Heterogeneity in Lateral Distribution of Polycations at the Surface of Lipid Membrane: From the Experimental Data to the Theoretical Model
Abstract
1. Introduction
2. Electrostatic Contribution of Polymers’ Interaction with Phospholipids
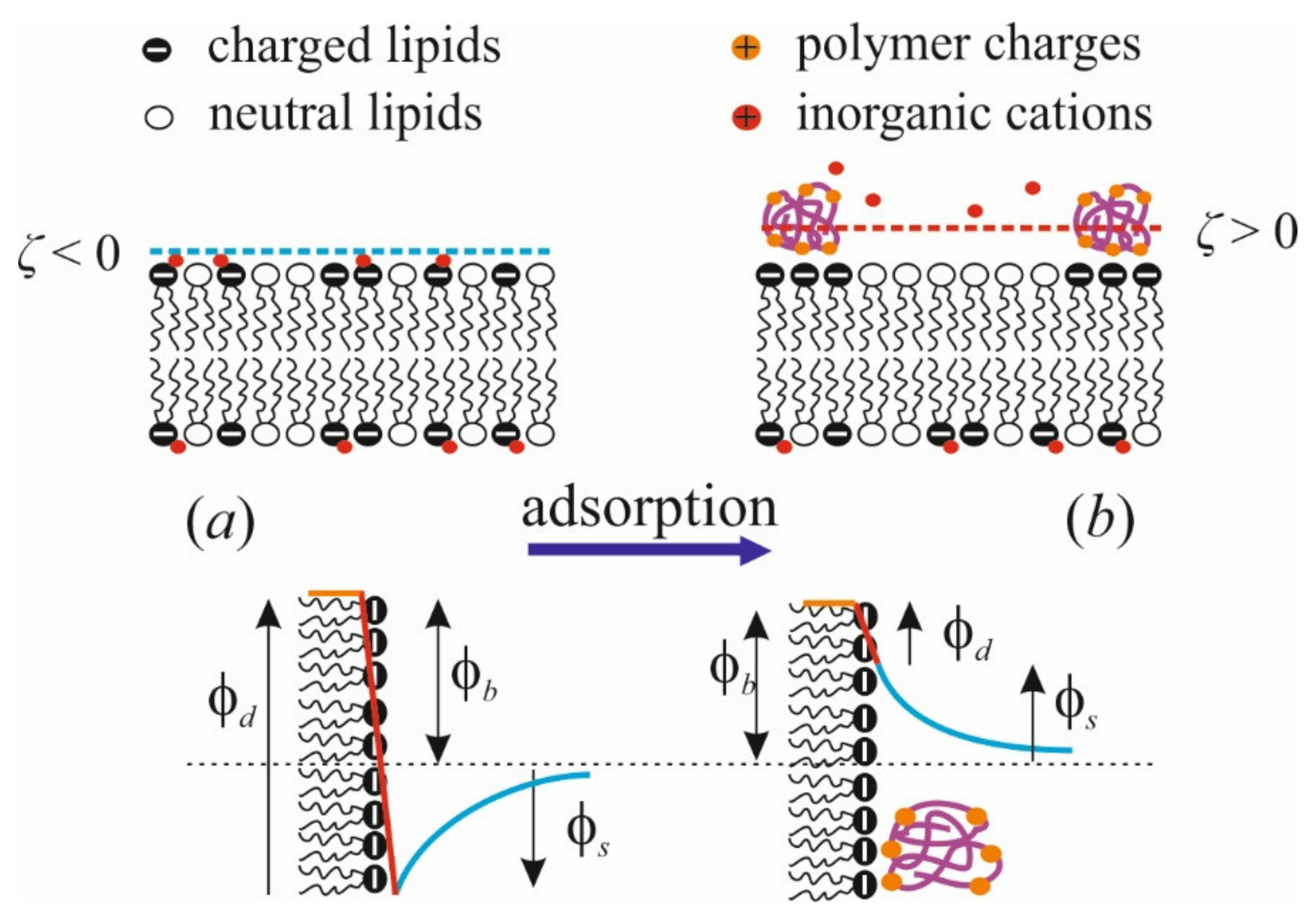
3. Inorganic Cations and Polycations in the Electric Fields at the Membrane Boundary
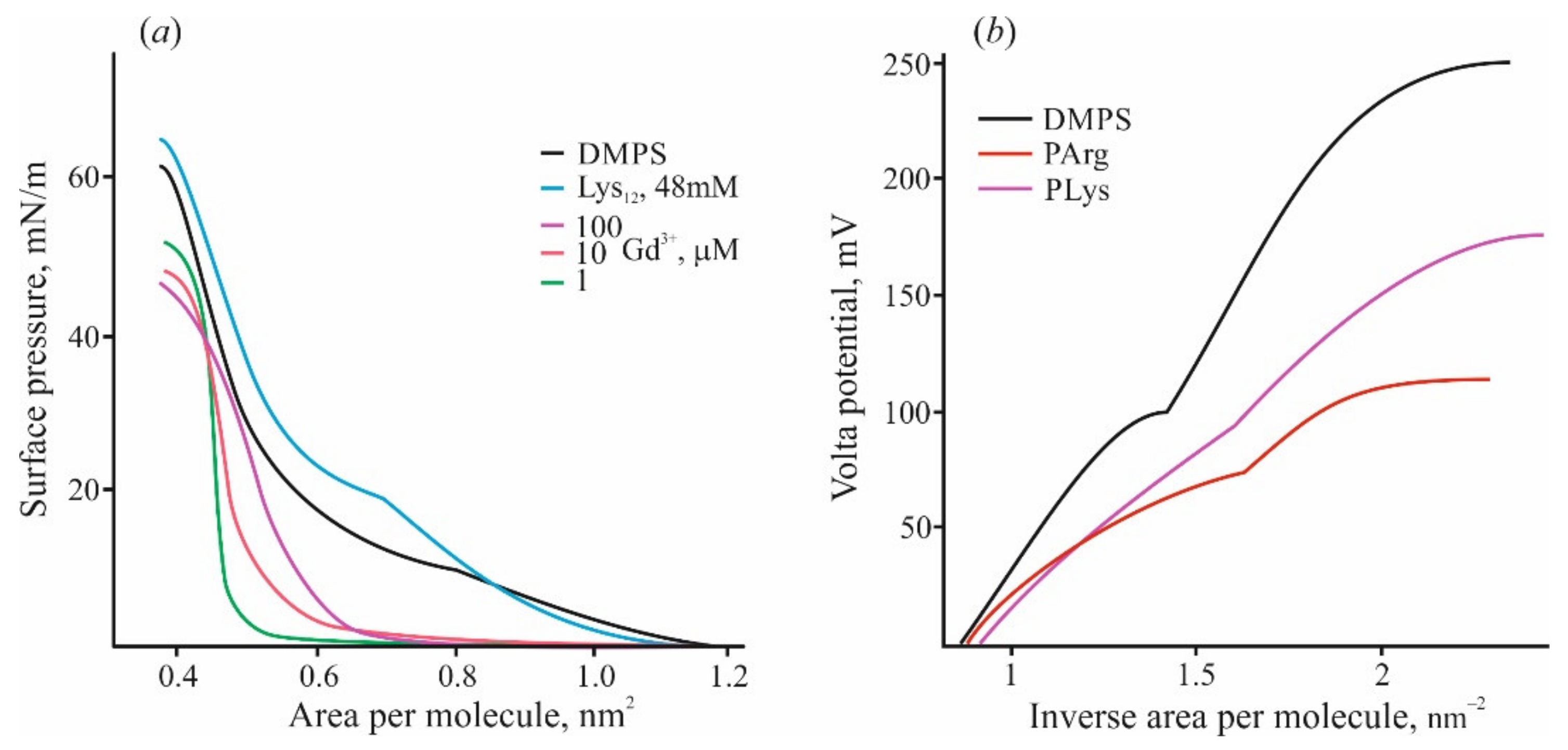
4. Polymer Layer at the Membranes of Mixed Composition
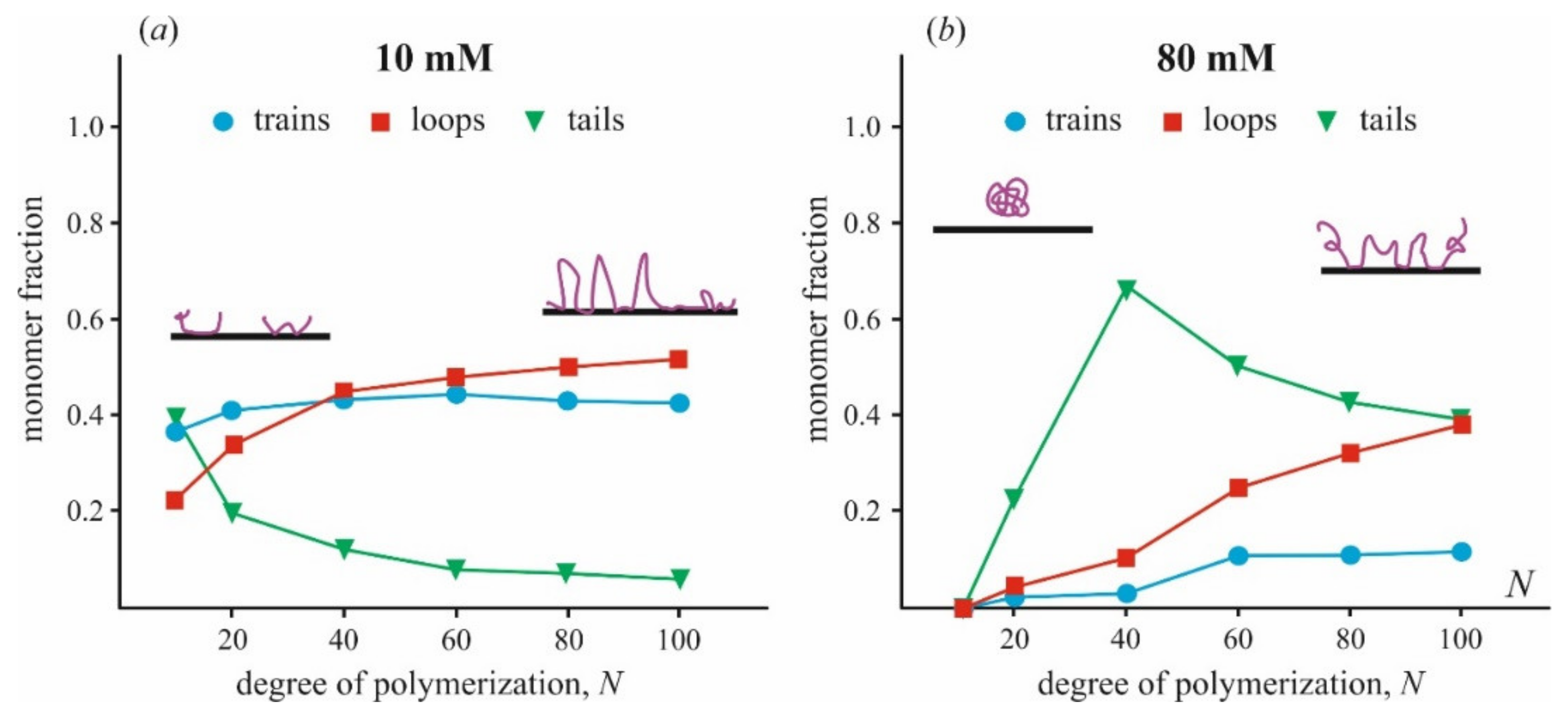
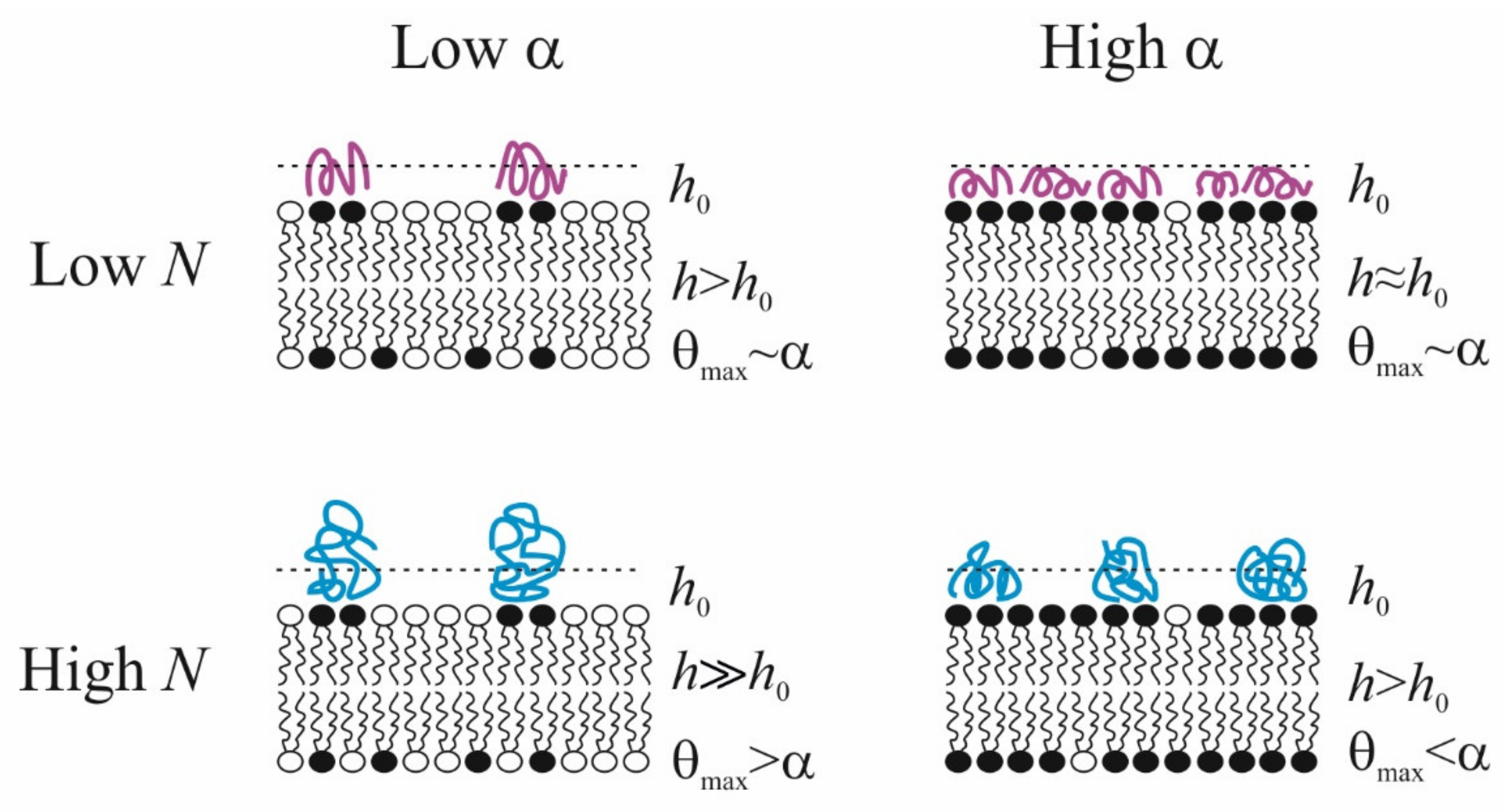
5. Conformations of Linear Polycation at the Charged Surface
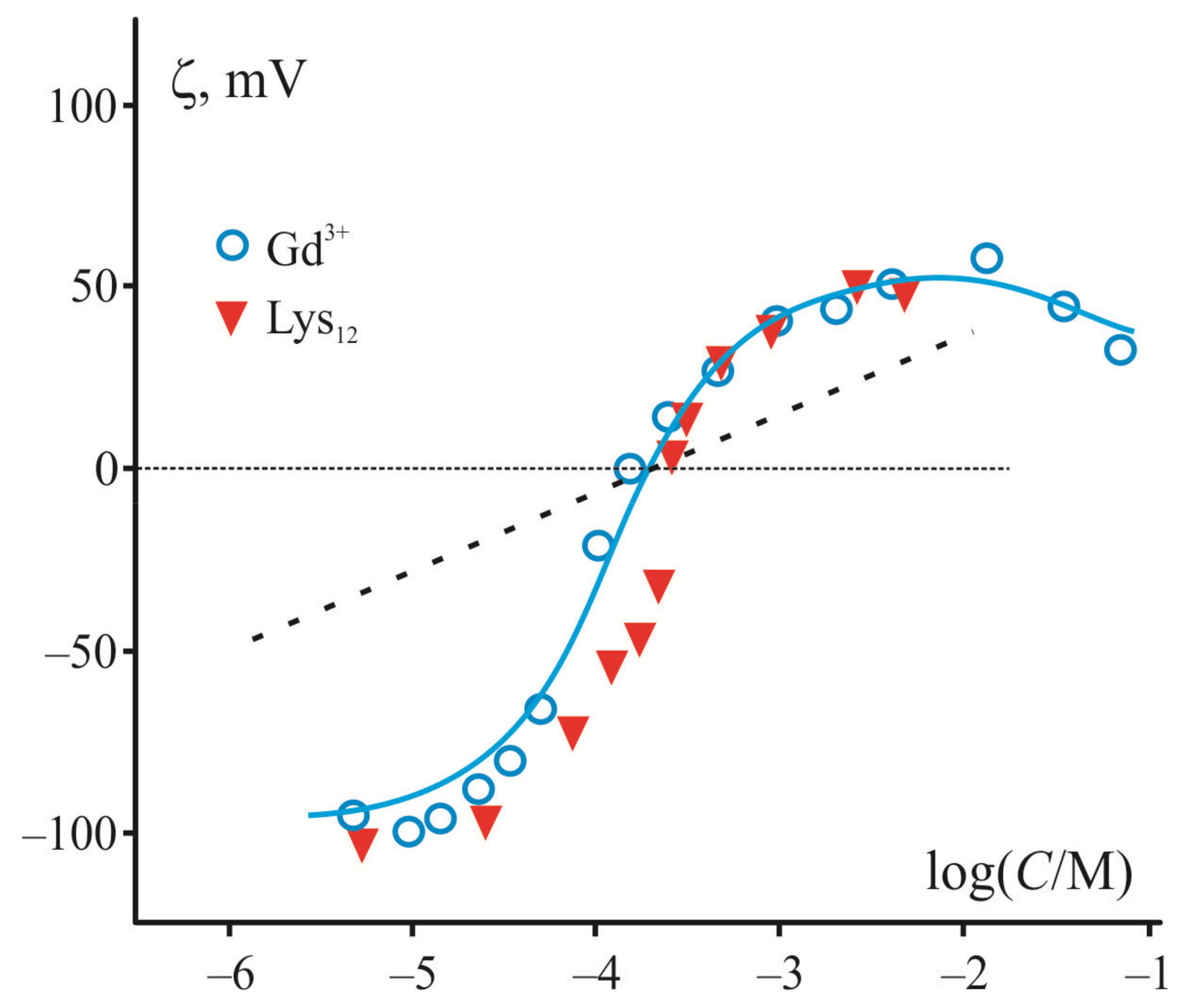
6. Lateral Heterogeneity of Polymer Layer as It Follows from the Analysis of Electrokinetic Data
7. Concluding Remarks and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Biricova, V.; Laznickova, A. Dendrimers: Analytical characterization and applications. Bioorg. Chem. 2009, 37, 185–192. [Google Scholar] [CrossRef]
- Lee, C.C.; MacKay, J.A.; Fréchet, J.M.J.; Szoka, F.C. Designing dendrimers for biological applications. Nat. Biotechnol. 2005, 23, 1517–1526. [Google Scholar] [CrossRef]
- Timofeeva, L.; Kleshcheva, N. Antimicrobial polymers: Mechanism of action, factors of activity, and applications. Appl. Microbiol. Biotechnol. 2011, 89, 475–492. [Google Scholar] [CrossRef]
- Ai, H.; Jones, S.A.; Lvov, Y.M. Biomedical Applications of Electrostatic Layer-by-Layer Nano-Assembly of Polymers, Enzymes, and Nanoparticles. Cell Biochem. Biophys. 2003, 39, 23–44. [Google Scholar] [CrossRef]
- Svenson, S.; Tomalia, D.A. Dendrimers in biomedical applications—Reflections on the field. Adv. Drug Deliv. Rev. 2012, 64, 102–115. [Google Scholar] [CrossRef]
- Renault, F.; Sancey, B.; Badot, P.-M.; Crini, G. Chitosan for coagulation/flocculation processes—An eco-friendly approach. Eur. Polym. J. 2009, 45, 1337–1348. [Google Scholar] [CrossRef]
- Ikeda, T.; Ledwith, A.; Bamford, C.H.; Hann, R.A. Interaction of a polymeric biguanide biocide with phospholipid membranes. Biochim. Biophys. Acta-Biomembr. 1984, 769, 57–66. [Google Scholar] [CrossRef]
- Xue, Y.; Xiao, H.; Zhang, Y. Antimicrobial Polymeric Materials with Quaternary Ammonium and Phosphonium Salts. Int. J. Mol. Sci. 2015, 16, 3626–3655. [Google Scholar] [CrossRef]
- Carmona-Ribeiro, A.; de Melo Carrasco, L. Cationic Antimicrobial Polymers and Their Assemblies. Int. J. Mol. Sci. 2013, 14, 9906–9946. [Google Scholar] [CrossRef]
- Cho, Y.W.; Kim, J.-D.; Park, K. Polycation gene delivery systems: Escape from endosomes to cytosol. J. Pharm. Pharmacol. 2003, 55, 721–734. [Google Scholar] [CrossRef]
- Kabanov, A.V. Taking polycation gene delivery systems from in vitro to in vivo. Pharm. Sci. Technol. Today 1999, 2, 365–372. [Google Scholar] [CrossRef]
- Park, T.; Jeong, J.; Kim, S. Current status of polymeric gene delivery systems. Adv. Drug Deliv. Rev. 2006, 58, 467–486. [Google Scholar] [CrossRef]
- Azevedo, M.M.; Ramalho, P.; Silva, A.P.; Teixeira-Santos, R.; Pina-Vaz, C.; Rodrigues, A.G. Polyethyleneimine and polyethyleneimine-based nanoparticles: Novel bacterial and yeast biofilm inhibitors. J. Med. Microbiol. 2014, 63, 1167–1173. [Google Scholar] [CrossRef]
- Beyth, N.; Houri-Haddad, Y.; Baraness-Hadar, L.; Yudovin-Farber, I.; Domb, A.J.; Weiss, E.I. Surface antimicrobial activity and biocompatibility of incorporated polyethylenimine nanoparticles. Biomaterials 2008, 29, 4157–4163. [Google Scholar] [CrossRef]
- Illergård, J.; Römling, U.; Wågberg, L.; Ek, M. Biointeractive antibacterial fibres using polyelectrolyte multilayer modification. Cellulose 2012, 19, 1731–1741. [Google Scholar] [CrossRef]
- Timofeeva, L.M.; Kleshcheva, N.A.; Shleeva, M.O.; Filatova, M.P.; Simonova, Y.A.; Ermakov, Y.A.; Kaprelyants, A.S. Nonquaternary poly(diallylammonium) polymers with different amine structure and their biocidal effect on Mycobacterium tuberculosis and Mycobacterium smegmatis. Appl. Microbiol. Biotechnol. 2015, 99, 2557–2571. [Google Scholar] [CrossRef]
