Potassium Complexes of Quercetin-5′-Sulfonic Acid and Neutral O-Donor Ligands: Synthesis, Crystal Structure, Thermal Analysis, Spectroscopic Characterization and Physicochemical Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of KQSA Complexes
2.3. Methods
3. Results and Discussion
3.1. Morphology of Crystals
3.2. Crystal Structure of Compound 1
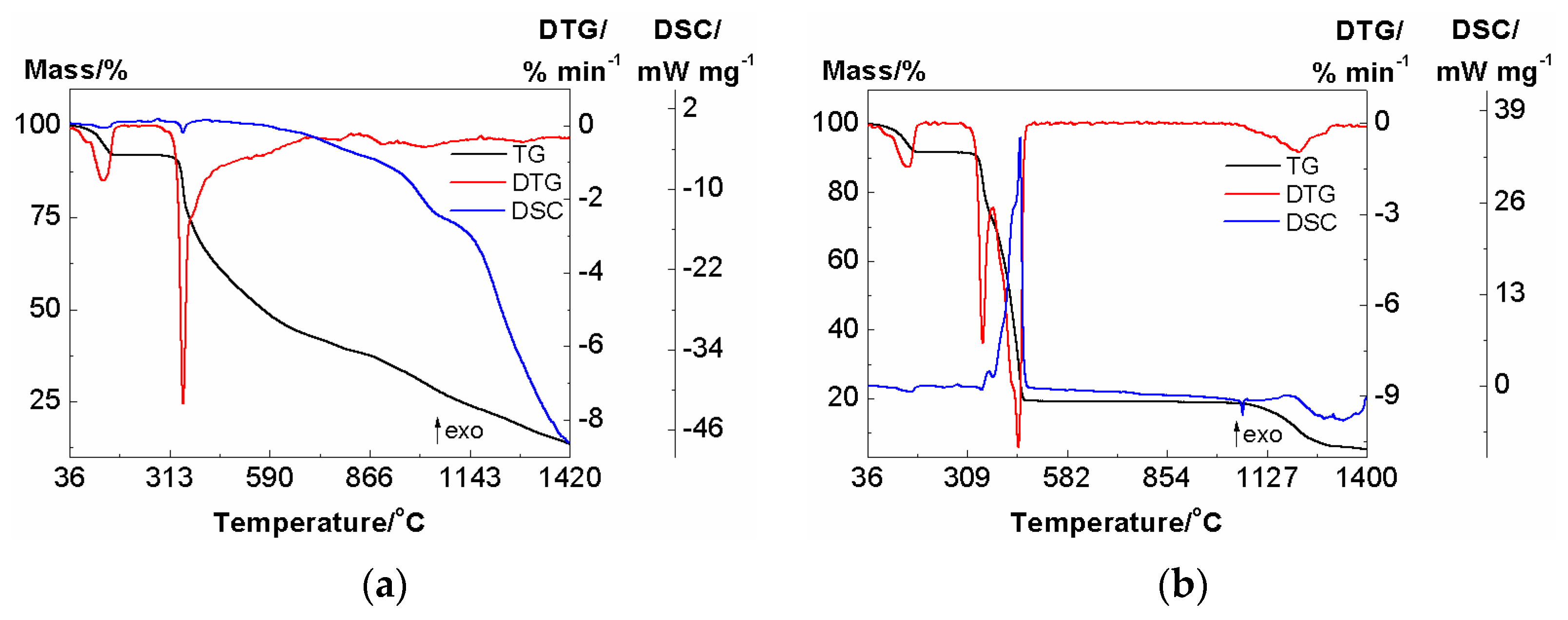
3.3. Thermal Analysis of Complex 1
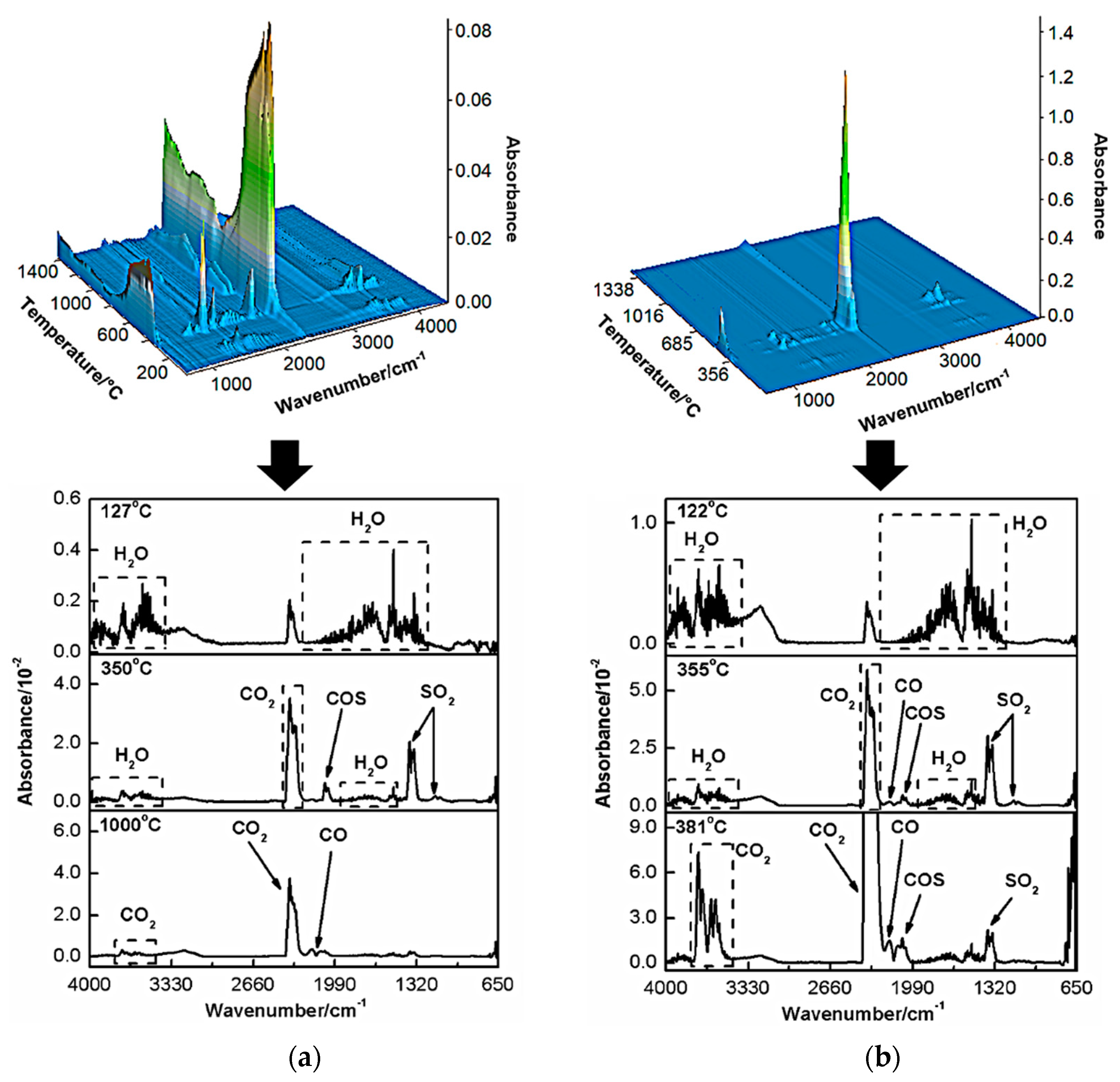
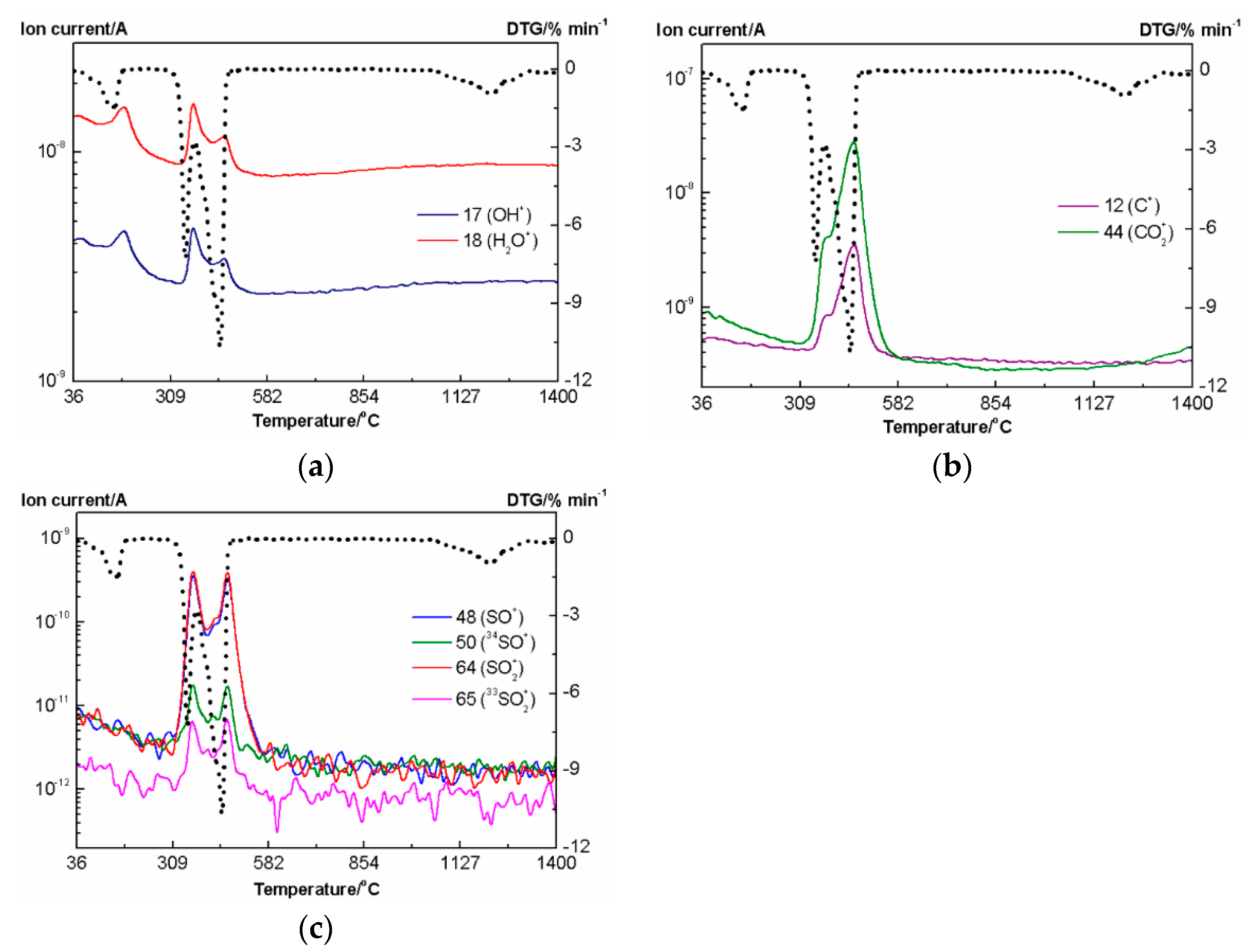
3.3.1. Thermal Analysis in Helium Atmosphere
3.3.2. Thermal Analysis in Air Atmosphere
3.4. Dissolution Tests of Complex 1
3.5. Crystal Structure of Compound 3
3.6. FT-IR and FT-Raman Spectroscopy
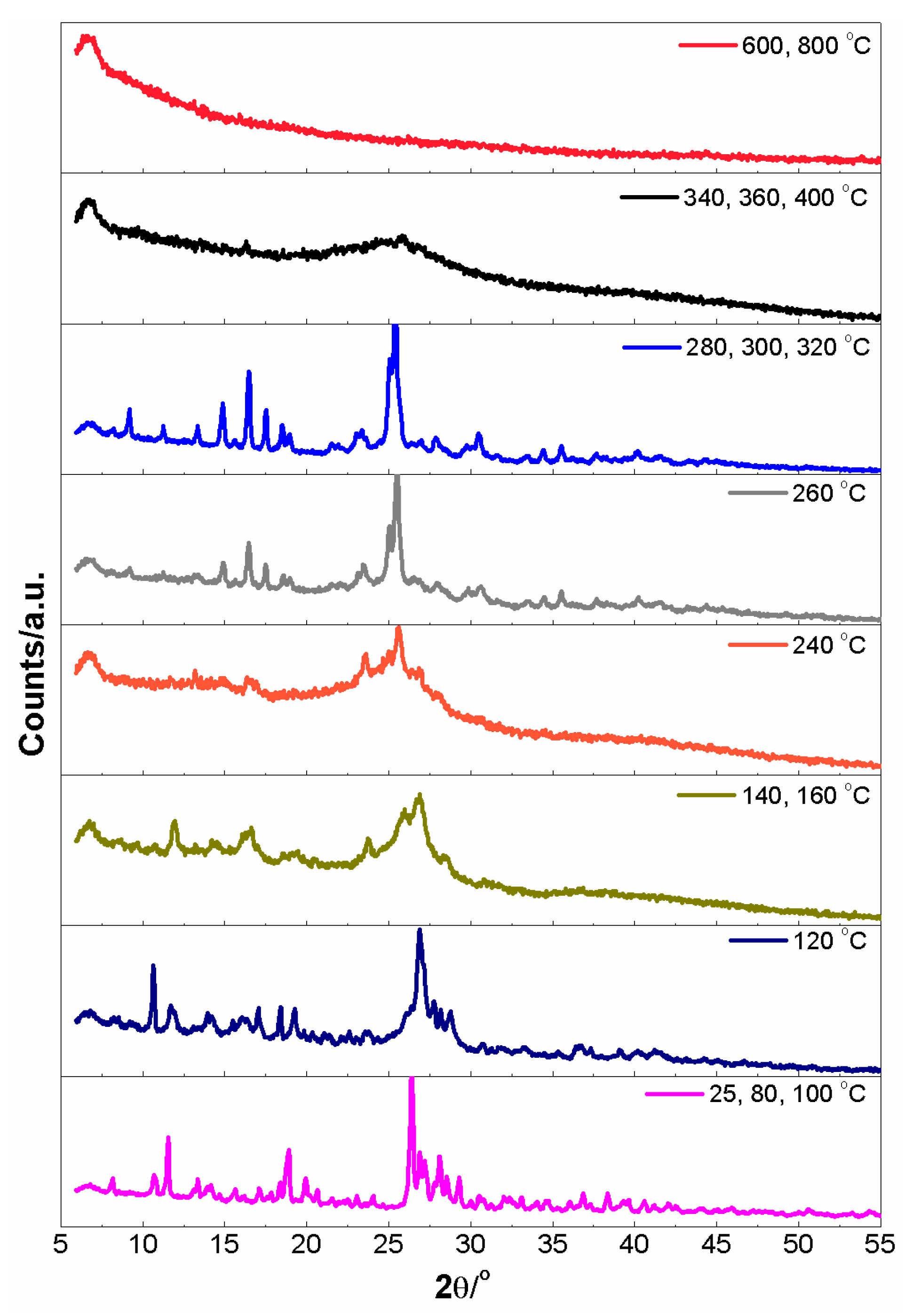
3.7. Desolvation of Crystals 1 and 3 in a Vacuum
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khater, M.; Ravishankar, D.; Greco, F.; Osborn, H.M. Metal complexes of flavonoids: Their synthesis, characterization and enhanced antioxidant and anticancer activities. Future Med. Chem. 2019, 11, 2845–2867. [Google Scholar] [CrossRef]
- Kasprzak, M.M.; Erxleben, A.; Ochocki, J. Properties and applications of flavonoid metal complexes. RSC Adv. 2015, 5, 45853–45877. [Google Scholar] [CrossRef]
- Selvaraj, S.; Krishnaswamy, S.; Devashya, V.; Sethuraman, S.; Krishnan, U.M. Flavonoid-metal ion complexes: A novel class of therapeutic agents. Med. Res. Rev. 2014, 34, 677–702. [Google Scholar] [CrossRef]
- Uivarosi, V.; Munteanu, A.C. Flavonoid Complexes as Promising Anticancer Metallodrugs. In Flavonoids—From Biosynthesis to Human Health; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef]
- Caddeo, C.; Gabriele, M.; Fernàndez-Busquets, X.; Valenti, D.; Fadda, A.M.; Pucci, L.; Manconi, M. Antioxidant activity of quercetin in Eudragit-coated liposomes for intestinal delivery. Int. J. Pharm. 2019, 565, 64–69. [Google Scholar] [CrossRef]
- Piovezana Bossolani, G.D.; Silva, B.T.; Colombo Martins Perles, J.V.; Lima, M.M.; Vieira Frez, F.C.; Garcia de Souza, S.R.; Sehaber-Sierakowski, C.C.; Bersani-Amado, C.A.; Zanoni, J.N. Rheumatoid arthritis induces enteric neurodegeneration and jejunal inflammation, and quercetin promotes neuroprotective and anti-inflammatory actions. Life Sci. 2019, 238, 116956. [Google Scholar] [CrossRef]
- Güran, M.; Şanlıtürk, G.; Kerküklü, N.R.; Altundağ, E.M.; Süha Yalçın, A. Combined effects of quercetin and curcumin on anti-inflammatory and antimicrobial parameters in vitro. Eur. J. Pharmacol. 2019, 859, 172486. [Google Scholar] [CrossRef]
