Investigation of Photon Radiation Attenuation Capability of Different Clay Materials
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Esra Kavaz, E.H.; Ghanim, A.S. Abouhaswa, Optical, structural and nuclear radiation security properties of newly fabricated V2O5-SrO-PbO glass system. J. Non-Cryst. Solids 2020, 538, 120045. [Google Scholar] [CrossRef]
- Sayyed, M.I.; Mahmoud, K.A.; Tashlykov, O.L. Mayeen Uddin Khandaker, M.R.I. Faruque, 2021. Enhancement of the Shielding Capability of Soda–Lime Glasses with Sb2O3 Dopant: A Potential Material for Radiation Safety in Nuclear Installations. Appl. Sci. 2021, 11, 326. [Google Scholar] [CrossRef]
- Obaid, S.S.; Gaikwad, D.K.; Pawar, P.P. Determination of gamma ray shielding parameters of rocks and concrete. Radiat. Phys. Chem. 2018, 144, 356–360. [Google Scholar]
- Gökçe, H.S.; Yalçınkaya, Ç.; Tuyan, M. Optimization of reactive powder concrete by means of barite aggregate for neutrons and gamma rays. Constr. Build. Mater. 2018, 189, 470–477. [Google Scholar] [CrossRef]
- Yasmin, S.; Barua, B.S.; Khandaker, M.U.; Rashid, M.A.; Bradley, D.A.; Olatunji, M.A.; Kamal, M. Studies of ionizing radiation shielding effectiveness of silica-based commercial glasses used in Bangladeshi dwellings. Results Phys. 2018, 9, 541–549. [Google Scholar] [CrossRef]
- Sayyed, M.I.; Jecong, J.F.M.; Hila, F.C.; Balderas, C.V.; Alhuthali, A.M.; Guillermo, N.R.D.; Al-Hadeethi, Y. Radiation shielding characteristics of selected ceramics using the EPICS2017 library. Ceram. Int. 2021, 47, 13181–13186. [Google Scholar] [CrossRef]