- Baumgart, T.; Offenhäusser, A. Polysaccharide-Supported Planar Bilayer Lipid Model Membranes. Langmuir 2003, 19, 1730–1737. [Google Scholar] [CrossRef]
- Diamanti, E.; Gregurec, D.; Rodríguez-Presa, M.J.; Gervasi, C.A.; Azzaroni, O.; Moya, S.E. High Resistivity Lipid Bilayers Assembled on Polyelectrolyte Multilayer Cushions: An Impedance Study. Langmuir 2016, 32, 6263–6271. [Google Scholar] [CrossRef]
- Smith, H.L.; Jablin, M.S.; Vidyasagar, A.; Saiz, J.; Watkins, E.; Toomey, R.; Hurd, A.J.; Majewski, J. Model Lipid Membranes on a Tunable Polymer Cushion. Phys. Rev. Lett. 2009, 102, 228102. [Google Scholar] [CrossRef]
- Van Meer, G.; Voelker, D.R.; Feigenson, G.W. Membrane lipids: Where they are and how they behave. Nat. Rev. Mol. Cell Biol. 2008, 9, 112–124. [Google Scholar] [CrossRef]
- Yaroslavov, A.; Efimova, A.; Lobyshev, V.; Kabanov, V. Reversibility of structural rearrangements in the negative vesicular membrane upon electrostatic adsorption/desorption of the polycation. Biochim. Biophys. Acta-Biomembr. 2002, 1560, 14–24. [Google Scholar] [CrossRef]
- Antunes, F.E.; Marques, E.F.; Miguel, M.G.; Lindman, B. Polymer–vesicle association. Adv. Colloid Interface Sci. 2009, 147, 18–35. [Google Scholar] [CrossRef]
- Kahveci, Z.; Martínez-Tomé, M.; Esquembre, R.; Mallavia, R.; Mateo, C. Selective Interaction of a Cationic Polyfluorene with Model Lipid Membranes: Anionic versus Zwitterionic Lipids. Materials 2014, 7, 2120–2140. [Google Scholar] [CrossRef]
- Ding, L.; Chi, E.Y.; Schanze, K.S.; Lopez, G.P.; Whitten, D.G. Insight into the Mechanism of Antimicrobial Conjugated Polyelectrolytes: Lipid Headgroup Charge and Membrane Fluidity Effects. Langmuir 2010, 26, 5544–5550. [Google Scholar] [CrossRef]
- Kiss, É.; Heine, E.T.; Hill, K.; He, Y.C.; Keusgen, N.; Pénzes, C.B.; Schnöller, D.; Gyulai, G.; Mendrek, A.; Keul, H.; et al. Membrane Affinity and Antibacterial Properties of Cationic Polyelectrolytes With Different Hydrophobicity. Macromol. Biosci. 2012, 12, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Kozon, D.; Mierzejewska, J.; Kobiela, T.; Grochowska, A.; Dudnyk, K.; Głogowska, A.; Sobiepanek, A.; Kuźmińska, A.; Ciach, T.; Augustynowicz-Kopeć, E.; et al. Amphiphilic Polymethyloxazoline–Polyethyleneimine Copolymers: Interaction with Lipid Bilayer and Antibacterial Properties. Macromol. Biosci. 2019, 19, 1900254. [Google Scholar] [CrossRef]
- Eisenberg, M.; Gresalfi, T.; Riccio, T.; McLaughlin, S. Adsorption of Monovalent Cations to Bilayer Membranes Containing Negative Phospholipids. Biochemistry 1979, 8, 5213–5223. [Google Scholar] [CrossRef] [PubMed]
- Ermakov, Y.A. Ion equilibrium near lipid membranes: Empirical analysis of the simplest model. Colloid J. 2000, 62, 389–400. [Google Scholar]
- Douglas, J.F.; Schneider, H.M.; Frantz, P.; Lipman, R.; Granick, S. The origin and characterization of conformational heterogeneity in adsorbed polymer layers. J. Phys. Condens. Matter 1997, 9, 7699–7718. [Google Scholar] [CrossRef]
- Mbamala, E.C.; Ben-Shaul, A.; May, S. Domain Formation Induced by the Adsorption of Charged Proteins on Mixed Lipid Membranes. Biophys. J. 2005, 88, 1702–1714. [Google Scholar] [CrossRef]
- Tribet, C.; Vial, F. Flexible macromolecules attached to lipid bilayers: Impact on fluidity, curvature, permeability and stability of the membranes. Soft Matter 2008, 4, 68–81. [Google Scholar] [CrossRef]
- Kim, J.; Mosior, M.; Chung, L.A.; Wu, H.; McLaughlin, S. Binding of peptides with basic residues to membranes containing acidic phospholipids. Biophys. J. 1991, 60, 135–148. [Google Scholar] [CrossRef]
- Samoshina, Y.; Nylander, T.; Shubin, V.; Bauer, R.; Eskilsson, K. Equilibrium Aspects of Polycation Adsorption on Silica Surface: How the Adsorbed Layer Responds to Changes in Bulk Solution. Langmuir 2005, 21, 5872–5881. [Google Scholar] [CrossRef] [PubMed]