- Kundur, S.; Prayag, A.; Selvakumar, P.; Nguyen, H.; McKee, L.; Cruz, C.; Srinivasan, A.; Shoyele, S.; Lakshmikuttyamma, A. Synergistic anticancer action of quercetin and curcumin against triple-negative breast cancer cell lines. J. Cell Physiol. 2019, 234, 11103–11118. [Google Scholar] [CrossRef]
- Ding, Y.; Li, C.; Zhang, Y.; Ma, P.; Zhao, T.; Che, D.; Cao, J.; Wang, J.; Liu, R.; Zhang, T.; et al. Quercetin as a Lyn kinase inhibitor inhibits IgE-mediated allergic conjunctivitis. Food Chem. Toxicol. 2020, 135, 110924. [Google Scholar] [CrossRef]
- Wang, Y.; Tao, B.; Wan, Y.; Sun, Y.; Wang, L.; Sun, J.; Li, C. Drug delivery based pharmacological enhancement and current insights of quercetin with therapeutic potential against oral diseases. Biomed. Pharmacother. 2020, 128, 110372. [Google Scholar] [CrossRef]
- Woźnicka, E.; Kuźniar, A.; Nowak, D.; Nykiel, E.; Kopacz, M.; Gruszecka, J.; Golec, K. Comparative study on the antibacterial activity of some flavonoids and their sulfonic derivatives. Acta Pol. Pharm. Drug Res. 2013, 70, 567–571. [Google Scholar]
- Król, W.; Dworniczak, S.; Pietsz, G.; Czuba, Z.P.; Kunicka, M.; Kopacz, M.; Nowak, D. Synthesis and tumoricidal activity evaluation of new morin and quercetin sulfonic derivatives. Acta Pol. Pharm. 2002, 59, 77–79. [Google Scholar]
- Chlebda, E.; Magdalan, J.; Merwid-Lad, A.; Trocha, M.; Kopacz, M.; Kuźniar, A.; Nowak, D.; Szelag, A. Influence of water-soluble flavonoids, quercetin-5′-sulfonic acid sodium salt and morin-5′-sulfonic acid sodium salt, on antioxidant parameters in the subacute cadmium intoxication mouse model. Exp. Toxicol. Pathol. 2010, 62, 105–108. [Google Scholar] [CrossRef]
- Trocha, M.; Merwid-Lad, A.; Szuba, A.; Sozański, T.; Magdalan, J.; Szelag, A.; Kopacz, M.; Kuźniar, A.; Nowak, D. Effect of quercetin-5′-sulfonic acid sodium salt on SOD activity and ADMA/DDAH pathway in extracorporeal liver perfusion in rats. Adv. Clin. Exp. Med. 2012, 21, 423–431. [Google Scholar]
- Magdalan, J.; Szelag, A.; Chlebda, E.; Merwid-Lad, A.; Trocha, M.; Kopacz, M.; Kuźniar, A.; Nowak, D. Quercetin-5′-sulfonic acid solution salt and morin-5′-sulfonic acid sodium salt as antidotes in the subacute cadmium intoxication in mice. Pharmacol. Rep. 2007, 59, 210–216. [Google Scholar]
- Mazur, L.; Tokarska, J.; Koziol, A.E.; Kopacz, M. Ammonium quercetin-5′-sulfonate formamide solvate. Acta Crystallogr. 2004, E60, o779–o781. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Chen, P.; Han, T.; Zhao, W. Sodium quercetin-8-sulfonate trihydrate. Acta Crystallogr. 2010, E66, m1036–m1037. [Google Scholar] [CrossRef]
- Liu, B.; Yang, B.L. Syntheses and crystal structures of [Na(H2O)1/2]X and NH2(CH2CH3)2X and antioxidant activity of the former. Chin. J. Struct. Chem. 2009, 28, 1112–1120. [Google Scholar]