- Yasmin, S.; Rozaila, Z.S.; Khandaker, M.U.; Barua, B.S.; Chowdhury, F.U.Z.; Rashid, M.A.; Bradley, D.A. The radiation shielding offered by the commercial glass installed in Bangladeshi dwellings. Radiat. Eff. Defects Solids 2018, 173, 657–672. [Google Scholar] [CrossRef]
- Almuqrin, A.H.; Sayyed, M.I. Radiation shielding characterizations and investigation of TeO2–WO3–Bi2O3 and TeO2–WO3–PbO glasses. Appl. Phys. A 2021, 127, 190. [Google Scholar]
- Abouhaswa, A.S.; Kavaz, E. A novel B2O3-Na2O-BaO-HgO glass system: Synthesis, physical, optical and nuclear shielding features. Ceram. Int. 2020, 46, 16166–16177. [Google Scholar]
- Mhareb, M.H.A. Physical, optical and shielding features of Li2O–B2O3–MgO–Er2O3 glasses co-doped of Sm2O3. Appl. Phys. A 2020, 126, 71. [Google Scholar] [CrossRef]
- Sayyed, M.I.; Mahmoud, K.A.; Lacomme, E.; AlShammari, M.M.; Dwaikat, N.; Alajerami, Y.S.M.; Alqahtani, M.; El-bashir, B.O.; Mhareb, M.H.A. Development of a novel MoO3-doped borate glass network for gamma-ray shielding applications. Eur. Phys. J. Plus 2021, 136, 108. [Google Scholar] [CrossRef]
- Dong, M.; Xue, X.; Yang, H.; Li, Z. Highly cost-effective shielding composite made from vanadium slag and boron-rich slag and its properties. Radiat. Phys. Chem. 2017, 141, 239–244. [Google Scholar] [CrossRef]
- Aygün, B. High alloyed new stainless steel shielding material for gamma and fast neutron radiation. Nucl. Eng. Technol. 2020, 52, 647–653. [Google Scholar]