- Shubin, V. Adsorption of Cationic Polymer onto Negatively Charged Surfaces in the Presence of Anionic Surfactant. Langmuir 1994, 10, 1093–1100. [Google Scholar] [CrossRef]
- Dobrynin, A.V.; Rubinstein, M. Theory of polyelectrolytes in solutions and at surfaces. Prog. Polym. Sci. 2005, 30, 1049–1118. [Google Scholar] [CrossRef]
- Tzlil, S.; Ben-Shaul, A. Flexible Charged Macromolecules on Mixed Fluid Lipid Membranes: Theory and Monte Carlo Simulations. Biophys. J. 2005, 89, 2972–2987. [Google Scholar] [CrossRef]
- Netz, R.R.; Andelman, D. Neutral and charged polymers at interfaces. Phys. Rep. 2003, 380, 1–95. [Google Scholar] [CrossRef]
- Gurtovenko, A.A. Molecular-Level Insight into the Interactions of DNA/Polycation Complexes with Model Cell Membranes. J. Phys. Chem. B 2019, 123, 6505–6514. [Google Scholar] [CrossRef]
- Khomich, D.A.; Nesterenko, A.M.; Kostritskii, A.Y.; Kondinskaia, D.A.; Ermakov, Y.A.; Gurtovenko, A.A. Independent adsorption of monovalent cations and cationic polymers at PE/PG lipid membranes. J. Phys. Conf. Ser. 2017, 794, 012010. [Google Scholar] [CrossRef]
- Kostritskii, A.Y.; Kondinskaia, D.A.; Nesterenko, A.M.; Gurtovenko, A.A. Adsorption of Synthetic Cationic Polymers on Model Phospholipid Membranes: Insight from Atomic-Scale Molecular Dynamics Simulations. Langmuir 2016, 32, 10402–10414. [Google Scholar] [CrossRef]
- Tzlil, S.; Murray, D.; Ben-Shaul, A. The “Electrostatic-Switch” Mechanism: Monte Carlo Study of MARCKS-Membrane Interaction. Biophys. J. 2008, 95, 1745–1757. [Google Scholar] [CrossRef]
- Chodanowski, P.; Stoll, S. Polyelectrolyte Adsorption on Charged Particles in the Debye−Hückel Approximation. A Monte Carlo Approach. Macromolecules 2001, 34, 2320–2328. [Google Scholar] [CrossRef]
- Silva, R.A.; Urzúa, M.D.; Petri, D.F.S.; Dubin, P.L. Protein Adsorption onto Polyelectrolyte Layers: Effects of Protein Hydrophobicity and Charge Anisotropy. Langmuir 2010, 26, 14032–14038. [Google Scholar] [CrossRef] [PubMed]
- Alvares, D.S.; dos Santos Cabrera, M.P.; Ruggiero Neto, J. Strategies for exploring electrostatic and nonelectrostatic contributions to the interaction of helical antimicrobial peptides with model membranes. Adv. Biomembr. Lipid Self-Assem. 2016, 24, 43–73. [Google Scholar] [CrossRef]
- Zhao, H.; Mattila, J.P.; Holopainen, J.M.; Kinnunen, P.K.J. Comparison of the membrane association of two antimicrobial peptides, magainin 2 and indolicidin. Biophys. J. 2001, 81, 2979–2991. [Google Scholar] [CrossRef]
- Matos, P.M.; Franquelim, H.G.; Castanho, M.A.R.B.; Santos, N.C. Quantitative assessment of peptide–lipid interactions. Biochim. Biophys. Acta-Biomembr. 2010, 1798, 1999–2012. [Google Scholar] [CrossRef]
- Chieng, Y.Y.; Chen, S.B. Complexation of cationic polyelectrolyte with anionic phospholipid vesicles: Concentration, molecular weight and salt effects. J. Colloid Interface Sci. 2011, 354, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Leroueil, P.R.; Janus, E.K.; Peters, J.L.; Kober, M.M.; Islam, M.T.; Orr, B.G.; Baker, J.R.; Banaszak Holl, M.M. Interaction of polycationic polymers with supported lipid bilayers and cells: Nanoscale hole formation and enhanced membrane permeability. Bioconjugate Chem. 2006, 17, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Nievergelt, A.P.; Erickson, B.W.; Hosseini, N.; Adams, J.D.; Fantner, G.E. Studying biological membranes with extended range high-speed atomic force microscopy. Sci. Rep. 2015, 5, 11987. [Google Scholar] [CrossRef]
- Sybachin, A.V.; Tsarkova, L.A.; Yaroslavov, A.A. Atomic force microscopy of supported lipid membranes and their complexes with polycations. Biochem. Suppl. Ser. A Membr. Cell Biol. 2010, 4, 240–246. [Google Scholar] [CrossRef]
- Ruths, J.; Essler, F.; Decher, G.; Riegler, H. Polyelectrolytes I: Polyanion/Polycation Multilayers at the Air/Monolayer/Water Interface as Elements for Quantitative Polymer Adsorption Studies and Preparation of Hetero-superlattices on Solid Surfaces. Langmuir 2000, 16, 8871–8878. [Google Scholar] [CrossRef]