- Liu, B.; Yang, B.L. Poly[(μ-5,7-dihydr-oxy-4-oxo-2-phenyl-4H-chromene-8-sulfonato)potassium(I)]. Acta Crystallogr. 2009, E65, m66–m77. [Google Scholar] [CrossRef]
- Liu, B.; Yang, B.L. Hexaaqua (5,7-dihydroxy-4-oxo-2-phenyl-4H-chromene-8-sulfonato)calcium(II) 5,7-dihydroxy-4-oxo-2-phenyl-4H-chromene-8-sulfonate trihydrate. Acta Crystallogr. 2008, E64, m1569–m1570. [Google Scholar] [CrossRef]
- Zhang, Z.T.; Shi, J.; He, Y.; Guo, Y.N. Self-assembly and crystal structure of a barium sulfonate chrysin coordination polimer. Inorg. Chem. Commun. 2006, 9, 579–581. [Google Scholar] [CrossRef]
- Li, W.W.; Zhang, Z.T. Synthesis, crystal structure and antitumor activity of tectochrysin-6-sulfonate. Chin. J. Struct. Chem. 2018, 1, 97. [Google Scholar]
- Li, W.W.; Zhang, Z.T. Hexaqua-cobalt(II) bis (5-hydroxy-7-methoxy-4-oxo-2-phenyl -4H-chromene-6-sulfonate) tetrahydrate. Acta Crystallogr. 2008, C64, m176–m178. [Google Scholar] [CrossRef]
- Yun, H. Tetraaqua(7-hydroxy-5-oxidoflavone-6-sulfonato-kappa-2O4,O5)nickel(II) dimethylformamide solvate monohydrate. Acta Crystallogr. 2006, E62, m469–m471. [Google Scholar] [CrossRef]
- Zhang, Z.T.; Shi, J. Self-assembly and crystal structure of a three-dimensional copper(II) complex. J. Coord. Chem. 2007, 60, 1485–1495. [Google Scholar] [CrossRef]
- Li, W.W.; Zheng, M.Y.; Gao, Y.H.; Zhang, Z.T. Argentum 5-Hydroxy-7-methoxy-2-phenyl-4H-chromen-4-one-6-sulfonate:Synthesis, Crystal Structure and Antitumor Activity. Chin. J. Struct. Chem. 2020, 39, 1898–1905. [Google Scholar]
- Pieniazek, E.; Kalembkiewicz, J.; Dranka, M.; Woznicka, E. Syntheses, crystal structures and antioxidant study of Zn(II) complexes with morin-5′-sulfonic acid (MSA). J. Inorg. Biochem. 2014, 141, 180–187. [Google Scholar] [CrossRef]
- Shi, J.; Zhang, Z. Crystal Structure and Photoluminescence of a Tetranuclear Cadmium Complex. Chin. J. Chem. 2012, 30, 1057–1062. [Google Scholar] [CrossRef]
- Jiang, R.W.; He, Z.D.; But, P.P.H.; Chan, Y.M.; Mak, T.C. A novel 1: 1 Complex of potassium mikanin-3-O-sulfate with methanol. Chem. Pharm. Bull. 2001, 49, 1166–1169. [Google Scholar] [CrossRef][Green Version]
- Liu, Q.G.; Zhang, Z.T.; Xue, D. Synthesis, crystal structure and activity of sulfated daidzein. Chem. J. Chin. U 2003, 24, 820–825. [Google Scholar]
- Zhang, X.L.; Zhang, Z.T.; Liang, Y. Synthesis and single crystal X-ray structure of [Na(H2O)2(C18H15O6SO3)]2. J. Chem. Crystallogr. 2008, 38, 861–865. [Google Scholar] [CrossRef]
- Zhang, Z.T.; Guo, Y.N.; Liu, Q.G. Syntheses and crystal structures of [Na(H2O)] (C17H13O6SO3)·2H2O and [Ni(H2O)6](C17H13O6SO3)2·4H2O. Chin. J. Chem. 2004, 22, 971–977. [Google Scholar] [CrossRef]