- Gökç, H.S.; Canbaz-Öztürk, B.; Çam, N.F.; Andiç-Çakır, Ö. Gamma-ray attenuation coefficients and transmission thickness of high consistency heavyweight concrete containing mineral admixture. Cem. Concr. Compos. 2018, 92, 56–69. [Google Scholar]
- Sayyed, M.I.; Al-Hadeethi, Y.; AlShammari, M.M.; Ahmed, M.; Al-Heniti, S.H.; Rammah, Y.S. Physical, optical and gamma radiation shielding competence of newly borotellurite based glasses: TeO2–B2O3–ZnO–Li2O3–Bi2O3. Ceram. Int. 2021, 47, 611–618. [Google Scholar] [CrossRef]
- Sayyed, M.I.; Mhareb, M.H.A.; Alajerami, Y.S.M.; Mahmoud, K.A.; Imheidat, M.A.; Alshahri, F.; Alqahtani, M.; Al-Abdullah, T. Optical and radiation shielding features for a new series of borate glass samples. Optik 2021, 239, 166790. [Google Scholar] [CrossRef]
- Dong, M.; Xue, X.; Yang, H.; Liu, D.; Wang, C.; Li, Z. A novel comprehensive utilization of vanadium slag: As gamma ray shielding material. J. Hazard. Mater. 2016, 318, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Sirin, M. The effect of titanium (Ti) additive on radiation shielding efficiency of Al25Zn alloy. Prog. Nucl. Energy 2020, 128, 103470. [Google Scholar]
- Sousa, L.; Barabasch, J.; Stein, K.J.; Siegesmund, S. Characterization and quality assessment of granitic building stone deposits: A case study of two different Portuguese granites. Eng. Geol. 2017, 221, 29–40. [Google Scholar]
- Huang, B.; Lu, W. Experimental investigation of the uniaxial compressive behavior of thin building granite. Constr. Build. Mater. 2021, 267, 120967. [Google Scholar]