- de Meijere, K.; Brezesinski, G.; Möhwald, H. Polyelectrolyte Coupling to a Charged Lipid Monolayer. Macromolecules 1997, 30, 2337–2342. [Google Scholar] [CrossRef]
- Pavinatto, F.J.; Pavinatto, A.; Caseli, L.; dos Santos, D.S.; Nobre, T.M.; Zaniquelli, M.E.D.; Oliveira, O.N. Interaction of Chitosan with Cell Membrane Models at the Air−Water Interface. Biomacromolecules 2007, 8, 1633–1640. [Google Scholar] [CrossRef] [PubMed]
- Freire, J.M.; Domingues, M.M.; Matos, J.; Melo, M.N.; Veiga, A.S.; Santos, N.C.; Castanho, M.A.R.B. Using zeta-potential measurements to quantify peptide partition to lipid membranes. Eur. Biophys. J. 2011, 40, 481–487. [Google Scholar] [CrossRef]
- Yaroslavov, A.A.; Yaroslavova, E.G.; Rakhnyanskaya, A.A.; Menger, F.M.; Kabanov, V.A. Modulation of interaction of polycations with negative unilamellar lipid vesicles. Colloids Surf. B Biointerfaces 1999, 16, 29–43. [Google Scholar] [CrossRef]
- Kabanov, V.; Yaroslavov, A. What happens to negatively charged lipid vesicles upon interacting with polycation species? J. Control. Release 2002, 78, 267–271. [Google Scholar] [CrossRef]
- Lorenz, C.D.; Faraudo, J.; Travesset, A. Hydrogen Bonding and Binding of Polybasic Residues with Negatively Charged Mixed Lipid Monolayers. Langmuir 2008, 24, 1654–1658. [Google Scholar] [CrossRef]
- Troiano, J.M.; McGeachy, A.C.; Olenick, L.L.; Fang, D.; Liang, D.; Hong, J.; Kuech, T.R.; Caudill, E.R.; Pedersen, J.A.; Cui, Q.; et al. Quantifying the Electrostatics of Polycation–Lipid Bilayer Interactions. J. Am. Chem. Soc. 2017, 139, 5808–5816. [Google Scholar] [CrossRef]
- Carrillo, J.-M.Y.; Dobrynin, A.V. Molecular Dynamics Simulations of Polyelectrolyte Adsorption. Langmuir 2007, 23, 2472–2482. [Google Scholar] [CrossRef]
- Dobrynin, A.V.; Deshkovski, A.; Rubinstein, M. Adsorption of Polyelectrolytes at Oppositely Charged Surfaces. Macromolecules 2001, 34, 3421–3436. [Google Scholar] [CrossRef]
- Dobrynin, A.V.; Rubinstein, M.; Joanny, J.-F. Adsorption of a Polyampholyte Chain on a Charged Surface. Macromolecules 1997, 30, 4332–4341. [Google Scholar] [CrossRef]
- Lasic, D.D. The Conformation of Polymers at Interfaces; ACS publications: Washington, DC, USA, 1997; pp. 31–44. [Google Scholar]
- May, S.; Harries, D.; Ben-Shaul, A. Lipid demixing and protein-protein interactions in the adsorption of charged proteins on mixed membranes. Biophys. J. 2000, 79, 1747–1760. [Google Scholar] [CrossRef]
- Mcgeachy, A.C.; Caudill, E.R.; Liang, D.; Cui, Q.; Pedersen, J.A.; Geiger, F.M. Counting charges on membrane-bound peptides. Chem. Sci. 2018, 9, 4285–4298. [Google Scholar] [CrossRef] [PubMed]
- Schwieger, C.; Blume, A. Interaction of poly(l-lysines) with negatively charged membranes: An FT-IR and DSC study. Eur. Biophys. J. 2007, 36, 437–450. [Google Scholar] [CrossRef]
- Quemeneur, F.; Rinaudo, M.; Maret, G.; Pépin-Donat, B. Decoration of lipid vesicles by polyelectrolytes: Mechanism and structure. Soft Matter 2010, 6, 4471–4481. [Google Scholar] [CrossRef]
- Quemeneur, F.; Rinaudo, M.; Pépin-Donat, B. Influence of Polyelectrolyte Chemical Structure on their Interaction with Lipid Membrane of Zwitterionic Liposomes. Biomacromolecules 2008, 9, 2237–2243. [Google Scholar] [CrossRef] [PubMed]
- Papahadjopoulos, D. Surface properties of acidic phospholipids: Interaction of monolayers and hydrated liquid crystals with uni- and bi-valent metal ions. Biochim. Biophys. Acta-Biomembr. 1968, 163, 240–254. [Google Scholar] [CrossRef]
- Tocanne, J.-F.; Teissié, J. Ionization of phospholipids and phospholipid-supported interfacial lateral diffusion of protons in membrane model systems. Biochim. Biophys. Acta-Rev. Biomembr. 1990, 1031, 111–142. [Google Scholar] [CrossRef]
- Zimmermann, R.; Freudenberg, U.; Schweiß, R.; Küttner, D.; Werner, C. Hydroxide and hydronium ion adsorption—A survey. Curr. Opin. Colloid Interface Sci. 2010, 15, 196–202. [Google Scholar] [CrossRef]