- Wang, Q.Y.; Zhang, Z.T. Synthesis and crystal structure of [Na(H2O)1.5][Na(H2O)3.5] X2·2H2O (X = 4′-methoxy-7-hydroxyisoflavone-3′-sulfonate). J. Chem. Crystallogr. 2011, 41, 1467–1471. [Google Scholar] [CrossRef]
- Zhang, Z.T.; Zhang, Z.T.; Wang, Q.Y.; He, Y.; Wang, X.B.; Xue, D.; Zheng, J.B. Syntheses, crystal structures and biological activity of bimethylation daidzein sulfonates. Chem. J. Chin. U 2005, 26, 2247–2253. [Google Scholar]
- Woźnicka, E.; Pieniążek, E.; Zapała, L.; Byczyński, Ł.; Trojnar, I.; Kopacz, M. New sulfonic derivatives of quercetin as complexing reagents: Synthesis, spectral, and thermal characterization. J. Therm. Anal. Calorim. 2015, 120, 351–361. [Google Scholar] [CrossRef]
- Czerwonka, A.; Maciołek, U.; Kałafut, J.; Mendyk, E.; Kuźniar, A.; Rzeski, W. Anticancer effects of sodium and potassium quercetin-5′-sulfonates through inhibition of proliferation, induction of apoptosis, and cell cycle arrest in the HT-29 human adenocarcinoma cell line. Bioorg. Chem. 2020, 94, 103426. [Google Scholar] [CrossRef]
- Masoud, M.S.; El-Enein, S.A.A.; Ramadan, A.M.; Goher, A.S. Thermal properties of some biologically active 5-(p-substituted phenylazo)-6-aminouracil complexes. J. Anal. Appl. Pyrol. 2008, 81, 45–51. [Google Scholar] [CrossRef]
- Ekawa, B.; Nunes, W.D.G.; Teixeira, J.A.; Cebim, M.A.; Ionashiro, E.Y.; Caires, F.J. New complexes of light lanthanides with the valsartan in the solid state: Thermal and spectroscopic studies. J. Anal. Appl. Pyrol. 2018, 135, 299–309. [Google Scholar] [CrossRef]
- Jendrzejewska, I.; Goryczka, T.; Pietrasik, E.; Klimontko, J.; Jampilek, J. X-ray and Thermal Analysis of Selected Drugs Containing Acetaminophen. Molecules 2020, 25, 5909. [Google Scholar] [CrossRef]
- Qi, S. Advances in Delivery Science and Technology. In Analytical Techniques in the Pharmaceutical Sciences; Müllertz, A., Perrie, Y., Rades, T., Eds.; Springer: New York, NY, USA, 2016; pp. 363–387. [Google Scholar]
- Maciołek, U.; Mendyk, E.; Kosińska, M.; Sternik, D.; Drewniak, M.; Kozioł, A.E. Thermal study, identification of intermediate solid products and evolved gas analysis (EGA) during pyrolysis and oxidative decomposition of sodium complex of quercetin-5′-sulfonic acid (Na-5′-QSA). J. Anal. Appl. Pyrolysis 2020, 150, 104881. [Google Scholar] [CrossRef]
- Rigaku. CRYSALIS Software System; Rigaku: Oxford, UK, 2016. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, A71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8. [Google Scholar] [CrossRef]
- Sherje, A.; Desai, K. Spectrophotometric Determination of Poorly Water Soluble Drug Rosiglitazone Using Hydrotropic Solubilization technique. Indian J. Pharm. Sci. 2011, 73, 579–582. [Google Scholar] [CrossRef]