- El-Khatib, A.M.; Elsafi, M.; Almutiri, M.N.; Mahmoud, R.M.M.; Alzahrani, J.S.; Sayyed, M.I.; Abbas, M.I. Enhancement of Bentonite Materials with Cement for Gamma-Ray Shielding Capability. Materials 2021, 14, 4697. [Google Scholar] [CrossRef]
- Olukotun, S.; Gbenu, S.; Ibitoye, F.; Oladejo, O.; Shittu, H.; Fasasi, M.; Balogun, F. Investigation of gamma radiation shielding capability of two clay materials. Nucl. Eng. Technol. 2018, 50, 957–962. [Google Scholar] [CrossRef]
- ASTM Standards. Standard Test Methods for Apparent Porosity, Water Absorption, Apparent Specific Gravity, and Bulk Density of Burned Refractory Brick and Shapes by Boiling Water; ASTM C20-00; ASTM International: West Conshohocken, PA, USA, 2015. [Google Scholar]
- Saleh, H.M.; Bondouk, I.I.; Salama, E.; Esawii, H.A. Consistency and shielding efficiency of cement-bitumen composite for use as gamma-radiation shielding material. Prog. Nucl. Energy 2021, 137, 103764. [Google Scholar] [CrossRef]
- Mavi, B. Experimental investigation of c-ray attenuation coefficients for granite. Ann. Nucl. Energy 2012, 44, 22–25. [Google Scholar] [CrossRef]
- Ozyurt, O.; Altinsoy, N.; Karaaslan, Ş.İ.; Bora, A.; Buyuk, B.; Erk, İ. Calculation of gamma ray attenuation coefficients of some granite samples using a Monte Carlo simulation code. Radiat. Phys. Chem. 2018, 144, 271–275. [Google Scholar]
- Elsafi, M.; Alrashedi, M.F.; Sayyed, M.I.; Al-Hamarneh, I.F.; El-Nahal, M.A.; El-Khatib, M.; Khandaker, M.U.; Osman, H.; Askary, A.E. The Potentials of Egyptian and Indian Granites for Protection of Ionizing Radiation. Materials 2021, 14, 3928. [Google Scholar] [CrossRef]
