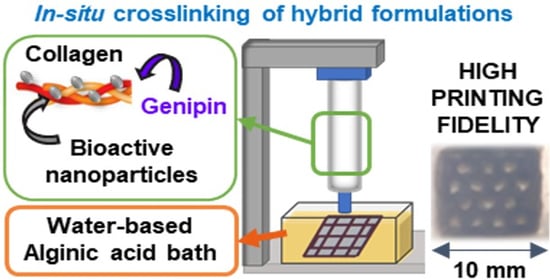
3D Printing in Alginic Acid Bath of In-Situ Crosslinked Collagen Composite Scaffolds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
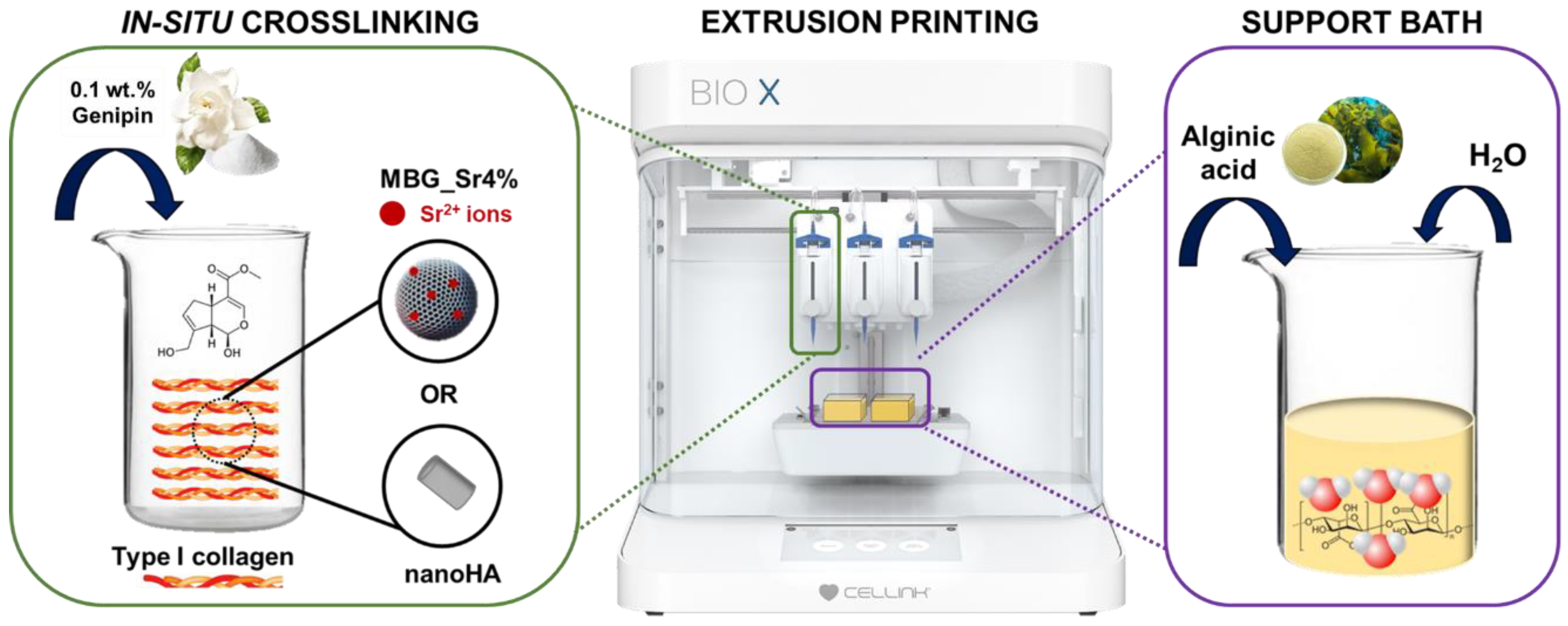
2.2. Preparation of Genipin-Containing Collagenous Suspensions: GEN-Coll, GEN-Coll/MBG_Sr4% and GEN-Coll/nanoHA
2.3. Preparation of the Alginic Acid Support Bath
2.4. Rheological Testing
2.5. Strontium Ion Release
2.6. 3D Printing of Collagen Composite Scaffolds
2.7. Field Emission Scanning Electron Microscopy (FE-SEM)
2.8. Statistics
3. Results
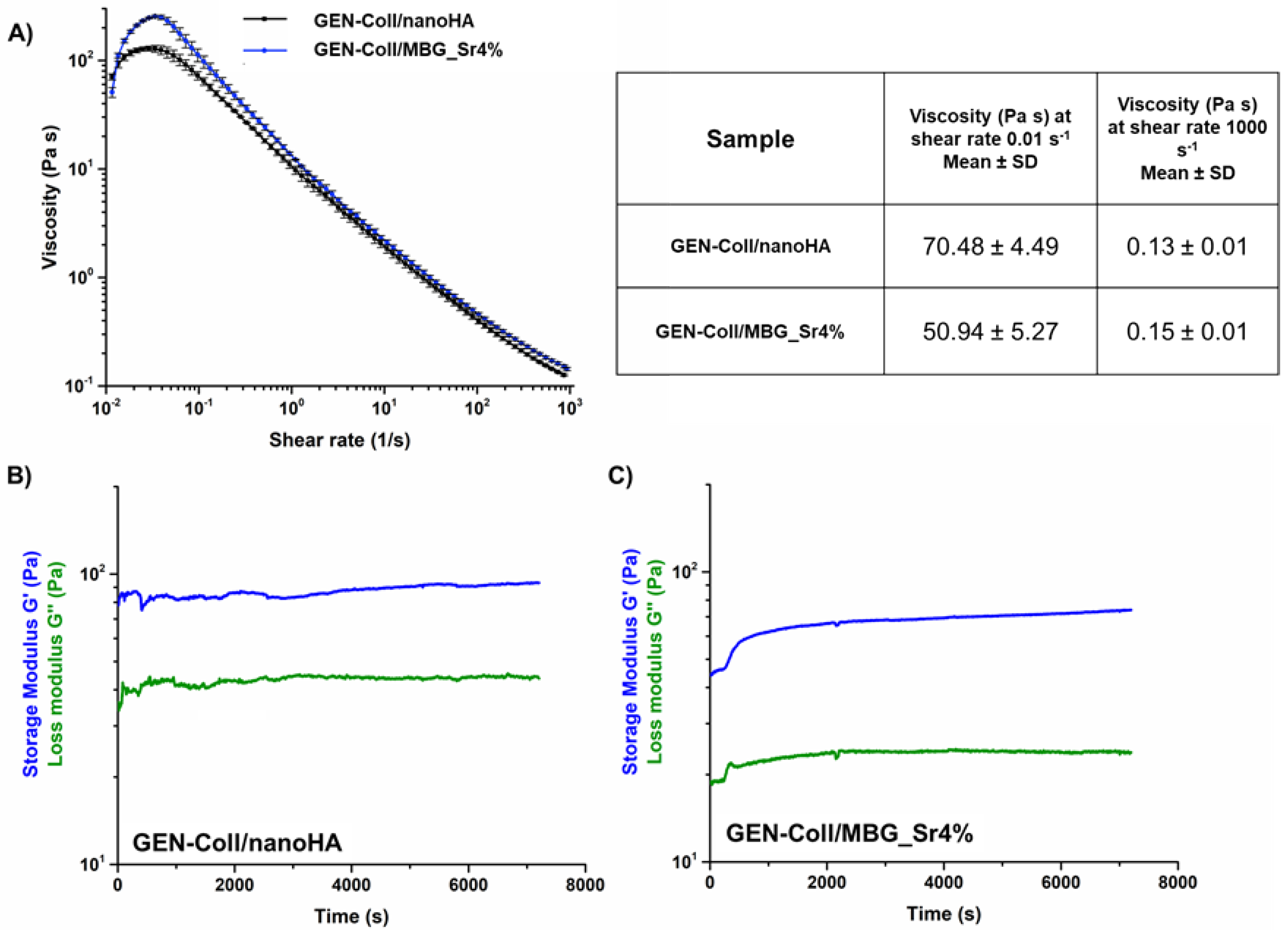
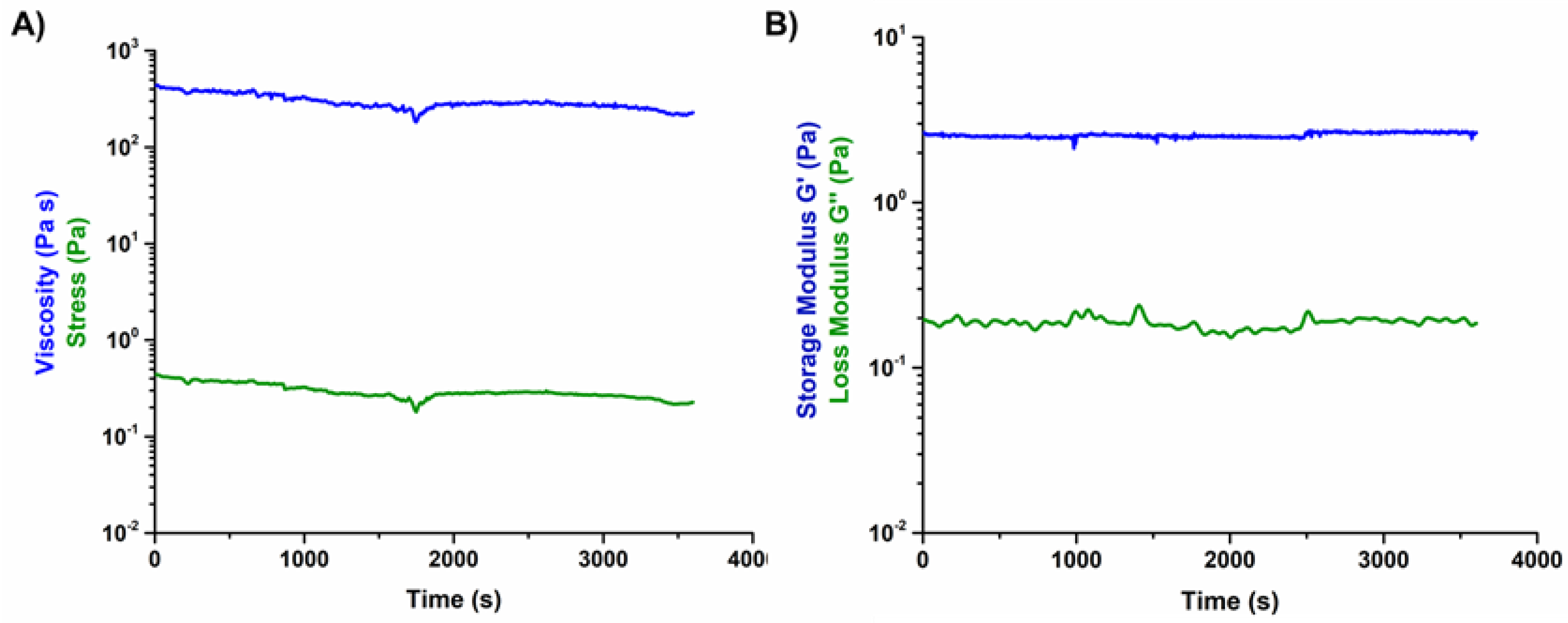
3.1. Printability of In-Situ Crosslinking Collagen Hybrid Formulations
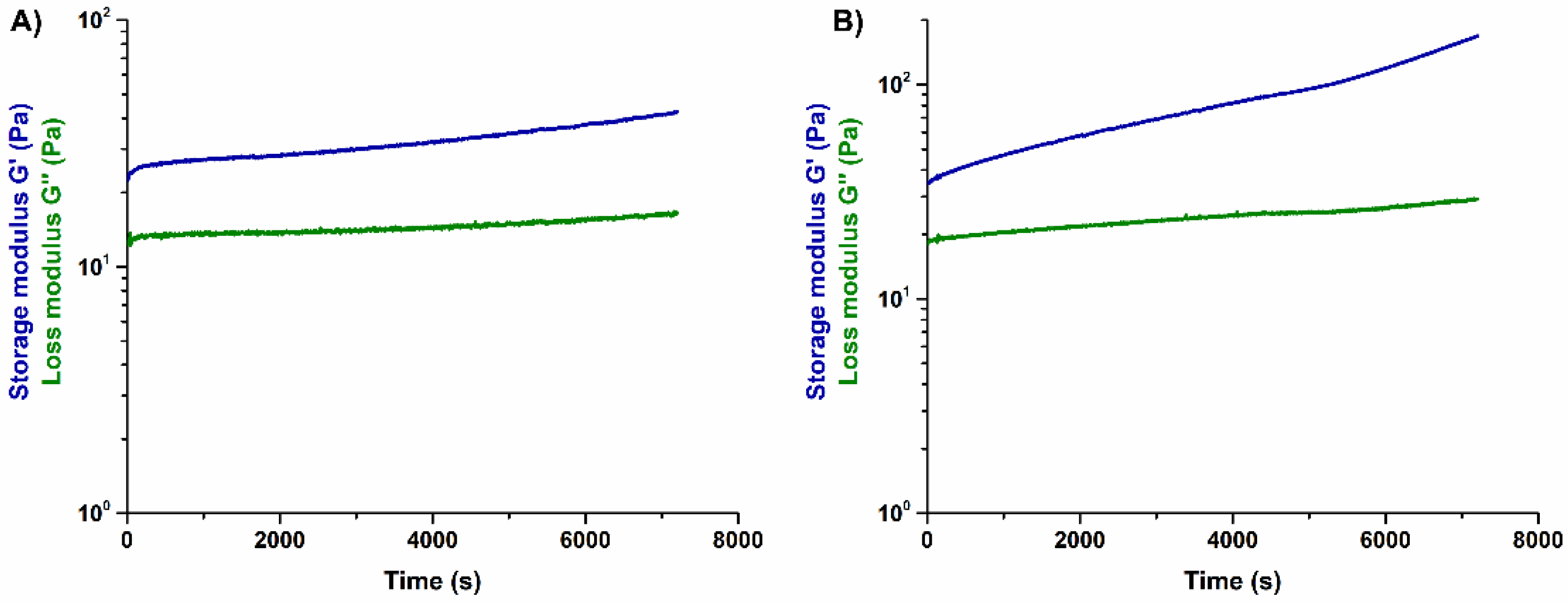
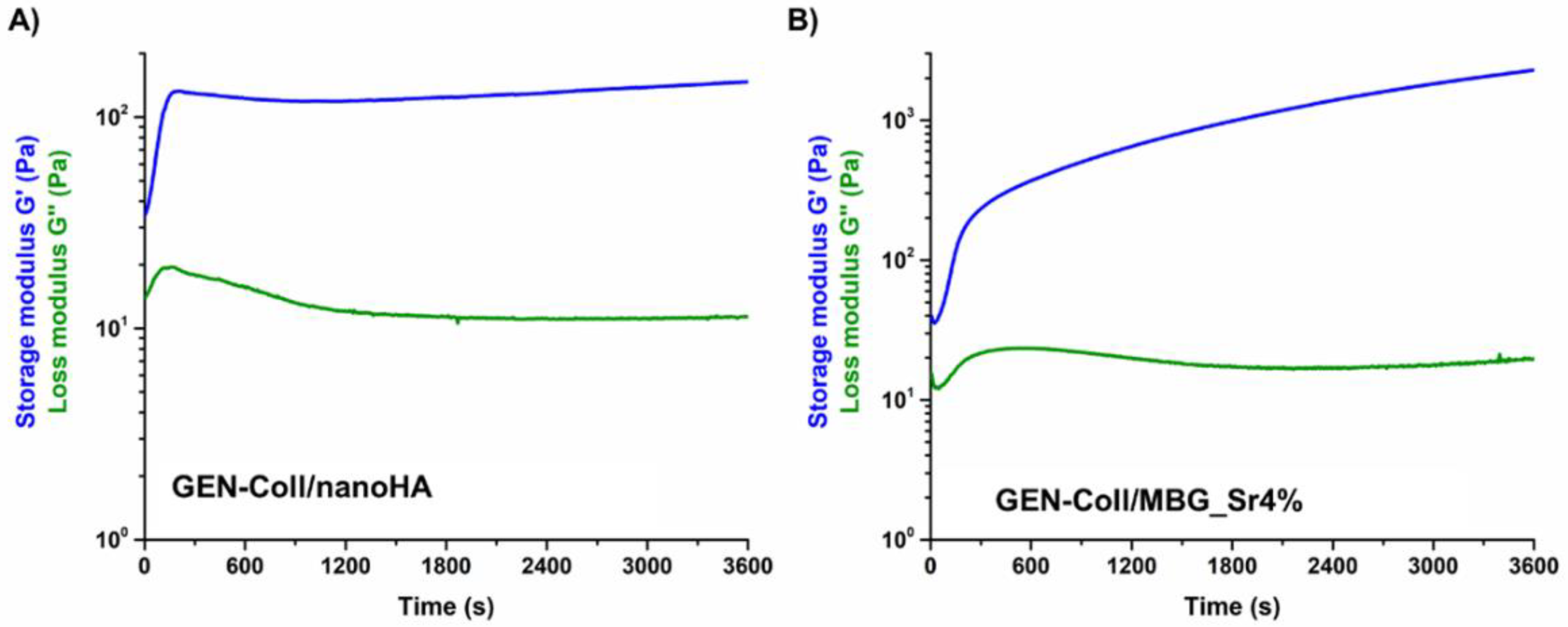
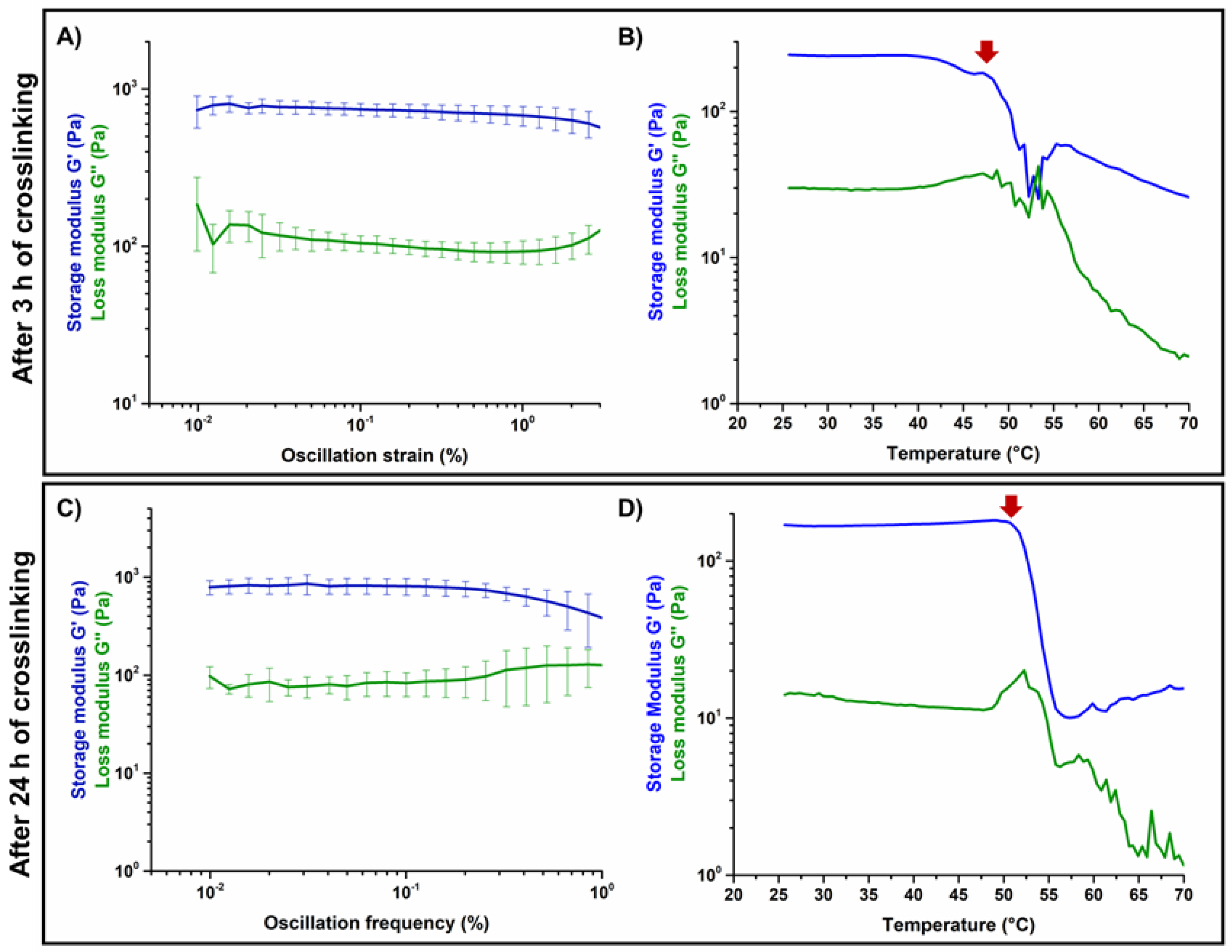
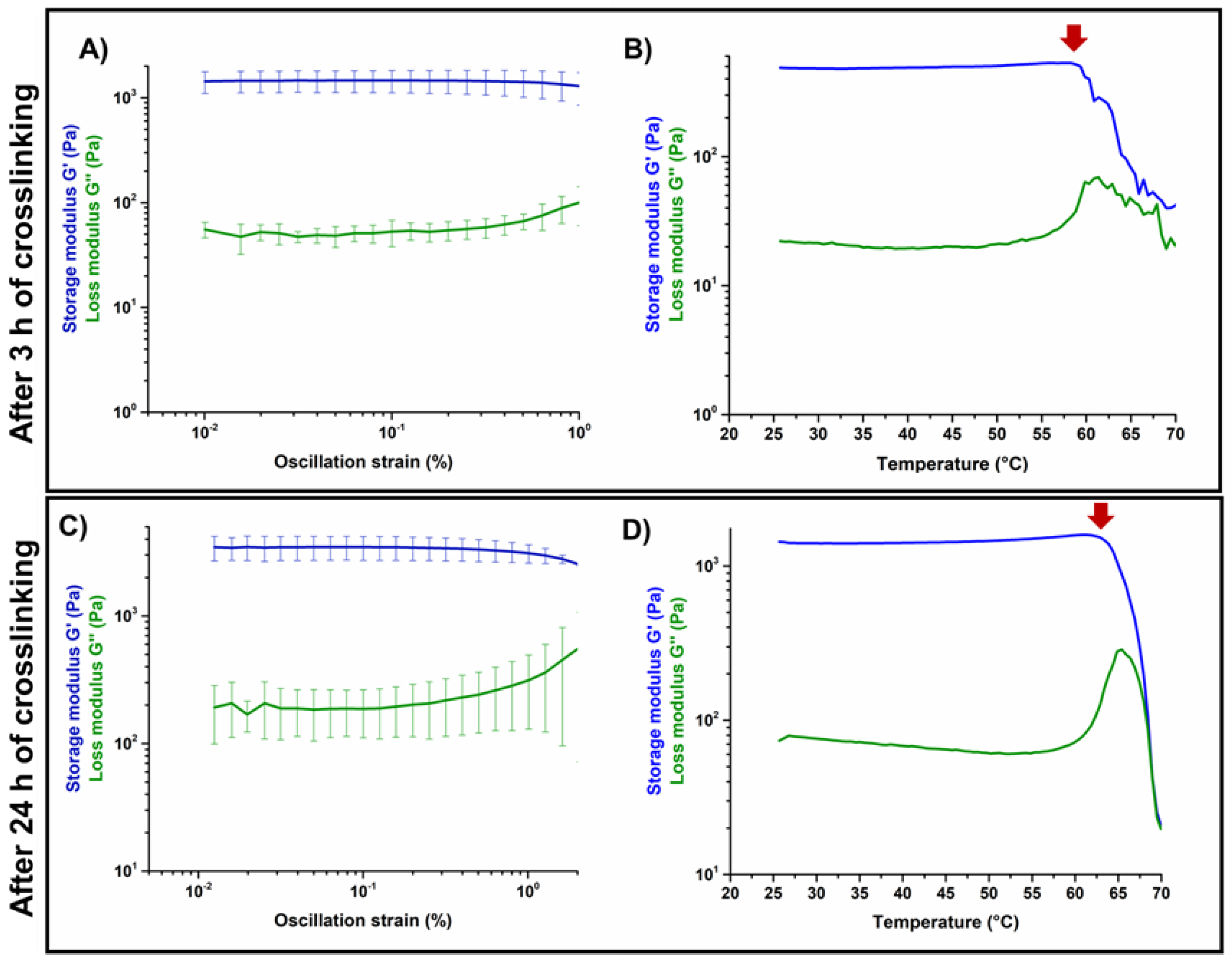
3.2. Effects of Genipin Crosslinking on the Visco-Elastic and Thermal Properties of the Systems upon Reconstitution at 37 °C
3.3. Strontium Release of the MBG_Sr4% Containing Formulations
3.4. Printability of the Hybrid Formulations in Aqueous Alginic Acid Bath (15 wt.%)
3.5. Morphological Assessment of the Resulting 3D Scaffolds
4. Discussion
4.1. Influence of Genipin Content on the Rheological Behavior of the Hybrid Formulations
4.2. Suitability of the Alginic Bath for the Printing Process