- Czakis-Sulikowska, D.; Radwańska-Doczekalska, J.; Czyłkowska, A.; Gołuchowska, J. TG-MS, TG, DTG and DTA methods in study of thermal decomposition of some d-metal complexes with 4,4′-bpy and propionates. J. Therm. Anal. Calorim. 2004, 78, 501–511. [Google Scholar] [CrossRef]
- Risoluti, R.; Piazzese, D.; Napoli, A.; Materazzi, S. Study of [2-(2′-pyridyl)imidazole] complexes to confirm two main characteristic thermoanalytical behaviors of transition metal complexes based on imidazole derivatives. J. Anal. Appl. Pyrolysis. 2016, 117, 82–87. [Google Scholar] [CrossRef]
- Silverstein, R.M.; Webster, F.X.; Kiemle, D.J. Spectrometric Identification of Organic Compound, 7th ed.; John Wiley and Sons: Hoboken, NJ, USA, 2005. [Google Scholar]
- Anoop, M.R.; Jisha, K.R.; Suma, S.; Sudarsanakumar, M.R. Synthesis, spectral characterization, thermal and biological studies of lanthanide(III) complexes of oxyphenbutazone. J. Rare Earths 2014, 32, 43–51. [Google Scholar] [CrossRef]
- Guerra, R.B.; Gálico, D.A.; Holanda, B.B.C.; Bannach, G. Solid-state thermal and spectroscopic studies of the antiinflammatory drug sulindac using UV–Vis, MIR, NIR, DSC, simultaneous TG–DSC, and the coupled techniques TG-EGA-MIR and DSC–optical microscopy. J. Therm. Anal. Calorim. 2016, 123, 2523–2530. [Google Scholar] [CrossRef]
- Otto, K.; Bombicz, P.; Madarász, J.; Acik, I.O.; Krunks, M.; Pokol, G. Structure and evolved gas analyses (TG/DTA-MS and TG-FTIR) of mer-trichlorotris(thiourea)-indium(III), a precursor for indium sulfide thin films. J. Therm. Anal. Calorim. 2011, 105, 83–91. [Google Scholar] [CrossRef]
- Franzé, J.A.; Carvalho, T.F.; Gaglieri, C.; Caires, F.J.; Bannach, G.; Castro, R.C.; Treu-Filho, O.; Ionashiro, M.; Mendes, R.A. Synthesis, characterization, thermal and spectroscopic studies and bioactivity of complexes of meloxicam with some bivalent transition metals. J. Therm. Anal. Calorim. 2017, 127, 1393–1405. [Google Scholar] [CrossRef]
- Madarász, J. Evolved gas analyses on a mixed valence copper(I,II) complex salt with thiosulfate and ammonia by in situ TG-EGA-FTIR and TG/DTA-EGA-MS. J. Therm. Anal. Calorim. 2009, 97, 111–116. [Google Scholar] [CrossRef]
- Hanuza, J. Vibrational Dynamics of the Bent Symmetric and Asymmetric Oxygen Bridges and Terminal Metal=Oxygen Bond. Bull. Pol. Acad. Sci. Chem. 1994, 42, 255–267. [Google Scholar]
- Xu, G.R.; In, M.Y.; Yuan, Y.; Lee, J.J.; Kim, S. In situ Spectroelectrochemical Study of Quercetin Oxidation and Complexation with Metal Ions in Acidic Solutions. Bull. Korean Chem. Soc. 2007, 28, 889–892. [Google Scholar] [CrossRef]