- Zimmermann, R.; Küttner, D.; Renner, L.; Kaufmann, M.; Zitzmann, J.; Müller, M.; Werner, C. Charging and structure of zwitterionic supported bilayer lipid membranes studied by streaming current measurements, fluorescence microscopy, and attenuated total reflection Fourier transform infrared spectroscopy. Biointerphases 2009, 4, 1–6. [Google Scholar] [CrossRef]
- Tian, C.S.; Shen, Y.R. Structure and charging of hydrophobic material/water interfaces studied by phase-sensitive sum-frequency vibrational spectroscopy. Proc. Natl. Acad. Sci. USA 2009, 106, 15148–15153. [Google Scholar] [CrossRef]
- Hartvig, R.A.; van de Weert, M.; Østergaard, J.; Jorgensen, L.; Jensen, H. Protein Adsorption at Charged Surfaces: The Role of Electrostatic Interactions and Interfacial Charge Regulation. Langmuir 2011, 27, 2634–2643. [Google Scholar] [CrossRef]
- Weichselbaum, E.; Österbauer, M.; Knyazev, D.G.; Batishchev, O.V.; Akimov, S.A.; Hai Nguyen, T.; Zhang, C.; Knör, G.; Agmon, N.; Carloni, P.; et al. Origin of proton affinity to membrane/water interfaces. Sci. Rep. 2017, 7, 4553. [Google Scholar] [CrossRef] [PubMed]
- Kopec, W.; Żak, A.; Jamróz, D.; Nakahata, R.; Yusa, S.; Gapsys, V.; Kepczynski, M. Polycation–Anionic Lipid Membrane Interactions. Langmuir 2020, 36, 12435–12450. [Google Scholar] [CrossRef] [PubMed]
- Dalchand, N.; Cui, Q.; Geiger, F.M. Electrostatics, Hydrogen Bonding, and Molecular Structure at Polycation and Peptide:Lipid Membrane Interfaces. ACS Appl. Mater. Interfaces 2020, 12, 21149–21158. [Google Scholar] [CrossRef]
- Wilkosz, N.; Jamróz, D.; Kopeć, W.; Nakai, K.; Yusa, S.; Wytrwal-Sarna, M.; Bednar, J.; Nowakowska, M.; Kepczynski, M. Effect of Polycation Structure on Interaction with Lipid Membranes. J. Phys. Chem. B 2017, 121, 7318–7326. [Google Scholar] [CrossRef] [PubMed]
- Carrier, D.; Pezolet, M. Investigation of polylysine-dipalmitoylphosphatidylglycerol interactions in model membranes. Biochemistry 1986, 25, 4167–4174. [Google Scholar] [CrossRef] [PubMed]
- Fleming, E.; Maharaj, N.P.; Chen, J.L.; Nelson, R.B.; Elmore, D.E. Effect of lipid composition on buforin II structure and membrane entry. Proteins Struct. Funct. Bioinform. 2008, 73, 480–491. [Google Scholar] [CrossRef]
- Kwolek, U.; Jamróz, D.; Janiczek, M.; Nowakowska, M.; Wydro, P.; Kepczynski, M. Interactions of Polyethylenimines with Zwitterionic and Anionic Lipid Membranes. Langmuir 2016, 32, 5004–5018. [Google Scholar] [CrossRef] [PubMed]
- Marukovich, N.I.I.; Nesterenko, A.M.M.; Ermakov, Y.A.Y.A. Structural factors of lysine and polylysine interaction with lipid membranes. Biochem. Suppl. Ser. A Membr. Cell Biol. 2015, 9, 40–47. [Google Scholar] [CrossRef]
- Ermakov, Y.A.; Averbakh, A.Z.; Arbuzova, A.; Sukharev, S.I. Lipid and cell membranes in the presence of gadolinium and other ions with high affinity to lipids. 2. A dipole component of the boundary potential on membranes with different surface charge. Membr. Cell Biol. 1998, 12, 411–426. [Google Scholar] [PubMed]
- Finogenova, O.A.; Filinsky, D.V.; Ermakov, Y.A. Electrostatic effects upon adsorption and desorption of polylysines on the surface of lipid membranes of different composition. Biochem. Suppl. Ser. A Membr. Cell Biol. 2008, 2, 181–188. [Google Scholar] [CrossRef]
- Finogenova, O.A.; Batischev, O.V.; Indenbom, A.V.; Zolotarevsky, V.I.; Ermakov, Y.A. Molecular distribution and charge of polylysine layers at the surface of lipid membranes and mica. Biochem. Suppl. Ser. A Membr. Cell Biol. 2009, 3, 496–503. [Google Scholar] [CrossRef]
- Hunter, R. Zeta Potential in Colloid Science: Principles and Applications; Ottewill, R.H., Rowell, R.L., Eds.; Academic Press Inc.: London, UK, 1981. [Google Scholar]
- Duan, X.; Zhang, Y.; Li, L.; Zhang, R.; Ding, M.; Huang, Q.; Xu, W.-S.; Shi, T.; An, L. Effects of Concentration and Ionization Degree of Anchoring Cationic Polymers on the Lateral Heterogeneity of Anionic Lipid Monolayers. J. Phys. Chem. B 2017, 121, 984–994. [Google Scholar] [CrossRef]