4.3. Printability of the In-Situ Crosslinking Suspensions in the Alginic Acid Bath (15 wt.%) and Post-Processing
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Iaquinta, M.R.; Mazzoni, E.; Manfrini, M.; D’Agostino, A.; Trevisiol, L.; Nocini, R.; Trombelli, L.; Barbanti-Brodano, G.; Martini, F.; Tognon, M. Innovative Biomaterials for Bone Regrowth. Int. J. Mol. Sci. 2019, 20, 618. [Google Scholar] [CrossRef] [Green Version]
- Dzobo, K.; Thomford, N.E.; Senthebane, D.A.; Shipanga, H.; Rowe, A.; Dandara, C.; Pillay, M.; Motaung, K.S.C.M. Advances in Regenerative Medicine and Tissue Engineering: Innovation and Transformation of Medicine. Stem Cells Int. 2018, 2018, 2495848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adamovic, D.; Ristic, B.; Zivic, F. Review of existing biomaterials—method of material selection for specific applications in orthopedic. In Biomaterials in Clinical Practice: Advances in Clinical Research and Medical Devices; Zivic, F., Affatato, S., Trajanovic, M., Schnabelrauch, M., Grujovic, N., Choy, K.L., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 47–99. ISBN 978-3-319-68025-5. [Google Scholar]
- Zeng, J.-H.; Liu, S.-W.; Xiong, L.; Qiu, P.; Ding, L.-H.; Xiong, S.-L.; Li, J.-T.; Liao, X.-G.; Tang, Z.-M. Scaffolds for the repair of bone defects in clinical studies: A systematic review. J. Orthop. Surg. Res. 2018, 13, 33. [Google Scholar] [CrossRef]