- Symonowicz, M.; Kolanek, M. Flavonoids and Their Properties to form Chelate Complexes. Biotechnol. Food Sci. 2012, 76, 35–41. [Google Scholar]
- Teslova, T.; Corredor, C.; Livingstone, R.; Spataru, R.; Birke, R.L.; Lombardi, J.R.; Cañamares, M.V.; Leona, M. Raman and surface-enhanced Raman spectra of flavone and several hydroxy derivatives. J. Raman Spectrosc. 2007, 38, 802–818. [Google Scholar] [CrossRef]
- Rossi, M.; Rickles, L.F.; Halpin, W.A. The crystal and molecular structure of quercetin: A biologically active and naturally occurring flavonoid. Bioorg. Chem. 1986, 14, 55–69. [Google Scholar] [CrossRef]
- Numata, Y.; Tanaka, H. Quantitative analysis of quercetin using Raman spectroscopy. Food Chem. 2011, 126, 751–755. [Google Scholar] [CrossRef]
- Hanuza, J.; Godlewska, P.; Kucharska, E.; Ptak, M.; Kopacz, M.; Mączka, M.; Hermanowicz, K.; Macalik, L. Molecular Structure and Vibrational Spectra of Quercetin and Quercetin-5′-Sulfonic Acid A. Vib. Spectrosc. 2017, 88, 94–105. [Google Scholar] [CrossRef]
- Dimitrić Marković, J.M.; Marković, Z.S.; Krstić, J.B.; Milenković, D.; Lučić, B.; Amić, D. Interpretation of the IR and Raman spectra of morin by density functional theory and comparative analysis. Vib. Spectrosc. 2013, 64, 1–9. [Google Scholar] [CrossRef]
- Pompeu, D.R.; Larondelle, Y.; Rogez, H.; Abbas, O.; Pierna, J.A.F.; Baeten, V. Characterization and discrimination of phenolic compounds using Fourier transform Raman spectroscopy and chemometric tools. Biotechnol. Agron. Soc. Environ. 2018, 22, 13–28. [Google Scholar] [CrossRef]
- Heneczkowski, M.; Kopacz, M.; Nowak, D.; Kuźniar, A. Infrared spectrum analysis of some flavonoids. Acta Pol. Pharm. 2001, 58, 415–420. [Google Scholar]
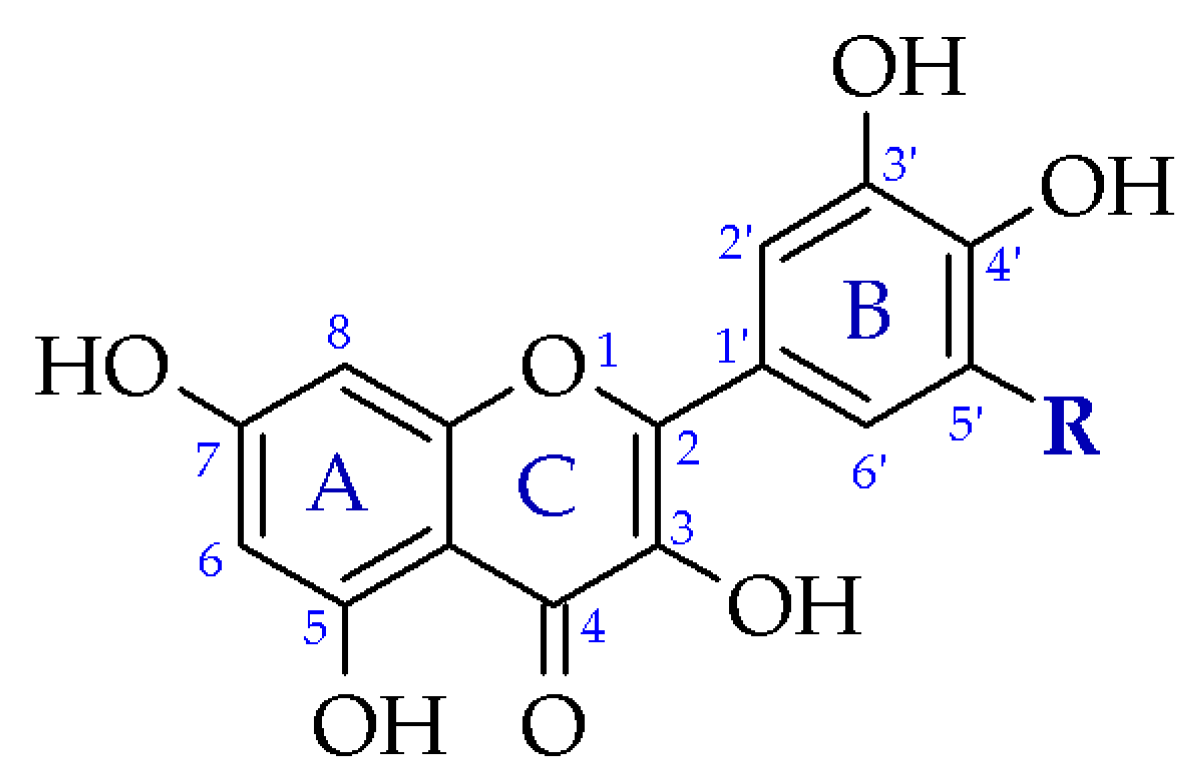
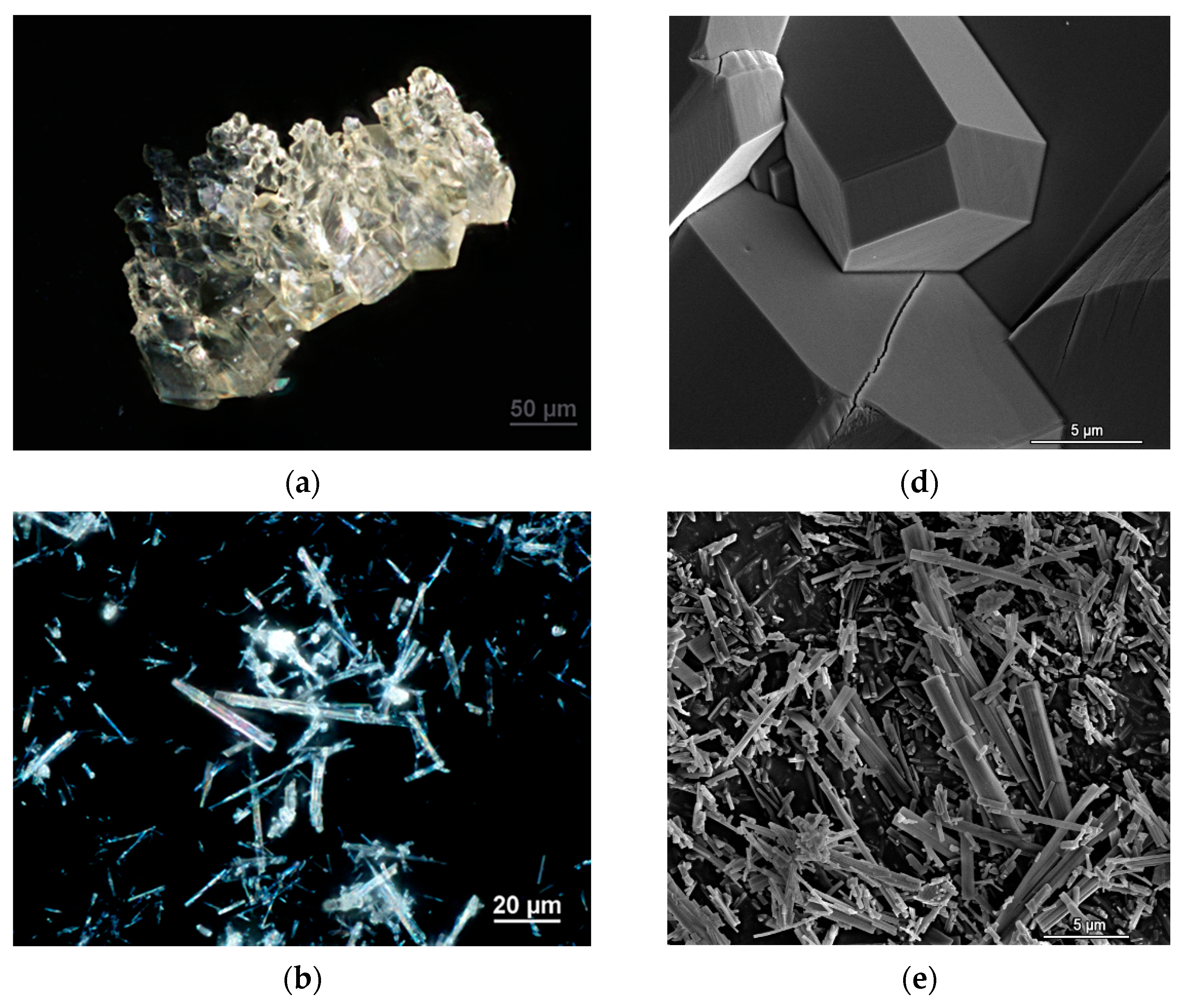
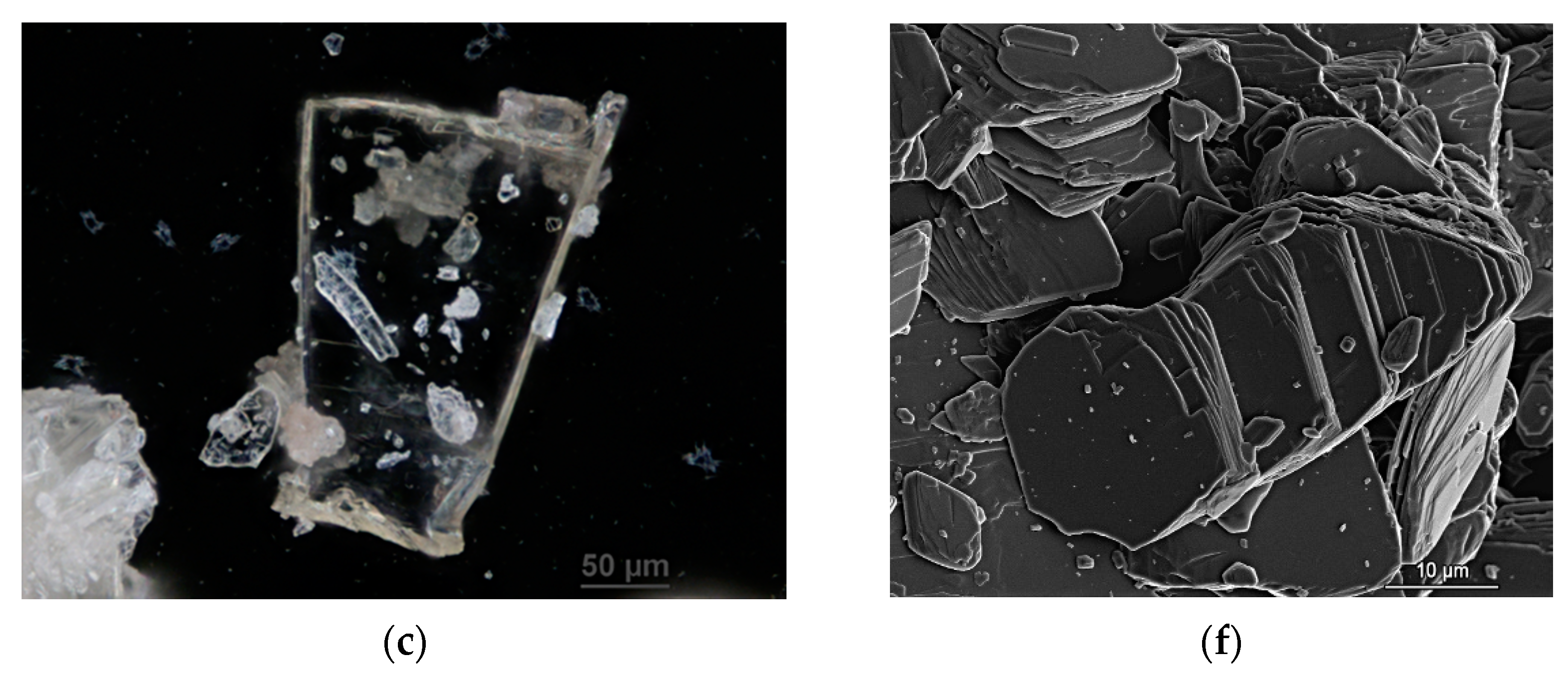
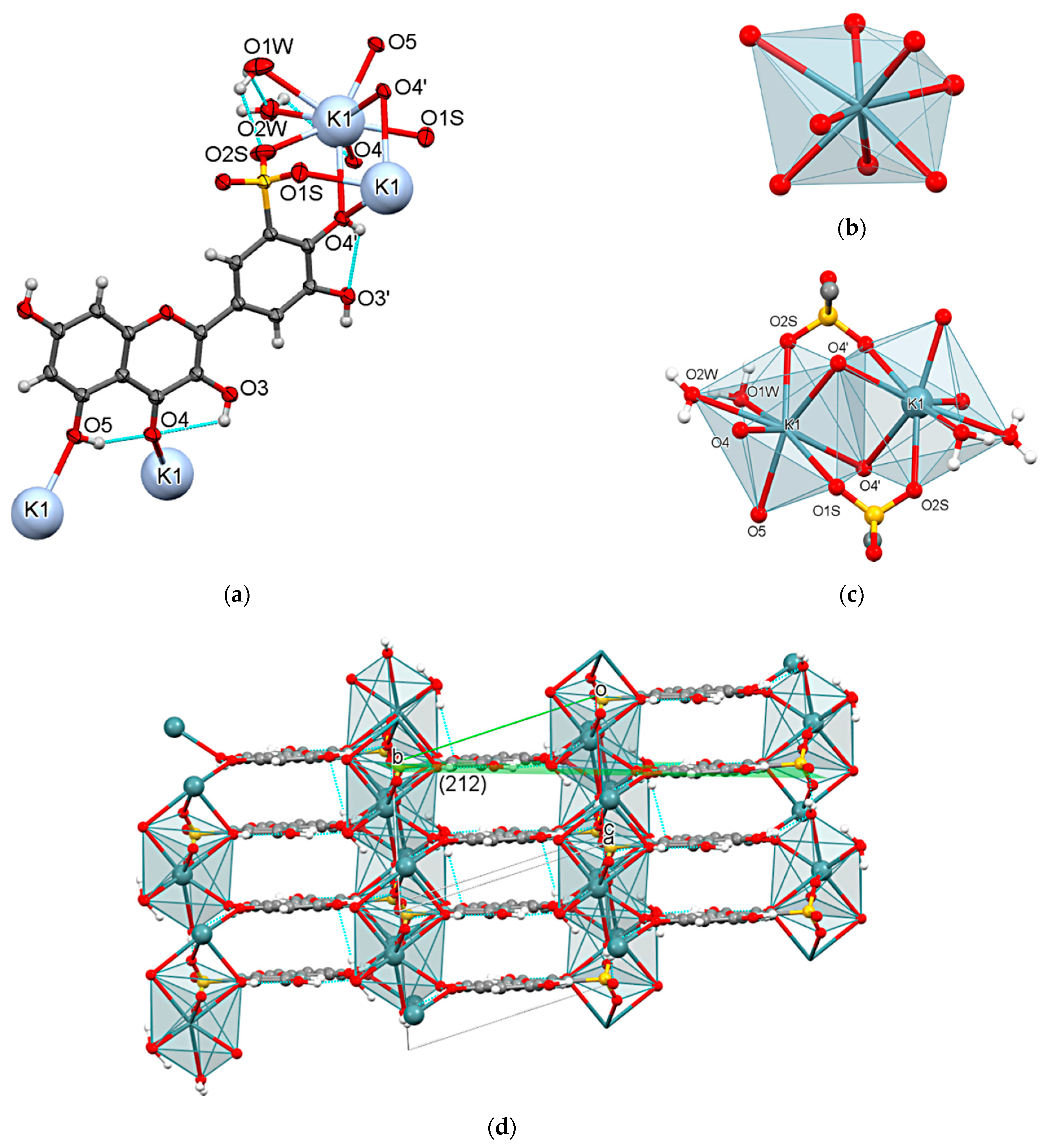

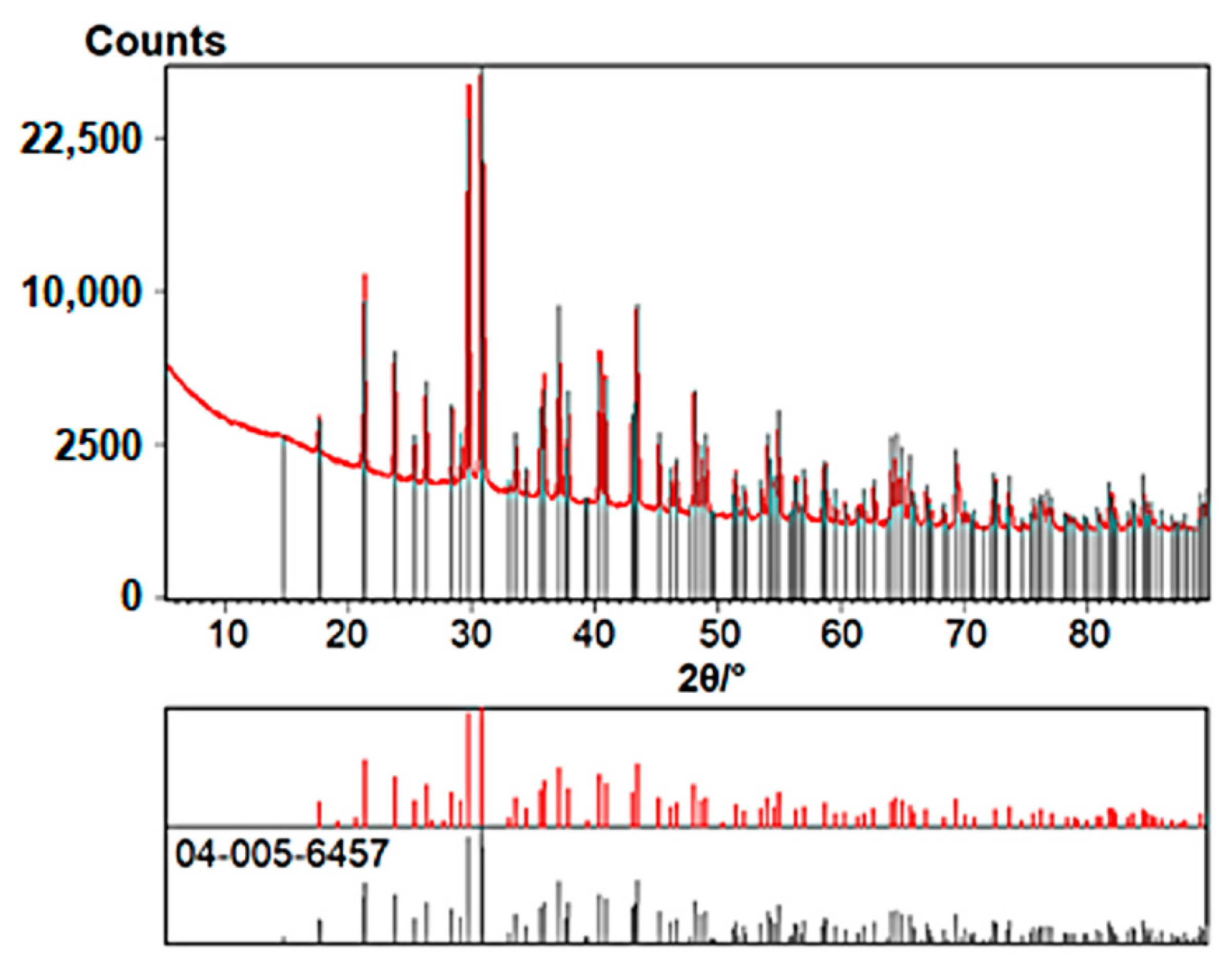
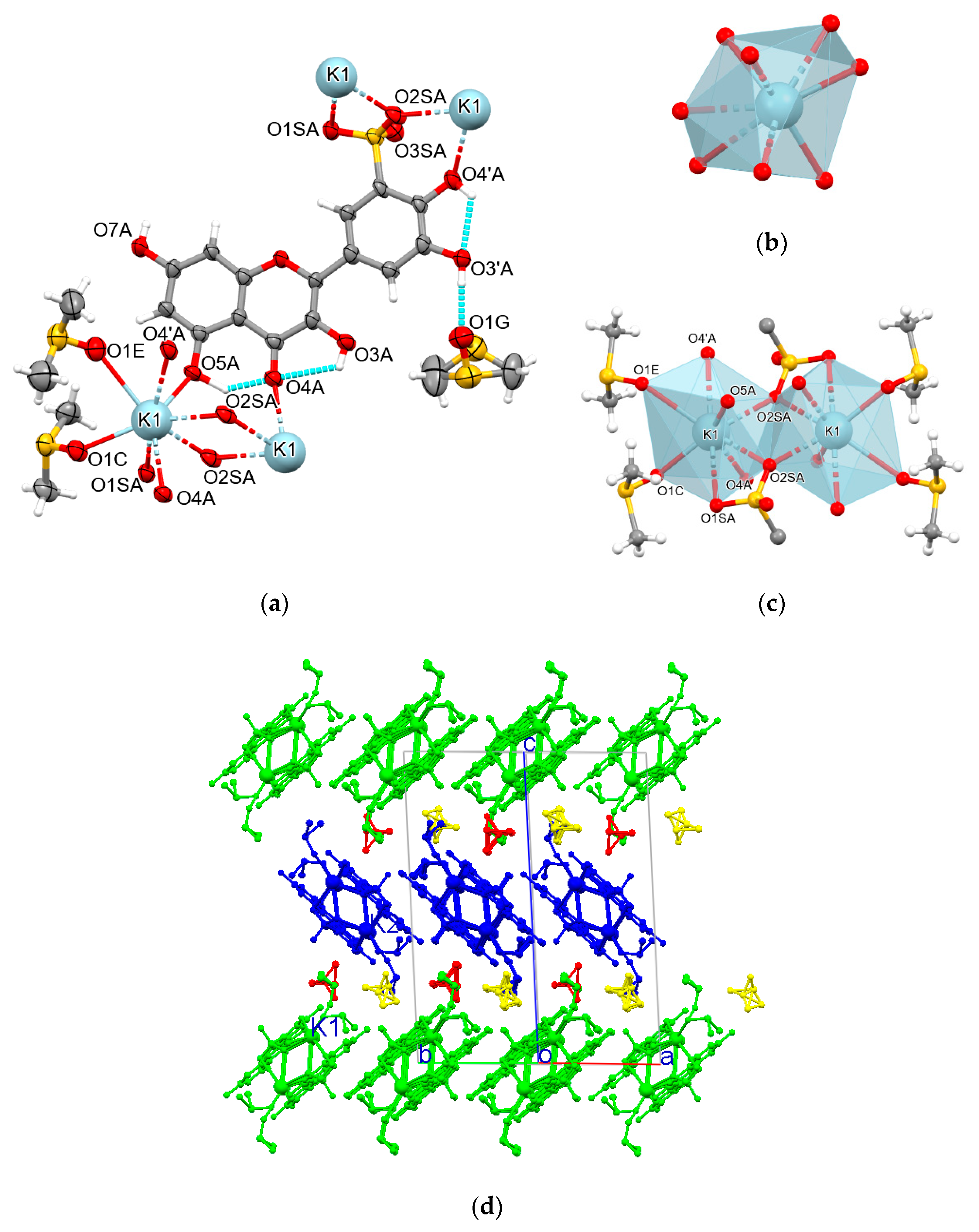

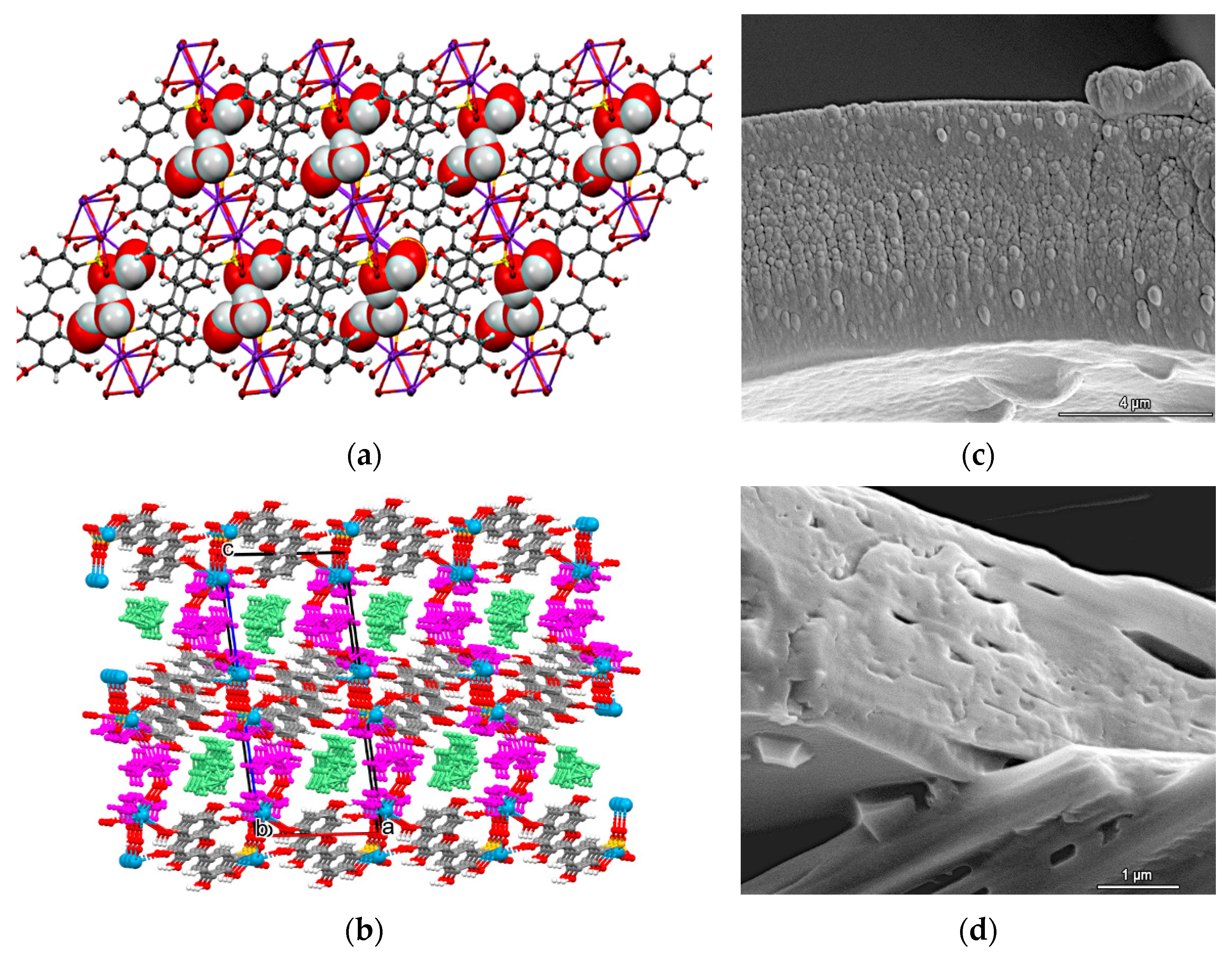
| Cation | CSD Refcode/SO3− Substitution | Coordination Sphere | Coordinating SO3− Group | Type of Complex | Reference |
|---|---|---|---|---|---|
| Na | DURTEQ/C8 | CN = 6, NaO6 | yes | 1D polymer | [17] |
| SULFOV/C8 | CN = 5, NaO5 CN = 5, NaO5 | yes | 3D polymer | [18] | |
| K | QOLLAF/C8 | CN = 5, KO5 | yes | 3D polymer | [19] |
| Ca * | POMDUR/C8 | CN = 7, CaO7 | yes | mononuclear | [20] |
| Ba | PEHROK/C6 | CN = 10, BaO10 | yes | 1D polymer | [21] |
| Mn * | YELTAN/C6 | CN = 6, MnO6 | no | mononuclear | [22] |
| Co * | WIZGIW/C6 | CN = 6, CoO6 | no | mononuclear | [23] |
| Ni * | WEPPAJ/C6 | CN = 6, NiO6 | no | mononuclear | [24] |
| YELSUG/C6 | CN = 6, NiO6 | no | mononuclear | [22] | |