- Duan, X.; Zhang, R.; Li, Y.; Shi, T.; An, L.; Huang, Q. Monte Carlo study of polyelectrolyte adsorption on mixed lipid membrane. J. Phys. Chem. B 2013, 117, 989–1002. [Google Scholar] [CrossRef]
- Cevc, G.; Marsh, D. Phospholipid Bilayers: Physical Principles and Models; Bittar, E., Ed.; John Wiley & Sons, Ltd.: New York, NY, USA, 1987. [Google Scholar]
- Ermakov, Y.A. Boundary Potential and the Energy of Lipid Monolayer Compression at the Liquid Expanded State. Biochem. Suppl. Ser. A Membr. Cell Biol. 2021, 15, 130–141. [Google Scholar] [CrossRef]
- Ermakov, Y.A.; Averbakh, A.Z.; Yusipovich, A.I.; Sukharev, S. Dipole Potentials Indicate Restructuring of the Membrane Interface Induced by Gadolinium and Beryllium Ions. Biophys. J. 2001, 80, 1851–1862. [Google Scholar] [CrossRef]
- Vorotyntsev, M.A.; Ermakov, Y.A.; Markin, V.S.; Rubashkin, A.A. Distribution of the interfacial potential drop in a situation when ionic solution components enter a surface layer of finite thickness with fixed space charge. Russ. Electrochem. 1993, 29, 730–748. [Google Scholar]
- Ermakov, Y.A.; Kamaraju, K.; Sengupta, K.; Sukharev, S. Gadolinium Ions Block Mechanosensitive Channels by Altering the Packing and Lateral Pressure of Anionic Lipids. Biophys. J. 2010, 98, 1018–1027. [Google Scholar] [CrossRef] [PubMed]
- Hoernke, M.; Schwieger, C.; Kerth, A.; Blume, A. Binding of cationic pentapeptides with modified side chain lengths to negatively charged lipid membranes: Complex interplay of electrostatic and hydrophobic interactions. Biochim. Biophys. Acta-Biomembr. 2012, 1818, 1663–1672. [Google Scholar] [CrossRef]
- Ermakov, Y.A. Boundary potential of lipid bilayers: Methods, interpretations and biological applications. J. Phys. Conf. Ser. 2017, 794, 012007. [Google Scholar] [CrossRef]
- Ermakov, Y.A.; Nesterenko, A.M. Boundary potential of lipid bilayers: Methods and interpretations. J. Phys. Conf. Ser. 2017, 780, 012002. [Google Scholar] [CrossRef]
- Ermakov, Y.A.; Sokolov, V.S. Boundary Potentials of Bilayer Lipid Membranes: Methods and Interpretations; Science Direct: Amsterdam, The Netherlands, 2003; pp. 109–141. [Google Scholar]
- Marukovich, N.; McMurray, M.; Finogenova, O.; Nesterenko, A.; Batishchev, O.; Ermakov, Y. Interaction of Polylysines with the Surface of Lipid Membranes. The Electrostatic and Structural Aspects. Adv. Planar Lipid 2013, 17, 139–166. [Google Scholar] [CrossRef]
- Molotkovsky, R.J.; Galimzyanov, T.R.; Khomich, D.A.; Nesterenko, A.M.; Ermakov, Y.A. Inhomogeneity of polylysine adsorption layers on lipid membranes revealed by theoretical analysis of electrokinetic data and molecular dynamics simulations. Bioelectrochemistry 2021, 141, 107828. [Google Scholar] [CrossRef] [PubMed]
- Ermakov, Y.A.; Kamaraju, K.; Dunina-Barkovskaya, A.; Vishnyakova, K.S.; Yegorov, Y.E.; Anishkin, A.; Sukharev, S. High-Affinity Interactions of Beryllium(2+) with Phosphatidylserine Result in a Cross-Linking Effect Reducing Surface Recognition of the Lipid. Biochemistry 2017, 56, 5457–5470. [Google Scholar] [CrossRef]
- McLaughlin, S.; Mulrine, N.; Gresalfi, T.; Vaio, G.; McLaughlin, A. Adsorption of divalent cations to bilayer membranes containing phosphatidylserine. J. Gen. Physiol. 1981, 77, 445–473. [Google Scholar] [CrossRef] [PubMed]
- Decher, G. Fuzzy Nanoassemblies: Toward Layered Polymeric Multicomposites. Science 1997, 277, 1232–1237. [Google Scholar] [CrossRef]
- Sukhorukov, G.B.; Donath, E.; Davis, S.; Lichtenfeld, H.; Caruso, F.; Popov, V.I.; Möhwald, H. Stepwise polyelectrolyte assembly on particle surfaces: A novel approach to colloid design. Polym. Adv. Technol. 1998, 9, 759–767. [Google Scholar] [CrossRef]
- Ferreira, M.; Cheung, J.H.; Rubner, M.F. Molecular self-assembly of conjugated polyions: A new process for fabricating multilayer thin film heterostructures. Thin Solid Film. 1994, 244, 806–809. [Google Scholar] [CrossRef]