- Guvendiren, M.; Molde, J.; Soares, R.M.D.; Kohn, J. Designing biomaterials for 3D printing. ACS Biomater. Sci. Eng. 2016, 2, 1679–1693. [Google Scholar] [CrossRef] [PubMed]
- McCormack, A.; Highley, C.B.; Leslie, N.R.; Melchels, F.P.W. 3D Printing in Suspension Baths: Keeping the Promises of Bioprinting Afloat. Trends Biotechnol. 2020, 38, 584–593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montalbano, G.; Borciani, G.; Cerqueni, G.; Licini, C.; Banche-Niclot, F.; Janner, D.; Sola, S.; Fiorilli, S.; Mattioli-Belmonte, M.; Ciapetti, G.; et al. Collagen hybrid formulations for the 3d printing of nanostructured bone scaffolds: An optimized genipin-crosslinking strategy. Nanomaterials 2020, 10, 1681. [Google Scholar] [CrossRef] [PubMed]
- Montalbano, G.; Molino, G.; Fiorilli, S.; Vitale-Brovarone, C. Synthesis and incorporation of rod-like nano-hydroxyapatite into type I collagen matrix: A hybrid formulation for 3D printing of bone scaffolds. J. Eur. Ceram. Soc. 2020, 40, 3689–3697. [Google Scholar] [CrossRef]
- Montalbano, G.; Fiorilli, S.; Caneschi, A.; Vitale-Brovarone, C. Type I collagen and strontium-containing mesoporous glass particles as hybrid material for 3D printing of bone-like materials. Materials 2018, 11, 700. [Google Scholar] [CrossRef] [Green Version]
- Fiorilli, S.; Molino, G.; Pontremoli, C.; Iviglia, G.; Torre, E.; Cassinelli, C.; Morra, M.; Vitale-Brovarone, C. The Incorporation of Strontium to Improve Bone-Regeneration Ability of Mesoporous Bioactive Glasses. Material 