- Elsafi, M.; El-Nahal, M.A.; Alrashedi, M.F.; Olarinoye, O.I.; Sayyed, M.I.; Khandaker, M.U.; Osman, H.; Alamri, S.; Abbas, M.I. Shielding Properties of Some Marble Types: A Comprehensive Study of Experimental and XCOM Results. Materials 2021, 14, 4194. [Google Scholar] [CrossRef] [PubMed]
- Sayyed, M.I.; Albarzan, B.; Almuqrin, A.H.; El-Khatib, A.M.; Kumar, A.; Tishkevich, D.I.; Trukhanov, A.V.; Elsafi, M. Experimental and Theoretical Study of Radiation Shielding Features of CaO-K2O-Na2O-P2O5 Glass Systems. Materials 2021, 14, 3772. [Google Scholar] [CrossRef] [PubMed]
- Al-Harbi, N.; Sayyed, M.I.; Al-Hadeethi, Y.; Kumar, A.; Elsafi, M.; Mahmoud, K.A.; Khandaker, M.U.; Bradley, D.A. A novel CaO-K2O-Na2O-P2O5 Glass Systems for Radiation Shielding Applications. Radiat. Phys. Chem. 2021, 188, 109645. [Google Scholar] [CrossRef]
- Knoll, G.F. Radiation Detection and Measurement, 3rd ed.; John Wiley and Sons: New York, NY, USA, 2000. [Google Scholar]
- Kumar, A. Gamma ray shielding properties of PbO-Li2O-B2O3 glasses. Radiat. Phys. Chem. 2017, 136, 50–53. [Google Scholar] [CrossRef]
- Mann, H.S.; Brar, G.S.; Mudahar, G.S. Gamma ray shielding effectiveness of novel light weight clay flyash bricks. Radiat. Phys. Chem. 2016, 127, 97–101. [Google Scholar]
- Bashter, I. Calculation of radiation attenuation coefficients for shielding concretes. Ann. Nucl. Energy 1997, 24, 1389–1401. [Google Scholar] [CrossRef]