- Borukhov, I.; Andelman, D.; Orland, H. Effect of Polyelectrolyte Adsorption on Intercolloidal Forces. J. Phys. Chem. B 1999, 103, 5042–5057. [Google Scholar] [CrossRef][Green Version]
- de Vos, W.M.; Lindhoud, S. Overcharging and charge inversion: Finding the correct explanation(s). Adv. Colloid Interface Sci. 2019, 274, 102040. [Google Scholar] [CrossRef]
- Shin, Y.; Roberts, J.E.; Santore, M.M. The Relationship between Polymer/Substrate Charge Density and Charge Overcompensation by Adsorbed Polyelectrolyte Layers. J. Colloid Interface Sci. 2002, 247, 220–230. [Google Scholar] [CrossRef][Green Version]
- Schlenoff, J.B.; Dubas, S.T. Mechanism of Polyelectrolyte Multilayer Growth: Charge Overcompensation and Distribution. Macromolecules 2001, 34, 592–598. [Google Scholar] [CrossRef]
- Joanny, J.F. Polyelectrolyte adsorption and charge inversion. Eur. Phys. J. B 1999, 9, 117–122. [Google Scholar] [CrossRef]
- Joanny, J.-F.; Castelnovo, M.; Netz, R. Adsorption of charged polymers. J. Phys. Condens. Matter 2000, 12, A1–A7. [Google Scholar] [CrossRef]
- Brockman, H. Dipole potential of lipid membranes. Chem. Phys. Lipids 1994, 73, 57–79. [Google Scholar] [CrossRef]
- Ermakov, Y.A.; Asadchikov, V.E.; Volkov, Y.O.; Nuzhdin, A.D.; Roshchin, B.S.; Honkimaki, V.; Tikhonov, A.M. Electrostatic and Structural Effects at the Adsorption of Polylysine on the Surface of the DMPS Monolayer. JETP Lett. 2019, 109, 334–339. [Google Scholar] [CrossRef]
- Yaroslavov, A.A.; Efimova, A.A.; Lobyshev, V.I.; Ermakov, Y.A.; Kabanov, V.A. Reversibility of structural rearrangements in lipid membranes induced by adsorption-desorption of a polycation. Biol. Membr. 1996, 13, 628–633. [Google Scholar]
- Heimburg, T.; Angerstein, B.; Marsh, D. Binding of peripheral proteins to mixed lipid membranes: Effect of lipid demixing upon binding. Biophys. J. 1999, 76, 2575–2586. [Google Scholar] [CrossRef]
- Yaroslavov, A.A.; Melik-Nubarov, N.S.; Menger, F.M. Polymer-Induced Flip-Flop in Biomembranes. Acc. Chem. Res. 2006, 39, 702–710. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Li, Y.; Zhang, R.; Shi, T.; An, L.; Huang, Q. Regulation of anionic lipids in binary membrane upon the adsorption of polyelectrolyte: A Monte Carlo simulation. AIP Adv. 2013, 3, 062128. [Google Scholar] [CrossRef]
- Breen, C. The characterisation and use of polycation-exchanged bentonites. Appl. Clay Sci. 1999, 15, 187–219. [Google Scholar] [CrossRef]
- Adachi, Y.; Matsumoto, T. Dynamics of initial stage flocculation of polystyrene latex spheres with polyelectrolytes. Colloids Surf. A Physicochem. Eng. Asp. 1996, 113, 229–236. [Google Scholar] [CrossRef]
- Aoki, K.; Adachi, Y. Kinetics of polyelectrolyte adsorption onto polystyrene latex particle studied using electrophoresis: Effects of molecular weight and ionic strength. J. Colloid Interface Sci. 2006, 300, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Stuart, M.C.; Adachi, Y. Dynamics of polyelectrolyte adsorption and colloidal flocculation upon mixing studied using mono-dispersed polystyrene latex particles. Adv. Colloid Interface Sci. 2015, 226, 101–114. [Google Scholar] [CrossRef]
- Perel, V.; Shklovskii, B. Screening of a macroion by multivalent ions: A new boundary condition for the Poisson–Boltzmann equation and charge inversion. Phys. A Stat. Mech. Its Appl. 1999, 274, 446–453. [Google Scholar] [CrossRef]
- Shklovskii, B.I. Wigner Crystal Model of Counterion Induced Bundle Formation of Rodlike Polyelectrolytes. Phys. Rev. Lett. 1999, 82, 3268–3271. [Google Scholar] [CrossRef]
- Morga, M.; Adamczyk, Z.; Gödrich, S.; Oćwieja, M.; Papastavrou, G. Monolayers of poly-l-lysine on mica—Electrokinetic characteristics. J. Colloid Interface Sci. 2015, 456, 116–124. [Google Scholar] [CrossRef]
- Morga, M.; Adamczyk, Z. Monolayers of cationic polyelectrolytes on mica—Electrokinetic studies. J. Colloid Interface Sci. 2013, 407, 196–204. [Google Scholar] [CrossRef]