2018, 11, 678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hinton, T.J.; Jallerat, Q.; Palchesko, R.N.; Park, J.H.; Grodzicki, M.S.; Shue, H.J.; Ramadan, M.H.; Hudson, A.R.; Feinberg, A.W. Three-dimensional printing of complex biological structures by freeform reversible embedding of suspended hydrogels. Sci. Adv. 2015, 1, e1500758. [Google Scholar] [CrossRef] [Green Version]
- Meng, L.; Arnoult, O.; Smith, M.; Wnek, G.E. Electrospinning of in situ crosslinked collagen nanofibers. J. Mater. Chem. 2012, 22, 19412–19417. [Google Scholar] [CrossRef]
- Paxton, N.; Smolan, W.; Böck, T.; Melchels, F.; Groll, J.; Jungst, T. Proposal to assess printability of bioinks for extrusion-based bioprinting and evaluation of rheological properties governing bioprintability. Biofabrication 2017, 9, 44107. [Google Scholar] [CrossRef]
- Duchi, S.; Onofrillo, C.; O’Connell, C.D.; Blanchard, R.; Augustine, C.; Quigley, A.F.; Kapsa, R.M.I.; Pivonka, P.; Wallace, G.; Di Bella, C.; et al. Handheld Co-Axial Bioprinting: Application to in situ surgical cartilage repair. Sci. Rep. 2017, 7, 5837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouyang, L.; Highley, C.B.; Sun, W.; Burdick, J.A. A Generalizable Strategy for the 3D Bioprinting of Hydrogels from Nonviscous Photo-crosslinkable Inks. Adv. Mater. 2017, 29, 1604983. [Google Scholar] [CrossRef] [PubMed]