- Kaçal, M.R.; Akman, F.; Sayyed, M.I. Evaluation of gamma-ray and neutron attenuation properties of some polymers. Nucl. Eng. Technol. 2019, 51, 818–824. [Google Scholar] [CrossRef]

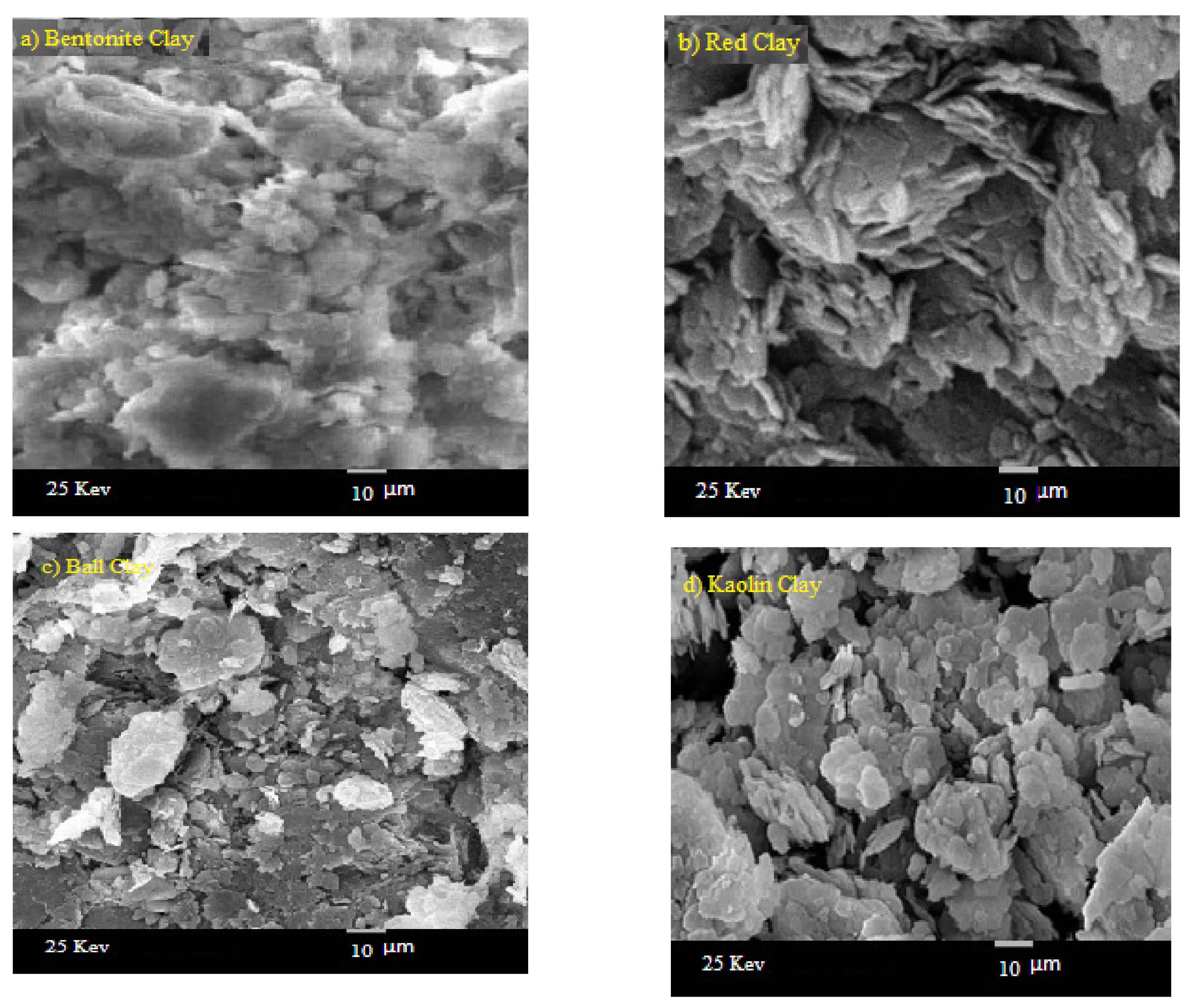

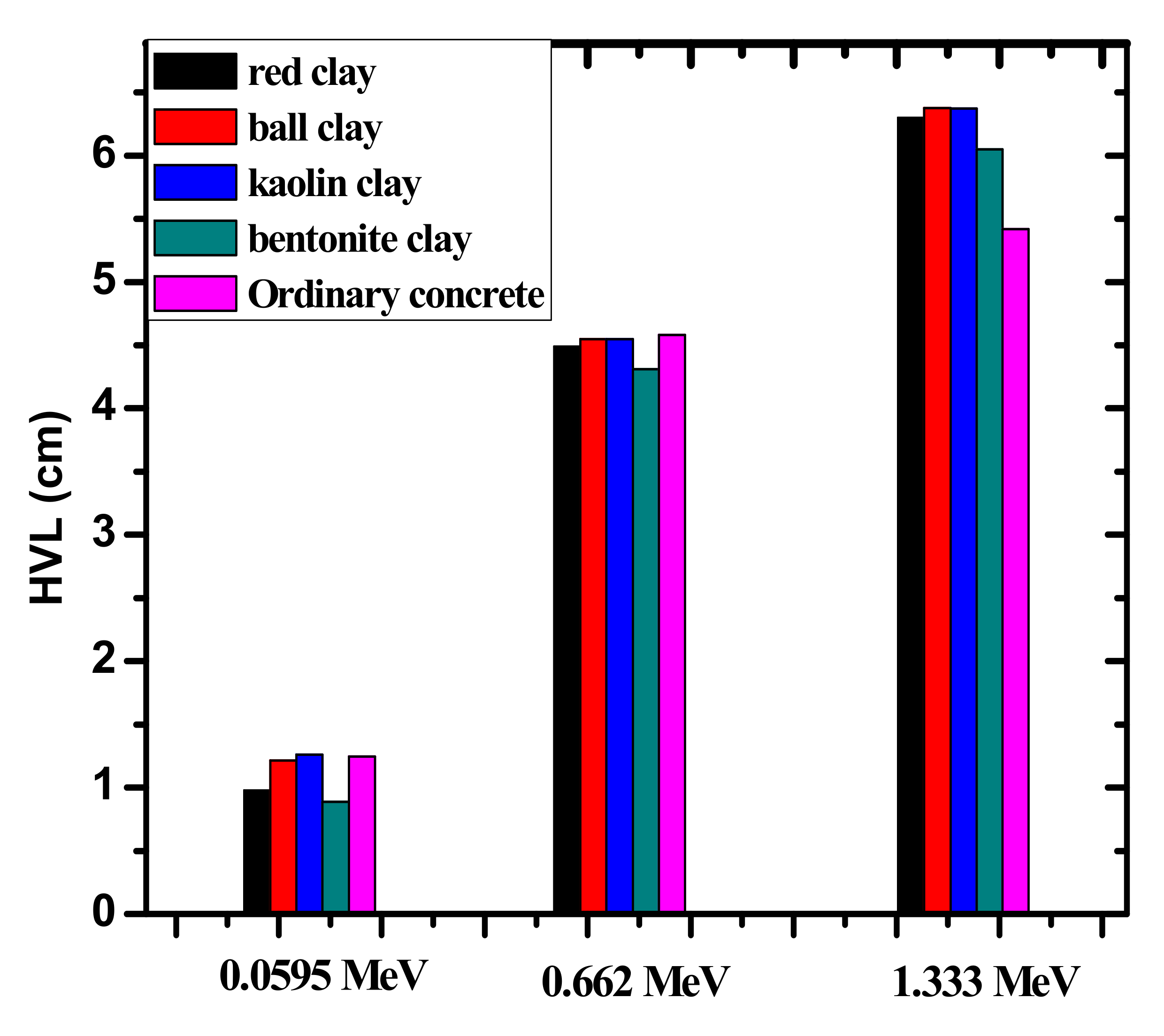
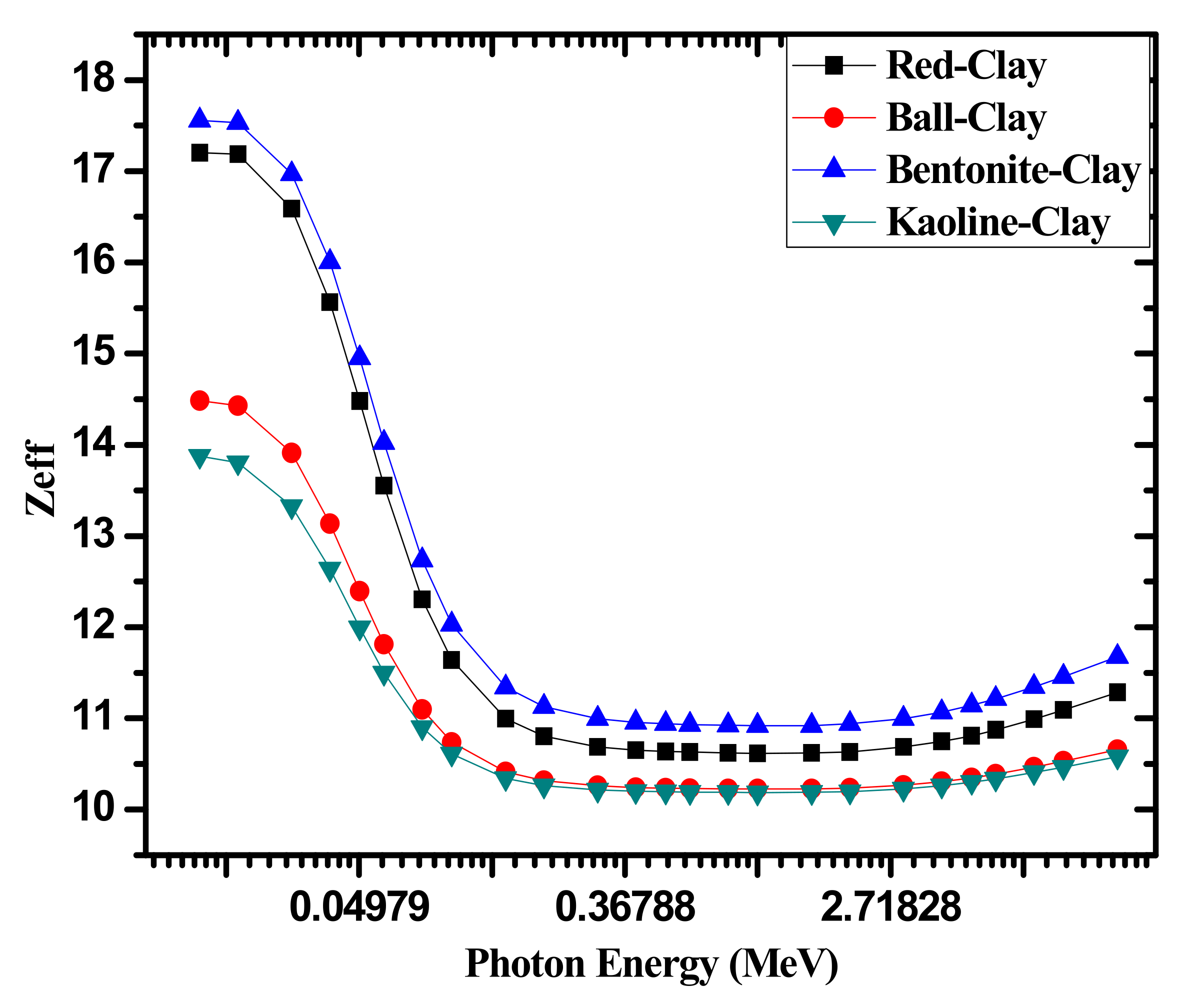
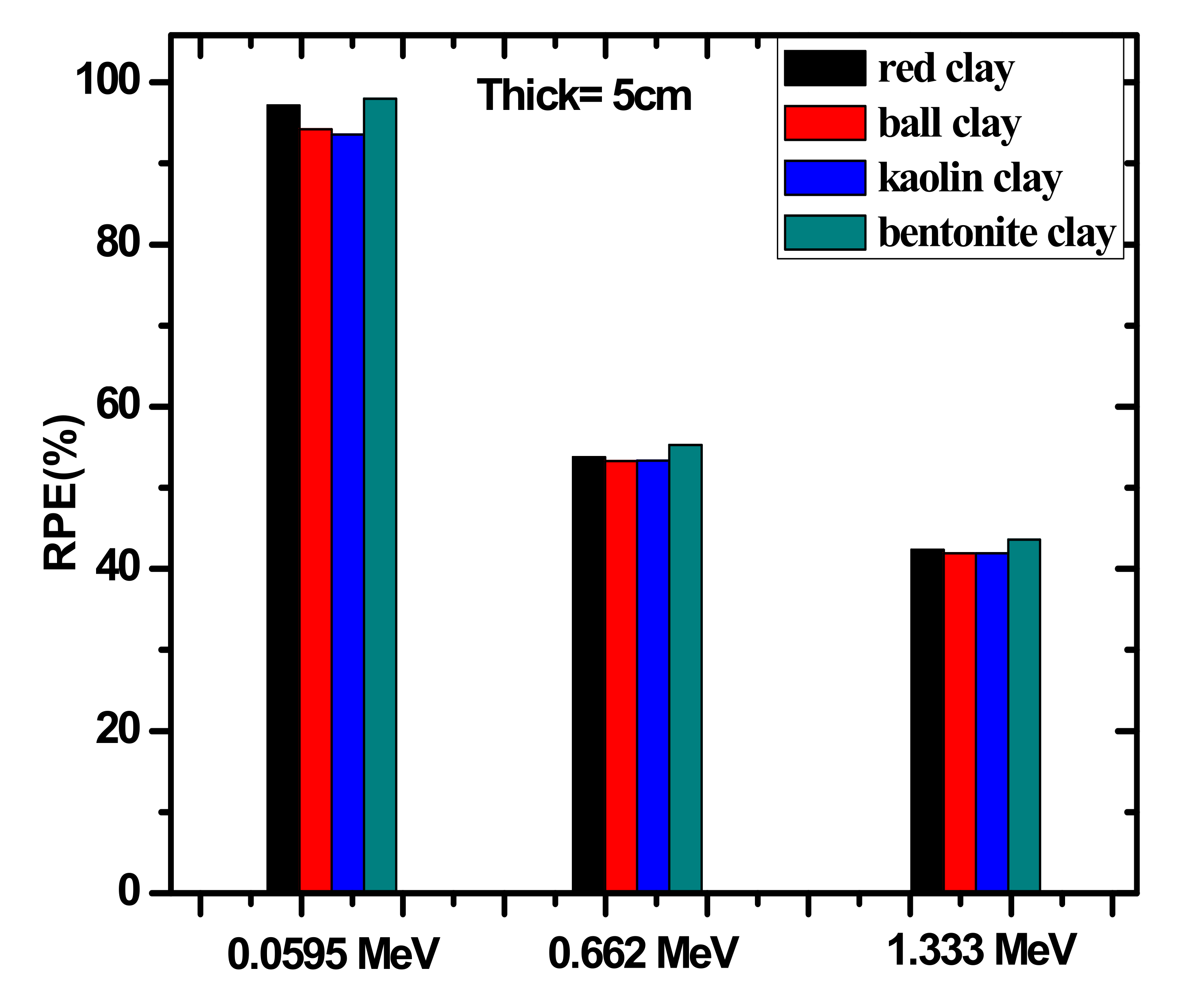
| Sample | Red Clay 2.02 g/cm3 | Ball Clay 1.99 g/cm3 | Bentonite 2.1 g/cm3 | Kaolin 1.99 g/cm3 |
|---|---|---|---|---|
| Na2O | 0 | 0 | 1.3 ± 0.11 | 0 |
| MgO | 0.89 ± 0.32 | 0 | 1.18 ± 0.21 | 2.99 ± 0.16 |
| Al2O3 | 27.34 ± 0.24 | 35.08 ± 0.21 | 20.35 ± 0.14 | 35.53 ± 0.24 |
| SiO2 | 55.95 ± 0.11 | 58.26 ± 0.21 | 49.65 ± 0.22 | 55.26 ± 0.11 |
| SO3 | 0 | 0 | 1.96 ± 0.46 | 0 |
| K2O | 0.98 ± 0.25 | 0 | 1.28 ± 0.13 | 0 |
| CaO | 0 | 0 | 10.92 ± 0.25 | 1.24 ± 0.42 |
| TiO2 | 2.43 ± 0.41 | 2.5 ± 0.21 | 2.74 ± 0.14 | 2.76 ± 0.25 |
| FeO | 12.41 ± 0.02 | 4.16 ± 0.21 | 10.62 ± 0.19 | 2.22 ± 0.17 |
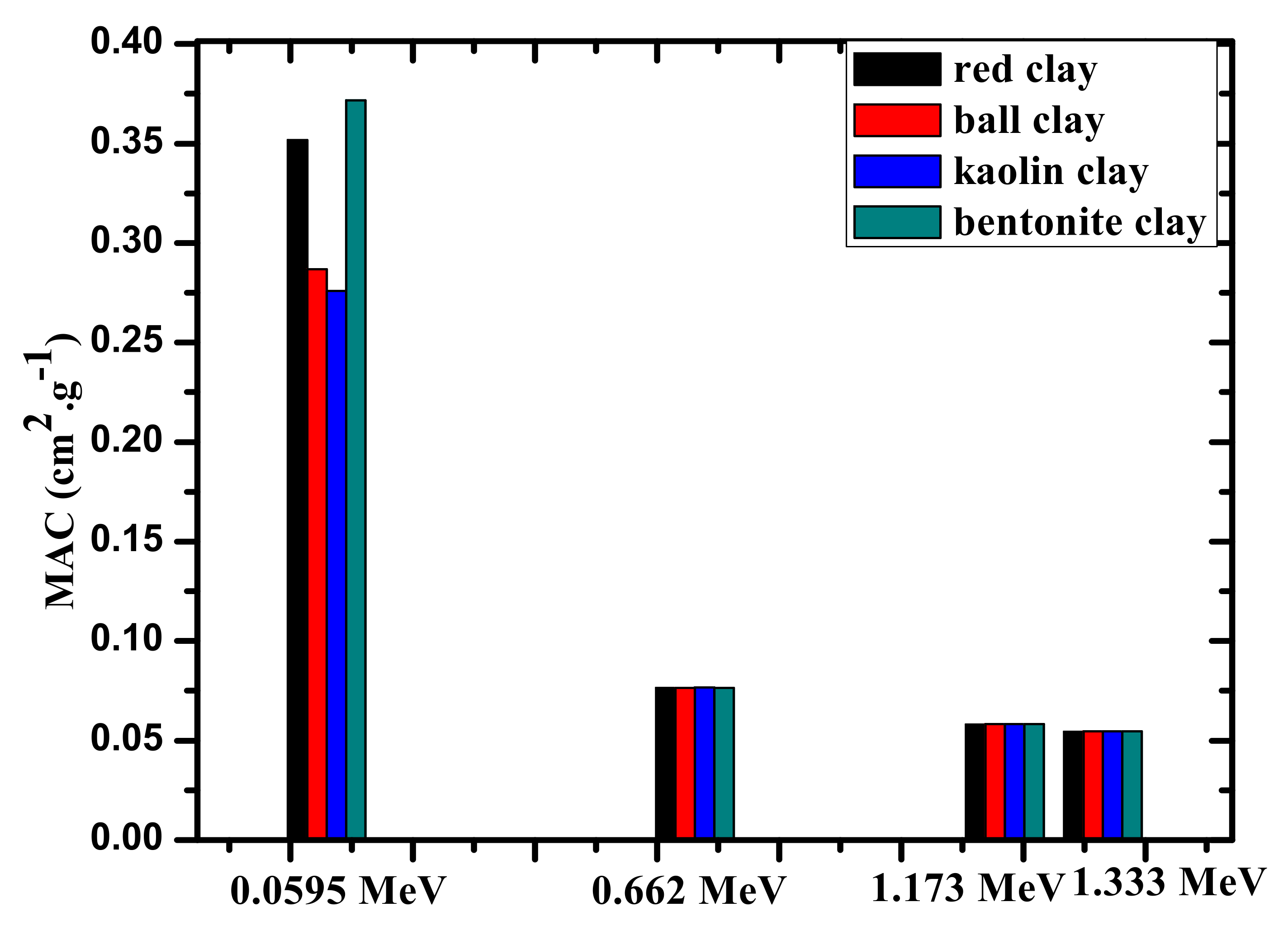
| Clay Type | Nuclide | Energy (MeV) | MAC (cm2.g−1) | R.D (∆%) | |
|---|---|---|---|---|---|
| XCOM | Experimental | ||||
| red clay | Am (241) | 0.060 | 0.352 | 0.346 ± 0.006 | 1.55 |
| Cs (137) | 0.662 | 0.076 | 0.077 ± 0.007 | −2.01 | |
| Co (60) | 1.172 | 0.058 | 0.056 ± 0.001 | 1.95 | |
| 1.333 | 0.054 | 0.053 ± 0.005 | 0.98 | ||
| ball clay | Am (241) | 0.060 | 0.287 | 0.282 ± 0.006 | 1.54 |
| Cs (137) | 0.662 | 0.077 | 0.076 ± 0.001 | 1.22 | |
| Co (60) | 1.172 | 0.058 | 0.058 ± 0.005 | −0.88 | |
| 1.333 | 0.055 | 0.054 ± 0.004 | 1.11 | ||
| kaolin clay | Am (241) | 0.060 | 0.276 | 0.270 ± 0.005 | 2.05 |