| Cu * | YIGZOE/C6 | CN = 5, CuO5 | no | mononuclear | [25] |
| Ag | PAGVUS/C6 | CN = 4, AgO4 CN = 4, AgO4 | yes | tetranuclear | [26] |
| Zn | ZUJTIJ/C5′ | CN = 6, ZnO6 | yes | 1D polymer | [27] |
| Cd | XESPES/C6 | CN = 7, CdO7 CN = 7, CdO7 CN = 7, CdO7 CN = 7, CdO7 | yes | tetranuclear | [28] |
| Na, Zn | ZUJGUI/C5′ | CN = 6, ZnO6 CN = 6, NaO6 | no yes | 1D polymer 1D polymer | [27] |
| K-O | Symmetry Transformations | d(K-O) [Å] |
|---|---|---|
| * K(1)-O(4’)#2 | #1 −x, −y + 1, −z + 1 #2 −x, −y, −z + 2 #3 x − 1, y − 1, z + 1 | 3.016(2) |
| *$ K(1)-O(4’) | 3.101(2) | |
| $ K(1)-O(2S) | 2.754(2) | |
| K(1)-O(1S)#2 | 2.754(2) | |
| K(1)-O(4)#1 | 2.713(2) | |
| K(1)-O(5)#3 | 2.759(2) | |
| K(1)-O(1W) | 3.034(2) | |
| K(1)-O(2W) | 3.029(2) |
| D-H...A | d(D-H) [Å] | d(H...A) [Å] | d(H...A) [Å] | <(DHA) [°] |
|---|---|---|---|---|
| O(1W)-H(2W1)…O(2W) | 0.89(5 | 2.05(5) | 2.849(3) | 150(3) |
| O(1W)-H(1W1)…O(2S) | 0.83(4) | 2.03(4) | 2.766(2) | 147(3) |
| O(4’)-H(4’)...O(3’) | 0.77(3) | 2.21(3) | 2.653(2) | 118(2) |
| O(3)-H(3)...O(4) | 0.84(3) | 2.29(3) | 2.718(2) | 112(2) |
| O(5)-H(5)...O(4) | 0.88(3) | 1.78(3) | 2.610(2) | 156(3) |
| O(2W)-H(1W2)...O(3S)#a | 0.83(4) | 2.02(4) | 2.829(2) | 164(3) |
| O(2W)-H(2W2)...O(3S)#b | 0.92(4) | 1.90(4) | 2.814(2) | 173(3) |
| O(3’)-H(3’)...O(1W)#c | 0.86(3) | 1.78(3) | 2.628(2) | 169(3) |
| O(4’)-H(4’)...O(7)#d | 0.77(3) | 2.09(3) | 2.764(2) | 146(3) |
| O(7)-H(7)...O(1S)#e | 0.83(3) | 1.98(3) | 2.783(2) | 161(3) |
| O(3)-H(3)...O(2W)#f | 0.84(3) | 2.03(3) | 2.823(2) | 158(2) |
| Medium | Stage | tpeak [°C] | Thermogravimetry | ||
|---|---|---|---|---|---|
| Temp.Range; TDTG(mx) [°C] | Δm [%] Found (calc.) | Residue [%] Found (calc.) | |||
| air | I | 154 (−) | 25−300; 144 | 8.24 (7.89) | 19.31 (19.09) 0.5 K2SO4 |
| 280 (+) | |||||
| II | 367 (+) | 300−400; 351 | 20.00 | ||
| III | 453 (+) | 400−600; 449 | 52.45 | ||
| IV | 1062 (−) | 800−1400; 1215 | 14.13 | 5.18 | |
| helium | I | 130 (−) | 25−300; 127 | 8.13 (7.89) | 42.79 (42.72) 0.5 K2C2 + 12C |
| 280 (+) | |||||
| II | 350 (−) | 300−700; 350 | 49.08 | ||
| III | 700−1420; - | 29.30 | 13.49 | ||
| Solvent | QUE·2H2O | 1 | ||
|---|---|---|---|---|
| [g/L] | [mol/L] | [g/L] | [mol/L] | |
| Water | 0.002 | 5.91·10−6 | 0.09 | 1.97 × 10−4 |
| Acetone | 3.69 | 1.10·10−2 | 0.25 | 5.48 × 10−4 |
| Ethanol | 8.11 | 2.40·10−2 | 0.06 | 1.31 × 10−4 |
| DMSO | 685 | 2.03 | 167 | 0.37 |
| K(1)-O | Symmetry Transformations | d(K-O) [Å] | K(2)-O | Symmetry Transformations | d(K-O) [Å] |
|---|---|---|---|---|---|
| K(1)-O(1C) | #1 1 − x, 1 − y, −z #2 2 − x, 2 − y, −z #3 x + 1, y + 1, z | 2.672(5) | K(2)-O(5B) | #4 1 − x, 2 – y, 1 – z #5 −x, 1 − y, 1 – z #6 x − 1, y − 1, z | 2.685(5) |