- Fessel, G.; Cadby, J.; Wunderli, S.; van Weeren, R.; Snedeker, J.G. Dose- and time-dependent effects of genipin crosslinking on cell viability and tissue mechanics—Toward clinical application for tendon repair. Acta Biomater. 2014, 10, 1897–1906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montalbano, G.; Borciani, G.; Pontremoli, C.; Ciapetti, G.; Mattioli-Belmonte, M.; Fiorilli, S.; Vitale-Brovarone, C. Development and biocompatibility of collagen-based composites enriched with nanoparticles of strontium containing mesoporous glass. Materials 2019, 12, 3719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vozzi, G.; Corallo, C.; Carta, S.; Fortina, M.; Gattazzo, F.; Galletti, M.; Giordano, N. Collagen-gelatin-genipin-hydroxyapatite composite scaffolds colonized by human primary osteoblasts are suitable for bone tissue engineering applications: In vitro evidences. J. Biomed. Mater. Res. Part A 2014, 102, 1415–1421. [Google Scholar] [CrossRef] [PubMed]
- Ovsianikov, A.; Yoo, J.; Mironov, V. 3D Printing and Biofabrication; Springer International Publishing: Cham, Switzerland, 2018; ISBN 9783319454436. [Google Scholar]
- Rabotyagova, O.S.; Cebe, P.; Kaplan, D.L. Collagen Structural Hierarchy and Susceptibility to Degradation by Ultraviolet Radiation. Mater. Sci. Eng. C Mater. Biol. Appl. 2008, 28, 1420–1429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, A.M.; Gentile, P.; Chiono, V.; Ciardelli, G. Collagen for bone tissue regeneration. Acta Biomater. 2012, 8, 3191–3200. [Google Scholar] [CrossRef]