| Cs (137) | 0.662 | 0.077 | 0.075 ± 0.005 | 1.95 | |
| Co (60) | 1.172 | 0.058 | 0.059 ± 0.007 | −2.85 | |
| 1.333 | 0.055 | 0.054 ± 0.001 | 1.66 | ||
| bentonite clay | Am (241) | 0.060 | 0.372 | 0.383 ± 0.006 | −3.02 |
| Cs (137) | 0.662 | 0.077 | 0.078 ± 0.002 | −1.55 | |
| Co (60) | 1.172 | 0.058 | 0.057 ± 0.005 | 0.88 | |
| 1.333 | 0.055 | 0.054 ± 0.006 | 0.78 | ||
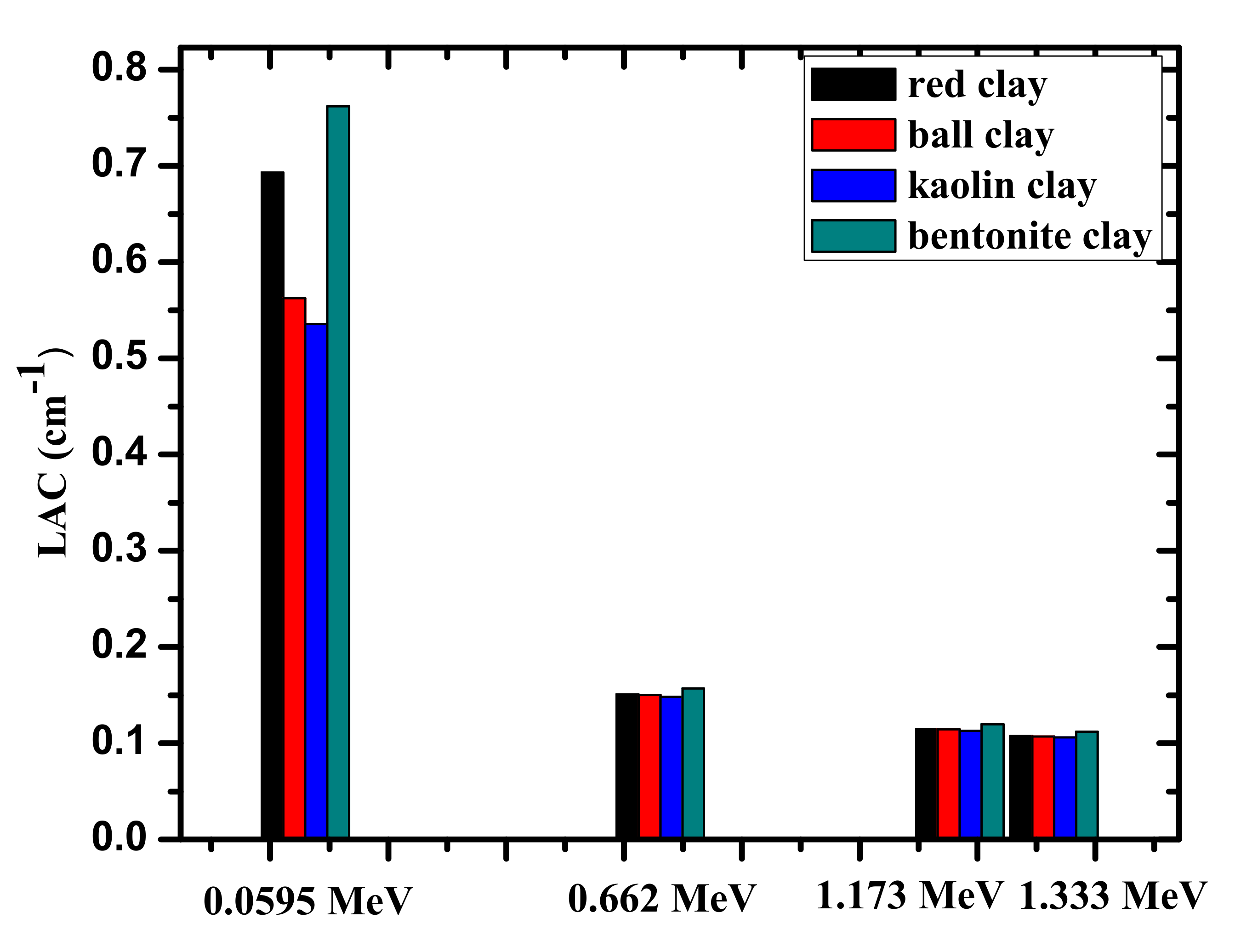
| Attenuation Parameters | Energy (MeV) | This Work | [21] | [22] | [34] | ||||
|---|---|---|---|---|---|---|---|---|---|
| Bentonite Clay | Red Clay | Ball Clay | Kaolin Clay | Bentonite/Cement | Ball Clay | Kaolin Clay | Ordinary Concrete | ||
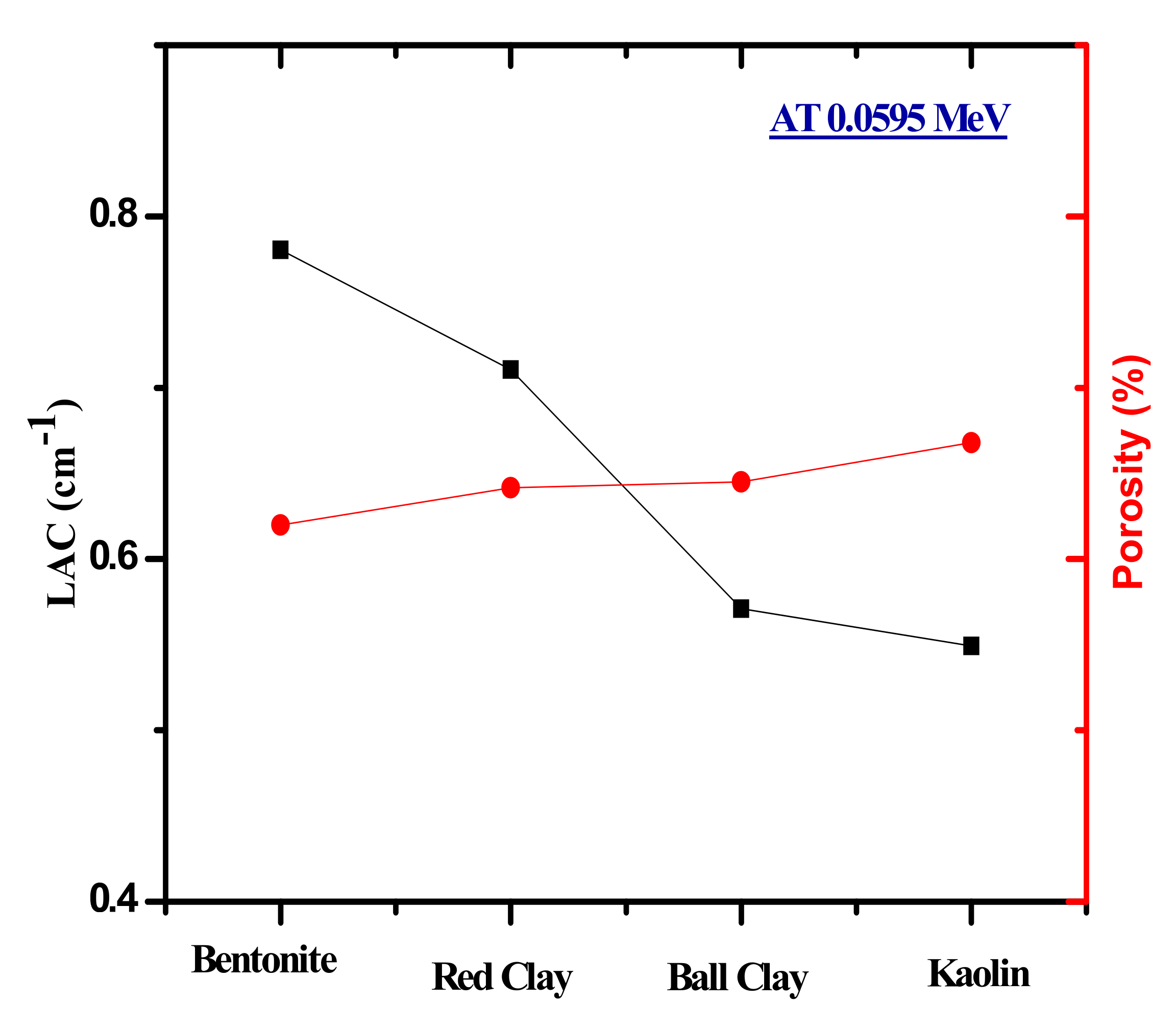
| LAC | 0.060 | 0.7806 | 0.7106 | 0.5709 | 0.5492 | 0.5911 | 0.5631 | 0.5421 | 0.5559 |
| 0.662 | 0.1608 | 0.1544 | 0.1524 | 0.1525 | 0.1511 | 0.1513 | 0.1504 | 0.1513 | |
| 1.170 | 0.1224 | 0.1175 | 0.1161 | 0.1161 | 0.1241 | 0.1196 | 0.1196 | 0.1366 | |
| 1.330 | 0.1146 | 0.1101 | 0.1087 | 0.1088 | 0.1161 | 0.1124 | 0.1124 | 0.1279 | |
| HVL | 0.060 | 0.8880 | 0.9754 | 1.2141 | 1.2620 | 1.1726 | 1.231 | 1.279 | 1.2469 |
| 0.662 | 4.3101 | 4.4896 | 4.5484 | 4.5466 | 4.5873 | 4.581 | 4.609 | 4.5813 | |
| 1.170 | 5.6645 | 5.8990 | 5.9725 | 5.9694 | 5.5854 | 5.796 | 5.796 | 5.0743 | |
| 1.330 | 6.0475 | 6.2973 | 6.3759 | 6.3724 | 5.9703 | 6.167 | 6.167 | 5.4194 | |
| MFP | 0.060 | 1.2811 | 1.4072 | 1.7515 | 1.8207 | 1.6918 | 1.776 | 1.845 | 1.7989 |
| 0.662 | 6.2182 | 6.4772 | 6.5619 | 6.5594 | 6.6181 | 6.609 | 6.649 | 6.6094 | |
