol. 2014, 18, 272–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varga, S.; Spalj, S.; Lapter Varga, M.; Anic Milosevic, S.; Mestrovic, S.; Slaj, M. Maximum Voluntary Molar Bite Force in Subjects with Normal Occlusion. Eur. J. Orthod. 2011, 33, 427–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tovar-Vargas, D.; Roitero, E.; Anglada, M.; Jiménez-Piqué, E.; Reveron, H. Mechanical Properties of Ceria-Calcia Stabilized Zirconia Ceramics with Alumina Additions. J. Eur. Ceram. Soc. 2021, 41, 5602–5612. [Google Scholar] [CrossRef]
- Mundhe, K.; Jain, V.; Pruthi, G.; Shah, N. Clinical Study to Evaluate the Wear of Natural Enamel Antagonist to Zirconia and Metal Ceramic Crowns. J. Prosthet. Dent. 2015, 114, 358–363. [Google Scholar] [CrossRef]
- Chai, H.; Lee, J.J.W.; Mieleszko, A.J.; Chu, S.J.; Zhang, Y. On the Interfacial Fracture of Porcelain/Zirconia and Graded Zirconia Dental Structures. Acta Biomater. 2014, 10, 3756–3761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
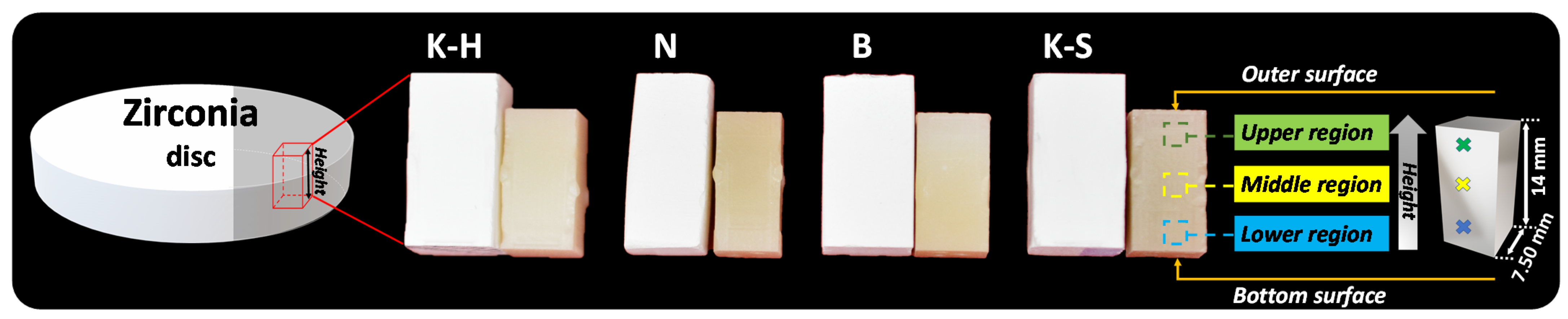



| Material | Manufacturer | Type | Codification |
|---|---|---|---|
| Zirconia | Kuraray Noritake Inc. | KatanaTM Zirconia HTML | K-H |
| Novadent/Dentaltechnik | NOVAZir® Fusion float® ml | N | |
| Bloomden Bioceramics | 3D PRO Zirconia | B | |
| Kuraray Noritake Inc. | KatanaTM Zirconia STML | K-S |
| Material | Relative Frequency | Average Grain Size Area (μm2) | |||||
|---|---|---|---|---|---|---|---|
| 0.10–0.50 | 0.51–1.00 | 1.01–2.00 | 2.01–3.00 | >3.01 | |||
| K-H | 87.01% | 9.10% | 3.26% | 0.49% | 0.15% | 0.22 | |
| N | 92.10% | 5.58% | 2.09% | 0.23% | - | 0.20 | |
| B | Up | 44.26% | 21.11% | 23.65% | 6.59% | 4.39% | 0.89 |
| Mid | 84.81% | 8.33% | 4.75% | 1.40% | 0.70% | 0.35 | |
| Low | 73.65% | 20.37% | 5.82% | 0.17% | - | 0.37 | |
| K-S | Up | 59.66% | 11.82% | 12.01% | 8.63% | 7.88% | 0.95 |
| Mid | 56.49% | 11.72% | 13.39% | 5.65% | 12.76% | 1.06 | |
| Low | 56.74% | 12.36% | 13.86% | 7.12% | 9.93% | 0.97 | |
| Materials | Force at Failure (kN) |
|---|---|
| K-H | 1.63 (±0.11) |
| N | 1.31 (±0.17) |
| B | 2.00 (±0.14) |
| K-S | 0.94 (±0.15) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fischer, P.; Barbu, H.M.; Fischer, C.A.I.; Pantea, M.; Baciu, F.; Vranceanu, D.M.; Cotrut, C.M.; Spinu, T.C. Bending Fracture of Different Zirconia-Based Bioceramics for Dental Applications: A Comparative Study. Materials 2021, 14, 6887. https://doi.org/10.3390/ma14226887
Fischer P, Barbu HM, Fischer CAI, Pantea M, Baciu F, Vranceanu DM, Cotrut CM, Spinu TC. Bending Fracture of Different Zirconia-Based Bioceramics for Dental Applications: A Comparative Study. Materials. 2021; 14(22):6887. https://doi.org/10.3390/ma14226887
Chicago/Turabian StyleFischer, Peter, Horia Mihail Barbu, Caroline Adela Ingrid Fischer, Mihaela Pantea, Florin Baciu, Diana Maria Vranceanu, Cosmin Mihai Cotrut, and Tudor Claudiu Spinu. 2021. "Bending Fracture of Different Zirconia-Based Bioceramics for Dental Applications: A Comparative Study" Materials 14, no. 22: 6887. https://doi.org/10.3390/ma14226887
APA StyleFischer, P., Barbu, H. M., Fischer, C. A. I., Pantea, M., Baciu, F., Vranceanu, D. M., Cotrut, C. M., & Spinu, T. C. (2021). Bending Fracture of Different Zirconia-Based Bioceramics for Dental Applications: A Comparative Study. Materials, 14(22), 6887. https://doi.org/10.3390/ma14226887