- Kim, Y.B.; Lee, H.; Kim, G.H. Strategy to Achieve Highly Porous/Biocompatible Macroscale Cell Blocks, Using a Collagen/Genipin-bioink and an Optimal 3D Printing Process. ACS Appl. Mater. Interfaces 2016, 8, 32230–32240. [Google Scholar] [CrossRef] [PubMed]
- Ficai, A.; Andronescu, E.; Voicu, G.; Ghitulica, C.; Vasile, B.S.; Ficai, D.; Trandafir, V. Self-assembled collagen/hydroxyapatite composite materials. Chem. Eng. J. 2010, 160, 794–800. [Google Scholar] [CrossRef]
- Ficai, A.; Andronescu, E.; Ghitulica, C.; Voicu, G.; Trandafir, V.; Mânzu, D.; Ficai, M.; Pall, S. Colagen/Hydroxyapatite Interactions in Composite Biomaterials. Mater. Plast. 2009, 46, 11–15. [Google Scholar]
- Chen, L.; Hu, J.; Ran, J.; Shen, X.; Tong, H. Preparation and evaluation of collagen-silk fibroin/hydroxyapatite nanocomposites for bone tissue engineering. Int. J. Biol. Macromol. 2014, 65, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Chan, B.P.; So, K.-F. Photochemical crosslinking improves the physicochemical properties of collagen scaffolds. J. Biomed. Mater. Res. Part A 2005, 75A, 689–701. [Google Scholar] [CrossRef] [PubMed]
- Pontremoli, C.; Izquierdo-Barba, I.; Montalbano, G.; Vallet-Regí, M.; Vitale-Brovarone, C.; Fiorilli, S. Strontium-releasing mesoporous bioactive glasses with anti-adhesive zwitterionic surface as advanced biomaterials for bone tissue regeneration. J. Colloid