| 1.170 | 8.1721 | 8.5104 | 8.6165 | 8.6120 | 8.0580 | 8.361 | 8.361 | 7.3206 | |
| 1.330 | 8.7246 | 9.0851 | 9.1985 | 9.1934 | 8.6133 | 8.897 | 8.897 | 7.8186 | |
| TVL | 0.060 | 2.9499 | 3.2402 | 4.0330 | 4.1923 | 3.8954 | 4.089 | 4.248 | 4.1421 |
| 0.662 | 14.3180 | 14.9142 | 15.1094 | 15.1035 | 15.2388 | 15.219 | 15.310 | 15.2187 | |
| 1.170 | 18.8170 | 19.5959 | 19.8402 | 19.8300 | 18.5543 | 19.252 | 19.252 | 16.8564 | |
| 1.330 | 20.0892 | 20.9193 | 21.1803 | 21.1686 | 19.8328 | 20.486 | 20.486 | 18.0030 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elsafi, M.; Koraim, Y.; Almurayshid, M.; Almasoud, F.I.; Sayyed, M.I.; Saleh, I.H. Investigation of Photon Radiation Attenuation Capability of Different Clay Materials. Materials 2021, 14, 6702. https://doi.org/10.3390/ma14216702
Elsafi M, Koraim Y, Almurayshid M, Almasoud FI, Sayyed MI, Saleh IH. Investigation of Photon Radiation Attenuation Capability of Different Clay Materials. Materials. 2021; 14(21):6702. https://doi.org/10.3390/ma14216702
Chicago/Turabian StyleElsafi, Mohamed, Yousry Koraim, Mansour Almurayshid, Fahad I Almasoud, M. I. Sayyed, and I. H. Saleh. 2021. "Investigation of Photon Radiation Attenuation Capability of Different Clay Materials" Materials 14, no. 21: 6702. https://doi.org/10.3390/ma14216702
APA StyleElsafi, M., Koraim, Y., Almurayshid, M., Almasoud, F. I., Sayyed, M. I., & Saleh, I. H. (2021). Investigation of Photon Radiation Attenuation Capability of Different Clay Materials. Materials, 14(21), 6702. https://doi.org/10.3390/ma14216702