| K(1)-O(1E) | 3.037(7) | K(2)-O(3SB)#4 | 2.657(5) | ||
| K(1)-O(5A) | 2.730(5) | K(2)-O(4’B)#4 | 2.934(5) | ||
| * K(1)-O(2SA)#3 | 2.807(6) | K(2)-O(4B)#5 | 2.725(5) | ||
| *$$ K(1)-O(2SA)#1 | 2.661(5) | K(2)-O(3SB)#6 | 2.796(6) | ||
| $ K(1)-O(4’A)#1 | 2.835(5) | K(2)-O(1SB)#6 | 3.019(5) | ||
| $ K(1)-O(1SA)#3 | 3.167(6) | K(2)-O(1F) | 2.681(6) | ||
| K(1)-O(4A)#2 | 2.675(5) | K(2)-O(1D) | 2.962(7) |
| D-H...A | d(D-H) [Å] | d(H...A) [Å] | d(H...A) [Å] | <(DHA) [°] |
|---|---|---|---|---|
| O(3A)-H(3A)...O(4A) | 0.84 | 2.23 | 2.688(6) | 115 |
| O(5A)-H(5A)...O(4A) | 1.19 | 1.65 | 2.671(7) | 139 |
| O(4′A)-H(4′A)...O(3′A) | 0.90 | 2.14 | 2.695(7) | 112 |
| O(7A)-H(7A)...O(3SA)#a | 0.79 | 1.90 | 2.673(7) | 163 |
| O(3A)-H(3A)...O(1C)#b | 0.84 | 2.04 | 2.770(7) | 144 |
| O(4′A)-H(4′A)...O(1E)#c | 0.90 | 1.98 | 2.653(7) | 130 |
| O(3′A)-H(3′A)...O(1G) | 0.84 | 1.82 | 2.661(8) | 177 |
| O(3B)-H(3B)...O(4B) | 0.96 | 2.06 | 2.687(7) | 121 |
| O(5B)-H(5B)...O(4B) | 0.93 | 1.86 | 2.665(7) | 144 |
| O(7B)-H(7B)...O(2SB)#d | 0.88 | 1.84 | 2.697(7) | 165 |
| O(4′B)-H(4′B)...O(1D)#e | 0.88 | 1.90 | 2.649(8) | 142 |
| O(3B)-H(3B)...O(1F)#f | 0.96 | 2.05 | 2.796(7) | 133 |
| O(3’B)-H(3’B)...O(1H)#e | 0.84 | 1.85 | 2.684(9) | 172 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maciołek, U.; Mendyk, E.; Kosińska-Pezda, M.; Kamiński, D.M.; Kozioł, A.E. Potassium Complexes of Quercetin-5′-Sulfonic Acid and Neutral O-Donor Ligands: Synthesis, Crystal Structure, Thermal Analysis, Spectroscopic Characterization and Physicochemical Properties. Materials 2021, 14, 6798. https://doi.org/10.3390/ma14226798
Maciołek U, Mendyk E, Kosińska-Pezda M, Kamiński DM, Kozioł AE. Potassium Complexes of Quercetin-5′-Sulfonic Acid and Neutral O-Donor Ligands: Synthesis, Crystal Structure, Thermal Analysis, Spectroscopic Characterization and Physicochemical Properties. Materials. 2021; 14(22):6798. https://doi.org/10.3390/ma14226798
Chicago/Turabian StyleMaciołek, Urszula, Ewaryst Mendyk, Małgorzata Kosińska-Pezda, Daniel M. Kamiński, and Anna E. Kozioł. 2021. "Potassium Complexes of Quercetin-5′-Sulfonic Acid and Neutral O-Donor Ligands: Synthesis, Crystal Structure, Thermal Analysis, Spectroscopic Characterization and Physicochemical Properties" Materials 14, no. 22: 6798. https://doi.org/10.3390/ma14226798
APA StyleMaciołek, U., Mendyk, E., Kosińska-Pezda, M., Kamiński, D. M., & Kozioł, A. E. (2021). Potassium Complexes of Quercetin-5′-Sulfonic Acid and Neutral O-Donor Ligands: Synthesis, Crystal Structure, Thermal Analysis, Spectroscopic Characterization and Physicochemical Properties. Materials, 14(22), 6798. https://doi.org/10.3390/ma14226798