Interface Sci. 2020, 563, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Roger, S.; Yip Cheung Sang, Y.; Bee, A.; Perzynski, R.; Meglio, J.; Ponton, A. Structural and multi-scale rheophysical investigation of diphasic magneto-sensitive materials based on biopolymers. Eur. Phys. J. E. Soft Matter 2015, 38, 173. [Google Scholar] [CrossRef]
- Capron, I.; Costeux, S.; Djabourov, M. Water in water emulsions: Phase separation and theology of biopolymer solutions. Rheol. Acta 2001, 40, 441–456. [Google Scholar] [CrossRef]
- Zhang, X.; Weng, L.; Liu, Q.; Li, D.; Deng, B. Facile fabrication and characterization on alginate microfibres with grooved structure via microfluidic spinning. R. Soc. Open Sci. 2019, 6, 181928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naghieh, S.; Sarker, M.D.; Sharma, N.K.; Barhoumi, Z.; Chen, X. Printability of 3D Printed Hydrogel Scaffolds: Influence of Hydrogel Composition and Printing Parameters. Appl. Sci. 2020, 10, 292. [Google Scholar] [CrossRef] [Green Version]


| Trial | Composition | Geometry | Speed (mm/s) | Pressure (kPa) | Layer Thickness (mm) |
|---|---|---|---|---|---|
| T1 | GEN-Coll/MBG_Sr4% | Grid | 8 | 50 | 0.18 |
| T2 | 8 | 50 | 0.17 | ||
| T3 | GEN-Coll/nanoHA | Honeycomb | 8 | 50 | 0.17 |
| T4 | 7 | 40 | 0.18 |
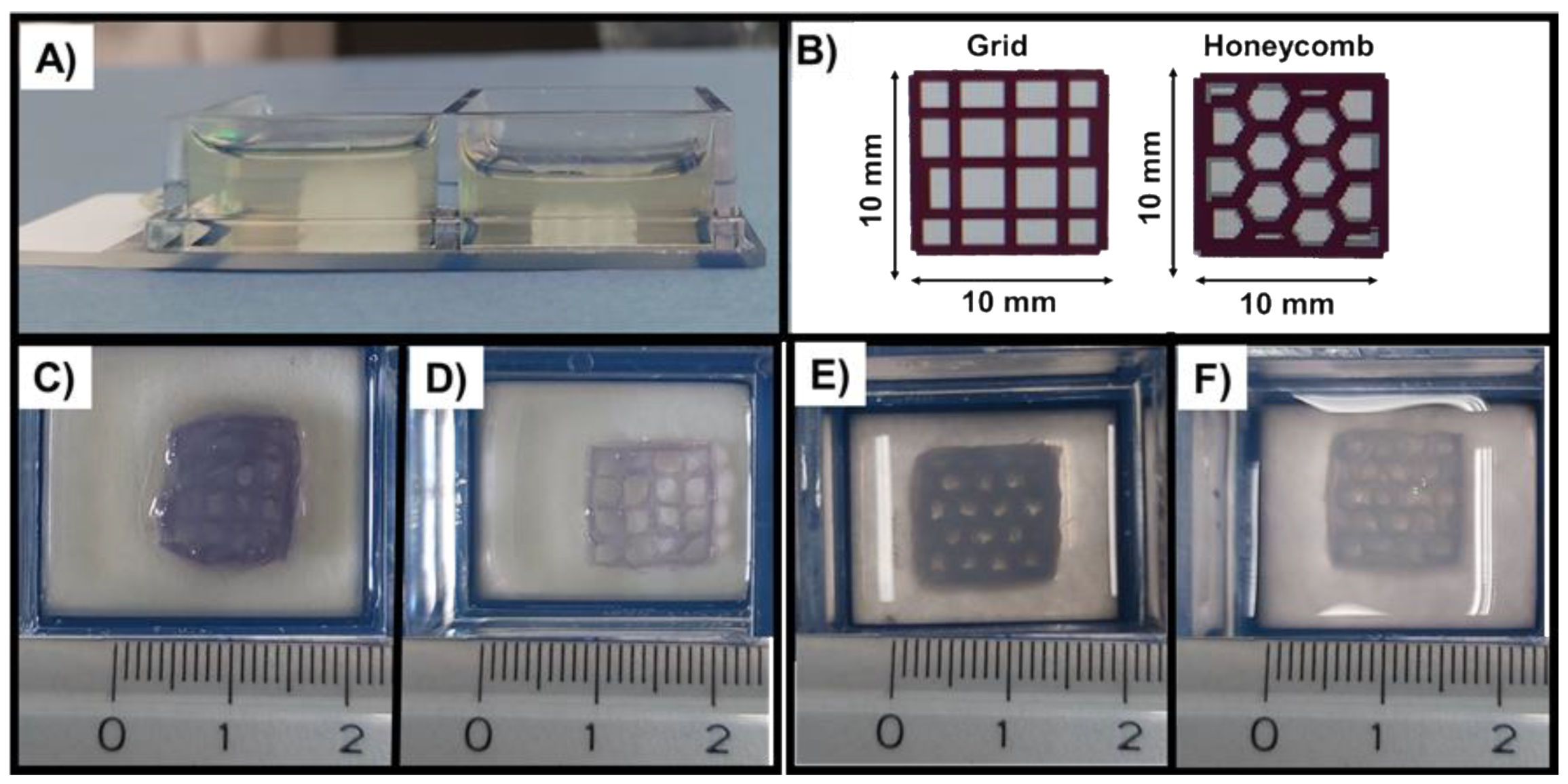
| Samples | Sides (mm) | Pores (mm) Mean ± SD | Strand (mm) Mean ± SD | |||
|---|---|---|---|---|---|---|