- Morga, M.; Adamczyk, Z.; Kosior, D.; Kujda-Kruk, M. Kinetics of Poly-L-lysine Adsorption on Mica and Stability of Formed Monolayers: Theoretical and Experimental Studies. Langmuir 2019, 35, 12042–12052. [Google Scholar] [CrossRef]
- Finessi, M.; Sinha, P.; Szilágyi, I.; Popa, I.; Maroni, P.; Borkovec, M. Charge Reversal of Sulfate Latex Particles by Adsorbed Linear Poly(ethylene imine) Probed by Multiparticle Colloidal Probe Technique. J. Phys. Chem. B 2011, 115, 9098–9105. [Google Scholar] [CrossRef] [PubMed]
- Szilagyi, I.; Trefalt, G.; Tiraferri, A.; Maroni, P.; Borkovec, M. Polyelectrolyte adsorption, interparticle forces, and colloidal aggregation. Soft Matter 2014, 10, 2479–2502. [Google Scholar]
- Porus, M.; Maroni, P.; Borkovec, M. Structure of Adsorbed Polyelectrolyte Monolayers Investigated by Combining Optical Reflectometry and Piezoelectric Techniques. Langmuir 2012, 28, 5642–5651. [Google Scholar] [CrossRef]
- Molotkovsky, R.J.; Galimzyanov, T.R.; Ermakov, Y.A. Polypeptides on the Surface of Lipid Membranes. Theoretical Analysis of Electrokinetic Data. Colloid J. 2019, 81, 125–135. [Google Scholar] [CrossRef]
- Anderson, J.L. Effect of nonuniform zeta potential on particle movement in electric fields. J. Colloid Interface Sci. 1985, 105, 45–54. [Google Scholar] [CrossRef]
- Ivashkov, O.V.; Sybachin, A.V.; Efimova, A.A.; Pergushov, D.V.; Orlov, V.N.; Schmalz, H.; Yaroslavov, A.A. The Influence of the Chain Length of Polycations on their Complexation with Anionic Liposomes. ChemPhysChem 2015, 16, 2849–2853. [Google Scholar] [CrossRef] [PubMed]
- Molotkovsky, R.J.; Galimzyanov, T.R.; Ermakov, Y.A. Influence of Ionic Strength on Adsorption of Polypeptides on Lipid Membranes: Theoretical Analysis. Biochem. Suppl. Ser. A Membr. Cell Biol. 2021, 15, 175–183. [Google Scholar] [CrossRef]
- Markin, V.S.; Volkova-Gugeshashvili, M.I.; Volkov, A.G. Adsorption at Liquid Interfaces: The Generalized Langmuir Isotherm and Interfacial Structure. J. Phys. Chem. B 2006, 110, 11415–11420. [Google Scholar] [CrossRef]
- Donath, E.; Kuzmin, P.; Krabi, A.; Voigt, A. Electrokinetics of structured interfaces with polymer depletion—A theoretical study. Colloid Polym. Sci. 1993, 271, 930–939. [Google Scholar] [CrossRef]
- Ohshima, H. Electrophoretic mobility of soft particles. Colloids Surf. A Physicochem. Eng. Asp. 1995, 103, 249–255. [Google Scholar] [CrossRef]
- Hill, R.J. Hydrodynamics and electrokinetics of spherical liposomes with coatings of terminally anchored poly(ethylene glycol): Numerically exact electrokinetics with self-consistent mean-field polymer. Phys. Rev. E 2004, 70, 051406. [Google Scholar] [CrossRef] [PubMed]
- Hill, R.J.; Saville, D.A. ‘Exact’ solutions of the full electrokinetic model for soft spherical colloids: Electrophoretic mobility. Colloids Surf. A Physicochem. Eng. Asp. 2005, 267, 31–49. [Google Scholar] [CrossRef][Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molotkovsky, R.J.; Galimzyanov, T.R.; Ermakov, Y.A. Heterogeneity in Lateral Distribution of Polycations at the Surface of Lipid Membrane: From the Experimental Data to the Theoretical Model. Materials 2021, 14, 6623. https://doi.org/10.3390/ma14216623
Molotkovsky RJ, Galimzyanov TR, Ermakov YA. Heterogeneity in Lateral Distribution of Polycations at the Surface of Lipid Membrane: From the Experimental Data to the Theoretical Model. Materials. 2021; 14(21):6623. https://doi.org/10.3390/ma14216623
Chicago/Turabian StyleMolotkovsky, Rodion J., Timur R. Galimzyanov, and Yury A. Ermakov. 2021. "Heterogeneity in Lateral Distribution of Polycations at the Surface of Lipid Membrane: From the Experimental Data to the Theoretical Model" Materials 14, no. 21: 6623. https://doi.org/10.3390/ma14216623
APA StyleMolotkovsky, R. J., Galimzyanov, T. R., & Ermakov, Y. A. (2021). Heterogeneity in Lateral Distribution of Polycations at the Surface of Lipid Membrane: From the Experimental Data to the Theoretical Model. Materials, 14(21), 6623. https://doi.org/10.3390/ma14216623