| Original grid | 9.44 | 10.16 | 9.47 | 10.03 | 1.89 ± 0.36 | 0.56 ± 0.07 |
| Grid (Figure 9C) | 11.58 | 10.65 | 10.41 | 10.31 | 1.33 ± 0.15 | 1.19 ± 0.12 |
| Grid (Figure 9D) | 10.17 | 10.44 | 10.35 | 10.26 | 1.91 ± 0.11 | 0.62 ± 0.14 |
| Original honeycomb | 9.52 | 9.95 | 9.44 | 10 | 1.86 ± 0.26 | 0.72 ± 0.19 |
| Honeycomb (Figure 9E) | 12.01 | 12.14 | 11.65 | 11.61 | 1.33 ± 0.17 | 1.38 ± 0.15 |
| Honeycomb (Figure 9D) | 11.25 | 10.89 | 12.14 | 11.43 | 1.30 ± 0.15 | 0.82 ± 0.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melo, P.; Montalbano, G.; Fiorilli, S.; Vitale-Brovarone, C. 3D Printing in Alginic Acid Bath of In-Situ Crosslinked Collagen Composite Scaffolds. Materials 2021, 14, 6720. https://doi.org/10.3390/ma14216720
Melo P, Montalbano G, Fiorilli S, Vitale-Brovarone C. 3D Printing in Alginic Acid Bath of In-Situ Crosslinked Collagen Composite Scaffolds. Materials. 2021; 14(21):6720. https://doi.org/10.3390/ma14216720
Chicago/Turabian StyleMelo, Priscila, Giorgia Montalbano, Sonia Fiorilli, and Chiara Vitale-Brovarone. 2021. "3D Printing in Alginic Acid Bath of In-Situ Crosslinked Collagen Composite Scaffolds" Materials 14, no. 21: 6720. https://doi.org/10.3390/ma14216720
APA StyleMelo, P., Montalbano, G., Fiorilli, S., & Vitale-Brovarone, C. (2021). 3D Printing in Alginic Acid Bath of In-Situ Crosslinked Collagen Composite Scaffolds. Materials, 14(21), 6720. https://doi.org/10.3390/ma14216720